Abstract
Collagen is the central structural component of extracellular connective tissue, which provides elastic qualities to tissues. For skeletal muscle, extracellular connective tissue transmits contractile force to the tendons and bones. Connective tissue proteins are in a constant state of remodeling and have been shown to express a high level of plasticity. Dietary-protein ingestion increases muscle protein synthesis rates. High-quality, rapidly digestible proteins are generally considered the preferred protein source to maximally stimulate myofibrillar (contractile) protein synthesis rates. In contrast, recent evidence demonstrates that protein ingestion does not increase muscle connective tissue protein synthesis. The absence of an increase in muscle connective tissue protein synthesis after protein ingestion may be explained by insufficient provision of glycine and/or proline. Dietary collagen contains large amounts of glycine and proline and, therefore, has been proposed to provide the precursors required to facilitate connective tissue protein synthesis. This literature review provides a comprehensive evaluation of the current knowledge on the proposed benefits of dietary collagen consumption to stimulate connective tissue remodeling to improve health and functional performance.
Keywords: collagen, hydrolysate, muscle, peptides, protein
INTRODUCTION
Skeletal muscle tissue is in a constant state of remodeling, with mixed muscle protein turnover rates of between 1% and 2% per day.1 Physical activity and food ingestion are the main anabolic stimuli for muscle tissue. Dietary-protein ingestion leads to a rapid increase in plasma amino acid concentrations, thereby increasing mixed muscle protein synthesis rates by 40%–50%.2 A single bout of exercise sensitizes skeletal muscle tissue to the anabolic properties of ingested protein. When combined, exercise and protein ingestion can increase muscle protein synthesis rates by as much as 100%.3,4 The combined effects of exercise and sufficient dietary-protein provision support muscle conditioning, allowing greater gains in muscle mass and strength after prolonged resistance-type exercise training.5–7 Over the past decade, evidence has indicated that rapidly digestible, high-quality protein sources (ie, those containing high essential amino acid concentrations, such as dairy and other animal-derived proteins) are effective in stimulating postprandial mixed muscle protein synthesis and can facilitate training-induced increases in muscle mass and strength.6,8,9
The generation and transmission of force from muscle tissue to the bone involves several factors, including the muscle extracellular matrix (ECM). The collagenous tissues of the ECM within skeletal muscle have an important functional role as they provide tissue elasticity and transmit contractile force from myofibrillar proteins in skeletal muscle fibers toward the tendons, ligaments, and bones. Connective tissue function is largely determined by collagen content and cross-linking between collagen fibers. Collagen originally was thought to be inert and resistant to remodeling.10 However, more recent evidence has proven that collagen and connective tissue protein networks in various musculoskeletal tissues are in a constant state of remodeling.11,12 Collagen remodeling is regulated by collagen protein synthesis, collagen protein breakdown, and cross-linking activity (enzymatic and nonenzymatic). Physical activity potently increases connective tissue synthesis rates, leading to enhanced remodeling and more effective transfer of contractile force.13–16 On the other hand, physical inactivity reduces connective tissue synthesis rates.17,18 Impairments in connective tissue remodeling may lead to structural changes in tissues that compromise their mechanical properties and contribute to the development of musculoskeletal injury. For example, senescent muscle contains more collagen and greater cross-linking,19 which contribute to greater muscle stiffness.20 Stiffer connective tissues impair muscle-fiber contractility,21 compromise force transmission,22,23 and reduce muscle strength.24
The impact of dietary-protein ingestion on connective tissue remodeling remains to be fully defined. Recent studies demonstrated that the ingestion of free essential amino acids or high-quality, rapidly digestible protein sources (ie, whey or casein) does not increase muscle connective tissue protein synthesis rates in young (18–35 y) or older individuals (>65 y).11,25–28 However, there is a discrepancy between the amino acid profiles of the provided high-quality proteins (ie, low glycine and proline) (Table 1)29,30 and the amino acid profile of muscle connective tissue (ie, high proline and glycine) (Table 2).31 Insufficient delivery of these amino acid precursors may prevent an increase in connective tissue protein synthesis. Collagen-derived protein sources (eg, gelatin and collagen peptides) contain substantially greater amounts of proline and glycine (Table 3).32,33 Considering the high proline and glycine contents of connective tissues, it has been proposed that these collagen-derived protein sources may have the capacity to stimulate and/or support connective tissue protein synthesis rates and promote connective tissue remodeling. However, so far, there seems to be very little evidence to support the anabolic properties of collagen-derived protein sources, despite consistent claims being made in the popular media.
Table 1.
Amino acid composition of different animal-based protein sourcesa
| Amino acid | Milkb | Caseinb | Wheyb | Eggb | Beefc |
|---|---|---|---|---|---|
| Alanine | 2.6 | 2.0 | 4.2 | 2.6 | 1.2 |
| Arginine | 2.6 | 2.1 | 1.7 | 2.6 | 1.2 |
| Cysteine | 0.2 | 0.1 | 0.8 | 0.4 | 0.2 |
| Glutamic acid | 16.7 | 13.9 | 15.5 | 5.1 | 2.8 |
| Glycine | 1.5 | 1.2 | 1.5 | 1.4 | 1.3 |
| Histidine | 1.9 | 1.7 | 1.4 | 0.9 | 0.6 |
| Isoleucine | 2.9 | 2.3 | 3.8 | 1.6 | 0.8 |
| Leucine | 7.0 | 5.8 | 8.6 | 3.6 | 1.5 |
| Lysine | 5.9 | 4.6 | 7.1 | 2.7 | 1.5 |
| Methionine | 2.1 | 1.6 | 1.8 | 1.4 | 0.5 |
| Phenylalanine | 3.5 | 3.1 | 2.5 | 2.3 | 0.7 |
| Proline | 7.3 | 6.5 | 4.8 | 1.8 | 0.9 |
| Serine | 4.0 | 3.4 | 4.0 | 3.3 | 0.7 |
| Threonine | 3.5 | 2.6 | 5.4 | 2.0 | 0.7 |
| Tyrosine | 3.8 | 3.4 | 2.4 | 1.8 | 0.5 |
| Valine | 3.6 | 3.0 | 3.5 | 2.0 | 0.9 |
Table 2.
Amino acid composition of collagen subtype α chains a
| Amino acid | α1(I) | α2(I) | α1(II) | α1(III) | α1(IV) | α2(IV) |
|---|---|---|---|---|---|---|
| Alanine | 115 | 102 | 103 | 96 | 30 | 47 |
| Arginine | 50 | 50 | 50 | 46 | 22 | 42 |
| Aspartic acid | 42 | 44 | 43 | 42 | 45 | 49 |
| Glutamic acid | 73 | 68 | 89 | 71 | 78 | 65 |
| Glycine | 333 | 338 | 333 | 350 | 334 | 324 |
| Cysteine | 0 | 0 | 0 | 2 | 0 | 2 |
| Histidine | 3 | 12 | 2 | 6 | 6 | 6 |
| Hydroxylysine | 9 | 12 | 20 | 5 | 50 | 36 |
| Hydroxyproline | 109 | 94 | 99 | 125 | 123 | 111 |
| Leucine | 19 | 30 | 26 | 22 | 52 | 56 |
| Lysine | 26 | 18 | 15 | 30 | 6 | 7 |
| Methionine | 7 | 5 | 10 | 8 | 15 | 14 |
| Phenylalanine | 12 | 12 | 13 | 8 | 27 | 36 |
| Proline | 124 | 113 | 120 | 107 | 85 | 73 |
| Serine | 34 | 30 | 25 | 39 | 38 | 30 |
| Threonine | 16 | 19 | 23 | 13 | 19 | 30 |
| Tyrosine | 1 | 4 | 2 | 3 | 5 | 7 |
| Valine | 21 | 35 | 18 | 14 | 33 | 27 |
Values are number of amino acids per 1000 amino acids.31
Table 3.
Amino acid composition of different sources of collagen a
| Amino acid | Ox hide gelatinb | Commercial bone gelatinb | Pig skin gelatinb | Whaleskin gelatineb | Ox-bone gelatinb | Human bone collagenb | Human tendon acid extractb | Wallaby tendonb | Sturgeon swim-bladder collagenc | Cod-bone gelatinc | Shark skin gelatinc | Lung-fish skin collagenc | Lung-fish skin gelatinc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alanine | 11.0 | 11.3 | 10.7 | 10.8 | 10.5 | 10.9 | 10.3 | 10.7 | 11.6 | 10.4 | 11.2 | 11.7 | 11.9 |
| Arginine | 8.8 | 9.0 | 9.1 | 9.5 | 9.2 | 8.8 | 8.9 | 9.5 | 10.0 | 9.1 | 9.3 | 9.1 | 9.9 |
| Aspartic acid | 6.7 | 6.7 | 6.7 | 6.7 | 7.1 | 6.7 | 6.7 | 7.0 | 6.9 | 7.5 | 6.0 | 6.6 | 6.2 |
| Cystine | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Glutamic acid | 11.4 | 11.6 | 11.3 | 11.2 | 11.9 | 11.4 | 11.1 | 11.5 | 11.4 | 11.4 | 10.3 | 11.9 | 11.9 |
| Glycine | 27.5 | 27.2 | 26.4 | 26.7 | 25.3 | 25.8 | 25.4 | 25.7 | 27.7 | 28.2 | 26.5 | 24.0 | 26.1 |
| Histidine | 0.8 | 0.7 | 1.0 | 1.0 | 1.0 | 1.0 | 0.9 | 0.8 | 0.8 | 1.2 | 1.3 | 0.8 | 0.8 |
| Hydroxylysine | 1.0 | 0.8 | 1.0 | 1.0 | 1.1 | 0.6 | 1.5 | 1.4 | 1.9 | 1.4 | 0.8 | 0.9 | 1.1 |
| Hydroxyproline | 14.1 | 13.3 | 13.5 | 12.8 | 14.1 | 14.1 | 12.6 | 13.0 | 11.8 | 8.3 | 10.9 | 9.8 | 10.8 |
| Isoleucine | 1.7 | 1.5 | 1.4 | 1.6 | 1.7 | 1.9 | 1.5 | 1.3 | 1.7 | 1.6 | 2.7 | 1.6 | 1.3 |
| Leucine | 3.3 | 3.5 | 3.3 | 3.6 | 3.9 | 3.6 | 3.5 | 3.7 | 2.6 | 3.3 | 3.3 | 3.4 | 2.8 |
| Lysine | 4.5 | 4.4 | 4.1 | 4.1 | 4.1 | 4.4 | 3.3 | 3.8 | 3.5 | 3.7 | 3.8 | 3.6 | 3.6 |
| Methionine | 0.9 | 0.6 | 0.9 | 0.8 | 0.8 | 0.8 | 0.9 | 1.1 | 1.4 | 2.3 | 1.6 | 0.6 | 0.5 |
| Phenylalanine | 2.2 | 2.5 | 2.6 | 2.3 | 2.9 | 2.5 | 2.5 | 2.8 | 2.5 | 2.0 | 2.4 | 2.6 | 2.4 |
| Proline | 16.4 | 15.5 | 16.2 | 16.2 | 14.7 | 15.3 | 15.2 | 14.7 | 12.8 | 12.4 | 13.9 | 14.8 | 15.8 |
| Serine | 4.2 | 3.7 | 4.1 | 4.7 | 4.2 | 4.1 | 4.1 | 4.4 | 5.8 | 7.9 | 5.0 | 4.7 | 4.7 |
| Threonine | 2.2 | 2.4 | 2.2 | 3.1 | 2.5 | 2.4 | 2.3 | 2.6 | 3.8 | 3.2 | 3.2 | 3.2 | 3.0 |
| Tyrosine | 0.3 | 0.2 | 0.6 | 0.7 | 0.6 | 0.9 | 0.7 | 0.8 | 0.5 | 0.6 | 0.3 | 0.2 | 0.1 |
| Valine | 2.6 | 2.8 | 2.8 | 2.6 | 2.7 | 3.0 | 3.1 | 2.9 | 2.3 | 2.3 | 2.7 | 2.6 | 2.2 |
A more complete understanding of collagen network function and its consequences is required to identify potential strategies to improve collagen structure in musculoskeletal tissues. In this review, we provide an overview of the existing literature on collagen structure and function within muscle tissue and focus on the question of whether dietary protein, and collagen protein in particular, could play a role in the conditioning of muscle connective tissue.
COLLAGEN STRUCTURE AND REMODELING
Collagen structure
Collagen primarily acts as an integral structural component of the ECM within various tissues. Collagen is ubiquitous within various tissues, constituting approximately 25%–30% of all body protein. At least 28 different types of collagen proteins have been identified.34,35 However, collagen types I, II, III, and IV are most abundant in musculoskeletal tissues.36
All collagen types share a similar right-handed, triple-helix sequence consisting of 3 α chains of peptides, which can be homogenous (eg, type II: 3 α-1 chains) or heterogenous (eg, type I: 2 α-1 chains and 1 α-2 chain) in composition (Table 4).37 The α chains contain approximately 1000 amino acid residues arranged in the repeating peptide sequence Gly-X-Y. Therefore, glycine occupies every third position along the α chain. The X and Y positions can be occupied by any amino acid, although most frequently they are occupied by proline and hydroxyproline, respectively. Because of the repeating peptide sequence, collagen proteins contain high concentrations of glycine (∼33%), proline (∼10%), and hydroxyproline (∼13.5%) relative to other proteins. Glycine has a small molecular footprint, which adds stability to the α chain and permits close orientation with other α chains. The amino acid structures of proline and hydroxyproline allow twisting of the α chain, and the hydroxyl group of hydroxyproline stabilizes the triple helix structure at body temperature. The hydroxylated form of lysine (ie, hydroxylysine) is also characteristically found in collagen α chains and permits the formation of collagen fibrils by binding α chains to each other through cross-linking (described in the section Enzymatic cross-linking). Slight differences in amino acid composition exist between the different α chains and, thus, collagen types (Table 2). However, all collagen types are similar in that they contain very low amounts of cysteine and no tryptophan.
Table 4.
Collagen subtypes, function, and anatomic location a
| Molecular type | Key aspects | Constitution | Synthesizing cells | Function | Location (tissue) |
|---|---|---|---|---|---|
| I (Fibril forming) | Most abundant collagen type | 2 α1(I) chains, 1 α2(I) chain | Fibroblasts, osteoblasts, odontoblasts, cementoblasts | Resists tension | Dermis, tendon, ligaments, capsules of organs, bone, dentin, cementum |
| II (Fibril forming) | 3 α1(II) chains | Chondroblasts | Resists tension | Hyaline cartilage, elastic cartilage | |
| III (Fibril forming) | Highly glycosylated, known as reticular fibers | 3 α1(III) chains | Fibroblasts, reticular cells, smooth-muscle cells, hepatocytes | Forms structural framework of spleen, liver, lymph nodes, smooth muscle, adipose tissue | Lymphatic system, spleen, liver, cardiovascular system, lung, skin |
| IV (Network forming) | 2 α1(IV) chains, 1 α2(IV) chain | Epithelial cells, muscle cells, Schwann cells | Forms meshwork of the lamina densa of the basal lamina to provide support and filtration | Basal lamina | |
| V (Fibril forming) | 2 α1(V) chains, 1 α2(V) chain | Fibroblasts, mesenchymal cells | Associated with type I collagen and with placental ground substance | Dermis, tendon, ligaments, capsules of organs, bone, cementum, placenta |
Data extracted from Gartner.37
Collagen synthesis
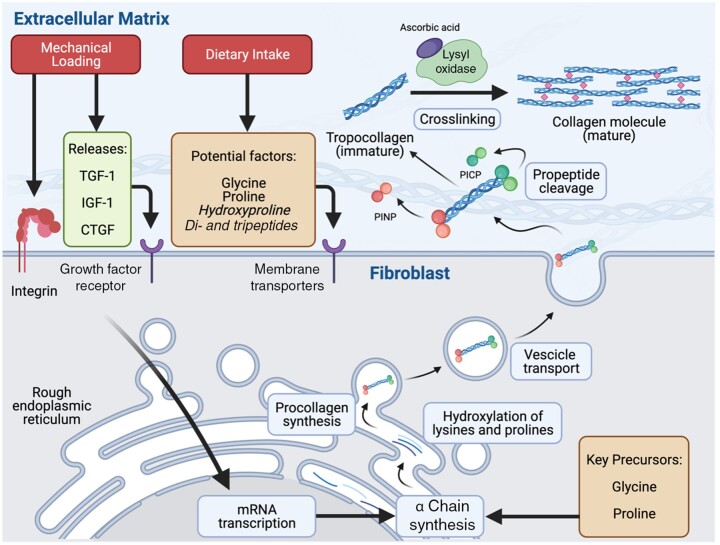
A graphical representation of collagen synthesis is presented in Figure 1. Collagen turnover is estimated to occur at a rate between 0.5% and 2% per day, which is within a similar range as myofibrillar proteins.1,11,12,15,38,39 This rate of collagen turnover translates to a half-life of approximately 2–5 months. Collagen synthesis originates in fibroblasts, which are embedded within the ECM of various tissues. Active fibroblasts are near collagen fibers, lying in a parallel orientation along the longitudinal axis of the collagen fibers.40 Collagen synthesis begins intracellularly with the assembly of the precursor to mature collagen, termed procollagen. The assembly of procollagen occurs primarily in the rough endoplasmic reticulum.
Figure 1.
Graphical representation of the key stages in collagen synthesis. Fibroblasts are embedded in a longitudinal orientation within the extracellular matrix (ECM) of various tissues (eg, skeletal muscle, tendon). Mechanical loading results in increased tension within the connective tissue, resulting in signal transduction through integrin activation and/or the release and binding of growth factors (eg, TGF-1, IGF-1, CTGF) on their respective membrane receptors. These signals are transduced to the nuclei of the fibroblast, resulting in increased mRNA transcription and α chain synthesis, which occurs in the rough endoplasmic reticulum. Part of the prolines and lysines in the α chains are hydroxylated into hydroxyproline and lysine, respectively, and 3 α chains are assembled into procollagen. Procollagen is packaged into vesicles and exported to the ECM. Extracellularly, procollagen peptidases cleave the propeptide regions (eg, procollagen type I N-terminal propeptide, and procollagen type I carboxy-terminal propeptide), allowing the resulting collagen monomers (tropocollagen) to stack spontaneously in an overlapping and parallel fashion. Lysyl oxidase activity, which requires ascorbic acid as a cofactor, forms cross-links between tropocollagen molecules, resulting in the formation of mature collagen within the extracellular network. Food ingestion, and collagen peptides in particular, has been proposed to deliver anabolic stimuli (eg, hydroxyproline, peptides) and key precursor amino acids (eg, glycine and proline) required to increase collagen synthesis. However, the proposed anabolic properties of food intake on muscle connective tissue synthesis rates in humans appear to have no stimulatory role (eg, leucine-rich protein) or have not yet been examined (eg, hydroxyproline, glycine, proline, peptides). CTGF, connective tissue growth factor; IGF-1, insulin-like growth factor-1; TGF-1, transforming growth factor-1
Translation is followed by multiple stages of post-translational modification including: 1) post-translational hydroxylation of lysine and proline on monomer α chains into hydroxylysine and hydroxyproline, respectively; 2) subsequent, additional post-translational glycosylation of hydroxylysine; and 3) aggregation of monomer α-collagen chains through cross-linking into a triple-helix procollagen structure. Both ends of the procollagen structure contain propeptide regions that contain disulfide bonds between α chains to stabilize the region. For type I procollagen, these regions are termed procollagen type I N-terminal propeptide and procollagen type I carboxy-terminal propeptide for the carboxyl terminal, respectively. Once formed, the procollagen structures are transported to the Golgi apparatus, where they are packaged into vesicles and transported out of the fibroblast. Extracellularly, procollagen peptidases cleave the procollagen type I N-terminal propeptide and procollagen type I carboxy-terminal propeptide regions, allowing the resulting collagen monomers, termed tropocollagen molecules, to stack spontaneously in an overlapping and parallel fashion.
Enzymatic cross-linking
The formation of covalent bonds between lysine and hydroxylysine residues of neighboring tropocollagen molecules facilitates formation of mature collagen fibrils. The formation of these so-called cross-links is enzymatically catalyzed by lysyl oxidase, which forms pyridinoline between (hydroxy)lysine residues of adjacent collagen fibrils. The positioning of pyridinoline cross-links facilitates a staggered arrangement of collagen fibrils, each overlapping another by one-third to provide strength to the entire collagen molecule. The staggered configuration, along with a greater abundance of cross-links within collagen fibrils, results in greater tissue stiffness. The degree of cross-linking relative to collagen abundance varies among different tissues and generally relates to the functional properties of the tissue. For example, the pyridinoline-to-collagen ratio is especially high in tendon, compared with bone.41 In animal models, inhibition of lysyl oxidase, and therefore a reduction in collagen cross-linking, results in collagen fibrils and tendons with reduced strength.42
Nonenzymatic cross-linking
A separate form of cross-links, known as advanced glycation end products, exist in connective tissues. The accumulation of advanced glycation end products is the result of the long-lived nature (ie, slow turnover) of mature collagen and prolonged exposure to monosaccharides. With prolonged exposure, spontaneous nonenzymatic bonds are formed between a reducing sugar and a protein residue on mature collagen (eg, lysine side chains). The development of nonenzymatic cross-links (eg, pentosidine, glucosepane) follows a complex series of reactions, known as the Maillard reaction, over the course of months or years.43 The presence of advanced glycation end products increases with increased plasma glucose concentrations (eg, diabetes) and with advanced aging.19,43–46 Similar to enzymatic cross-linking, nonenzymatic cross-linking increases the strength and stiffness of the connective tissue.20,47,48 Although this leads to greater failure loads, the uncontrolled nature of nonenzymatic cross-linking can be problematic, because overly stiff connective tissues are more prone to injury (eg, tissue rupture)49 and limit tissue compliance. Low compliance of the ECM restricts radial expansion of contracting muscle, limiting the force-generating capacity of muscle.21
Collagen degradation
Collagen degradation follows a 3-step process that occurs extracellularly, primarily by matrix metalloproteinases (MMPs). In the first step, MMPs are activated and bind to collagen fibrils. In the second step, the bound MMPs unwind the triple-helix structure to allow better access to the individual α strands. In the last step, MMPs cleave the individual strands at predictable regions. In collagen types I, II, and III, cleavage occurs at specific glycine-isoleucine bonds of the α-1 chains and a specific glycine-leucine bond of the α-2 chain.50–55 Cleavage at these bonds generates characteristic N-terminal and C-terminal fragments that are, respectively, three-fourths and one-fourth the length of the original α-chains.50 The fragments are unstable at body temperature, resulting in denaturation before further degradation by gelatinases (MMP-2, MMP-9) or other nonspecific proteases.56
The activation of MMPs is regulated by 1) gene transcription; 2) disruption of the thiol-Zn2+ interaction, which exposes the catalytic binding region; and 3) interaction with inhibitors such as the tissue inhibitors of metalloproteinases. There is substantial overlap in the substrate specificity of different members of the MMP family.57 However, MMP activity is selectively targeted toward the intended substrate through differences in enzyme affinity and compartmentalization. Compartmentalization of MMPs is achieved by the presence of a molecular component (eg, anchoring to cell membranes), which restricts the MMP activity to the immediate environment. MMP-1 (collagenase-1), MMP-8 (collagenase-2), MMP-13 (collagenase-3), MMP-18 (collagenase-4), MMP-2 (gelatinase-A), and MMP-14 (MT1-MMP) can cleave collagen types I, II, and III58 and are, therefore, most relevant for musculoskeletal tissues.
THE IMPACT OF PHYSICAL ACTIVITY ON COLLAGEN REMODELING
Mechanical loading as a main stimulus
Mechanical loading has been shown in vitro to stimulate fibroblasts to increase collagen synthesis over an acute period.59 In connective tissues, fibroblasts are generally arranged longitudinally along the collagen fibers. The fibroblasts are typically bound to the ECM by way of membrane-bound integrins. This configuration exposes fibroblasts to the forces transmitted though the muscle and the ECM, in particular. Fibroblasts sense the mechanical load and upregulate molecular processes (ie, synthesis) to facilitate appropriate tissue remodeling.59 In particular, it has been suggested that mechanical loading stimulates the release of growth factors (eg, transforming growth factor-1, connective tissue growth factor, and insulin-like growth factor-1), some of which (namely, transforming growth factor-1, connective tissue growth factor) act directly on fibroblasts to upregulate collagen synthesis.60–64 The anabolic impact of these growth factors on collagen synthesis has been demonstrated in various overload models and in response to a bout of treadmill running in rodents.63,65–67 However, it remains unclear whether these findings translate to humans, because growth factor expression has been shown to increase,68 remain unchanged,69,70 or even decrease69,70 in tendon tissue after exercise. Discrepancies between these findings may be due to differences in exercise duration and/or intensity, or differences in tissue sampling location. Mechanical loading has also been shown to upregulate transcriptional activity of lysyl oxidase after resistance-type exercise training in rats, suggesting that prolonged training may lead to greater cross-linking and stiffening of connective tissue.65
Exercise-induced adaptations in intramuscular connective tissue
Both endurance-71,72 and resistance-type exercise13,14,25,27,28,73 increase muscle connective tissue protein synthesis in humans. Lengthening contractions more robustly increase postexercise intramuscular connective tissue protein synthesis rates than do shortening contractions, reflecting the potent stimulatory effect of increased tension within contracting muscle tissue.39,74 The impact of prolonged exercise training on intramuscular connective tissue protein content has not been well explored in humans, though animal work has suggested that prolonged exercise training increases intramuscular connective tissue protein (ie, collagen) contents.75–77 Overall, exercise training–induced increases in collagen content and the level of cross-linking are likely critical to allow transmission of greater contractile forces from the muscle to tendon, ligament, and bone. In support, findings from recent work demonstrated that enhanced ECM remodeling is associated with increases in muscle mass and strength in overloaded rodent plantaris muscle.16 More exploration is required to determine whether the association between enhanced ECM remodeling and increases in muscle mass and strength following resistance-type exercise training in humans is both real and causal.
THE IMPACT OF DIETARY PROTEIN ON COLLAGEN REMODELING
Dietary-protein ingestion to support (muscle) tissue adaptation
It is well known that food ingestion is one of the most potent stimuli that promotes and/or supports musculoskeletal tissue adaptation. In particular, the ingestion of dietary protein potently increases muscle protein synthesis rates.38 The postprandial increase in plasma (essential) amino acids is a key factor for driving the increase in postprandial muscle protein synthesis rates.78 This anabolic response seems to be largely driven by the postprandial increase in circulating leucine levels and is further supported by the ample availability of amino acids as precursors for de novo muscle protein synthesis. In particular, leucine co-ingested with a suboptimal dose of protein further stimulates an increase in muscle protein synthesis rates at rest79–83 and during postexercise recovery.79–82,84,85 However, the increased muscle protein synthesis rates cannot be sustained without the delivery of other amino acids to serve as precursors.86 Together, these findings illustrate that the ingestion of dietary protein not only provides the anabolic signal to upregulate muscle protein synthesis (ie, leucine) but also delivers the amino acid precursors that are required to sustain muscle protein synthesis rates.
The properties of nutrition, and dietary protein in particular, that may specifically enhance connective tissue adaptation in the musculoskeletal tissues have not been fully elucidated. Seminal work by Babraj et al11 demonstrated that the ingestion of a 20-g mixture of essential amino acids did not increase intramuscular collagen protein synthesis rates in young and older individuals nor did it increase tendon collagen synthesis rates in young individuals. The absence of an impact of amino acid administration to enhance connective tissue protein remodeling was corroborated by Mikkelsen et al28 and Dideriksen et al,25 who demonstrated that the ingestion of 20–38 g of whey protein did not increase intramuscular collagen protein synthesis rates in older individuals at rest. Recently, we demonstrated that the ingestion of a larger, 40-g dose of casein protein does not result in increase in intramuscular collagen protein synthesis rates over a more prolonged postprandial period during overnight sleep (7.5 h).13
Dietary-protein ingestion and postexercise connective tissue protein synthesis rates
Although the combination of exercise and dietary-protein ingestion is well known to have an additive effect on stimulating muscle protein synthesis rates, dietary-protein intake does not appear to further increase the postexercise increase in connective tissue protein synthesis rates. For example, Holm et al27 showed that a pulse protein-feeding pattern (2–3 g of a soy or milk protein mixture ingested every 30 min) does not further increase postexercise intramuscular collagen protein synthesis rates during the early (ie, 30 min to 3 h) or late (3–5.5 h) phase of recovery from a bout of high- or low-intensity resistance-type exercise. More recent work by Holm et al74 suggested that the ingestion of an ∼18 g bolus of whey protein further increased intramuscular connective tissue protein synthesis rates during the later phase (2–5 h) of postexercise recovery when compared with the ingestion of carbohydrate. This finding suggests that protein ingestion may have a more delayed impact on increasing intramuscular collagen protein synthesis rates. However, recent work by Trommelen et al14 contests this hypothesis by demonstrating that the ingestion of a larger bolus of high-quality protein (30 g of casein) does not further increase postexercise intramuscular collagen protein synthesis rates throughout the subsequent 7.5 h of overnight sleep.
Although dietary-protein ingestion does not appear to stimulate connective tissue protein synthesis rates, the recent application of intrinsically labeled foods has indicated that protein ingestion delivers dietary-derived amino acids for incorporation in connective tissue proteins. The ingestion of specifically produced highly l-[1-13C]-phenylalanine–enriched (> 35 mole percent excess) casein protein combined with tissue sampling allows for the assessment of the metabolic fate of dietary protein–derived amino acids into newly synthesized proteins, termed de novo protein synthesis.87,88 Using this approach, Trommelen et al14 demonstrated for the first time that dietary protein–derived amino acids are, indeed, incorporated into de novo intramuscular connective tissue protein. Follow-up work demonstrated that the utilization of the ingested protein-derived amino acids for de novo intramuscular connective tissue protein synthesis is further enhanced during recovery from resistance exercise.13 Together, the observations that dietary protein–derived amino acids are incorporated into connective tissue protein and modulated with physical activity implies that dietary provision of amino acids is required and that there may be conditions where intramuscular connective tissue remodeling can be facilitated or compromised. For instance, it has been speculated that protein sources delivering large amounts of the key amino acids used for connective tissue protein synthesis (ie, glycine and proline) may be more suitable for facilitating increased connective tissue protein synthesis rates.13,14,89,90 This hypothesis is discussed in the following sections.
Dietary collagen ingestion to enhance connective tissue remodeling
Because the ingestion of essential amino acids, whey, or casein does not appear to stimulate intramuscular connective tissue protein synthesis rates, dietary-protein sources with alternative properties have been proposed to be more suitable to promote intramuscular connective tissue protein remodeling. For instance, rodent and ex vivo tissue engineering models have indicated that the provision of free glycine and proline promotes an increase in collagen synthesis.91,92 As such, dietary-protein sources that are rich in glycine and proline may be more suitable than dairy protein to promote tissue collagen synthesis. Collagen-derived dietary-protein sources, such as gelatin and collagen hydrolysate, provide large amounts of proline and glycine (Table 3). It has been proposed, therefore, that collagen-derived protein sources may be preferred to support connective tissue protein remodeling.89 At present, however, the digestion and absorption properties of collagen-derived dietary proteins and the biological impact on connective tissue remodeling in various human tissues remain to be elucidated.
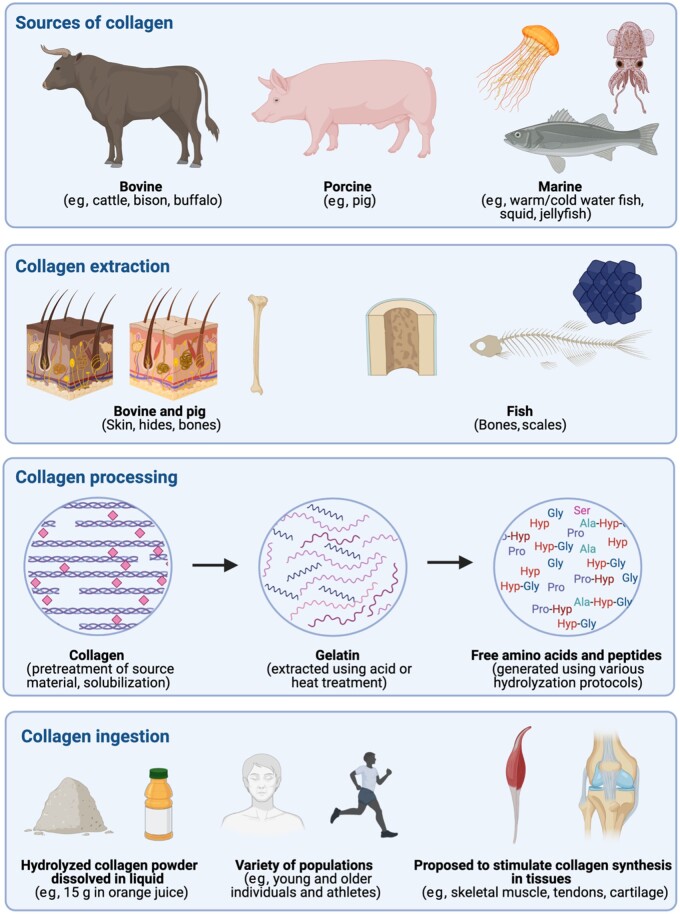
Collagen extraction and industrial processing for human consumption
A graphical representation of collagen extraction and industrial processing is presented in Figure 2. Collagen-derived dietary proteins are most commonly extracted from porcine skin (45%), bovine hide (30%), and the bones of both animals (23%).93 Chicken (bones) and fish (scales and bones) byproducts are also used as production sources of collagen-derived dietary protein. Collagen derived from fish sources have generally been shown to contain lower proline and hydroxyproline contents than collagen derived from pig or bovine sources, though warm-water fish tend to contain the highest proline and hydroxyproline contents among the different species.94,95 The process of collagen extraction varies slightly depending on the matrix of the collagen source (eg, bone has a high mineral content, whereas porcine skin has a high fat content). In general, however, collagen extraction involves trimming and cleaning of the source material; pretreatment to remove minerals, fats, or other noncollagen material; extraction; filtration; concentration; sterilization; and drying. The extraction of gelatin requires exposure to high temperatures (50–100°C), which denature the triple-helix structure of collagen and result in more soluble peptide strands that are lower in molecular weight (∼100 kDa) than intact collagen (300 kDa). The extraction of a more intact form of collagen is achieved when the source material is exposed to various acids at lower temperatures (2–8°C).95
Figure 2.
Graphical representation of the industrial sources of dietary collagen, the extraction procedures used to produce hydrolyzed collagen, and the proposed applications of collagen peptide ingestion in humans. The currently established industrial sources of collagen are of bovine (eg, cattle, bison buffalo), porcine (ie, pig), and marine (eg, warm- and cold-water fish, squid, jellyfish) origin. Collagen source materials from these animals include skin, bones, and scales, which are considered byproducts generated from processing for other purposes (eg, food production). The source materials undergo pretreatment, including cleaning, hair removal, and collagen solubilization using chemical treatment. Collagen is further extracted using acid or heat treatment, which results in gelatin release. Gelatin consists of denatured and unbound collagen fibers. Gelatin may undergo various hydrolyzation processes that result in the liberation of free amino acids and peptides, which may resist complete hydrolyzation. It has been suggested that certain peptides have stimulatory or inhibitory properties, though, to our knowledge, no human in vivo evidence has been generated to support such claims. Hydrolyzed collagen is soluble in liquid and, therefore, is more readily available for application as a dietary supplement (eg, 15 g of collagen hydrolysate dissolved in orange juice). Current claims for the benefits of collagen supplementation include application in younger (18–35 y) and older (>65 y) individuals and athletes to enhance collagen remodeling in skeletal muscle, tendons, ligaments, cartilage, and skin.
Extracted collagen and gelatin can be further processed using additional acid-exposure steps or enzymatic hydrolysis to produce collagen hydrolysates. Depending on the collagen source and hydrolyzation protocol, collagen hydrolysates can vary in size, with molecular weights ranging from 2 to 20 kDa, though most commonly < 6 kDa.96,97 Collagen hydrolysates of different molecular weights possess different properties, which are relevant when used as ingredients in food preparation (eg, emulsifiers, stabilizers, and enhancers that bind other ingredients). The processing of collagen may also have significant bearing on the digestion and absorption kinetics of collagen-derived dietary proteins and their subsequent biological impact on connective tissue remodeling in various human tissues (eg, skeletal muscle, tendons, ligaments, bones, and skin).98,99when used
Digestion and absorption of collagen-derived dietary proteins
Native collagen is resistant to peptide cleavage by digestive enzymes, resulting in poor absorption (as low as ∼10%) into the circulation.100 Production into gelatin increases digestion and absorption, though perhaps not to the extent of other dietary proteins. For instance, work using rodent models has shown that relatively more nitrogen is recovered from the intestine of rats fed gelatin in comparison with casein or egg albumin.101 Chen et al102 built on this finding by measuring intestinal contents and determined that gelatin feeding resulted in longer peptides (6.0 amino acid residues/peptide) than did casein feeding (2.7 amino acid residues/peptide) and a nitrogen-free diet (2.4 amino acid residues/peptide), suggesting less complete gelatin digestion. In humans, Shaw et al89 demonstrated that the ingestion of 5 g and 15 g of gelatin increases plasma glycine, proline, and hydroxyproline concentrations in comparison with placebo ingestion. Gelatin contains high(er) concentrations of glycine, proline, and hydroxyproline; thus, these findings suggest that gelatin is digested and subsequently absorbed into the circulation. The ingestion of 15 g of gelatin resulted in greater peak plasma concentrations of glycine (∼1.75-fold), proline (∼1.6-fold), and hydroxyproline (∼2-fold) than did the ingestion of 5 g of gelatin. The greater plasma concentrations of these amino acids remained elevated over the 3 -hour postprandial period in comparison with the ingestion of 5 g gelatin. These findings indicate a dose-dependent pattern of gelatin ingestion on peak plasma amino acid concentrations and amino acid availability. Others have reported plasma amino acid concentrations after the ingestion of 35 g of hydrolyzed collagen103 similar to those reported after the ingestion of 15 g gelatin.89 The similarity between peak plasma concentrations may suggest that the ingestion of ∼15 g dietary collagen-derived protein ingestion results in near-maximal rates of amino acid absorption from gelatin. However, digestion and absorption kinetics after collagen-derived protein ingestion (eg, the relative release into the plasma circulation and splanchnic uptake) remain to be determined in the human in vivo setting.
Digestion and absorption of hydrolyzed collagen
The hydrolyzation of dietary protein into peptides increases the postprandial rate of dietary protein–derived amino acid appearance into the plasma circulation after protein (hydrolysate) ingestion. For instance, Koopman et al98 demonstrated that hydrolyzed casein ingestion resulted in a greater rate of dietary protein–derived amino acid appearance, resulting in greater plasma amino acid availability and subsequent delivery to tissues, in comparison with the ingestion of intact casein. Oesser et al104 used rodent models to demonstrate that an orally administered dose of 14C-labelled hydrolyzed gelatin (3–6 kDa) was released to a similar extent (95% of ingested protein) in comparison with a matching oral dose of 14C-labelled free proline over a relatively prolonged 12-hour postprandial period. It has been proposed, therefore, that the hydrolyzation of collagen protein sources may enhance collagen protein digestion and amino acid absorption. However, Alcock et al90 have recently examined plasma amino acid profiles in human volunteers after the ingestion of either 20 g of gelatin or 20 g of hydrolyzed collagen. The ingestion of both collagen sources resulted in similar peak glycine concentrations, and no differences were observed in total plasma amino acid availability (incremental area under the curve), despite the apparently lower molecular weight of hydrolyzed collagen. Lis and Baar105 corroborated these findings, demonstrating that the ingestion of 15 g of hydrolyzed collagen and gelatin results in nearly identical postprandial plasma glycine, lysine, proline, hydroxyproline, but not leucine, concentrations at 1 hour after protein ingestion. The absence of differences in postprandial plasma amino acid profiles after the ingestion of gelatin vs hydrolyzed collagen may be due to protein processing. For instance, different hydrolyzation protocols are known to produce peptides of larger molecular weights.95 Skov et al103 recently demonstrated that ingestion of 35 g of enzymatically hydrolyzed collagen protein results in greater plasma glycine, proline, and hydroxyproline availability (incremental area under the curve) in comparison with the ingestion of 35 g of nonenzymatically hydrolyzed collagen. Work comparing digestibility after different hydrolyzation protocols of the same source of collagen-derived protein is required to identify characteristics that maximize the uptake of collagen protein-derived amino acids into the circulation.
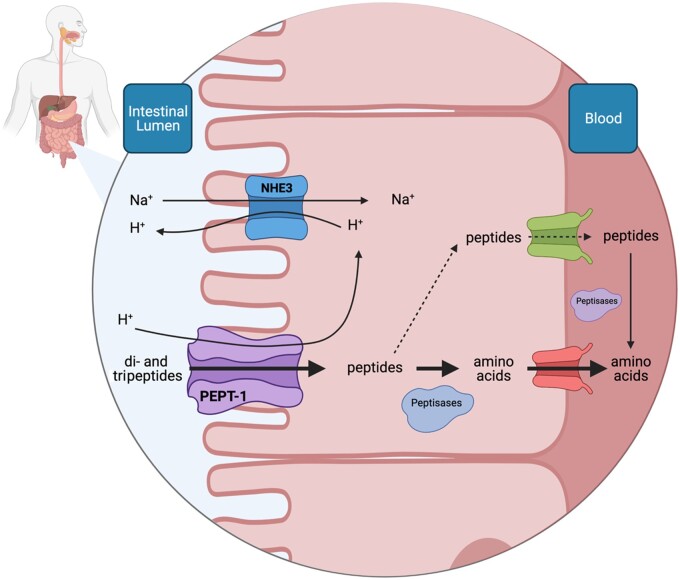
Potential mechanisms involved in collagen peptide digestion and absorption
It has been proposed that the hydrolyzation of collagen-derived proteins produces peptides, which may act to further enhance connective tissue remodeling in musculoskeletal and dermal tissues.104,106,107 To have any stimulatory or inhibitory properties, the ingested peptides must be absorbed in their intact form into the circulation and taken up into target tissues. Peptide absorption in the gut may occur through 4 possible pathways: 1) through the tight junctions between enterocytes, 2) by passive diffusion through the enterocytes, 3) by endocytosis through the enterocyte, and 4) through transmembrane transporter protein systems.108 However, no in vivo evidence has been generated to directly support the transport of peptides through tight junctions, passive diffusion, or endocytosis.108 With regard to active transport, a central transmembrane peptide transporter, PEPT-1, has been identified and its functionality has been defined (Figure 3). The PEPT-1 transporter is located on the brush border of the intestinal epithelium,109 and several studies have determined that it facilitates transport of only di- and tripeptides, as opposed to free amino acids and peptides longer than 4 amino acids, into the cytosolic space of the enterocyte.110–112 However, further transport of di- or tripeptides through the enterocyte into the hepatic portal vein is low.113–115 This is due to the presence of peptidases in the cytosolic space of the enterocyte that further hydrolyze peptides into their amino acid constituents,116 which are then transported into the hepatic portal vein. In the case that di- and tripeptides are, indeed, transported from the gut into the circulation, they are subjected to further hydrolysis by exposure to vascular endothelial tissue and plasma peptidases.114,117,118 However, although most (if not all) peptides are hydrolyzed during transport into the circulation, there is some evidence to suggest that specific collagen-derived di- and tripeptides can be absorbed intact, with those containing hydroxyproline and/or proline proposed to resist peptidase activity.119 In support of this proposition, early work demonstrated that the ingestion of 30 g of gelatin increased hydroxyproline-containing dipeptides, but not free hydroxyproline, in the urine of human participants.120 These findings were later corroborated by Weiss and Klein,121 who demonstrated that the administration of 14C-labeled proline-hydroxyproline (Pro-Hyp) in gelatin and in peptide form could be detected in the urine, suggesting intact Pro-Hyp absorption and excretion. Pro-Hyp is the most abundant peptide contained in collagen hydrolysate,122–124 and has been shown in vitro to be resistive to peptidase action in plasma.123
Figure 3.
Graphical representation of collagen-derived di- and tripeptide absorption from the intestinal lumen into the blood circulation. Various hydrolyzation procedures during industrial processing of collagen produce peptides, which have been proposed to have stimulatory or inhibitory properties within various tissues. Hydrolyzed collagen ingestion delivers peptides to the intestinal lumen. Collagen-derived peptides, and those containing hydroxyproline in particular, appear to be resistant to further intestinal peptide cleavage into free amino acids. These peptides may be transported through a transmembrane peptide transporter (PEPT1) into the enterocyte. Peptides transported to the cytosol of the enterocyte are either cleaved by peptidases into free amino acids and transported further into the circulation or are transported in their intact form into the circulation. Once in the circulation, peptides are subjected to cleavage through exposure to vascular endothelial tissue or peptidase activity. Overall, the existing evidence suggests that intact dietary-derived peptide uptake into the circulation is low. Whether collagen peptides, some of which are proposed to be more resistant to cleavage, have a stimulatory impact on connective tissue remodeling remains to be determined in humans
Current evidence regarding dietary collagen peptide absorption into plasma
The proposed absorption of collagen-derived peptides remains poorly described, primarily due to the analytical challenges of producing labeled internal standards for quantifying concentrations of the many different peptides within plasma samples. In early studies, researchers were able to determine that Hyp-containing peptide concentrations increased in the plasma circulation after gelatin ingestion.123–125 More recently, investigators were able to assess the presence of more-specific peptides in plasma samples. These studies have identified that the ingestion of ∼26 g of hydrolyzed collagen derived from fish scales resulted in a peak Pro-Hyp plasma concentrations of 60 μmol/L.122 After the ingestion of 10 g of hydrolyzed collagen, peak plasma concentration of Pro-Hyp reached ∼20 μmol/L at 60 minutes.126 These data suggest a dose-dependent response of collagen hydrolysate ingestion and subsequent peptide release, which has also been observed more generally by other investigators.127 Taga et al126 demonstrated that after the ingestion of 10 g of porcine gelatin, peak Pro-Hyp concentrations were nearly 8-fold greater than the next highest collagen-derived peptide (Hyp-Gly), though 14- and 5-fold lower than peak free Pro and Hyp concentrations, respectively. This indicates that although specific dipeptides may reach the plasma circulation after gelatin ingestion, their concentration is substantially lower in comparison to the postprandial increase in free amino acid availability.
Effect of collagen-derived protein supplementation on tissue remodeling
Protein ingestion can stimulate and/or support tissue adaptation through 1) the provision of a dietary protein–derived anabolic stimulus that upregulates protein synthesis (eg, leucine for myofibrillar protein synthesis)83,85; 2) the provision of dietary protein–derived amino acids as precursors for de novo tissue protein synthesis86; and/or 3) through the stimulation of more global postprandial responses (eg, insulin release, enhanced blood flow).128 Shaw et al89 demonstrated that the plasma milieu after the ingestion of 15 g of gelatin promoted greater collagen synthesis in ex vivo engineered ligament tissue constructs in comparison with the ingestion of 0 g and 5 g of gelatin. To date, however, the impact of collagen supplementation on connective tissue protein synthesis in vivo in humans has been evaluated in only 1 study. Oikawa et al129 applied deuterated water methodology and demonstrated that collagen peptide supplementation did not further increase intramuscular collagen protein synthesis rates in comparison with the supplementation of an equivalent dose of whey protein during 3 days of free-living conditions (ie, rest) or during 3 days after a single bout of resistance-type exercise in older women. Without a direct comparison of intramuscular collagen synthesis rates with and without collagen supplementation, it remains unclear whether collagen peptide supplementation provided an anabolic stimulus.
Collagen-derived protein supplementation on long-term clinical outcomes
Some evidence exists to support the impact of collagen-derived protein supplementation on the remodeling of various musculoskeletal tissues in healthy and clinical populations. For tendon, researchers found in a recent pilot study that oral collagen peptide supplementation improved symptoms and tendon vascularization in patients with chronic Achilles tendinopathy who were performing a structured exercise program.130 In bone, König et al131 recently reported that 12 weeks of collagen peptide supplementation (5 g/d) increased bone mineral density and markers of bone collagen synthesis in postmenopausal women. However, these findings are in partial contrast to those of Cúneo et al,132 who reported no impact of 24 weeks of collagen peptide supplementation (10 g/d) on comparable markers primarily reflecting bone metabolism in postmenopausal women. The discrepancy between these findings may be related to the nutritional status of the women, differences in the plasma markers measured, and/or production differences of the collagen provided in the supplements. Clifford et al133 reported no impact of collagen peptide supplementation (20 g/d) on circulating markers of bone metabolism (procollagen type I N-terminal propeptide, CTX-I) after a single bout of exercise.
Although the role of collagen supplementation on bone remodeling remains unclear, it is noteworthy to highlight that early stable isotope work indicated that intravenous infusion of lipids, glucose, and free amino acids stimulates an increase in bone collagen synthesis in humans.134 These data suggest a potential role of nutritional interventions to enhance bone remodeling. There is clearly a need for work to directly evaluate the impact and potential mechanisms of action of collagen ingestion and more prolonged collagen supplementation on bone collagen synthesis in vivo in humans.
Few studies have evaluated the long-term impact of collagen supplementation on skeletal muscle mass and strength with and without exercise training. Recently, Mertz et al135 demonstrated no impact of 20 g of collagen plus 10 g of carbohydrate supplementation vs 30 g of carbohydrate supplementation over 1 year on muscle mass or strength in healthy older men and women. However, dietary-protein supplementation has been shown to augment the gains in skeletal muscle mass and strength after prolonged resistance-type exercise training.5,136 In line with these findings, 2 long-term, resistance-type exercise training studies have reported that collagen peptide supplementation further augments training-induced skeletal muscle mass and strength gains. Zdzieblik et al137 showed that daily 15-g collagen peptide supplementation resulted in an average increase of 4.2 kg of fat-free mass vs 2.9 kg in the placebo-supplemented group after resistance-type exercise training in men with sarcopenia. It has been recently highlighted138 that the increase in fat-free mass observed in the collagen-supplemented group was exceptionally high (2.7-fold) in comparison with the increase in fat-free mass reported in recent meta-analyses examining the impact of protein supplementation on gains in fat-free mass after prolonged resistance exercise training.5,136 However, the anabolic impact of collagen peptide supplementation was also more recently demonstrated by Oertzen-Hagemann et al,139 who showed that 15 g of collagen peptide supplementation resulted in an average increase of 2.6 kg fat-free mass vs 0.7 kg in the placebo-supplemented group after resistance-type exercise training in young, healthy men. Though the reported gains in muscle mass and strength do not seem realistic, these findings underline the importance of performing additional studies with a well-controlled dietary intake approach to confirm the potential impact of collagen peptide ingestion on training-induced gains in muscle mass and strength.
Finally, Oikawa et al140 recently demonstrated that collagen peptide supplementation (30 g, twice daily, ∼45% of total protein intake, 1.6 g protein/kg body mass/d) did not attenuate the loss of lower limb fat-free mass during energy intake restriction (–500 kcal/d) and a reduction in physical activity (< 750 steps/d) in older men and women. These findings seem to add to the general understanding that the anabolic potential of protein sources with low essential amino acid content (ie, collagen) are minimal.
Proposed mechanisms of action of collagen-derived protein supplementation
Glycine and proline as precursors for collagen synthesis.
Provision of specific amino acids and peptides represent the 2 most likely mechanisms by which collagen-derived protein ingestion may upregulate tissue collagen synthesis and/or improve connective tissue function. As mentioned, the ingestion of relatively large doses of essential amino acids, casein, or whey do not seem to stimulate intramuscular connective tissue protein synthesis rates in vivo in humans.11,14,25,28 Connective tissue proteins contain high levels of proline (12%) and glycine (25%) (Table 2) relative to other proteins within skeletal muscle.32 Presumably, collagen protein synthesis rates will remain low or submaximal in the absence of sufficient glycine and proline availability. Early work has demonstrated that a reduction in glycine availability, as in the case of malnourished children,141 may limit the synthesis of key proteins and peptides, such as glutathione.142 More recently, Meléndez-Hevia et al143 estimated that glycine availability from endogenous sources (ie, synthesized from serine and protein breakdown) and provided by a regular diet falls short of the estimated metabolic requirements, including glycine required to support tissue collagen synthesis. It may be suggested, therefore, that the amount of glycine (and proline) provided by a regular diet is insufficient for facilitating increased rates of tissue collagen synthesis. Therefore, the ingestion of protein sources rich in proline and glycine (Table 3) may be more suitable than high-quality protein sources, such as casein or whey protein (providing only ∼6% proline and ∼2% glycine) (Table 1) for supplying the specific amino acid precursors required to support de novo connective tissue protein synthesis.29 In support of this, exposure to growth media containing proline and ascorbic acid has been shown to increase collagen content and improve the mechanical properties of engineered ligaments in an in vitro setting.91 In rats, Vieira et al92 showed that a diet rich in glycine further improved hydroxyproline content and maximal tolerable load in an Achilles tendonitis model, suggesting enhanced tendon recovery. Aside from its potential role as an amino acid precursor for collagen synthesis, glycine administration restored the activity of key translational signaling proteins (ie, mTORC1 pathway) in response to leucine administration in a rodent inflammation model.144,145 These findings may indicate the potential for glycine (or collagen-derived protein) supplementation to increase the muscle protein synthetic response to food intake in clinically compromised individuals with low-grade inflammation.146
Collagen-derived protein uniquely contains hydroxyproline (13.5%). The role of hydroxyproline provision in facilitating connective tissue protein synthesis remains unclear. No codon exists for hydroxyproline, and its content in collagen is the result of a posttranslational modification of proline. Therefore, a postprandial increase in plasma free hydroxyproline due to collagen-derived protein ingestion cannot be used as precursor for incorporation into newly synthesized procollagen.147 Whether increased hydroxyproline availability possesses stimulatory properties for collagen synthesis remains unknown. Ultimately, the impact of greater glycine, proline, and hydroxyproline provision through dietary collagen-derived protein supplementation on in vivo connective tissue reconditioning in humans remains to be addressed.
Collagen peptides to facilitate connective tissue remodeling.
It has been proposed that ingestion of collagen-derived peptides may directly enhance connective tissue remodeling. As described previously, evidence has been presented in some studies to suggest that collagen-derived di- and tripeptides (eg, Pro-Hyp, Hyp-Gly, Pro-Hyp-Gly) are absorbed in their intact form, albeit in low concentrations, into the circulation. However, currently, no human studies have evaluated the impact of the ingestion of these collagen-derived peptides on connective tissue synthesis or other in vivo remodeling processes (ie, cross-linking, breakdown), to our knowledge. Collagen-derived peptide administration has been shown to stimulate collagen synthesis and cross-linking activity and decrease breakdown using in vivo rodent and in vitro models. Oesser et al104 showed, with the use of specially produced 14C-labelled collagen peptides, that amino acids derived from orally administered peptides are incorporated in skin, liver, spleen, and skeletal muscle tissue in mice. However, the amino acids derived from administered peptides were most effectively delivered to cartilage tissue, as evidenced by nearly 2-fold greater specific radioactivity in comparison with oral administration of free 14C-proline.104 On the basis of these findings, it was suggested that amino acids ingested in peptide form are more effectively incorporated into cartilage than are their free amino acid counterparts. Most studies evaluating the impact of collagen peptides on connective tissue remodeling responses have been performed using skin models. For instance, Zague et al148 showed that 4 weeks of collagen hydrolysate supplementation increases collagen types I and IV concentrations and decreased MMP-2 activity in rat skin. More recently, Zague et al149 showed that exposure to collagen peptides increases collagen I synthesis and inhibits MMP-1 and MMP-2 activity in human skin collected during elective surgery. Edgar et al106 showed that collagen peptide exposure to human-derived skin cells increases elastin synthesis and decreases synthesis of MMP-1 and MMP-3 along with the elastin degradation product desmosine. The impact of collagen peptide exposure to enhance remodeling may extend to other tissues. For instance, Yamada et al150 showed that fish-derived collagen peptide exposure increases mixed collagen content along with COL1A2 mRNA expression in osteoblasts. The mRNA expression of several lysyl oxidase isoforms were also evaluated, revealing upregulation of some (namely, LOX-2, -3, and -4), but not the predominant isoforms (LOX, LOX-1).
Ohara et al.151 evaluated 9 different collagen-derived peptides in vitro and showed that only exposure to Pro-Hyp stimulated proliferation of human dermal fibroblasts. This finding aligns with those of Shigemura et al,152 who showed that exposure to Pro-Hyp stimulated the growth of mouse skin fibroblasts when embedded in a collagen gel and exposed to various growth factors. However, it is important to note that no stimulatory effect was demonstrated after exposure of 50 μmol/L Pro-Hyp, which has been reported in humans as the peak plasma concentration after the ingestion of as much as 26 g of collagen peptides derived from fish scales.122 Any stimulatory effect of collagen-derived peptides on fibroblasts may be restricted to only Pro-Hyp; 2 studies reported no impact of mixed collagen peptide exposure on human dermal fibroblast149 or mouse osteoblast proliferation.150
Although these studies suggest that collagen peptide provision may stimulate collagen synthesis and limit breakdown activity, the findings are unlikely to carry over to the human in vivo setting for various reasons. First, the in vitro setting tests fibroblasts directly on the culture plate or within a collagen gel. In the in vivo setting, fibroblasts are embedded in the ECM. Within the matrix, fibroblasts are situated optimally to sense and respond to metabolic and mechanical stimuli, such as an elevation in growth hormone concentrations or amino acids released from collagen breakdown. In the musculoskeletal tissues, fibroblasts are embedded in a longitudinal orientation to sense mechanical loading during physical activity. The in vitro experiments are often conducted in the absence of mechanical loading, under the administration of supraphysiological concentrations of nutrients and growth factors known to upregulate fibroblast proliferation, activity, and collagen synthesis. The importance of the in vivo environment is exemplified by the work of Shigemura et al,152 who clearly demonstrated that peptide administration increased collagen synthesis only when fibroblasts were incubated for at least 96 hours in optimized growth medium (ie, 5%–10% fetal bovine serum, rich in platelet-derived growth factor and FGF-1). As such, the absence of mechanical stimuli known to enhance fibroblast proliferation, activity, and/or collagen synthesis and breakdown in the in vitro model likely affected the results. Second, the concentrations of collagen-derived peptides provided to cells in nearly all studies are > 4-fold higher than what has been reported to be released in the plasma circulation after the ingestion of ∼25 g collagen peptides in humans.152 Shigemura et al152 did not detect an effect of Hyp-Pro on fibroblast proliferation when Hyp-Pro was administered in an amount that more closely reflected peak plasma concentrations. Last, incubation times of peptides in these in vitro studies generally ranged between 24 hours and 6 days.106,149,151,152 In humans, the postprandial period of protein digestion and absorption is far shorter, with several studies demonstrating that peptide concentrations may only be increased from 4 to 6 hours after ingestion.126,153,154 Therefore, it is expected that any stimulatory effect of peptide ingestion is minor and/or short-lived in comparison with the responses reported in the in vitro setting. Of course, no conclusions on the proposed impact of collagen peptide ingestion on connective tissue remodeling can be reached until evidence is gathered in the in vivo human setting.
CONCLUSIONS
Musculoskeletal connective tissue networks are in a constant state of remodeling. Exercise increases intramuscular connective tissue protein synthesis rates, demonstrating that connective tissue protein possesses a high level of plasticity. Despite the anabolic properties of dietary protein on muscle tissue remodeling, no study has demonstrated the impact of dietary protein ingestion to increase connective tissue synthesis rates. However, these studies have only evaluated the connective tissue protein synthetic response to essential amino acid and dairy protein ingestion. It has been suggested that the ingestion of collagen-derived protein sources, such as collagen peptides or gelatin, may be more suitable for stimulating connective tissue protein synthesis. Collagen-derived proteins contain ample amounts of glycine and proline, which may facilitate an increase in connective tissue protein synthesis rates. Some have speculated that collagen-derived protein sources also contain peptides with stimulatory or inhibitory properties. Although some in vitro evidence exists to support this hypothesis, there are no data, to our knowledge, to support the claim that the ingestion of collagen-derived peptides may stimulate connective tissue protein synthesis rates. Research is warranted to establish the proposed benefits of collagen-derived protein ingestion as a means to support connective tissue remodeling within musculoskeletal tissues. Such evidence is necessary for the potential development of nutritional intervention strategies to increase muscle strength and function in a variety of populations, including athletes and older individuals.
Acknowledgments
Author contributions. A.M.H. and L.J.C.vL conceived the idea of the review. A.M.H. compiled the articles and extracted the data, wrote the manuscript, and generated tables and figures. L.J.C.vL critically revised the manuscript. Both authors read and approved the final manuscript. Figures were created with Biorender.com.
Funding. A.M.H and L.J.C.vL have received funding from PB Leiner, part of Tessenderlo Group, to study the role of dietary collagen on skeletal muscle health. L.J.C.vL and his laboratory have received research grants, consulting fees, speaking honoraria, or a combination of these for work on postprandial protein muscle metabolism; a full overview is provided at https://www.maastrichtuniversity.nl/l.vanloon.
Funders did not play a role in the conception, design, performance, and approval of the presented work.
Declaration of interest. The authors have no relevant interests to declare.
Contributor Information
Andrew M Holwerda, NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht University Medical Centre+, Maastricht, The Netherlands.
Luc J C van Loon, NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht University Medical Centre+, Maastricht, The Netherlands.
References
- 1. Holwerda AM, Paulussen KJM, Overkamp M, et al. Daily resistance-type exercise stimulates muscle protein synthesis in vivo in young men. J Appl Physiol. 2018;124:66–75. [DOI] [PubMed] [Google Scholar]
- 2. Groen BBL, Horstman AM, Hamer HM, et al. Post-prandial protein handling: you are what you just ate. PLoS One. 2015;10:e0141582.doi:10.1371/journal.pone.0141582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burd NA, Gorissen SH, van Vliet S, et al. Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: a randomized controlled trial. Am J Clin Nutr. 2015;102:828–836. [DOI] [PubMed] [Google Scholar]
- 4. Pennings B, Koopman R, Beelen M, et al. Exercising before protein intake allows for greater use of dietary protein–derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr. 2011;93:322–331. [DOI] [PubMed] [Google Scholar]
- 5. Cermak NM, Res PT, de Groot LC, et al. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96:1454–1464. [DOI] [PubMed] [Google Scholar]
- 6. Snijders T, Res PT, Smeets JS, et al. Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance-type exercise training in healthy young men. J Nutr. 2015;145:1178–1184. [DOI] [PubMed] [Google Scholar]
- 7. Tieland M, van de Rest O, Dirks ML, et al. Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13:720–726. [DOI] [PubMed] [Google Scholar]
- 8. Volek JS, Volk BM, Gómez AL, et al. Whey protein supplementation during resistance training augments lean body mass. J Am Coll Nutr. 2013;32:122–135. [DOI] [PubMed] [Google Scholar]
- 9. Wilkinson SB, Tarnopolsky MA, MacDonald MJ, et al. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85:1031–1040. [DOI] [PubMed] [Google Scholar]
- 10. Bishop JE, Laurent GJ. Collagen turnover and its regulation in the normal and hypertrophying heart. Eur Heart J. 1995;16(suppl C): 38–44. [DOI] [PubMed] [Google Scholar]
- 11. Babraj JA, Cuthbertson DJR, Smith K, et al. Collagen synthesis in human musculoskeletal tissues and skin. Am J Physiol-Endocrinol Metab. 2005;289:E864–E869. [DOI] [PubMed] [Google Scholar]
- 12. Moore DR, Phillips SM, Babraj JA, et al. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol-Endocrinol Metab. 2005;288:E1153–E1159. [DOI] [PubMed] [Google Scholar]
- 13. Holwerda AM, Trommelen J, Kouw IWK, et al. Exercise plus presleep protein ingestion increases overnight muscle connective tissue protein synthesis rates in healthy older men. Int J Sport Nutr Exerc Metab. 2021;31:217–226. [DOI] [PubMed] [Google Scholar]
- 14. Trommelen J, Holwerda AM, Senden JM, et al. Casein ingestion does not increase muscle connective tissue protein synthesis rates. Med Sci Sports Exerc. 2020;52:1983–1991. doi:10.1249/MSS.0000000000002337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller BF, Olesen JL, Hansen M, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise: coordinated response of muscle and tendon. J Physiol. 2005;567:1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stantzou A, Relizani K, Morales‐Gonzalez S, et al. Extracellular matrix remodelling is associated with muscle force increase in overloaded mouse plantaris muscle. Neuropathol Appl Neurobiol. 2021;47:218–235. [DOI] [PubMed] [Google Scholar]
- 17. De Boer MD, Selby A, Atherton P, et al. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse: decreased protein maintenance of muscle and tendon during atrophy immobilization. J Physiol. 2007;585:241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dideriksen K, Boesen AP, Reitelseder S, et al. Tendon collagen synthesis declines with immobilization in elderly humans: no effect of anti-inflammatory medication. J Appl Physiol. 2017;122:273–282. [DOI] [PubMed] [Google Scholar]
- 19. Haus JM, Carrithers JA, Trappe SW, et al. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol. 2007;103:2068–2076. [DOI] [PubMed] [Google Scholar]
- 20. Wood LK, Kayupov E, Gumucio JP, et al. Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. J Appl Physiol (1985). 2014;117:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Azizi E, Deslauriers AR, Holt NC, Eaton CE. Resistance to radial expansion limits muscle strain and work. Biomech Model Mechanobiol. 2017;16:1633–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramaswamy KS, Palmer ML, van der Meulen JH, et al. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats: lateral transmission of force in skeletal muscles of mice and rats. J Physiol. 2011;589:1195–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang C, Gao Y. Effects of aging on the lateral transmission of force in rat skeletal muscle. J Biomech. 2014;47:944–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kragstrup TW, Kjaer M, Mackey AL. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging: skeletal muscle ECM and aging. Scand J Med Sci Sports. 2011;21:749–757. [DOI] [PubMed] [Google Scholar]
- 25. Dideriksen K, Reitelseder S, Malmgaard-Clausen NM, et al. No effect of anti-inflammatory medication on postprandial and postexercise muscle protein synthesis in elderly men with slightly elevated systemic inflammation. Exp Gerontol. 2016;83:120–129. [DOI] [PubMed] [Google Scholar]
- 26. Dideriksen K, Reitelseder S, Petersen SG, et al. Stimulation of muscle protein synthesis by whey and caseinate ingestion after resistance exercise in elderly individuals: milk proteins and resistance exercise. Scand J Med Sci Sports. 2011;21:e372–e383. [DOI] [PubMed] [Google Scholar]
- 27. Holm L, van Hall G, Rose AJ, et al. Contraction intensity and feeding affect collagen and myofibrillar protein synthesis rates differently in human skeletal muscle. Am J Physiol-Endocrinol Metab. 2010;298:E257–E269. [DOI] [PubMed] [Google Scholar]
- 28. Mikkelsen UR, Dideriksen K, Andersen MB, et al. Preserved skeletal muscle protein anabolic response to acute exercise and protein intake in well-treated rheumatoid arthritis patients. Arthritis Res Ther. 2015;17:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gorissen SHM, Crombag JJR, Senden JMG, et al. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids. 2018;50:1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Department of Agriculture. Beef, ground, 85% lean meat/15% fat, raw. FoodData Central. 2019. Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171796/nutrients.
- 31. Cunningham LW, Frederiksen DW, eds. Structural and Contractile Proteins. New York: Academic Press; 1982:31. [Google Scholar]
- 32. Eastoe JE. The amino acid composition of mammalian collagen and gelatin. Biochem J. 1955;61:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eastoe JE. The amino acid composition of fish collagen and gelatin. Biochem J. 1957;65:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gelse K, Pöschl E, Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. [DOI] [PubMed] [Google Scholar]
- 35. Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3:a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix: skeletal muscle ECM. Muscle Nerve. 2011;44:318–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gartner LP. Textbook of Histology. 4th ed. Amsterdam, the Netherlands: Elsevier; 2017. [Google Scholar]
- 38. Wall BT, Gorissen SH, Pennings B, et al. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS One. 2015;10:e0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cuthbertson DJ, Babraj J, Smith K, et al. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol-Endocrinol Metab. 2006;290:E731–E738. [DOI] [PubMed] [Google Scholar]
- 40. Elsdale TR. Parallel orientation of fibroblasts in vitro. Exp Cell Res. 1968;51:439–450. [DOI] [PubMed] [Google Scholar]
- 41. Kjaer M, Langberg H, Miller BF, et al. Metabolic activity and collagen turnover in human tendon in response to physical activity. J Musculoskelet Neuronal Interact. 2005;5:41–52. [PubMed] [Google Scholar]
- 42. Haut RC. The effect of a lathyritic diet on the sensitivity of tendon to strain rate. J Biomech Eng. 1985;107:166–174. doi:10.1115/1.3138537 [DOI] [PubMed] [Google Scholar]
- 43. Monnier VM, Mustata GT, Biemel KL, et al. Cross-linking of the extracellular matrix by the maillard reaction in aging and diabetes: an update on “a puzzle nearing resolution”. Ann N Y Acad Sci. 2005;1043:533–544. [DOI] [PubMed] [Google Scholar]
- 44. Alt N, Carson JA, Alderson NL, et al. Chemical modification of muscle protein in diabetes. Arch Biochem Biophys. 2004;425:200–206. [DOI] [PubMed] [Google Scholar]
- 45. Brown SM, Smith DM, Alt N, et al. Tissue-specific variation in glycation of proteins in diabetes: evidence for a functional role of amadoriase enzymes. Ann N Y Acad Sci. 2005;1043:817–823. [DOI] [PubMed] [Google Scholar]
- 46. Reddy GK. Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit Achilles tendon. Exp Diabesity Res. 2004;5:143–153. doi:10.1080/15438600490277860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Andreassen TT, Seyer-Hansen K, Bailey AJ. Thermal stability, mechanical properties and reducible cross-links of rat tail tendon in experimental diabetes. Biochim Biophys Acta BBA - Gen Subj. 1981;677:313–317. [DOI] [PubMed] [Google Scholar]
- 48. Galeski A, Kastelic J, Baer E, et al. Mechanical and structural changes in rat tail tendon induced by alloxan diabetes and aging. J Biomech. 1977;10:775–782. [DOI] [PubMed] [Google Scholar]
- 49. Fox AJS, Bedi A, Deng X-H, et al. Diabetes mellitus alters the mechanical properties of the native tendon in an experimental rat model. J Orthop Res. 2011;29:880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. J Biol Chem. 1995;270:5872–5876. [DOI] [PubMed] [Google Scholar]
- 51. Fields GB. A model for interstitial collagen catabolism by mammalian collagenases. J Theor Biol. 1991;153:585–602. [DOI] [PubMed] [Google Scholar]
- 52. Miller EJ, Harris ED, Chung E, et al. Cleavage of type I1 and I11 collagens with mammalian collagenase: site of cleavage and primary structure at the NH2-terminal portion of the smaller fragment released from both collagens. Biochemistry. 1976;15:787–792. [DOI] [PubMed] [Google Scholar]
- 53. Mitchell PG, Magna HA, Reeves LM, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ohuchi E, Imai K, Fujii Y, et al. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–2451. [DOI] [PubMed] [Google Scholar]
- 55. Shimada T, Nakamura H, Ohuchi E, et al. Characterization of a truncated recombinant form of human membrane type 3 matrix metalloproteinase. Eur J Biochem. 1999;262:907–914. [DOI] [PubMed] [Google Scholar]
- 56. Mackay R, Hartzler L, Pelina M D, et al. Studies on the ability of 65-kDa and 92-kDa tumor cell gelatinases to degrade type IV collagen. J Biol Chem. 1990;265:21929–21934. [PubMed] [Google Scholar]
- 57. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi:10.1146/annurev.cellbio.17.1.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lauer-Fields JL, Juska D, Fields GB. Matrix metalloproteinases and collagen catabolism. Biopolymers. 2002;66:19–32. [DOI] [PubMed] [Google Scholar]
- 59. Chiquet M, Gelman L, Lutz R, et al. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta. 2009;1793:911–920. doi:10.1016/j.bbamcr.2009.01.012 [DOI] [PubMed] [Google Scholar]
- 60. Abrahamsson S-O, Lohmander S. Differential effects of insulin-like growth factor-I on matrix and DNA synthesis in various regions and types of rabbit tendons. J Orthop Res. 1996;14:370–376. [DOI] [PubMed] [Google Scholar]
- 61. Schild C, Trueb B. Mechanical stress is required for high-level expression of connective tissue growth factor. Exp Cell Res. 2002;274:83–91. [DOI] [PubMed] [Google Scholar]
- 62. Yang G, Crawford RC, Wang JH-C. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J Biomech. 2004;37:1543–1550. [DOI] [PubMed] [Google Scholar]
- 63. Hansson H-A, Engström AMC, Holm S, et al. Somatomedin C immunoreactivity in the Achilles tendon varies in a dynamic manner with the mechanical load. Acta Physiol Scand. 1988;134:199–208. [DOI] [PubMed] [Google Scholar]
- 64. Kessler D, Dethlefsen S, Haase I, et al. Fibroblasts in mechanically stressed collagen lattices assume a “synthetic” phenotype. J Biol Chem. 2001;276:36575–36585. [DOI] [PubMed] [Google Scholar]
- 65. Heinemeier KM, Olesen JL, Haddad F, et al. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types: collagen and TGF-β-1 expression in exercised tendon and muscle. J Physiol. 2007;582:1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Heinemeier KM, Olesen JL, Schjerling P, et al. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol. 2007;102:573–581. [DOI] [PubMed] [Google Scholar]
- 67. Olesen JL, Heinemeier KM, Haddad F, et al. Expression of insulin-like growth factor I, insulin-like growth factor binding proteins, and collagen mRNA in mechanically loaded plantaris tendon. J Appl Physiol (1985). 2006;101:183–188. [DOI] [PubMed] [Google Scholar]
- 68. Dideriksen K, Sindby AKR, Krogsgaard M, et al. Effect of acute exercise on patella tendon protein synthesis and gene expression. SpringerPlus 2013;2:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Heinemeier KM, Bjerrum SS, Schjerling P, Kjaer M. Expression of extracellular matrix components and related growth factors in human tendon and muscle after acute exercise: Exercise effect on tendon and muscle matrix. Scand J Med Sci Sports. 2013;23: E 150–e161. [DOI] [PubMed] [Google Scholar]
- 70. Sullivan BE, Carroll CC, Jemiolo B, et al. Effect of acute resistance exercise and sex on human patellar tendon structural and regulatory mRNA expression. J Appl Physiol. 2009;106:468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hansen M, Miller BF, Holm L, et al. Effect of administration of oral contraceptives in vivo on collagen synthesis in tendon and muscle connective tissue in young women. J Appl Physiol. 2009;106:1435–1443. [DOI] [PubMed] [Google Scholar]
- 72. Miller BF, Hansen M, Olesen JL, et al. No effect of menstrual cycle on myofibrillar and connective tissue protein synthesis in contracting skeletal muscle. Am J Physiol-Endocrinol Metab. 2006;290:E163–E168. [DOI] [PubMed] [Google Scholar]
- 73. Hansen M, Skovgaard D, Reitelseder S, et al. Effects of estrogen replacement and lower androgen status on skeletal muscle collagen and myofibrillar protein synthesis in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2012;67:1005–1013. [DOI] [PubMed] [Google Scholar]
- 74. Holm L, Rahbek SK, Farup J, et al. Contraction mode and whey protein intake affect the synthesis rate of intramuscular connective tissue. Muscle Nerve. 2017;55:128–130. [DOI] [PubMed] [Google Scholar]
- 75. Ducomps C, Mauriege P, Darche B, et al. Effects of jump training on passive mechanical stress and stiffness in rabbit skeletal muscle: role of collagen. Acta Physiol Scand. 2003;178:215–224 [DOI] [PubMed] [Google Scholar]
- 76. Kovanen V, Suominen H, Peltonen L. Effects of aging and life-long physical training on collagen in slow and fast skeletal muscle in rats: a morphometric and immuno-histochemical study. Cell Tissue Res. 1987;248:247–255. [DOI] [PubMed] [Google Scholar]
- 77. Perhonen M, Han X, Wang W, et al. Skeletal muscle collagen type I and III mRNA, [corrected] prolyl 4-hydroxylase, and collagen in hypobaric trained rats. J Appl Physiol. 1996;80:2226–2233. [DOI] [PubMed] [Google Scholar]
- 78. Volpi E, Kobayashi H, Sheffield-Moore M, et al. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bukhari SSI, Phillips BE, Wilkinson DJ, et al. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am J Physiol-Endocrinol Metab. 2015;308:E1056–E1065. doi:10.1152/ajpendo.00481.2014 [DOI] [PubMed] [Google Scholar]
- 80. Churchward-Venne TA, Breen L, Di Donato DM, et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr. 2014;99:276–286. [DOI] [PubMed] [Google Scholar]
- 81. Devries MC, McGlory C, Bolster DR, et al. Protein leucine content is a determinant of shorter- and longer-term muscle protein synthetic responses at rest and following resistance exercise in healthy older women: A randomized, controlled trial. Am J Clin Nutr. 2018;107:217–226. [DOI] [PubMed] [Google Scholar]
- 82. Devries MC, McGlory C, Bolster DR, et al. Leucine, not total protein, content of a supplement is the primary determinant of muscle protein anabolic responses in healthy older women. J Nutr 2018;148:1088–1095. [DOI] [PubMed] [Google Scholar]
- 83. Wall BT, Hamer HM, de Lange A, et al. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin Nutr. 2013;32:412–419. [DOI] [PubMed] [Google Scholar]
- 84. Atherton PJ, Kumar V, Selby AL, et al. Enriching a protein drink with leucine augments muscle protein synthesis after resistance exercise in young and older men. Clin Nutr. 2017;36:888–895. [DOI] [PubMed] [Google Scholar]
- 85. Holwerda AM, Paulussen KJM, Overkamp M, et al. Leucine coingestion augments the muscle protein synthetic response to the ingestion of 15 g of protein following resistance exercise in older men. Am J Physiol-Endocrinol Metab. 2019;317:E473–E482. [DOI] [PubMed] [Google Scholar]
- 86. Fuchs CJ, Hermans WJH, Holwerda AM, et al. Branched-chain amino acid and branched-chain ketoacid ingestion increases muscle protein synthesis rates in vivo in older adults: a double-blind, randomized trial. Am J Clin Nutr. 2019;110:862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Trommelen J, Holwerda AM, Pinckaers PJM, et al. Comprehensive assessment of post-prandial protein handling by the application of intrinsically labelled protein in vivo in human subjects. Proc Nutr Soc. 2021;80:221–229. [DOI] [PubMed] [Google Scholar]
- 88. van Loon LJC, Boirie Y, Gijsen AP, et al. The production of intrinsically labeled milk protein provides a functional tool for human nutrition research. J Dairy Sci. 2009;92:4812–4822. [DOI] [PubMed] [Google Scholar]
- 89. Shaw G, Lee-Barthel A, Ross ML, et al. Vitamin C–enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am J Clin Nutr. 2017;105:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Alcock RD, Shaw GC, Tee N, et al. Plasma amino acid concentrations after the ingestion of dairy and collagen proteins in healthy active males . Front Nutr 2019;6:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Paxton JZ, Grover LM, Baar K. Engineering an in vitro model of a functional ligament from bone to bone. Tissue Eng Part A. 2010;16:3515–3525. [DOI] [PubMed] [Google Scholar]
- 92. Vieira CP, Oliveira LPD, Guerra FDR, et al. Glycine improves biochemical and biomechanical properties following inflammation of the Achilles tendon: glycine improves the tendon inflammation. Anat Rec (Hoboken). 2015;298:538–545. [DOI] [PubMed] [Google Scholar]
- 93. Gómez-Guillén MC, Giménez B, López-Caballero ME, et al. Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocoll. 2011;25:1813–1827. [Google Scholar]
- 94. Bae I, Osatomi K, Yoshida A, et al. Biochemical properties of acid-soluble collagens extracted from the skins of underutilised fishes. Food Chem. 2008;108:49–54. [Google Scholar]
- 95. Regenstein JM, Zhou P. Collagen and gelatin from marine by-products. In: Shahidi F, ed. Maximising the Value of Marine by-Products. Cambridge, England: Elsevier; 2007:279–303. [Google Scholar]
- 96. Li Z, Wang B, Chi C, et al. Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatis akjei and Raja porosa. Food Res Int. 2013;51:283–293. [Google Scholar]
- 97. Zhang G, Sun A, Li W, et al. Mass spectrometric analysis of enzymatic digestion of denatured collagen for identification of collagen type. J Chromatogr A. 2006;1114:274–277. [DOI] [PubMed] [Google Scholar]
- 98. Koopman R, Crombach N, Gijsen AP, et al. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr. 2009;90:106–115. [DOI] [PubMed] [Google Scholar]
- 99. Pennings B, Boirie Y, Senden JM, et al. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. 2011;93:997–1005. [DOI] [PubMed] [Google Scholar]
- 100. Moskowitz RW. Role of collagen hydrolysate in bone and joint disease. Semin Arthritis Rheum. 2000;30:87–99. [DOI] [PubMed] [Google Scholar]
- 101. Rogers QR, Chen M-L, Peraino C, et al. Observations on protein digestion in vivo: recovery of nitrogen from the stomach and small intestine at intervals after feeding diets containing different proteins. J Nutr. 1960;72:331–339. [DOI] [PubMed] [Google Scholar]
- 102. Chen M-L, Rogers QR, Harper AE. Observations on protein digestion in vivo: further observations on the gastrointestinal contents of rats fed different dietary proteins. J Nutr. 1962;76:235–241. [DOI] [PubMed] [Google Scholar]
- 103. Skov K, Oxfeldt M, Thøgersen R, et al. Enzymatic hydrolysis of a collagen hydrolysate enhances postprandial absorption rate—a randomized controlled trial. Nutrients. 2019;11:1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Oesser S, Adam M, Babel W, et al. Oral administration of 14C labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL). J Nutr. 1999;129:1891–1895. [DOI] [PubMed] [Google Scholar]
- 105. Lis DM, Baar K. Effects of different vitamin C–enriched collagen derivatives on collagen synthesis. Int J Sport Nutr Exerc Metab. 2019;29:526–531. [DOI] [PubMed] [Google Scholar]
- 106. Edgar S, Hopley B, Genovese L, et al. Effects of collagen-derived bioactive peptides and natural antioxidant compounds on proliferation and matrix protein synthesis by cultured normal human dermal fibroblasts. Sci Rep. 2018;8:10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Proksch E, Schunck M, Zague V, et al. Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin Pharmacol Physiol. 2014;27:113–119. [DOI] [PubMed] [Google Scholar]
- 108. Miner-Williams WM, Stevens BR, Moughan PJ. Are intact peptides absorbed from the healthy gut in the adult human? Nutr Res Rev. 2014;27:308–329. [DOI] [PubMed] [Google Scholar]
- 109. Ogihara H, Saito H, Shin BC, et al. Immuno-localization of H+/peptide cotransporter in rat digestive tract. Biochem Biophys Res Commun. 1996;220:848–852. [DOI] [PubMed] [Google Scholar]
- 110. Vig BS, Stouch TR, Timoszyk JK, et al. Human PEPT1 pharmacophore distinguishes between dipeptide transport and binding. J Med Chem. 2006;49:3636–3644. [DOI] [PubMed] [Google Scholar]
- 111. Terada T, Sawada K, Irie M, et al. Structural requirements for determining the substrate affinity of peptide transporters PEPT1 and PEPT2. Pflugers Arch. 2000;440:679–684. [DOI] [PubMed] [Google Scholar]
- 112. Thwaites DT, Brown CD, Hirst BH, et al. Transepithelial glycylsarcosine transport in intestinal Caco-2 cells mediated by expression of H(+)-coupled carriers at both apical and basal membranes. J Biol Chem. 1993;268:7640–7642. [PubMed] [Google Scholar]
- 113. Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol. 2004;66:361–384. [DOI] [PubMed] [Google Scholar]
- 114. Deacon CF, Nauck MA, Toft-Nielsen M, et al. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131. [DOI] [PubMed] [Google Scholar]
- 115. van der Pijl PC, Kies AK, Ten Have GAM, et al. Pharmacokinetics of proline-rich tripeptides in the pig. Peptides. 2008;29:2196–2202. [DOI] [PubMed] [Google Scholar]
- 116. Andria G, Cucchiara S, Vizia BD, et al. Brush border and cytosol peptidase activities of human small intestine in normal subjects and celiac patients. Pediatr Res. 1980;14:812–818. [DOI] [PubMed] [Google Scholar]
- 117. Vermeirssen V, Camp JV, Verstraete W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br J Nutr. 2004;92:357–366. [DOI] [PubMed] [Google Scholar]
- 118. Shigemura Y, Akaba S, Kawashima E, et al. Identification of a novel food-derived collagen peptide, hydroxyprolyl-glycine, in human peripheral blood by pre-column derivatisation with phenyl isothiocyanate. Food Chem. 2011;129:1019–1024. [DOI] [PubMed] [Google Scholar]
- 119. Kim YS, Birtwhistle W, Kim YW. Peptide hydrolases in the brush border and soluble fractions of small intestinal mucosa of rat and man. J Clin Invest. 1972;51:1419–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hueckel HJ, Rogers QR. Urinary excretion of hydroxyproline-containing peptides in man, rat, hamster, dog and monkey after feeding gelatin. Comp Biochem Physiol. 1970;32:7–16. [DOI] [PubMed] [Google Scholar]
- 121. Weiss PH, Klein L. The quantitative relationship of urinary peptide hydroxyproline excretion to collagen degradation. J Clin Invest. 1969;48:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ichikawa S, Morifuji M, Ohara H, et al. Hydroxyproline-containing dipeptides and tripeptides quantified at high concentration in human blood after oral administration of gelatin hydrolysate. Int J Food Sci Nutr. 2010;61:52–60. [DOI] [PubMed] [Google Scholar]
- 123. Iwai K, Hasegawa T, Taguchi Y, et al. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J Agric Food Chem. 2005;53:6531–6536. [DOI] [PubMed] [Google Scholar]
- 124. Ohara H, Matsumoto H, Ito K, et al. Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J Agric Food Chem. 2007;55:1532–1535. [DOI] [PubMed] [Google Scholar]
- 125. Aito-Inoue M, Ohtsuki K, Nakamura Y, et al. Improvement in isolation and identification of food-derived peptides in human plasma based on precolumn derivatization of peptides with phenyl isothiocyanate. J Agric Food Chem. 2006;54:5261–5266. [DOI] [PubMed] [Google Scholar]
- 126. Taga Y, Iwasaki Y, Shigemura Y, et al. Improved in vivo tracking of orally administered collagen hydrolysate using stable isotope labeling and LC–MS techniques. J Agric Food Chem. 2019;67:4671–4678. [DOI] [PubMed] [Google Scholar]
- 127. Shigemura Y, Kubomura D, Sato Y, et al. Dose-dependent changes in the levels of free and peptide forms of hydroxyproline in human plasma after collagen hydrolysate ingestion. Food Chem. 2014;159:328–332. [DOI] [PubMed] [Google Scholar]
- 128. Greenhaff PL, Karagounis LG, Peirce N, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol-Endocrinol Metab. 2008;295:E595–E604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Oikawa SY, Kamal MJ, Webb EK, et al. Whey protein but not collagen peptides stimulate acute and longer-term muscle protein synthesis with and without resistance exercise in healthy older women: a randomized controlled trial. Am J Clin Nutr. 2020;111:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Praet SFE, Purdam CR, Welvaert M, et al. Oral supplementation of specific collagen peptides combined with calf-strengthening exercises enhances function and reduces pain in Achilles tendinopathy patients. Nutrients 2019;11:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. König D, Oesser S, Scharla S, et al. Specific collagen peptides improve bone mineral density and bone markers in postmenopausal women—a randomized controlled study. Nutrients. 2018;10:97.doi:10.3390/nu10010097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cúneo F, Costa-Paiva L, Pinto-Neto AM, et al. Effect of dietary supplementation with collagen hydrolysates on bone metabolism of postmenopausal women with low mineral density. Maturitas 2010;65:253–257. [DOI] [PubMed] [Google Scholar]
- 133. Clifford T, Ventress M, Allerton DM, et al. The effects of collagen peptides on muscle damage, inflammation and bone turnover following exercise: a randomized, controlled trial. Amino Acids. 2019;51:691–704. [DOI] [PubMed] [Google Scholar]
- 134. Babraj JA, Smith K, Cuthbertson DJ, et al. Human bone collagen synthesis is a rapid, nutritionally modulated process. J Bone Miner Res. 2005;20:930–937. [DOI] [PubMed] [Google Scholar]
- 135. Mertz KH, Reitelseder S, Bechshoeft R, et al. The effect of daily protein supplementation, with or without resistance training for 1 year, on muscle size, strength, and function in healthy older adults: a randomized controlled trial. Am J Clin Nutr. 2021;113:790–800. [DOI] [PubMed] [Google Scholar]
- 136. Morton RW, Murphy KT, McKellar SR, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zdzieblik D, Oesser S, Baumstark MW, et al. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: a randomised controlled trial. Br J Nutr. 2015;114:1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Phillips SM, Tipton KD, van Loon LJC, et al. Exceptional body composition changes attributed to collagen peptide supplementation and resistance training in older sarcopenic men. Br J Nutr. 2016;116:569–570. [DOI] [PubMed] [Google Scholar]
- 139. Oertzen-Hagemann V, Kirmse M, Eggers B, et al. Effects of 12 weeks of hypertrophy resistance exercise training combined with collagen peptide supplementation on the skeletal muscle proteome in recreationally active men. Nutrients. 2019;11:1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Oikawa SY, McGlory C, D'Souza LK, et al. A randomized controlled trial of the impact of protein supplementation on leg lean mass and integrated muscle protein synthesis during inactivity and energy restriction in older persons. Am J Clin Nutr. 2018;108:1060–1068. [DOI] [PubMed] [Google Scholar]
- 141. Persaud C, Forrester T, Jackson AA. Urinary excretion of 5-l-oxoproline (pyroglutamic acid) is increased during recovery from severe childhood malnutrition and responds to supplemental glycine. J Nutr 1996;126:8. [DOI] [PubMed] [Google Scholar]
- 142. Jackson AA, Badaloo AV, Forrester T, et al. Urinary excretion of 5-oxoproline (pyroglutamic aciduria) as an index of glycine insufficiency in normal man. Br J Nutr. 1987;58:207–214. [DOI] [PubMed] [Google Scholar]
- 143. Meléndez-Hevia E, de Paz-Lugo P, Cornish-Bowden A, et al. A weak link in metabolism: the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. J Biosci. 2009;34:853–872. [DOI] [PubMed] [Google Scholar]
- 144. Ham DJ, Gardner A, Kennedy TL, et al. Glycine administration attenuates progression of dystrophic pathology in prednisolone-treated dystrophin/utrophin null mice. Sci Rep. 2019;9:12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Ham DJ, Caldow MK, Chhen V, et al. Glycine restores the anabolic response to leucine in a mouse model of acute inflammation. Am J Physiol Endocrinol Metab. 2016;310:E970–E981. [DOI] [PubMed] [Google Scholar]
- 146. Mercier S, Breuillé D, Mosoni L, et al. Chronic inflammation alters protein metabolism in several organs of adult rats. J Nutr. 2002;132:1921–1928. [DOI] [PubMed] [Google Scholar]
- 147. Stetten MR. Some aspects of the metabolism of hydroxyproline, studied with the aid of isotopic nitrogen. J Biol Chem. 1949;181:31–37. [PubMed] [Google Scholar]
- 148. Zague V, de Freitas V, Rosa M da C, et al. Collagen hydrolysate intake increases skin collagen expression and suppresses matrix metalloproteinase 2 activity. J Med Food. 2011;14:618–624. [DOI] [PubMed] [Google Scholar]
- 149. Zague V, do Amaral JB, Rezende Teixeira P, et al. Collagen peptides modulate the metabolism of extracellular matrix by human dermal fibroblasts derived from sun-protected and sun-exposed body sites: collagen peptides modulate ECM metabolism. Cell Biol Int. 2018;42:95–104. [DOI] [PubMed] [Google Scholar]
- 150. Yamada S, Yoshizawa Y, Kawakubo A, et al. Early gene and protein expression associated with osteoblast differentiation in response to fish collagen peptides powder. Dent Mater J. 2013;32:233–240. [DOI] [PubMed] [Google Scholar]
- 151. Ohara H, Ichikawa S, Matsumoto H, et al. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J Dermatol. 2010;37:330–338. [DOI] [PubMed] [Google Scholar]
- 152. Shigemura Y, Iwai K, Morimatsu F, et al. Effect of prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on growth of fibroblasts from mouse skin. J Agric Food Chem. 2009;57:444–449. [DOI] [PubMed] [Google Scholar]
- 153. Taga Y, Kusubata M, Ogawa-Goto K, et al. Highly accurate quantification of hydroxyproline-containing peptides in blood using a protease digest of stable isotope-labeled collagen. J Agric Food Chem. 2014;62:12096–12102. [DOI] [PubMed] [Google Scholar]
- 154. Taga Y, Kusubata M, Ogawa-Goto K, et al. Identification of collagen-derived hydroxyproline (Hyp)-containing cyclic dipeptides with high oral bioavailability: efficient formation of cyclo(X-Hyp) from X-Hyp-Gly-type tripeptides by heating. J Agric Food Chem. 2017;65:9514–9521. [DOI] [PubMed] [Google Scholar]