Abstract
The antibacterial activities and target inhibition of 15 quinolones against grlA and gyrA mutant strains were studied. The strains were obtained from wild-type Staphylococcus aureus MS5935 by selection with norfloxacin and nadifloxacin, respectively. The antibacterial activities of most quinolones against both mutant strains were lower than those against the wild-type strain. The ratios of MICs for the gyrA mutant strain to those for the grlA mutant strain (MIC ratio) varied from 0.125 to 4. The ratios of 50% inhibitory concentrations (IC50s) of quinolones against topoisomerase IV to those against DNA gyrase (IC50 ratios) also varied, from 0.177 to 5.52. A significant correlation between the MIC ratios and the IC50 ratios was observed (r = 0.919; P < 0.001). These results suggest that the antibacterial activities of quinolones against the wild-type strain are involved not only in topoisomerase IV inhibition but also in DNA gyrase inhibition and that the target preference in the wild-type strain can be anticipated by the MIC ratios. Based on the MIC ratios, the quinolones were classified into three categories. Type I quinolones (norfloxacin, enoxacin, fleroxacin, ciprofloxacin, lomefloxacin, trovafloxacin, grepafloxacin, ofloxacin, and levofloxacin) had MIC ratios of <1, type II quinolones (sparfloxacin and nadifloxacin) had MIC ratios of >1, and type III quinolones (gatifloxacin, pazufloxacin, moxifloxacin, and clinafloxacin) had MIC ratios of 1. Type I and type II quinolones seem to prefer topoisomerase IV and DNA gyrase, respectively. Type III quinolones seem to target both enzymes at nearly the same level in bacterial cells (a phenomenon known as the dual-targeting property), and their IC50 ratios were approximately 2.
Quinolone antibacterial agents have potent activities against gram-positive and -negative bacteria, and they are currently used for the therapeutic treatment of various bacterial infections. Antibacterial activities of quinolones are involved in their inhibitory activities for DNA gyrase and topoisomerase IV (1, 6, 14). Both enzymes are members of the type II topoisomerase family that controls bacterial DNA topology by passing a DNA double helix through another, using a transient double-strand break. DNA gyrase catalyzes ATP-dependent negative supercoiling of DNA and is involved in DNA replication, recombination, and transcription. Topoisomerase IV is also involved in supporting DNA replication, and the primary function of this enzyme seems to be the decatenation of multiply linked daughter chromosomes during the terminal stages of DNA replication.
It has been proposed that the susceptibility of bacteria to quinolones is determined primarily by which one of the two target enzymes is more sensitive to quinolones (4, 6, 19). It has been reported that the primary target of many quinolones seems to be topoisomerase IV in Staphylococcus aureus (2–4, 6, 9, 19) and that the primary target in Streptococcus pneumoniae varies among the quinolones (5, 12, 13). Recently, some quinolones were reported to target both enzymes at nearly the same level in these bacterial species (4–6, 13, 17). Therefore, it is interesting to explore the contribution of secondary-target inhibition to the antibacterial activity of quinolones alongside that of primary-target inhibition.
In this study, we determined the antibacterial activities of 15 quinolones against the wild-type and grlA and gyrA mutant strains of S. aureus as well as the inhibitory activities of these quinolones against DNA gyrase and topoisomerase IV of S. aureus. Furthermore, we will discuss the relationship between antibacterial activity and target inhibition in S. aureus.
(A part of this work was presented at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 17 to 20 September 2000 [M. Takei, H. Fukuda, and M. Hosaka, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 748, 2000].)
MATERIALS AND METHODS
Antibacterial agents and bacterial strains.
Gatifloxacin and the other quinolones tested were synthesized at Kyorin Pharmaceutical Co., Ltd. (Tokyo, Japan), or were purchased from Sigma Chemical Co. (St. Louis, Mo.). The bacterial strains used in this study were the quinolone-susceptible clinical isolate S. aureus MS5935 (7) and its quinolone-resistant grlA and gyrA mutant strains, which were spontaneously obtained from the wild-type strain (S. aureus MS5935) by selection with quinolones. The grlA mutant strain harboring altered subunit A of topoisomerase IV (Ser80 → Phe) was obtained by selection with norfloxacin (7). The gyrA mutant strain harboring altered subunit A of DNA gyrase (Ser84 → Leu) was obtained by selection with twice the MIC of nadifloxacin in this study. Mutations of the quinolone resistance-determining region of the grlA and gyrA genes were determined by a method described previously (4).
Determination of MICs.
The MICs were determined by standard agar dilution methods recommended by the National Committee for Clinical Laboratory Standards (8) with Mueller-Hinton agar (Difco Laboratories, Detroit, Mich.). The MIC was defined as the lowest concentration of an antibacterial agent that inhibited visible growth after incubation for 14 h at 35°C.
Preparation of type II topoisomerases.
The gyrA and gyrB genes of S. aureus were amplified from S. aureus MS5935 genomic DNA by PCR on a Perkin-Elmer thermal cycler with eLONGase enzyme mix (GIBCO BRL, Gaithersburg, Md.) and the following oligonucleotide primers: for gyrA, 5′-GAAGGAGGGATCCTTGATGGCTGAATTACC-3′ and 5′-GAAGTCGGATCCTTTATTATTCTTCATCTG-3′; for gyrB, 5′-GTAACAGGGATCCATGGTGACTGCATTGTC-3′ and 5′-CAAAAGTTCAGGATCCAGCGCTTAGAAGTC-3′ (restriction sites are underlined). The basic PCR parameters were 30 cycles consisting of 95°C for 1 min, 55°C for 1 min, and 70°C for 2 min. The DNA fragments and pGEX-2T (Amersham Pharmacia Biotech) were digested with BamHI, ligated, and transformed into Escherichia coli DH5α (Toyobo, Tokyo, Japan). The GyrA and GyrB proteins of DNA gyrase were purified separately as fusion proteins with glutathione S-transferase from overproducing strains of E. coli. Transformants were cultured in 2x YTA medium (0.5% NaCl, 1.6% tryptone, 1% yeast extract, [pH 7.0]) containing 50 μg of ampicillin/ml at 37°C and induced at mid-exponential phase by the addition of isopropyl-1-thio-β-d(−)-galactopyranoside to a final concentration of 0.05 mM. After incubation for 16 to 19 h at 25°C, the cells were harvested and stored at −80°C until use. The cells were suspended in sonication buffer (50 mM Tris-HCl [pH 8.0], 50 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol [DTT]) and sonicated on ice. Triton X-100 was added to a final concentration of 1%, and the suspension was centrifuged at 30,000 × g for 30 min. The supernatant was loaded onto a glutathione-Sepharose 4B column previously equilibrated with a sonication buffer containing 0.5% Triton X-100. The column was washed with 30 volumes of sonication buffer plus 0.5% Triton X-100 and with 10 volumes of phosphate-buffered saline. The GyrA and GyrB proteins were eluted with 1 volume of thrombin solution (50 U of thrombin/ml in phosphate-buffered saline). Concentration and buffer exchange were carried out with Centriprep-10 or Centriprep-30 (Amicon, Beverly, Mass.) with a buffer containing 50 mM Tris-HCl (pH 8.0), 10% glycerol, 1 mM EDTA, and 1 mM DTT and stored at −80°C.
The GrlA and GrlB proteins of topoisomerase IV were prepared by a method described previously (15).
Topoisomerase reactions.
Both S. aureus DNA gyrase and topoisomerase IV were reconstituted by incubation of each A and B subunit of the enzymes (GyrA-GyrB or GrlA-GrlB) on ice for at least 30 min. The activities of topoisomerases were measured electrophoretically.
(i) Supercoiling activity of DNA gyrase.
The reaction mixtures (10 μl), which contained 50 mM Tris-HCl (pH 7.5), 6 mM MgCl2, 80 mM KCl, 3 mM ATP, 5 mM DTT, 2 mM spermidine HCl, 500 mM potassium glutamate, 50 μg of bovine serum albumin/ml, 40 μg of tRNA/ml, 1 U of reconstituted DNA gyrase, 50 ng of relaxed pBR322 DNA, and various concentrations of the quinolones tested, were incubated at 37°C for 1 h. The reaction was terminated by the addition of 200 μg of proteinase K/ml. After an additional 10 min of incubation at 37°C, one-fifth volume of a loading dye was added, and the reaction mixtures were electrophoresed on a 0.8% agarose gel. DNA quantification in agarose gels was carried out after ethidium bromide staining. The brightness of the bands corresponding to supercoiled pBR322 DNA was determined by densitometric analysis with an FMBIO II Multi View fluorescent image analyzer (Hitachi Software Engineering Co., Ltd., Yokohama, Japan). One unit of supercoiling activity was defined as the amount of enzyme required to effect full supercoiling in the reaction under the above-mentioned conditions.
(ii) Decatenating activity of topoisomerase IV.
The decatenating activity of topoisomerase IV, that is, the conversion of kinetoplast DNA (Topogene, Inc., Columbus, Ohio) to the monomer, was measured by a method described previously (15).
(iii) Inhibitory effects of quinolones.
The inhibitory effect of each quinolone against topoisomerases was assayed by determining the concentration required to inhibit 50% of the enzyme reaction (IC50).
Statistical analysis.
Correlations were determined by linear regression analysis. A P value of <0.05 was considered statistically significant.
RESULTS
Isolation of gyrA mutant strains of S. aureus.
The grlA mutant strain [Ser-80 (TCC) → Phe (TTC)] was obtained by selection with norfloxacin (7) and used as the representative strain. The gyrA mutant strains were spontaneously obtained by selection with twice the MIC of nadifloxacin, which was used commercially as a topical quinolone in Japan (18). The representative gyrA mutant strain possessing a single mutation at codon 84 [TCA (Ser) → TTA (Leu)] in gyrA was used in this study. Neither the grlA nor the gyrA mutant strain showed a change in susceptibility to ethidium bromide compared with that of the wild-type strain (M. Takei, H. Fukuda, and M. Hosaka, unpublished data), indicating that the NorA-like efflux system was not changed in these mutant strains.
Antibacterial activities against gyrA and grlA mutant strains.
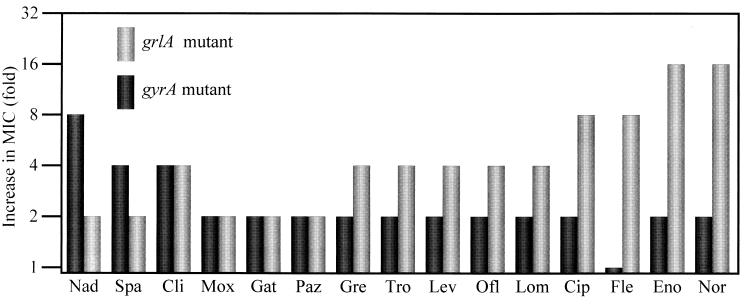
Table 1 presents the antibacterial activities of quinolones tested against both mutant strains. Compared with the quinolone MIC for the wild-type strain, most of the quinolone MICs for both the gyrA and grlA mutant strains were found to be higher. The increases in the MICs for the two mutant strains differed. Figure 1 summarizes the increases in the MICs of 15 quinolones for both the grlA and gyrA mutant strains. According to these results, the ratios of MICs for the gyrA mutant strain to those for the grlA mutant strain (MIC ratios) ranged from 0.125 to 4 (Table 1).
TABLE 1.
Quinolone antibacterial activities against target-altered mutant strains of S. aureus and inhibitory activities against type II topoisomerases
| Quinolone | MIC (μg/ml) for:
|
IC50 (μg/ml) against:
|
Ratio
|
||||
|---|---|---|---|---|---|---|---|
| Wild type | grlA mutant | gyrA mutant | DNA gyrase | Topoisomerase IV | MICa | IC50b | |
| Nadifloxacin | 0.016 | 0.031 | 0.125 | 3.44 | 19.0 | 4 | 5.52 |
| Sparfloxacin | 0.031 | 0.063 | 0.125 | 13.5 | 19.7 | 2 | 1.46 |
| Clinafloxacin | 0.016 | 0.063 | 0.063 | 0.915 | 1.62 | 1 | 1.77 |
| Gatifloxacin | 0.063 | 0.125 | 0.125 | 3.01 | 6.09 | 1 | 2.02 |
| Moxifloxacin | 0.031 | 0.063 | 0.063 | 3.44 | 7.84 | 1 | 2.28 |
| Pazufloxacin | 0.125 | 0.25 | 0.25 | 10.2 | 24.2 | 1 | 2.37 |
| Ofloxacin | 0.25 | 1 | 0.5 | 18.8 | 22.8 | 0.5 | 1.21 |
| Levofloxacin | 0.125 | 0.5 | 0.25 | 8.06 | 9.81 | 0.5 | 1.22 |
| Grepafloxacin | 0.031 | 0.125 | 0.063 | 28.4 | 23.3 | 0.5 | 0.820 |
| Lomefloxacin | 0.5 | 2 | 1 | 37.5 | 21.7 | 0.5 | 0.579 |
| Trovafloxacin | 0.016 | 0.063 | 0.031 | 7.13 | 3.02 | 0.5 | 0.424 |
| Ciprofloxacin | 0.25 | 2 | 0.5 | 13.5 | 5.76 | 0.25 | 0.427 |
| Fleroxacin | 0.5 | 4 | 0.5 | 82.6 | 31.6 | 0.125 | 0.383 |
| Enoxacin | 0.5 | 8 | 1 | 126 | 26.5 | 0.125 | 0.210 |
| Norfloxacin | 1 | 16 | 2 | 55.5 | 9.84 | 0.125 | 0.177 |
MIC ratio, MIC for gyrA mutant/MIC for grlA mutant.
IC50 ratio, IC50 against topoisomerase IV/IC50 against DNA gyrase.
FIG. 1.
Increases in MICs for target-altered mutant strains. Abbreviations: Nad, nadifloxacin; Spa, sparfloxacin; Cli, clinafloxacin; Gat, gatifloxacin; Mox, moxifloxacin; Paz, pazufloxacin; Gre, grepafloxacin; Lev, levofloxacin; Ofl, ofloxacin; Tro, trovafloxacin; Lom, lomefloxacin; Cip, ciprofloxacin; Fle, fleroxacin; Eno, enoxacin; Nor, norfloxacin.
The increases in the MICs of norfloxacin, enoxacin, fleroxacin, ciprofloxacin, lomefloxacin, trovafloxacin, grepafloxacin, ofloxacin, and levofloxacin for the grlA mutant strain were 4- to 16-fold, whereas the MIC increases for the gyrA mutant strain were small (one- to twofold) (Fig. 1). Since the increases in the MICs of these quinolones for the grlA mutant strain were greater than those for the gyrA mutant strain, the MIC ratios of these quinolones ranged from 0.125 to 0.5 (Table 1).
On the other hand, the increases in the MICs of sparfloxacin and nadifloxacin for the gyrA mutant strain were four- to eightfold, whereas those against the grlA mutant strain were twofold (Fig. 1). Therefore, the MIC ratios of these quinolones were 2 and 4, respectively (Table 1).
The same levels of increase in the MICs of gatifloxacin, pazufloxacin, moxifloxacin, and clinafloxacin for both the grlA and gyrA mutant strains were observed (Fig. 1). The MIC ratios of these quinolones were 1 (Table 1). The reproducibility of these results was confirmed by repeated experiments.
Inhibitory activities against type II topoisomerases of S. aureus.
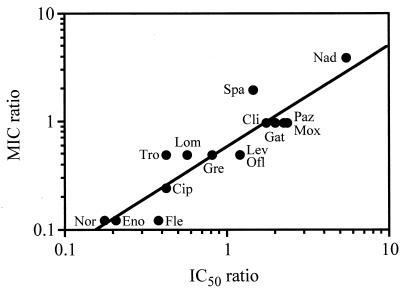
The IC50s of quinolones ranged from 0.915 to 126 μg/ml against DNA gyrase and from 1.62 to 31.6 μg/ml against topoisomerase IV (Table 1). The ratios of IC50s against topoisomerase IV to those against DNA gyrase (IC50 ratios) ranged from 0.177 to 5.52. The correlation between the IC50 ratios and the MIC ratios is shown in Fig. 2. They were significantly correlated (r = 0.919; P < 0.001).
FIG. 2.
Correlation between the MIC ratios and the IC50 ratios of quinolones tested (r = 0.919; P < 0.001). See the legend to Fig. 1 for abbreviations.
DISCUSSION
Bacterial type II topoisomerases (DNA gyrase and topoisomerase IV) are known to be essential enzymes for cell growth (10), and it has been proposed that the MICs of quinolones are determined mainly by the inhibitory activities against the primary (more susceptible) target (6). We and other researchers have reported that in S. pneumoniae, the primary target of trovafloxacin, levofloxacin, ciprofloxacin, and norfloxacin was topoisomerase IV and that the primary target of gatifloxacin and sparfloxacin was DNA gyrase (5, 12).
It has been reported that in S. aureus, the grlA (encoding the A subunit of topoisomerase IV) mutant strains were obtained from wild-type strains by selection with norfloxacin, ciprofloxacin, and ofloxacin and displayed a lower level of susceptibility to many quinolones than their parent strains (4). Therefore, the primary target of many quinolones in S. aureus seems to be topoisomerase IV (2, 4, 6, 9). In this study, we spontaneously obtained the gyrA (encoding the A subunit of DNA gyrase) mutant strains by selection with nadifloxacin, and the susceptibility of the gyrA mutant strain to nadifloxacin decreased more than that of the grlA mutant strain. These results suggest that the primary target of nadifloxacin in S. aureus is likely to be DNA gyrase. Therefore, we concluded that the primary target in S. aureus differs among the quinolones, as in S. pneumoniae.
It was observed that the antibacterial activities of most quinolones against both grlA mutant and gyrA mutant strains were lower than those against the wild-type strain (Table 1; Fig. 1). These results suggest that the antibacterial activities of quinolones can be attributed to DNA gyrase inhibition as well as to topoisomerase IV inhibition.
The MIC ratios were significantly correlated with the IC50 ratios (Fig. 2). We hypothesize that the target preference in the wild-type strain can be anticipated by the MIC ratios. Based on the MIC ratios, the quinolones were classified into three categories. Type I consisted of norfloxacin, enoxacin, fleroxacin, ciprofloxacin, lomefloxacin, trovafloxacin, grepafloxacin, ofloxacin, and levofloxacin. Quinolones of this type showed a greater increase in MICs for the grlA mutant strain than for the gyrA mutant strain relative to the MIC for the wild-type strain (i.e., they had an MIC ratio of <1). These results suggest that the antibacterial activities of this type of quinolone against the wild-type strain are influenced more by topoisomerase IV inhibition than by DNA gyrase inhibition (indicating a preference for topoisomerase IV).
The second type of quinolones (type II) comprised sparfloxacin and nadifloxacin. These quinolones showed a greater decrease in the activity against the gyrA mutant strain than against the grlA mutant strain (MIC ratio of >1). These results suggest that the antibacterial activities of this type of quinolone are influenced more by DNA gyrase inhibition than by topoisomerase IV inhibition (indicating a preference for DNA gyrase).
The third type of quinolones (type III) comprised gatifloxacin, pazufloxacin, moxifloxacin, and clinafloxacin. In this type of quinolone, the same level of increase in the MICs for both mutant strains (MIC ratio of 1) was observed. These results suggest that the antibacterial activities of this type of quinolone are equally influenced by DNA gyrase inhibition and topoisomerase IV inhibition (dual-targeting property). It has been reported that gatifloxacin selected the mutant strains of S. aureus less frequently, since this agent was thought to inhibit both DNA gyrase and topoisomerase IV at nearly the same level in bacterial cells (4). Our results agreed with this finding.
Some recent reports suggest that the IC50 ratio of the quinolones possessing the dual-targeting property is approximately 1 (11, 16). However, the IC50s were determined by an in vitro assay system, the conditions of which were different from those of an in vivo condition. In our system, the IC50 ratio of the quinolones that inhibited both enzymes at nearly the same level in bacterial cells seemed to be approximately 2. As a result of this study, we suggest that the antibacterial activities of quinolones are involved in the inhibition of both target enzymes and that the target preference of the quinolones in S. aureus can be anticipated by determination of the inhibitory activities against DNA gyrase and topoisomerase IV in addition to determination of the antibacterial activities against grlA and gyrA mutant strains.
REFERENCES
- 1.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrero L, Cameron B, Manse B, Lagneaux D, Crouzet J, Famechon A, Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferrero L, Cameron B, Crouzet J. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:1554–1558. doi: 10.1128/aac.39.7.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukuda H, Hori S, Hiramatsu K. Antibacterial activity of gatifloxacin (AM-1155, CG5501, BMS-206584), a newly developed fluoroquinolone, against sequentially acquired quinolone-resistant mutants and the norA transformant of Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:1917–1922. doi: 10.1128/aac.42.8.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukuda H, Hiramatsu K. Primary targets of fluoroquinolones in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:410–412. doi: 10.1128/aac.43.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooper D C. Mechanisms of action and resistance of older and newer fluoroquinolones. Clin Infect Dis. 2000;31:S24–S28. doi: 10.1086/314056. [DOI] [PubMed] [Google Scholar]
- 7.Hori S, Ohshita Y, Utsui Y, Hiramatsu K. Sequential acquisition of norfloxacin and ofloxacin resistance by methicillin-resistant and -susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:2278–2284. doi: 10.1128/aac.37.11.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7–A4. 4th ed. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 9.Ng E Y, Trucksis M, Hooper D C. Quinolone resistance mutations in topoisomerase IV: relationship to the flqA locus and genetic evidence that topoisomerase IV is the primary target and DNA gyrase is the secondary target of fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1881–1888. doi: 10.1128/aac.40.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nitiss J L. Roles of DNA topoisomerases in chromosomal replication and segregation. In: Liu L F, editor. DNA topoisomerases: biochemistry and molecular biology. San Diego, Calif: Academic Press, Inc.; 1994. pp. 103–134. [DOI] [PubMed] [Google Scholar]
- 11.Onodera Y, Uchida Y, Tanaka M, Sato K. Dual inhibitory activity of sitafloxacin (DU-6859α) against DNA gyrase and topoisomerase IV of Streptococcus pneumoniae. J Antimicrob Chemother. 1999;44:533–536. doi: 10.1093/jac/44.4.533. [DOI] [PubMed] [Google Scholar]
- 12.Pan X-S, Fisher L M. Targeting of DNA gyrase in Streptococcus pneumoniae by sparfloxacin: selective targeting of gyrase or topoisomerase IV by quinolones. Antimicrob Agents Chemother. 1997;41:471–474. doi: 10.1128/aac.41.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan X-S, Fisher L M. DNA gyrase and topoisomerase IV are dual targets of clinafloxacin action in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2810–2816. doi: 10.1128/aac.42.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen L L, Chu D T W. Type II DNA topoisomerases as antibacterial targets. Curr Pharm Des. 1996;2:195–208. [Google Scholar]
- 15.Takei M, Fukuda H, Yasue T, Hosaka M, Oomori Y. Inhibitory activities of gatifloxacin (AM-1155), a newly developed fluoroquinolone, against bacterial and mammalian type II topoisomerases. Antimicrob Agents Chemother. 1998;42:2678–2681. doi: 10.1128/aac.42.10.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka M, Onodera Y, Uchida Y, Sato K, Hayakawa I. Inhibitory activities of quinolones against DNA gyrase and topoisomerase IV purified from Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:2362–2366. doi: 10.1128/aac.41.11.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varon E, Janoir C, Kitzis M-D, Gutmann L. ParC and GyrA may be interchangeable initial targets of some fluoroquinolones in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:302–306. doi: 10.1128/aac.43.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogt K, Hahn H, Haustein U F, Blume U, Gollnick H, Orfanos C E. Antimicrobial evaluation of nadifloxacin (OPC-7251), a new topical quinolone, in acne vulgaris. Drugs. 1995;49:266–268. doi: 10.2165/00003495-199500492-00065. [DOI] [PubMed] [Google Scholar]
- 19.Yamagishi J-I, Kojima T, Oyamada Y, Fujimoto K, Hattori H, Nakamura S, Inoue M. Alterations in DNA topoisomerase IV grlA gene responsible for quinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1157–1163. doi: 10.1128/aac.40.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]