Abstract
Purpose
Platelet function test (PFT) results and genotype hold unique prognostic implications in East Asian patients. The aim of the PTRG-DES (Platelet function and genoType-Related long-term proGnosis in Drug-Eluting Stent-treated Patients with coronary artery disease) consortium is to assess the clinical impact thereof on long-term clinical outcomes in Korean patients with coronary artery disease during dual antiplatelet therapy (DAPT) including clopidogrel.
Materials and Methods
Searching publications on the PubMed, we reviewed clopidogrel treatment studies with PFT and/or genotype data for potential inclusion in this study. Lead investigators were invited to share PFT/genotype results, patient characteristics, and clinical outcomes to evaluate relationships among them.
Results
Nine registries from 32 academic centers participated in the PTRG-DES consortium, contributing individual patient data from 13160 patients who underwent DES implantation between July 2003 and August 2018. The PTRG-PFT cohort was composed of 11714 patients with available VerifyNow assay results. Platelet reactivity levels reached 218±79 P2Y12 reaction units (PRU), and high on-clopidogrel platelet reactivity based on a consensus-recommended cutoff (PRU >208) was observed in 55.9%. The PTRG-Genotype cohort consisted of 8163 patients with candidate genotypes related with clopidogrel responsiveness. Of those with cytochrome P450 (CYP) 2C19 genotype, frequencies of carrying one and two loss-of-function allele (s) (*2 or *3) were 47.9% (intermediate metabolizers) and 14.2% (poor metabolizers), respectively.
Conclusion
The PTRG-DES consortium highlights unique values for on-clopidogrel platelet reactivity and CYP2C19 phenotype that may be important to developing optimal antiplatelet regimens in East Asian patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT04734028.
Keywords: East Asia, platelet function, genotype, drug-eluting stent, coronary artery disease
INTRODUCTION
Following percutaneous coronary intervention (PCI) in patients with significant coronary artery disease (CAD), dual antiplatelet therapy (DAPT) consisting of aspirin and a P2Y12 receptor inhibitor is the cornerstone of pharmacologic treatment to improve clinical prognosis.1,2 Since platelet inhibition levels have been shown to be associated with the risks of post-PCI atherothrombotic and bleeding complications, tailored DAPT approaches guided by platelet function test (PFT) or genotyping (e.g., escalation, de-escalation, or switching) have been proposed and tested in multiple clinical trials.3
Compared with Caucasian patients, East Asian patients show unique antiplatelet effects and clinical outcomes (different therapeutic window of platelet inhibition), greater resistance to atherothrombotic events, and more vulnerability to bleeding at the same level of platelet reactivity (“East Asian Paradox”).4,5 Therefore, numerous clinical trials and analysis have suggested different efficacy and safety profiles during individual P2Y12 inhibitor treatment (clopidogrel, prasugrel or ticagrelor) in East Asian patients treated with PCI.4,6,7,8 However, consensus documents by expert groups recommend different strategies in selecting a DAPT regimen for East Asian patients.9,10
The PTRG-DES (Platelet function and genoType-Related long-term proGnosis in Drug-Eluting Stent-treated patients with coronary artery disease) consortium was established to determine the linkage of PFT and genotyping with long-term prognosis during clopidogrel treatment in a large-scale East Asian cohort treated with drug-eluting stent (DES). This article describes the framework for the organization and characteristics of the PTRG-DES consortium. Upcoming publications from various sub-analyses of this consortium is expected to divulge information important to developing individualized treatment regimens for East Asian patients requiring antiplatelet therapy.
MATERIALS AND METHODS
Study design and patients
An organizing committee of PTRG-DES investigators was established to define scientific goals. The organizing committee invited the lead investigators of clopidogrel-related prospective clinical registries published in the PubMed as of January 2018 to participate. Criteria for participation included available on-clopidogrel PFT or genotyping data and, for outcome analysis, availability of baseline characteristics and clinical prognosis in CAD patients treated with DES implantation.
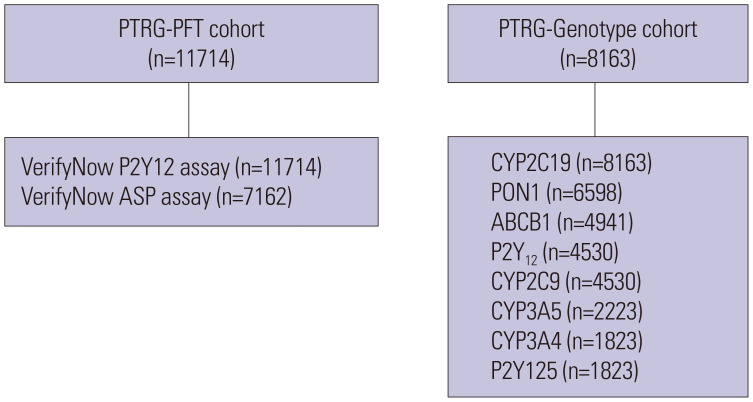
In total, nine prospective registries from 32 Korean academic centers have joined the PTRG-DES consortium, contributing data from 13160 DES patients treated between July 2003 and August 2018 in Supplementary Table 1 (only online). We obtained 11714 PFT results measured by the VerifyNow assay (PTRG-PFT cohort) and 8163 genotyping results related with clopidogrel responsiveness (PTRG-Genotype cohort) (Fig. 1). The type and number of patients in the PFT and/or genotyping datasets are provided in Supplementary Table 2 (only online). The PTRG-DES consortium is supported by the Platelet-Thrombosis Research Group under the Korean Society of Interventional Cardiology. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents. The institutional review board of each participating center approved the registry and waived the requirement for written informed consent for access to institutional registries. The study was performed in accordance with the Good Clinical Practice Guidelines and the principles of the Declaration of Helsinki.
Fig. 1. Cohorts of the PTRG-DES consortium. ABCB1, ATP Binding Cassette Subfamily B Member 1; CYP, cytochrome P450; PFT, platelet function test; PON1, Paraoxonase 1; PTRG-DES, Platelet function and genoType-Related long-term proGnosis in Drug-Eluting Stent-treated patients with coronary artery disease.
Procedures and test methods
Consecutive patients at each center who were successfully treated with one or more DESs approved by the US Food and Drug Administration (FDA) or CE mark and who were adequately loaded with aspirin and clopidogrel were eligible for enrollment, regardless of patient or lesion complexity. The major exclusion criteria were the occurrence of a major complication during the procedure, fibrinolytic therapy, and need for oral anticoagulant or potent P2Y12 inhibitor, such as ticagrelor or prasugrel.
All PCI procedures were performed according to the standard technique.11 Following a PCI procedure, patients were administered 100 mg of aspirin and 75 mg of clopidogrel a day. Patients were recommended to maintain aspirin indefinitely and clopidogrel for at least 1 year, and all other treatments were per standard of care. Clinical outcomes were evaluated until the last outpatient visit.
Platelet function test
Platelet reactivity was measured after an adequate period to ensure the full anti-platelet effect using the VerifyNow assay (Accriva, San Diego, CA, USA) during the peri-procedural period (from “just after the insertion of the arterial sheath” to “within 24 hr after DES implantation”).12 Aspirin was given as either 1) a coated oral dose of 300 mg at least 6 hr or 2) a dose of 100 mg at least 5 days before PCI. Clopidogrel was given as either 1) a dose of 600 mg at least 6 hr, 2) a dose of 300 mg at least 12 hr, or 3) a dose of 75 mg at least 5 days before PCI. If eptifibatide or tirofiban was used during PCI, a 24-hr washout period was required before VerifyNow testing. No patients receiving abciximab were enrolled because of a long washout period.
This VerifyNow assay is a whole-blood, point-of-care, turbidimetric optical detection assay designed to measure agonist-induced platelet aggregation. Blood samples were collected in 3.2% citrate Vacuette tubes (Greiner Bio-One Vacuette North America, Monroe, NC, USA). The measurement protocol followed the manufacturer’s recommendations, the details of which have been described elsewhere.13 We collected the following PFT data as continuous measures: VerifyNow P2Y12 baseline reactivity (BASE) and P2Y12 reaction units (PRU) and VerifyNow aspirin reaction units (ARU). Additionally, based on Western consensus documents in which cutoffs for high platelet reactivity (HPR) were identified, we initially defined the criteria of HPRs during DAPT with clopidogrel [>208 PRU to adenosine diphosphate (ADP) and >550 ARU to arachidonic acid].3
Genotyping
For genotyping, whole peripheral blood was obtained from patients. Genomic deoxyribonucleic acid (DNA) was extracted from mononuclear cells with the DNA kit and stored at -20℃ until ready for use. The genotype of each single nucleotide polymorphism (SNP) was determined by pyrosequencing using a PSQ 96MA Pyrosequencer (Pyrosequencing AB, Uppsala, Sweden)14 or ABI PRISM® 3100 genetic analyzer (Thermo Fisher Scientific Inc., Agawam, MA, USA).15 SNPs were measured at cytochrome P450 (CYP) 2C19*2 (rs4244285), CYP2C19*3 (rs4986893), CYP2C19*17 (rs12248560), CYP2C9*3 (rs1057910), ABCB1 (rs 1045642), paraoxonase-1 (PON1) (rs662), and P2Y12 (rs6809699).
The CYP2C19 phenotypes were classified into three groups according to the number of CYP2C19 loss-of-function (LOF) alleles: 1) extensive metabolizers, for individuals not carrying a LOF variant (*1/*1, *1/*17, or *17/*17); 2) intermediate metabolizers, for carriers of one LOF allele (*1/*2, *1/*3, *2/*17, or *3/*17); and 3) poor metabolizers, for carriers of two LOF alleles (*2/*2, *2/*3, or *3/*3).3
Clinical outcomes and definitions
An independent clinical events committee masked to PFT and genotyping results adjudicated all clinical events using original source documents. The primary ischemic endpoint was occurrence of major adverse cardiac and cerebrovascular events (MACCE), including all-cause death, myocardial infarction (MI), definite stent thrombosis (ST), or stroke. In addition, major bleeding was defined as Bleeding Academic Research Consortium (BARC) bleeding type 3–5 (Supplementary Table 3, only online).16
All deaths were considered to be of cardiovascular (CV) cause unless a definite non-CV cause could be established. MI was defined as increased cardiac troponin values with ischemic symptoms or ischemic changes on electrocardiogram or imaging evidence of recent loss of viable myocardium or new regional wall motion abnormality that were not related to the procedure. ST (definite) was defined according to Academic Research Consortium criteria.17 Stroke was defined as evidence of a neurological deficit requiring hospitalization and clinically documented lesions on brain computed tomography or magnetic resonance imaging.
Statistical analysis
Data at the individual patient level were used to calculate prevalence, mean values, and event rates for the total cohort with two-sided 95% confidence intervals (CIs). If there were crucial issues after validation of the PTRG-DES central database, individual data were sent to the principal investigator of the respective registry to confirm the data, perform corrections, and provide additional data.
The Kolmogorov-Smirnov test was performed to analyze the normal distribution of continuous variables. Continuous variables are expressed as a mean±SD or as a median (interquartile range), while categorical variables are presented as absolute numbers and frequencies (%). Student’s unpaired t-test for parametric continuous variables and the Mann-Whitney U test for non-parametric continuous variables were used. Comparisons between categorical variables were performed using the Pearson chi-square test or Fisher exact test when the Cochran rule was not met for categorical variables. To assess the relationship between the criteria of HPR and subsequent clinical outcomes, we compared time-to-event data with log-rank tests and presented them as Kaplan-Meier estimates. A p value <0.05 was considered statistically significant. All statistical analyses were performed with IBM/SPSS v24.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Patient characteristics
Between July 2003 to August 2018, 13160 patients treated with DESs in South Korea were enrolled (PTRG-PFT: n=11714 and PTRG-Genotype: n=8163). After DES implantation, clopidogrel was prescribed in all patients, and 97.5% (n=12831) of patients was discharged with aspirin.
A high-risk cohort was enrolled, with a large proportion of patients having diabetes (35.1%), index presentation with acute coronary syndrome (ACS) (56.8%), and complex lesion (American College of Cardiology/American Heart Association lesion B2 or C type: 57.2%) (Table 1). Most of these patients received 2nd generation DESs (n=11226, 85.3%), and stents were implanted for 1.6 lesions per patient, with a total stent length of 35.1 mm.
Table 1. Baseline Characteristics of the Patients (n=13160).
| Variables | Value | ||
|---|---|---|---|
| Index presentation | |||
| Stable angina | 5684 (43.2) | ||
| Unstable angina | 3750 (28.5) | ||
| Non-ST-segment elevation MI | 2009 (15.3) | ||
| ST-segment elevation MI | 1717 (13.0) | ||
| Age, yr | 64.4±10.9 | ||
| Male | 8848 (67.2) | ||
| Body mass index, kg/m2 | 24.5±3.1 | ||
| Risk factors* | |||
| Hypertension | 7933 (60.3) | ||
| Dyslipidemia | 8303 (63.1) | ||
| Smoking | 3578 (27.2) | ||
| Diabetes mellitus | 4619 (35.1) | ||
| Insulin-treated | 459 (3.5) | ||
| Chronic kidney disease | 2875 (21.8) | ||
| Current dialysis | 191 (1.5) | ||
| Anemia | 3345 (25.4) | ||
| Previous history | |||
| History of congestive heart failure | 1072 (8.1) | ||
| Previous MI | 971 (7.4) | ||
| Previous PCI | 1737 (13.2) | ||
| Previous CABG | 163 (1.2) | ||
| Previous stroke | 921 (7.0) | ||
| Laboratory measurements | |||
| LV ejection fraction, % | 58.8±10.6 | ||
| WBC, ×103/mm3 | 7.8±3.0 | ||
| Hemoglobin, g/dL | 13.6±1.8 | ||
| Platelet, ×103/mm3 | 243.7± 80.0 | ||
| GFR, mL/min/1.73 m2 (MDRD) | 77.3±26.6 | ||
| HbA1c, % | 6.5±1.4 | ||
| Total cholesterol, mg/dL | 173.6±44.0 | ||
| LDL-cholesterol, mg/dL | 106.5±42.6 | ||
| HDL-cholesterol, mg/dL | 43.9±12.5 | ||
| Triglyceride, mg/dL | 142.3± 96.6 | ||
| Angiographic feature | |||
| ACC/AHA lesion | |||
| A/B1 type | 5626 (42.8) | ||
| B2/C type | 7534 (57.2) | ||
| Number of diseased vessels | |||
| One | 7755 (58.9) | ||
| Two | 3517 (26.7) | ||
| Three | 1888 (14.3) | ||
| Multivessel disease | 5405 (41.1) | ||
| Bifurcation lesion | 1508 (11.5) | ||
| Chronic total occlusion lesion | 897 (6.8) | ||
| Procedural data | |||
| Multivessel PCI | 3234 (24.6) | ||
| Treated lesions | |||
| Left main coronary artery | 659 (5.0) | ||
| Left anterior descending artery | 7757 (58.9) | ||
| Left circumflex artery | 3933 (29.9) | ||
| Right coronary artery | 5018 (38.1) | ||
| Stent type† | |||
| 1st generation DES | 1934 (14.7) | ||
| ≥2nd generation DES | 11226 (85.3) | ||
| Number of stent, n | 1.6±0.8 | ||
| Stent length, mm | 35.1±21.9 | ||
| Stent diameter, mm | 3.03±0.44 | ||
| Concomitant medications | |||
| Aspirin | 12831 (97.5) | ||
| Clopidogrel | 13160 (100.0) | ||
| Cilostazol | 1292 (9.8) | ||
| Beta blocker | 7627 (58.0) | ||
| Angiotensin blockade | 8063 (61.3) | ||
| Calcium channel blocker | 3118 (23.7) | ||
| Statin | 11607 (88.2) | ||
| Proton pump inhibitor | 2235 (17.0) | ||
MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; LV, left ventricular; WBC, white blood cell; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; HbA1c, hemoglobin A1c; LDL, low density lipoprotein; HDL, high density lipoprotein; ACC, American College of Cardiology; AHA, American Heart Association; DES, drug-eluting stent.
Continuous variables are expressed as a mean±SD.
*Hypertension was diagnosed according to one of the following: 1) history of hypertension diagnosed and treated with medication, diet, and/or exercise; 2) blood pressure greater than 140 mm Hg systolic or 90 mm Hg diastolic on at least two occasions; or 3) currently on antihypertensive pharmacologic therapy. Dyslipidemia was diagnosed according to one of the following: 1) total cholesterol ≥200 mg/dL; 2) LDL-cholesterol ≥130 mg/dL; 3) HDL-cholesterol <40 mg/dL; or 4) triglycerides ≥150 mg/dL. Current smoker was defined as the use of tobacco within 1 year of admission; diabetes mellitus was diagnosed according to one of the following: 1) a history of diabetes, regardless of duration of disease, or need for antidiabetic agents; 2) a fasting blood glucose ≥126 mg/dL; or 3) glycosylated hemoglobin ≥6.5%. Chronic kidney disease was diagnosed according to one of the following: 1) GFR <60 mL/min/1.73 m2 (MDRD); 2) on dialysis; or 3) history of a renal transplantation. Anemia was defined as hemoglobin level <12 g/dL in women and 13 g/dL in men; †First-generation DES indicates durable polymer-based paclitaxel-eluting stents (PES: Taxus, Pico) or sirolimus-eluting stents (SES: Cypher). Second-generation DESs include next-generation DESs, including everolimus-eluting stent (EES: Promus, Xience), zotarolimus-eluting stent (ZES: Endeavor, Resolute, Onyx), biolimus-eluting stent (BES: Biolimus A9), and polymer-free SES. If a patient was treated with first- and second-generation DESs together, this patient was considered as implantation with first-generation DES.
Platelet function test and genotype
From the PTRG-PFT cohort, the mean values of the VerifyNow P2Y12 assay were 218±79 PRU (n=11714) and 298±59 PRU at BASE (n=10487), and that of the VerifyNow aspirin assay was 444±69 ARU (n=7162). The prevalences of HPR to ADP and arachidonic acid (consensus-recommended definition)3 were 55.9% (>208 PRU) and 10.9% (>550 ARU), respectively. The cutoffs of upper tertiles were 253 PRU and 461 ARU, respectively.
The distributions of genotypes and associated phenotypes in the PTRG-Genotype cohort (n=8163) are shown in Table 2. Like previous data from East Asian countries,14,15,18 more than 60% of the enrolled subjects carried at least one copy of the CYP2C19 LOF allele (62.0%). Extensive metabolizers represented 38.0% (n=3098), whereas intermediate and poor metabolizers accounted for 47.9% (n=3906) and 14.2% (n=1159), respectively.
Table 2. Distribution of CYP2C19 Alleles, Genotype and Phenotype (PTRG-Genotype Cohort: n=8163).
| Allele | Frequency no. (%) | Genotype | Frequency no. (%) | Phenotype | Frequency no. (%) |
|---|---|---|---|---|---|
| *1 | 9950 (60.9) | *1/*1 | 3011 (36.9) | Extensive metabolizer | 3098 (38.0) |
| *2 | 4486 (27.5) | *1/*2 | 2786 (34.1) | Intermediate metabolizer | 3906 (47.9) |
| *3 | 1738 (10.6) | *1/*3 | 1055 (12.9) | Poor metabolizer | 1159 (14.2) |
| *17 | 152 (0.9) | *1/*17 | 87 (1.1) | ||
| *2/*2 | 606 (7.4) | ||||
| *2/*3 | 440 (5.4) | ||||
| *2/*17 | 48 (0.6) | ||||
| *3/*3 | 113 (1.4) | ||||
| *3/*17 | 17 (0.2) | ||||
| *17/*17 | 0 (0) |
CYP, cytochrome P450.
Clinical outcomes
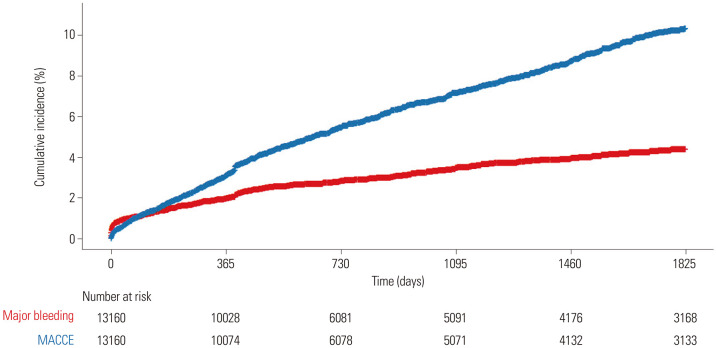
During the median follow-up duration of 21 months (interquartile range: 12 to 61 months) (up to 199 months), MACCE and major bleeding events occurred in 6.8% (n=899, mean time of 25 months) and 3.3% (n=431, mean time of 20 months), respectively (Fig. 2 and Table 3). The risk of MACCE increased over time, whereas events of major bleeding were observed more frequently in the early phase (up to 1 year).
Fig. 2. Kaplan-Meier analysis for MACCE and major bleeding events (PTRG-DES consortium; n=13160). The blue line represents cumulative event rates of MACCE and the red line represents cumulative event rates of major bleeding events during follow-up period in this consortium. MACCE, major adverse cardiac and cerebrovascular events; PTRG-DES, Platelet function and genoType-Related long-term proGnosis in Drug-Eluting Stent-treated Patients with coronary artery disease.
Table 3. Prevalence of MACCE and Major Bleeding Events during Follow-Up (PTRG-DES: n=13160).
| MACCE | Major bleeding | ||||
|---|---|---|---|---|---|
| All-cause death | 512 (3.9) | BARC type 3 | 414 (3.1) | ||
| CV death | 165 (1.3) | 3a | 297 (2.3) | ||
| Non-CV death | 347 (2.6) | 3b | 67 (0.5) | ||
| 3c | 50 (0.4) | ||||
| Non-fatal MI | 201 (1.5) | BARC type 4 | 7 (0.1) | ||
| Stent thrombosis (definite) | 69 (0.5) | BARC type 5 | 10 (0.1) | ||
| 5a | 3 (0.0) | ||||
| 5b | 7 (0.1) | ||||
| Non-fatal stroke | 238 (1.8) | ||||
MACCE, major adverse cardiac and cerebrovascular events; PTRG-DES, Platelet function and genoType-Related long-term proGnosis in Drug-Eluting Stent-treated Patients with coronary artery disease; BARC, Bleeding Academic Research Consortium; CV, cardiovascular; MI, myocardial infarction.
Data are presented as n (%).
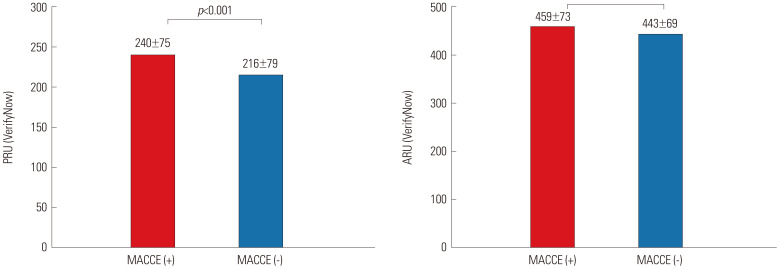
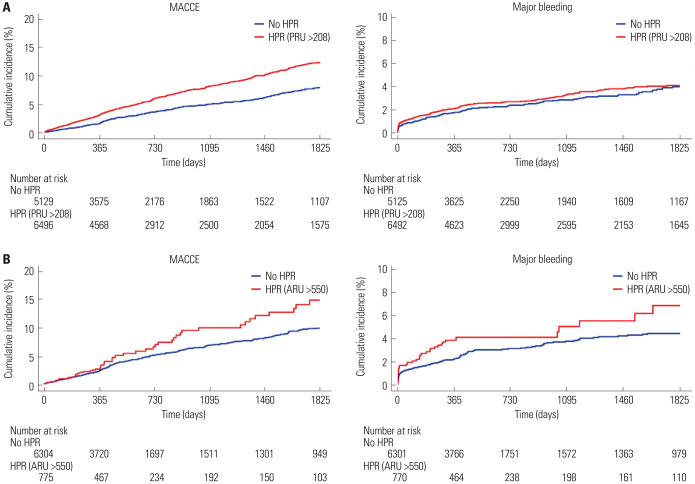
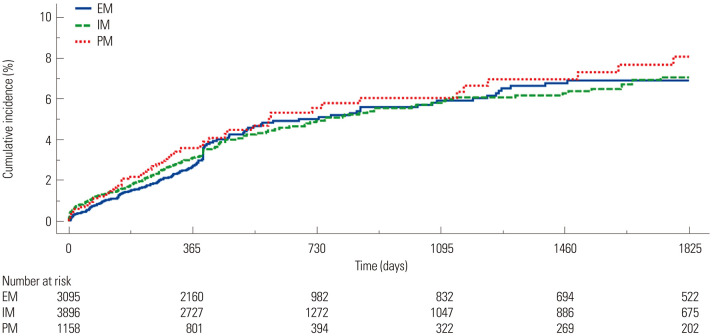
Patients who suffered from MACCE showed higher PRU and ARU levels than those without MACCE (Fig. 3). Subjects with HPR to ADP (>208 PRU) exhibited a higher risk of MACCE occurrence than those without HPR (8.4% vs. 4.9%, p<0.001), while there was no significant difference in MACCE risk between patients with vs. without HPR to arachidonic acid (>550 ARU) (6.4% vs. 5.0%, p=0.084) (Fig. 4). Rates of MACCE, however, did not differ across CYP2C19 phenotypes (4.4% vs. 4.6% vs. 5.3% in extensive vs. intermediate vs. poor metabolizers, p=0.261) (Fig. 5).
Fig. 3. PRU and ARU levels in patients with (red) vs. without (blue) ischemic events (MACCE). PRU, P2Y12 reaction unit; ARU, aspirin reaction unit; MACCE, major adverse cardiac and cerebrovascular events.
Fig. 4. Kaplan-Meier analysis for the association between HPR and MACCE and major bleeding events. (A) HPR to ADP (PRU >208) and MACCE and major bleeding events. (B) HPR to arachidonic acid (ARU >550) and MACCE and major bleeding events. HPR, high platelet reactivity; MACCE, major adverse cardiac and cerebrovascular events; ADP, adenosine diphosphate; PRU, P2Y12 reaction units; ARU, aspirin reaction units.
Fig. 5. Kaplan-Meier analysis for the association between CYP2C19 phenotype and MACCE. MACCE, major adverse cardiac and cerebrovascular events; EM, extensive metabolizers; IM, intermediate metabolizers; PM, poor metabolizers.
DISCUSSION
The PTRG-DES consortium, to our knowledge, is the largest PCI cohort with the longest clinical follow-up to evaluate the relation between platelet reactivity/genotype and clinical outcomes. The main findings of this consortium are as follows: 1) East Asian patients showed a higher level of platelet reactivity during clopidogrel treatment than Western patients (218 vs. 188 PRU in PTRG-DES vs. ADAPT-DES)13; 2) East Asian patients exhibited a higher prevalence of CYP2C19 LOF allele carriage (62%) than Western patients;19 and 3) East Asian patients showed different risk ratios (RR) of MACCE and major bleeding occurrence according to the time period during clopidogrel treatment.13
Following large-scale placebo-controlled randomized clinical trials (RCTs) showing a net clinical benefit for potent P2Y12 inhibitor (prasugrel or ticagrelor) vs. clopidogrel use in ACS patients,20,21 Jeong22 proposed a new concept for East Asian patients against Western guidelines. Based on a unique risk-benefit ratio (or therapeutic zone of platelet reactivity) and pharmacodynamic profile in East Asian patients,4,5 Jeong22 suggested that potent P2Y12 inhibitor vs. clopidogrel would have a different effect than that observed in the Western population. Subsequently, clinical data from several registries6,23,24 and a small-sized RCT8 from East Asian countries have suggested diminished clinical benefits and an increased bleeding risk for potent P2Y12 inhibitor use in East Asian patients with ACS, compared with clopidogrel.
Antiplatelet strategies for East Asian patients differ across consensus documents and clinical guidelines.9,10,25 Substantial clinical evidence from large-scale PFT/genotype-based registries and RCTs are required to establish unwavering treatment guidelines for East Asians patients. Therefore, we believe that this PTRG-DES consortium will be able to suggest reliable linkage of platelet function and genotyping with clinical outcomes in East Asian populations. Since the present consortium is the largest PFT cohort of DES-treated all-comers with a long-term follow-up, our results are expected to overcome the limitations of previous East Asian studies related with cohort size, follow-up duration, and heterogenicity of disease entity.
The use of guided antiplatelet strategies (platelet function and genetic testing) in PCI-treated patients with high-risk profiles has aimed to improve clinical outcomes by tailoring antiplatelet potency and duration to the individual patient.3 Early clinical RCTs did not find any clinical benefit with a guided approach, however. Caveats in trial designs, such as the inclusion of low-risk patients, inadequate identification of high-risk patients by a guided method, and undecided optimal selection of potent P2Y12 inhibitors, could contribute to these disappointing results. Nevertheless, a recent meta-analysis (n=20743; 11 RCTs and three observation studies) demonstrated that guided selection of antiplatelet therapy vs. standard therapy was associated with reductions in ischemic events (RR 0.78, 95% CI 0.63–0.95, p=0.015) and bleeding (RR 0.88, 95% CI 0.77–1.01, p=0.069).26
Compared with Western individuals, East Asian patients follow different guided cutoffs (e.g., high or low platelet reactivity) and enhanced platelet inhibition by standard-dose potent P2Y12 inhibitor.4,5 In addition, research suggests that the association between CYP2C19 genotyping and clinical outcomes during clopidogrel treatment can differ according to ethnicity. In the Tailored Antiplatelet Therapy Following PCI (TAILOR-PCI) trial, despite the same treatment with clopidogrel in patients carrying the CYP2C19 LOF allele, Caucasian patients showed an increased risk of ischemic events by about 1.8-fold than East Asian patients (7.3% vs. 4.1%).27 A genotype-guided strategy (mainly with ticagrelor) vs. conventional treatment reduced the risk of 1-year ischemic event after index PCI by 1.4% (2.7% vs. 4.1%) and 2.6% (4.7% vs. 7.3%) in the East Asians and Caucasians, respectively. There are unmet needs to establish cutoffs of high-risk phenotypes and optimal escalation strategies balancing clinical efficacy and safety.
In the present analysis, ARU (i.e., aspirin responsiveness) showed a limited relationship with clinical events. For aspirin resistance, recent data have mostly suggested an imprecise clinical impact on clinical outcomes,3 which may be related with the weak and indirect inhibition of thromboxane pathway by aspirin. Based on this observation, numerous clinical trials have tested the clinical benefit of early aspirin discontinuation from the initial DAPT regimen. In addition, CYP2C19 phenotype did not show a close relationship with clinical events in this cohort. Since genetic variants are just one influential factor affecting the antiplatelet effect of clopidogrel, their contribution to clinical events would be diminished in East Asian patients. East Asian patients show a unique risk-benefit ratio during antiplatelet treatment and a relatively weak relationship between platelet reactivity and atherothrombotic events.4,5,6
There are several limitations to this study that warrant consideration. This study has an innate limitation regarding its observational nature with registry data. However, with the extensive sensitivity analyses, confounders were adjusted to minimize bias from different baseline characteristics. Nonetheless, unmeasured confounders might still be present, and the extent to which unmeasured confounders contribute to the relation between platelet reactivity, genotyping, and adverse clinical outcomes is uncertain. One-point platelet function may not reflect dynamic changes over time. Lastly, the results of the present study may be limited to the boundaries of measurements and cannot be directly extended to the selection of an optimal antiplatelet strategy.
In conclusion, the PTRG-DES consortium indicates that East Asian patients have a higher level of on-clopidogrel platelet reactivity and higher prevalence of CYP2C19 LOF allele carriage, compared with Western patients, as well as a different RR of MACCE and major bleeding occurrence according to the time period during clopidogrel treatment. This registry will provide important “real-world” information regarding the linkage of platelet function and genotype with clinical events in a large cohort of clopidogrel-treated East Asian patients to better develop optimal antiplatelet strategies in this ethnicity.
ACKNOWLEDGEMENTS
The study was designed by the principal investigator and executive committee, and was sponsored by the Platelet-Thrombosis Research Group under the Korean Society of Intervention Cardiology.
Footnotes
Dr. Jeong has received honoraria for lectures from AstraZeneca, Daiichi Sankyo, Sanofi-Aventis, Han-mi Pharmaceuticals, and Yuhan Pharmaceuticals, as well as research grants or support from Yuhan Pharmaceuticals and U&I Corporation. Dr. Song has received honoraria for lectures from AstraZeneca, Daiichi Sankyo, Sanofi-Aventis, Bayer Korea, and Samjin Pharmaceutical. Dr. Joo has received honoraria for lectures from AstraZeneca, Hanmi, Samjin, Dong-A, HK inno. N Pharmaceuticals, and DIO Medical Ltd. The other authors have no potential conflicts of interest to disclose.
- Conceptualization: Ae-Young Her, Young-Hoon Jeong, Yongwhi Park, and Eun-Seok Shin.
- Data curation: Ae-Young Her, Young-Hoon Jeong, Hyung Joon Joo, and Yongwhi Park.
- Formal analysis: Ae-Young Her, Young-Hoon Jeong, and Jung Rae Cho.
- Funding acquisition: Moo Hyun Kim, Do-Sun Lim, and Eun-Seok Shin.
- Investigation: Ae-Young Her, Young-Hoon Jeong, Byeong-Keuk Kim, Kiyuk Chang, Young Bin Song, Sung Gyun Ahn, Jung-Won Suh, Sang Yeup Lee, Hyo-Soo Kim, Moo Hyun Kim, Do-Sun Lim, and Eun-Seok Shin.
- Methodology: Ae-Young Her, Young-Hoon Jeong, Hyung Joon Joo, Yongwhi Park, and Jung Rae Cho.
- Project administration: Yongwhi Park.
- Resources: Kiyuk Chang, Hyo-Soo Kim, Moo Hyun Kim, Do-Sun Lim, and Eun-Seok Shin.
- Software: Ae-Young Her and Young-Hoon Jeong.
- Supervision: Kiyuk Chang, Hyo-Soo Kim, Moo Hyun Kim, Do-Sun Lim, and Eun-Seok Shin.
- Validation: Byeong-Keuk Kim, Yongwhi Park, Young Bin Song, and Jung-Won Suh.
- Visualization: Ae-Young Her, Young-Hoon Jeong, and Eun-Seok Shin.
- Writing—original draft: Ae-Young Her and Young-Hoon Jeong.
- Writing—review & editing: Ae-Young Her, Young-Hoon Jeong, and Eun-Seok Shin.
- Approval of final manuscript: all authors.
DATA SHARING STATEMENT
The data generated in this study are available from the corresponding authors upon reasonable request.
SUPPLEMENTARY MATERIALS
List of Participating Registries (9 Registries, 32 Centers)
Method of Platelet Function Test and Genotyping in Each Registry
Definition of BARC Bleeding Type 3–5
References
- 1.Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2016;68:1082–1115. doi: 10.1016/j.jacc.2016.03.513. [DOI] [PubMed] [Google Scholar]
- 2.Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2017;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 3.Sibbing D, Aradi D, Alexopoulos D, Ten Berg J, Bhatt DL, Bonello L, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12:1521–1537. doi: 10.1016/j.jcin.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Kim HK, Tantry US, Smith SC, Jr, Jeong MH, Park SJ, Kim MH, et al. The East Asian paradox: an updated position statement on the challenges to the current antithrombotic strategy in patients with cardiovascular disease. Thromb Haemost. 2021;121:422–432. doi: 10.1055/s-0040-1718729. [DOI] [PubMed] [Google Scholar]
- 5.Levine GN, Jeong YH, Goto S, Anderson JL, Huo Y, Mega JL, et al. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol. 2014;11:597–606. doi: 10.1038/nrcardio.2014.104. [DOI] [PubMed] [Google Scholar]
- 6.Kang J, Han JK, Ahn Y, Chae SC, Kim YJ, Chae IH, et al. Third-generation P2Y12 inhibitors in East Asian acute myocardial infarction patients: a nationwide prospective multicentre study. Thromb Haemost. 2018;118:591–600. doi: 10.1055/s-0038-1626697. [DOI] [PubMed] [Google Scholar]
- 7.Kang J, Park KW, Palmerini T, Stone GW, Lee MS, Colombo A, et al. Racial differences in ischaemia/bleeding risk trade-off during anti-platelet therapy: individual patient level landmark meta-analysis from seven RCTs. Thromb Haemost. 2019;119:149–162. doi: 10.1055/s-0038-1676545. [DOI] [PubMed] [Google Scholar]
- 8.Park DW, Kwon O, Jang JS, Yun SC, Park H, Kang DY, et al. Clinically significant bleeding with ticagrelor versus clopidogrel in Korean patients with acute coronary syndromes intended for invasive management: a randomized clinical trial. Circulation. 2019;140:1865–1877. doi: 10.1161/CIRCULATIONAHA.119.041766. [DOI] [PubMed] [Google Scholar]
- 9.Kim HK, Ahn Y, Chang K, Jeong YH, Hahn JY, Choo EH, et al. 2020 Korean Society of Myocardial Infarction expert consensus document on pharmacotherapy for acute myocardial infarction. Korean Circ J. 2020;50:845–866. doi: 10.4070/kcj.2020.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan JW, Chew DP, Abdul Kader MAS, Ako J, Bahl VK, Chan M, et al. 2020 Asian Pacific Society of cardiology consensus recommendations on the use of P2Y12 receptor antagonists in the Asia-Pacific region. Eur Cardiol. 2021;16:e02. doi: 10.15420/ecr.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology appropriate use criteria task force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:2212–2241. doi: 10.1016/j.jacc.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Jeong YH, Bliden KP, Antonino MJ, Park KS, Tantry US, Gurbel PA. Usefulness of the VerifyNow P2Y12 assay to evaluate the antiplatelet effects of ticagrelor and clopidogrel therapies. Am Heart J. 2012;164:35–42. doi: 10.1016/j.ahj.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Stone GW, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann FJ, Metzger DC, et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet. 2013;382:614–623. doi: 10.1016/S0140-6736(13)61170-8. [DOI] [PubMed] [Google Scholar]
- 14.Joo HJ, Ahn SG, Park JH, Park JY, Hong SJ, Kim SY, et al. Effects of genetic variants on platelet reactivity and one-year clinical outcomes after percutaneous coronary intervention: a prospective multicentre registry study. Sci Rep. 2018;8:1229. doi: 10.1038/s41598-017-18134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HS, Chang K, Koh YS, Park MW, Choi YS, Park CS, et al. CYP2C19 poor metabolizer is associated with clinical outcome of clopidogrel therapy in acute myocardial infarction but not stable angina. Circ Cardiovasc Genet. 2013;6:514–521. doi: 10.1161/CIRCGENETICS.113.000109. [DOI] [PubMed] [Google Scholar]
- 16.Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 17.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction. Eur Heart J. 2018;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Zhang L, Wang H, Li S, Zhang Y, You L, et al. Gene variants in responsiveness to clopidogrel have no impact on clinical outcomes in Chinese patients undergoing percutaneous coronary intervention-A multicenter study. Int J Cardiol. 2017;240:360–366. doi: 10.1016/j.ijcard.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 20.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 21.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 22.Jeong YH. “East asian paradox”: challenge for the current antiplatelet strategy of “one-guideline-fits-all races” in acute coronary syndrome. Curr Cardiol Rep. 2014;16:485. doi: 10.1007/s11886-014-0485-4. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, Li C, Zhang L, Yu T, Ye H, Yu B, et al. Clinical outcomes after ticagrelor and clopidogrel in Chinese post-stented patients. Atherosclerosis. 2019;290:52–58. doi: 10.1016/j.atherosclerosis.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 24.You SC, Rho Y, Bikdeli B, Kim J, Siapos A, Weaver J, et al. Association of ticagrelor vs clopidogrel with net adverse clinical events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA. 2020;324:1640–1650. doi: 10.1001/jama.2020.16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura M, Kimura K, Kimura T, Ishihara M, Otsuka F, Kozuma K, et al. JCS 2020 guideline focused update on antithrombotic therapy in patients with coronary artery disease. Circ J. 2020;84:831–865. doi: 10.1253/circj.CJ-19-1109. [DOI] [PubMed] [Google Scholar]
- 26.Galli M, Benenati S, Capodanno D, Franchi F, Rollini F, D’Amario D, et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet. 2021;397:1470–1483. doi: 10.1016/S0140-6736(21)00533-X. [DOI] [PubMed] [Google Scholar]
- 27.Pereira NL, Farkouh ME, So D, Lennon R, Geller N, Mathew V, et al. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: the TAILOR-PCI randomized clinical trial. JAMA. 2020;324:761–771. doi: 10.1001/jama.2020.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of Participating Registries (9 Registries, 32 Centers)
Method of Platelet Function Test and Genotyping in Each Registry
Definition of BARC Bleeding Type 3–5
Data Availability Statement
The data generated in this study are available from the corresponding authors upon reasonable request.