Abstract
Cereal grains are the main dietary source of energy, carbohydrates, and plant proteins world-wide. Currently, only 41% of grains are used for human consumption, and up to 35% are used for animal feed. Cereals have been overlooked as a source of environmentally sustainable and healthy plant proteins and could play a major role in transitioning towards a more sustainable food system for healthy diets. Cereal plant proteins are of good nutritional quality, but lysine is often the limiting amino acid. When consumed as whole grains, cereals provide health-protecting components such as dietary fiber and phytochemicals. Shifting grain use from feed to traditional foods and conceptually new foods and ingredients could improve protein security and alleviate climate change. Rapid development of new grain-based food ingredients and use of grains in new food contexts, such as dairy replacements and meat analogues, could accelerate the transition. This review discusses recent developments and outlines future perspectives for cereal grain use.
Keywords: cereal food, health, nutrient, proteins, sustainable diet, whole grains
INTRODUCTION
The current global food system places unsustainable pressure on the environment through land use, freshwater depletion, increased greenhouse gas emissions, disruption of the nitrogen and phosphorus cycles, biodiversity loss, and pollution. At the same time, an unhealthy diet low in fruits, vegetables, legumes, whole grains, nuts and seeds, milk, seafood, n-3 fatty acids, n-6 fatty acids, calcium and fiber, combined with high intake of red and processed meat, sugar-sweetened beverages, trans-fatty acids, and sodium, poses a major risk of non-communicable diseases (NCDs) such as cardiovascular disease, type 2 diabetes, and some cancers.1 Low whole-grain intake has been identified as the major disease risk factor for NCDs in most WHO regions.1 Environmental, social, and economic sustainability are all highly interrelated with health. FAO/WHO provides guiding principles on what constitutes a “Sustainable Healthy Diet,” taking a holistic approach to diets by considering international nutrition recommendations, the environmental costs of food production and consumption, and adaptability to local, social, cultural, and economic contexts.2 Some countries already include environmental sustainability aspects in their food-based dietary guidelines,3–5 but a wider transition, including emphasis on the integration of the environmental, cultural, and economic dimensions of sustainability, is needed to reach the global nutrition and diet-related NCD targets in line with the United Nations Sustainable Development Goals.
Increased use of plant-based foods to replace animal-based foods is one feasible strategy for reaching the targets, particularly in the Western world.6 With increasing standard of living, economic growth, and globalization, animal protein intake from meat, milk, and dairy products has expanded,7 with negative impacts not only on health and environmental sustainability, but also on animal welfare. This transition has led to increased greenhouse gas emissions and extensive use of arable land for feed production.
Food production is responsible for 26% of global greenhouse gas emissions.8 Production of beef, lamb and mutton, and cheese requires the greatest acreage to produce 1000 kcal energy, followed by milk, pig, and poultry production.9 Ruminants, however, while producing large amounts of emissions themselves, are fed on grassland not always suitable for crop production.
Even small changes in food consumption patterns can have large impacts on ecosystems. Healthy diets consisting of foods produced in a sustainable and resilient food system need to be regionally adapted to meet consumer expectations and to exploit the advantages of differences in production conditions.3 Healthy cereal foods, especially whole grains,6 have unused potential in this respect.
At a global level, cereal grains are the major source of energy and carbohydrates (including dietary fiber) and one of the major plant protein sources in the human diet .10 Sustainable cereal production systems involve enhanced biodiversity, living soils, use of integrated pest management, and low greenhouse gas emissions, simultaneously producing high-quality food and maintaining food security.11 They produce a low environmental footprint compared with the production systems for many animal-based raw materials, and the production of affordable products that can be used in many different local contexts.12–14 The health effects of cereal foods depend on the type of product. The intake of whole-grain cereal foods is consistently associated with health benefits at the population level, and increased consumption is therefore warranted and advocated in official dietary guidelines in many countries.15 However, the potential of grains, especially whole grains, as an important animal protein alternative has been neglected by scientists and the wider community. Despite the significant protein content of cereals, a recent review of food-based dietary guidelines from 90 countries revealed that none of these mention cereals as source of proteins; instead, they specifically mention meat (53% of national guidelines), poultry (29%), fish (58%), eggs (31%), legumes (41%), and sometimes dairy (9%), nuts/seeds (8%), and insects (only Kenya).16
The aim of the present review was to highlight the potential for grains and cereal foods to replace animal-based proteins as a source of proteins, simultaneously providing high-quality carbohydrates and energy as part of a healthy diet with a low environmental footprint. A further aim was to identify the knowledge gaps in and prospects for more efficient use of cereals in sustainable and healthy diets.
CEREAL GRAINS – AN IMPORTANT COMPONENT OF A SUSTAINABLE DIET
Cereal grains are produced world-wide for direct food use and for animal feed. Wheat and maize are produced in the largest annual amounts (766 and 1148 million metric tons, respectively), followed by rice (755 million metric tons) (Figure 1A).17 Barley, sorghum, and oats are other globally important cereal crops. Up to 40% of all cereal grains produced annually is used as animal feed (Figure 1B). As grains are often used in a refined form in food products, large volumes of side streams are produced. The annual amount of bran produced is around 160 million metric tons,17 16 million metric tons of which is protein (assuming a moderate 10% protein content for bran). The current main use of these side streams is for animal feed. However, it is widely recognized that the use of food-grade raw materials as animal feed poses a sustainability problem, and that a reduction in the use of food-competing feedstuffs would be beneficial.18 It would be desirable to shift cereal grain use towards direct human consumption.
Figure 1.
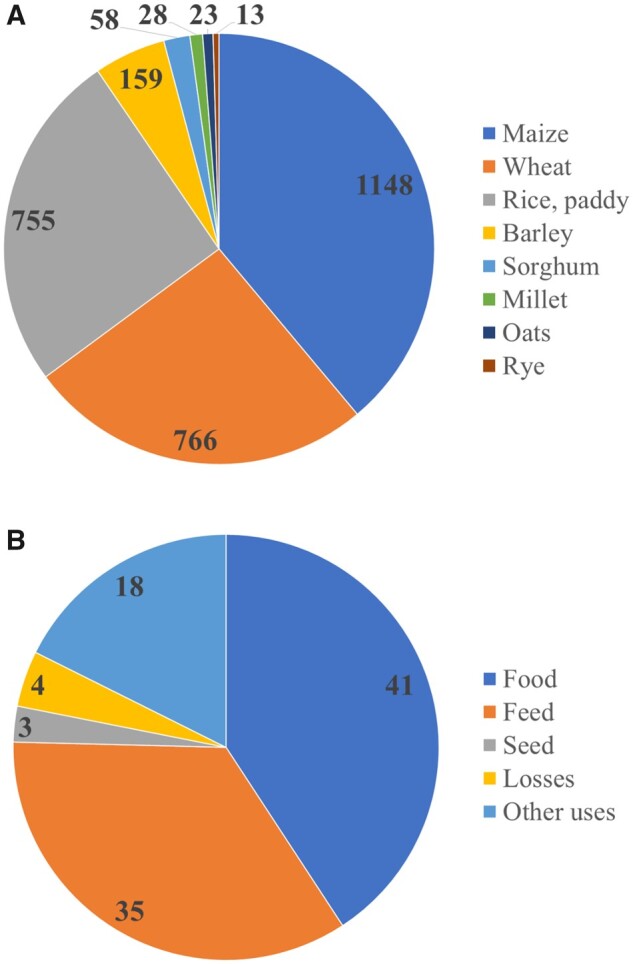
(A) Global production of cereal species (million metric tons/year). (B) Global uses and losses of cereals (%/year).
Data from FAOSTAT (2013, 2019).17
Cereals already constitute one of the most important protein sources in human diets.19 Grain production requires approximately 4.6 m2 of land per 100 g of protein , whereas 163.6 m2 are required to produce 100 g of beef protein (Table 1).9 From the perspective of greenhouse gas emissions, the production of 100 g of grain protein releases 2.7 kg CO2-equivalents of emissions, whereas 49.9 kg, 7.6 kg, and 5.7 kg of CO2-equivalents are released to produce 100 g of beef, pork, and poultry protein, respectively (Table 1). Replacing animal-based proteins with cereal grain proteins, especially in developed economies, would thus give significant reductions in greenhouse gas emissions and increased efficiency of land use.20
Table 1.
Environmental burden caused globally by production of 100 grams of protein from different sources
| Source | Land use, m2 | CO2-equivalents, kg | Freshwater withdrawals, L | Eutrophying emissions, g |
|---|---|---|---|---|
| Grains | 4.6 | 2.7 | ||
| − Maize | 3.1 | n.a. | 227 | 4.2 |
| − Rice | 3.9 | n.a. | 3167 | 49.4 |
| − Wheat and rye | 3.2 | n.a. | 531 | 5.9 |
| − Oatmeal | 5.8 | n.a. | 371 | 8.6 |
| Tofu | 2.2 | 1.98 | 93 | 3.9 |
| Peas | 3.4 | 0.44 | 178 | 3.4 |
| Groundnuts | 3.5 | 1.23 | 708 | 5.4 |
| Other legumes | 7.2 | 0.84 | 204 | 8.0 |
| Nuts | 7.9 | 0.26 | 2531 | 11.7 |
| Milk | 27.1 | 9.5 | 1904 | 32.3 |
| Cheese | 39.8 | 10.82 | 2539 | 44.6 |
| Dairy herd | 21.9 | 16.87 | 1375 | 185.1 |
| Beef herd | 163.6 | 49.89 | 728 | 151.2 |
| Pig meat | 10.7 | 7.61 | 1110 | 47.2 |
| Lamb and mutton | 184.8 | 19.85 | 901 | 48.5 |
| Poultry meat | 7.1 | 5.7 | 381 | 28.1 |
| Fish, farmed | 3.7 | 5.98 | 1619 | 103.1 |
| Prawns, farmed | 2.0 | 18.19 | 2380 | 153.8 |
| Eggs | 5.7 | 4.21 | 521 | 19.6 |
Data from Ritchie and Roser (2020)9 Abbreviations: n.a., not available.
CEREAL FOODS – A NUTRIENT-DENSE PROTEIN SOURCE
Grains as staple food
Cereal grains contribute about 50% of dietary energy globally, and the contribution is higher in developing countries.21 Global consumption of cereal foods is 176 kg per capita per year, or around 480 g per capita per day. Rice is the largest single cereal food consumed globally, followed by wheat and maize, which have relatively higher use as feed. Sorghum, millet, barley, rye, and oats are also used world-wide for human consumption (Table 2).17 With respect to protein intake, rice and wheat are clearly the most important sources (Table 2).
Table 2.
Global food supply of cereal grains and dietary protein in 2017
| Grain | Food consumption (kg per capita per year) | Protein provided (g per capita per day) | Food uses |
|---|---|---|---|
| Rice and products | 81.4 | 10.3 | Bakery products, breakfast cereals, extruded snacks, gluten-free foods, porridge, baby foods, fermented beverages, dairy analogues |
| Wheat and products | 65.3 | 16.0 | Bakery products, breakfast cereals, extruded snacks, pasta, porridge, baby foods, meat and dairy analogues |
| Maize and products | 20.0 | 3.9 | Bakery products, gluten-free foods, extruded snacks, breakfast cereals, baby foods |
| Sorghum and products | 3.3 | 0.8 | Bakery products, gluten-free foods, porridge |
| Millet and products | 2.8 | 0.6 | |
| Barley and products | 1.1 | 0.2 | Bakery products (bread and biscuits mainly) |
| Rye and products | 0.6 | 0.1 | Bakery products (mainly bread and crackers), extruded snacks, porridge |
| Oats and products | 0.6 | 0.1 | Bakery products, breakfast cereals and flakes, porridge, baby foods, gluten-free foods, meat and dairy analogues |
| Other cereals | 1.0 | 0.2 | |
| Total | 176.1 = 480 g/d | 32.2 |
Data from FAOSTAT (2017).17
There is large variation in the amount and type of grains used for food in different parts of the world. Porridge is one of the original processed cereal foods, and cereals in different forms are still an important breakfast item. Bread is a common staple food and an important snack item, especially in the Western world, whereas cooked rice forms the basis of the Asian kitchen. Pasta, noodles, and various cooked grains such as couscous are other globally important cereal foods.
Grains are most often milled prior to food use to remove the outer parts of the grain, which are used for feed or other non-food use. This means that dietary fiber, minerals, and phytochemicals are removed from food use, and refined grains are therefore less sustainable.6 Whole-grain food products, as the name indicates, contain all parts of the grain (endosperm, germ, bran), in the same relative proportions as in the intact caryopsis.22 Refined products are primarily based on the starchy endosperm and mainly contribute energy, with only small amounts of vitamins, minerals, fiber, and bioactive phytochemicals, which are mainly found in the germ and bran parts. Table 3 shows the compositional difference between whole-grain flour and refined flour, using wheat as an example.23 Whole-grain foods have differing national definitions22 and are included in many national dietary recommendations.15 Recommended intake is highest in the Scandinavian countries (approximately 75 g whole grains per 10 MJ or 2400 kcal), as is the estimated intake (41–58 g/d ).15 Based on data from 266 country-specific nutrition surveys, the global mean whole-grain intake in 2010 was 38 g/day (95% confidence interval [CI] 1.3–334.3 g/d).24 Only 7.6% of the global adult population was estimated to consume 50 g of whole grain daily in that year, with substantial regional variation.24 There is thus unexploited potential for increasing intake of whole grains.
Table 3.
Impact of refining on the nutrient content of wheat
| Wheat |
||
|---|---|---|
| Wholegraina | Refinedb | |
| Energy (kcal) | 332 | 364 |
| Protein (g) | 9.6 | 10.3 |
| Carbohydrate (g) | 74.5 | 76.3 |
| Fat (g) | 2.0 | 1.0 |
| Fiber (g) | 13 | 2.7 |
| Minerals | ||
| Calcium, Ca (mg) | 33 | 15 |
| Iron, Fe (mg) | 3.7 | 1.2 |
| Magnesium, Mg (mg) | 117 | 22 |
| Potassium, K (mg) | 394 | 107 |
| Sodium, Na (mg) | 3 | 2 |
| Zinc, Zn (mg) | 3.0 | 0.7 |
| Selenium, Se (mg) | 12.7 | 33.9 |
| Group B vitamins | ||
| Thiamin (mg) | 0.3 | 0.1 |
| Riboflavin (mg) | 0.2 | 0.04 |
| Niacin (mg) | 5.3 | 1.3 |
| Folate, total (µg) | 28 | 26 |
Data from USDA (2019).23
Wheat flour, whole-grain, soft wheat.
Wheat flour, white, all-purpose, unenriched.
Grains as source of nutrients
Cereal grains have long been recognized as the most important source of carbohydrates, including dietary fiber, in the human diet, but their contribution to protein intake has received little attention. Depending on the amount and quality of grains consumed within adult populations, grains are an important source of energy (30% of intake), proteins (25%–30%), carbohydrates (40%–45%), fiber (40%–60%), and vitamins and minerals such as thiamin (25%–35%), folate (30%–35%), iron (40%–45%), calcium (10%–30%), and selenium (20%).10,25 The bioavailability of these nutrients depends on the processing system, eg, fermentation can increase the bioavailability of nutrients in grain products.26 However, cereal foods can also be a major source of added sodium, depending on the product category consumed,27 with bread in particular being a major contributor.
Cereal foods are the largest plant protein source globally, providing on average more protein in the diet than meat products provide in Africa, Central America, Asia, and Europe (Table 4).17 Total average dietary protein intake ranges from 67.8 g/day (Africa) to 112.5 g/day (Northern America), while protein intake from cereals shows a narrower range, from 23.7 g/day (Oceania) to 34.2 g/day (Africa). The differences in animal protein intake on the different continents are thus larger than the differences in cereal protein intake. The recommended daily protein intake is about 50–70 g/day; thus, overconsumption of protein (and dietary energy) is common. Viewed within this context, a reduction in animal protein consumption without any replacement would add to environmental sustainability, and concurrently increase the share of grain protein in the diet.
Table 4.
Protein intake (g/capita/d) from different sources on different continents
| Area | Total protein intake | Animal | Meat | Plant | Cereal |
|---|---|---|---|---|---|
| Africa | 68.1 | 15.2 | 6.8 | 52.9 | 34.3 |
| Northern America | 112.8 | 71.8 | 40.0 | 41.0 | 24.1 |
| Central America | 85.6 | 39.7 | 19.5 | 46.0 | 32.5 |
| South America | 87.4 | 47.5 | 29.0 | 40.0 | 24.5 |
| Asia | 80.1 | 28.2 | 10.8 | 51.9 | 33.7 |
| Europe | 102.7 | 58.3 | 26.0 | 44.4 | 30.5 |
| Oceania | 101.3 | 64.7 | 34.3 | 36.6 | 23.0 |
Data from FAOSTAT (2018).17
Cereal fiber and phytochemicals
Cereal grains are a key source of dietary fiber and phytochemicals, and (whole) grain consumption is a key source of these components of the diet, beyond provision of energy and proteins. Grains contribute about half of the total dietary fiber intake in Western societies and contain about 10%–20% of dietary fiber, but the amount present in cereal foods depends on the refining process during milling. Dietary fiber is concentrated in the outer grain layers, and the content is thus higher in whole-grain foods than in refined cereal foods. Cereal fiber has various physiological functions throughout the gastrointestinal tract, and high fiber intake is considered health-promoting. On the other hand, short-chain oligosaccharides (such as fructans in, eg, wheat and rye) may cause bloating and flatulence in sensitive individuals, due to luminal effects such as absorbing water and serving as an easily fermentable substrate for the colon microbiota. These oligosaccharides belong to the group of fermentable mono-, di-, and oligosaccharides and polyols, referred to in combination as FODMAPS.28 FODMAPS have also been associated with a condition referred to as “non-celiac gluten sensitivity,” although the evidence for the association is weak.29 Grains contain various phytochemicals typically associated with the dietary fiber. From a dietary perspective, phytochemicals are regarded as non-nutrients but, since they possess various bioactivities, they are important for the metabolic implications of food and associated positive health impacts.30
All grains are rich sources of dietary phytochemicals, but the amounts and quality vary between cereal species. Whole-grain wheat contains, eg, alkylresorcinols, benzoxazinoids, lignans, phenolic acids, phytosterols, and tocols.31 The same compound classes are also present in rye, which (due to its primary use as a whole-grain product) is an important contributor to dietary phytochemical intake, especially in the hemiboreal region.32,33 Oats are rich in a unique group of alkaloids first discovered in oats, namely avenanthramides and avenacosides A and B.34 In addition, oat grains are rich in phenolic compounds, carotenoids, and phytosterols, including β-sitosterol, sitostanol, campesterol, and campestanol.35 Rice bran is particularly rich in a unique group of phytochemicals, oryzanols, that are conjugates of ferulic acid esters of phytosterols and triterpenoids.36
Various aspects affect the composition and bioavailability of phytochemicals in dietary cereals. Food processing steps such as baking, cooking, extrusion, and puffing offer the potential to enhance the content and bioavailability of phytochemicals.37 Once digested, the composition and function of the gut microbiota largely govern the form in which phytochemical compounds are eventually absorbed into the circulation and human metabolism.32
CEREAL PROTEINS
Composition and types
The protein content of cereal grains varies between 7% and 18% of dry matter (Table 5), depending on the species and variety.19,38–47 Most grain proteins are located in the endosperm, but the aleurone and subaleurone layers of the bran have the highest protein content.48 Cereal proteins are generally classified based on their solubility in water (albumins), saline (globulins), aqueous alcohol (prolamins), or acid/base solutions (glutelins).48 In wheat, barley, maize, sorghum, and millet, the major storage proteins are prolamins, while in oats and rice the main storage proteins belong to the legumin-like globulins and glutelins, respectively. Albumins and globulins contain more essential amino acids than prolamins and glutelins. For example, in wheat and rice, bran proteins contain three times more lysine than endosperm proteins.49–51 This means that production of refined flour not only alters the protein content, but also the quality of the proteins in the flour. In the context of sustainable diets, the selection of grain cultivars with high-yield production and reduced nitrogen requirement should be prioritized.52 Nitrogen fertilization increases grain yield and protein content in the grain. However, the consequences are not straightforward, and the effects vary among different cultivars due to genetic differences.53 In some studies, grain yield has even been negatively correlated with grain protein content.54
Table 5.
Protein types and digestible indispensable amino acid scores (DIAAS, %) of plant- and animal-based foods
| Food | Main protein types and protein content (%) in food groups | DIAAS, % | First limiting AA | Reference |
|---|---|---|---|---|
| Cereal grains | Albumins (1–12%), globulins (4–80%), prolamins (4–60%), glutelins (<10–80%) | 38 , 39 , 46 | ||
| Barley | 7–15 | 51 | Lys | 47 |
| Maize | 9–12 | 48 | Lys | 47 |
| Millet | 6–16 | 7a | Lys | 39 , 40 |
| Oats | 9–16 | 77 | Lys | 47 |
| Rice | 7–8 | 64 | Lys | 47 |
| Rye | 8–18 | 47 | Lys | 47 |
| Sorghum | 10–11 | 29 | Lys | 19 , 47 |
| Wheat | 8–18 | 43 | Lys | 47 |
| Legumes | Albumins (10–30%), globulins (45–70%) | 41 | ||
| Pea protein concentrate | 54 | 73 | SAA | 42 |
| Soy flour | 52 | 105 | SAA | 42 |
| Soy protein isolate | 93 | 98 | SAA | 42 |
| Meat | Sarcoplasmic proteins (30–35%), myofibrillar proteins (55–60%), stromal proteins (10–15%) | 43 | ||
| Beef jerky | 49 | 120 | SAA | 45 |
| Bologna (pork) | 12 | 128 | Leu | 45 |
| Ground beef, raw | 18 | 121 | Leu | 45 |
| Ground beef, cooked | 31 | 99 | Leu | 45 |
| Ribeye roast, 56°C | 24 | 111 | Val | 45 |
| Ribeye roast, 64°C | 26 | 130 | Val | 45 |
| Ribeye roast, 72°C | 26 | 107 | Val | 45 |
| Salami (pork) | 17 | 120 | Val | 45 |
| Dairy | Caseins (80%), whey proteins (14%), fat globule membrane proteins (2%) | 44 | ||
| Milk protein concentrate | 68 | 141 | SAA | 42 |
| Skimmed milk powder | 35 | 123 | SAA | 42 |
| Whey protein concentrate | 78 | 133 | His | 42 |
| Whey protein isolate | 85 | 125 | His | 42 |
Recommended amino acid (AA) score calculated for children older than 36 months, adolescents, and adults, using pig as the model organism.
Determined from cooked broomcorn millet (Panicum miliaceum L.) for a 0.5–3-year-old child.Abbreviations. His, histidine; Leu, leucine; Lys, lysine; SAA, sulfur amino acids; Val, valine. Davies (2020),19 Cervantes-Pahm et al. (2014),47 Mäkinen et al. (2016),38 Taylor and Taylor (2017),39 Han et al. (2019),40 Boye et al. (2010),41 Mathai et al. (2017),42 Yu et al. (2017),43 Bär et al. (2019),44 Bailey et al. (2020),45 Loveday (2019)46
Digestibility and bioavailability
Protein digestibility can be determined both in vitro and in vivo. In vitro, digestibility is measured by monitoring the amount of soluble proteins, changes in digesta pH (reflecting protein hydrolysis), or the level of increase in the nitrogen not incorporated in protein structures (ie, nonprotein nitrogen), eg.26 In vivo, protein digestion can be determined as true ileal digestibility; this approach compares the amount of ingested amino acids with the amount of amino acids recovered from human or animal ileal digesta.55 In addition, methods utilizing stabile isotope labels to evaluate the metabolic availability of cereal amino acids in vivo (ie, bioavailability) have been developed; these methods aim to trace the fate of limiting amino acids during digestion, absorption, and metabolic utilization.56,57
Proteins differ in their digestibility, depending on the content of essential amino acids and on the interplay of proteins with other polymers in the food matrix.46 For example, the ileal digestibility of proteins derived from raw polished white rice and from dehulled oat grains is higher than that of proteins derived from maize, barley, rye, and wheat, indicating that the legumin-like proteins, ie, globulins and glutelins, are more digestible than prolamin.47 Whole-grain rice contains many nutritionally valuable components,49 although it has been suggested that the lack of antinutritional factors, such as phytates, in white rice supports the metabolic availability of rice protein.56 Fiber and other plant tissue structures have been demonstrated to reduce protein digestibility in both in vitro and human trials, but this effect might differ between individuals and also depend on the type of fibre.58 Although whole-grain foods provide health benefits over refined grains, their proteins are likely less bioavailable.
Regardless of the source, different protein types are digested with different efficiencies. For example, in rice bran protein, prolamins show the lowest degree of hydrolysis and glutelins the highest, while albumins and globulins are hydrolyzed similarly, but to a lower degree than the glutelin fraction.59 Disulfide bonds may cause differences in the in vitro digestibility of cereal proteins.60 In addition, the level of antinutritional factors, protein localization, conformation, and environmental variables all affect protein digestibility. For example, irrigation has been shown to improve calculated amino acid indices and scores for winter wheat, while nitrogen fertilization has been suggested to decrease the values.61 In addition, the in vitro digestibility of protein in wheat varieties may differ depending on whether they have been cultivated organically or conventionally, with organic cultivation generally suggested to increase in vitro digestibility.62
Lysine often is the limiting amino acid in cereal grain foods, meaning that a low level of lysine suppresses efficient protein metabolism if no supplementary source of lysine is included in the meal or diet.40,47 The metabolic availability of individual amino acids within a food may also vary significantly, with the availability of the limiting amino acid determining the level to which all other amino acids can be utilized in protein synthesis and other metabolic activities.57 Thus, FAO recommends considering dietary amino acids as individual nutrients and utilizing digestible indispensable amino acid score (DIAAS) as an indicator of protein quality.55 DIAAS represents protein quality as the ratio of a digestible dietary indispensable amino acid in 1 g of the test protein to the same dietary indispensable amino acid in 1 g of a reference protein reflecting the amino acid composition of breast milk and human tissue protein.55 DIAAS (%) is calculated for each amino acid, and the amino acid with the lowest score is used as an indicator of protein quality.
Lysine in polished raw white rice and dehulled oat grains has a DIAAS score of 64% and 77%, respectively (Table 5). In comparison, DIAAS is above 100% for the first limiting amino acids in milk proteins.42 Most DIAAS values determined for cereal grains suggest that consuming cereals as the sole protein source could result in protein deficiency. However, pre-treatment of cereal raw materials can affect the DIAAS score.45 Pre-treatment can also affect the in vivo bioavailability. For example, in an indicator amino acid study on healthy young men, L-lysine in polished and cooked white rice was found to be highly available for human metabolism in vivo, with 97% being digested, absorbed, and used in protein synthesis.56 In a similar in vivo study on cooked white cornmeal, the metabolic availability of lysine was found to be 71% in healthy young men, while 80% of tryptophan was metabolically available.57
As discussed above, nitrogen fertilization affects total protein yield, but it also has a detrimental effect in protein quality, reducing the protein-digestibility-corrected amino acid score (PDCAAS) because the accumulation of Lys is lower than that of the total protein.61
Technological functionality
In addition to their nutritional role, proteins also have an essential structuring role in foods, due to their functional properties such as solubility, oil/water binding, texturizing, foaming, emulsification, gel formation, and fibrillation. These contribute to the overall food quality and to sensory attributes. Compared with animal proteins, the applicability of cereal proteins is restricted by their large molecular weight and limited solubility in water in the neutral and mildly acidic conditions typical of most food products.63 In general, high solubility enhances emulsification, foaming, and gelation of protein-rich ingredients. In addition to protein, other cereal components such as fiber and starch also have a considerable effect on the ingredient properties, so that the functionality of less-purified cereal ingredients deviates widely from that of highly pure animal-based proteins.46 The protein content in cereals (7%–18%) is lower than that in pulses (17%–30%), and pulses contain higher amounts of water and salt-soluble albumins and globulins.41,64 The functionality of cereal proteins is highly dependent on their specific types and relative amounts in the raw material (Table 5). For example, owing to the viscous protein gliadin and the elastic component glutenin, wheat proteins result in a protein network, yielding a cohesive and elastic mass when hydrated and mixed. This property can be further exploited because it forms an appealing structure and texture for bakery and extruded cereal foods, including meat analogues made from wheat fractions. Proteins in other cereal grains do not have these special properties and thus their techno-functional quality is limited.
CHALLENGES AND POSSIBILITIES OF INCREASING PROTEIN INTAKE FROM GRAINS
Consumer perspective
Shifting consumption behavior from animal to plant protein in economically strong cultures is a complex issue involving cultural, culinary, economic, and psychological factors.65 To succeed, it will be important to rely more on culinary and environmental aspects in order to effectively stimulate diet changes.65 Increasing the share of plant protein in the diet must become an integral part of regional and social processes in society, and not just rely on individual interest. This was demonstrated in a study in Finland, where eating motives were shown to be important for increasing plant protein intake, and the role of social aspects of eating in connection with certain foods was emphasized.66 Cereal foods can play an important role in the shift from animal to plant protein, but there are barriers that need to be overcome.
Although cereal foods are a staple item in the traditional diet of much of the world’s population, most food items are made of refined grains, which lack many of the nutrients of the outer grain layers. As awareness of the health benefits of eating whole-grain foods has increased, attention has turned towards increasing consumer uptake of whole-grain foods. Taste, convenience, and price are dominant motivators relevant to food choice, and the potential of applying the “food pleasure” concept in social marketing programs has been pointed out.67 The sensory characteristics of whole-grain foods may limit consumption. Proteolysis and Maillard reaction products, phenolic compounds, and lipid deterioration may cause bitterness during processing, while resistant cell wall structures influence the texture resulting from the processing of whole-grain foods.68 Flavor and texture design is thus important in determining the consumption of healthy cereal foods. Gradual addition of whole-grain ingredients to refined grain products has been suggested as a way to increase consumer acceptance and intake of whole grains.69 Consumer awareness of healthy whole-grain foods and their confidence in the health benefits is important, but difficulties in identifying, eg, whole-grain bread have been perceived as a barrier to consumption, especially among consumers with a lower education level.70
Effects of processing on proteins in the cereal food matrix
The nature of the processing during ingredient production and food manufacturing is critical for the applicability, digestibility, and nutritional quality of proteins. Processing also largely determines the sensory quality (flavor and texture) of foods, which is important for consumer acceptability. Most research on cereal protein food processing to date has focused on wheat, followed by maize and rice, but other cereal grains (eg, oats, sorghum, millet, barley) are gaining interest. The functionality of wheat gluten is well known, while the limited techno-functional properties of other cereal ingredients and proteins can be improved by physical (eg, milling, microfluidization, ultrasonication), hydrothermal (eg, steaming, extrusion, cooking), and biochemical (eg, chemical, enzymatic, fermentation) processing. Processing is also important in protein-enriched cereal ingredients produced using, eg, wet or dry separation technologies.
Physical tools applied in the functionalization of whole-grain cereal flours usually target modification of the structure of the raw materials. Dry milling results in particle size reduction and has been proven to have beneficial impacts on cereal bran through the liberation of physically entrapped proteins from inside insoluble fibrous cell wall structures, improving protein solubilization.50,71 For wheat bran, a median particle size of 400 µm has been shown to be optimal for in vitro protein digestibility.72 Harsh milling conditions have been linked with reduced protein solubility of rye proteins, due to heat generation or particle aggregation.73 In addition to milling, dry processing also includes dry fractionation, by sieving, air classification, or electrostatic separation, which can all be applied to increase the protein content of cereal ingredients. Air classification can enrich proteins from various cereal flours and brans, and improve the properties of ingredients compared with the raw materials or protein isolates.74,75 For example, an air-classified protein-enriched fraction from rice bran has been shown to exhibit higher protein solubility than the raw bran.76
Microfluidization for improved functionality of cereal protein ingredients has been less studied, but has been shown, eg, to improve the dispersion stability of wheat bran and aleurone.77 Ultrasound technology has been shown to alter the techno-functional properties of cereal protein ingredients via modifications in secondary, tertiary, or even primary protein structure.78–80 It can also increase cereal protein solubility, colloidal stability, surface hydrophobicity, and emulsifying and foaming properties.80–82 Sonication affects gluten properties, further improving eg, the quality of noodles.82
Sourdough fermentation is a traditional processing method used both for bran pre-treatment and in baking. Fermentation involves an interplay between lactic acid bacteria, yeast, and the endogenous enzymes in grain flour and causes acidification, together with other biochemical changes in grain constituents, including proteolysis. Such changes influence product texture and flavor, and can potentially change the bioavailability of nutrients and phytochemicals,83 increase the level of free amino acids, and decrease antinutritional components interfering with protein digestibility.26 Microbial fermentation of rye bran enables up to 40% bran supplementation in extrusion and delivers protein-rich extrudates with a higher degree of expansion and crispiness than achieved with refined flour.84,85 In general, hydrothermal treatment can significantly affect both the techno-functional properties and digestibility of proteins. For example, it has been shown that protein digestibility increases in proofing, but decreases again during oven baking.86
Similarly to fermentation, germination and malting of cereal grains activates the endogenous enzymes in the grains, with subsequent modifications in functional properties such as flavor and texture. Germination of oats and sorghum has also been shown to improve protein solubility,87,88 and to improve protein digestibility, either by increasing protein solubility or by removal of antinutrients.89
Exogenous enzymes are widely applied in food processing, eg, enzymatic crosslinking of oat proteins is used as a tool to improve their foam and colloidal stability, and the quality of oat bread.90 Controlled enzymatic hydrolysis has been shown to improve foaming, colloidal, and emulsifying characteristics of rice proteins.91,92 Phytase treatment improves both heat-induced gelation of rice bran proteins93 and the bioavailability of rye bran proteins.94
In general, the reported studies indicate that applying different processing techniques can pave the way for development of appealing plant protein and whole-grain foods with improved protein functionality and digestibility.95
Current and potential uses of grains in meat and dairy food alternatives
Increasing numbers of plant-based meat and dairy alternatives are now available on the market, and interest and demand for these types of products are increasing in many countries. The compound annual growth rate (CAGR) for meat alternatives is expected to be 12% between 2019 and 2026, while dairy alternatives are expected to have a CAGR of 11.4% between 2020 and 2025.96,97 Soybean is the main crop used for producing meat and dairy alternatives, but cereal-based alternatives are also emerging. In recent years, oats have attracted interest in this regard, and new oat-based product launches have expanded beyond cereal foods to a diverse category encompassing spoonable products, ready-to-eat meals, ice-cream, and even chocolate.
Structured plant-based products are commonly used as meat alternatives. These include several traditional foods from East Asia, such as seitan, tempeh, and tofu. The latter 2 are made from soybean, while seitan is made from wheat gluten, utilizing the protein’s ability to form viscoelastic networks when hydrated and subjected to mechanical processing.48 Wheat, oats, barley, and sorghum have also been used for the production of tempeh.98–100
A different approach for replacing meat with cereals is the development of meat analogues, ie, products that resemble meat in terms of functionality and organoleptic characteristics in burgers, crumbles, sausages, and other products where the aim is to imitate the fibrous structure of meat. Extrusion is the most commonly used technique to produce fibrous plant-based meat analogues, although alternatives such as wet spinning and electrospinning are being explored.101,102
Meat analogues can be produced by either high- or low-moisture extrusion. Typically, low-moisture extrusion yields texturized proteins, which are often rehydrated prior to production. They absorb water rapidly, turn into sponge-like elastic structures, and are used as an ingredient in food formulation (eg, sausages and patties). During high-moisture extrusion, the ingredients are hydrated in excessive water conditions (∼70%), and proteins are melted into a viscoelastic mass by extruding with a twin-screw co-rotating extruder at relatively high temperatures (130–180°C). An essential part of high-moisture extrusion is the presence of a long cooling die, in which the proteins are aligned into a fibrous structure resembling meat.
Another rapidly growing product segment for cereals such as oats, rice, and barley is as dairy alternatives. Cereal-based dairy alternatives typically have lower protein content than their dairy counterparts,103 and may suffer from off-flavors and a chalky texture due to large cell wall fragments, lowering their acceptability among consumers.104 However, the inferior protein content and sensory quality can be improved by mixed culture fermentation. A good example of this is the synergistic effect of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus during yogurt fermentation, where the proteolytic Lactobacillus strain benefits from the nonproteolyic S. thermophilus through release of peptides and free amino acids.105 Mixed-culture fermentation of oat protein concentrate (a side stream from β-glucan extraction) with S. thermophilus and L. delbrueckii subsp. bulgaricus improves gel formation, structure, and the consumer acceptability of non-dairy yoghurt-type products.106,107
Current commercial meat and dairy analogue products still have nutritional challenges, mainly associated with a lack of essential micronutrients, the presence of saturated fats, or a substantially low protein content in plant-based drinks. Therefore, future research on processing technologies should focus on designing healthy and appealing protein alternatives. In some regions, the price of cereal-based dairy analogues may be considerably higher than that of corresponding dairy products, providing a consumption barrier for some consumer segments.
CEREAL FOODS – AN IMPORTANT COMPONENT OF A HEALTHY DIET
Health effects of cereal foods – lack of evidence on cereal proteins
The association between cereal protein intake and incident risk of disease or mortality has rarely been addressed, although studies investigating the association of dietary plant proteins vs animal proteins with disease risk may at least partly reflect the impact of cereal protein. High animal protein intake has been associated with increased cardiovascular mortality, whereas high plant protein intake has been inversely associated with all-cause and cardiovascular mortality, even after adjusting for other dietary and lifestyle factors within the population at risk of developing noncommunicable diseases.108 Similar associations have been found in meta-analyses of 12 cohort studies,109 and in a Japanese prospective cohort study.110 To the best of our knowledge, there is one observational study in which the impact of cereal proteins on cardiovascular mortality was investigated, among other sources of proteins. Within this study, cereal protein intake was not associated with lower cardiovascular mortality, but a beneficial impact was associated with the protein intake from nuts and seeds.111 The association depends strongly on the consumption patterns of the target population. However, a large number of observational studies have shown consistent inverse associations between high whole-grain intake and the risk of developing noncommunicable diseases such as type 2 diabetes, cardiovascular disease, colorectal cancer, and total and cause-specific mortality.112,113
In addition to observational studies, more than 200 dietary intervention studies have shown – depending on the cereal species – reduced body weight, decreased total cholesterol, improved systolic blood pressure,113 improved postprandial glucose and insulin homeostasis,114 decreased inflammatory markers,115 and lowered total and low-density lipoprotein cholesterol,116,117 when whole grains have been consumed, although it should be acknowledged that some results are conflicting. However, only a few studies have examined the effects of high or low levels of cereal proteins in the diet on health risk factors. One showed that an 8-week low-gluten diet induced mild changes in the intestinal microbiota composition and the urine metabolome in healthy adults, and reduced body weight.118 Fermentation by the gut microbiota of cereal fiber and protein reaching the colon may partly contribute to the health effects seen in observational and intervention studies. Proteolytic fermentation in the distal colon yields metabolites (ammonia, certain phenols, branched-chain amino acids) that are usually regarded as harmful for the gut barrier and may activate pro-inflammatory mechanisms in the gut, while also predisposing the individual to noncommunicable diseases through systemic effects.119 However, the part of the dietary fiber that is fermented in the proximal colon alters the microbiota composition and increases its diversity, and induces gut barrier function associated with beneficial health outcomes. In addition, cereal fiber fermentation in the gut produces short-chain fatty acids (SCFAs) and several other metabolites, including derivatives of fiber-embedded phytochemicals that have been associated with health-supporting effects. For example, serum butyrate concentration has been shown to be inversely associated with the risk of type 2 diabetes.120
SCFAs are known for their protective effects on intestinal epithelial cells, with subsequent inhibition of pathogen proliferation, and modulation of microbial and host immunity and enzymatic activities.121–123 There are also indications that the metabolites produced by microbial fermentation of cereal fiber have an impact on regulation of appetite and satiety, and of mood.124 Dietary fiber phytochemicals are therefore important co-passengers of protein, especially in whole-grain cereal foods, and a clear nutritional benefit for this type of protein, overcoming any potential harmful effects of protein fermentation.
Novel research findings link tryptophan, an important amino acid within the cereal protein fraction, with health and risk of disease. Consumption of a healthy diet including whole-grain cereals (rye) increases the production of indolepropionic acid and decreases serotonin production, both of which are tryptophan metabolites, in the gut.125 Such shifts have been associated with a reduced risk of chronic diseases and reduced gut inflammation.126 Moreover, brans from different cereals may induce the growth of different microbiota species and produce different tryptophan metabolites, supporting balanced metabolism and activating, eg, bile acid metabolism via different mechanisms.127 This justifies the inclusion of various whole-grain types in dietary patterns, because they will induce complementary health effects resulting from the proteins, dietary fiber, and co-passengers.
Health concerns about cereal proteins
According to current knowledge, adverse health effects caused by intake of cereal protein mainly affect the immune system. Gluten proteins (wheat) and gluten-like proteins, such as hordeins (barley) and secalins (rye), may have detrimental effects in genetically predisposed individuals. Prolamins, particularly gliadin subtypes, are the most immunogenic compounds. These peptides contain repetitive amino acid sequences that are recognized by the human transglutaminase family of enzymes and, once processed, they may trigger an autoimmune response.128 Transglutaminase is activated by calcium,129 which may explain why, eg, people in the Nordic countries, with high intakes of calcium-rich dairy products, have a high incidence of celiac-type gluten intolerance. Depending on specific genetic susceptibility and the type of transglutaminase involved (TG2–TG6), a few classes of symptoms may be present.128 In most cases, the dominant transglutaminase in the intestine, TG2, is affected, a condition referred to as celiac disease.
The classical symptoms of celiac disease are pain, diarrhea, and nutrient malabsorption triggered by the ingestion of gluten-containing foods. Approximately 20%–30% of the population world-wide is genetically predisposed to celiac disease, but only about 0.75% of people in the U.S. (in 2012) and 1%–2% in Northern Europe (in 2003) develop active disease,130,131 indicating that there are several determinants for initiation.132,133
Allergic responses to gluten proteins are rare, with the exception of wheat-dependent exercise-induced anaphylaxis caused by ingestion of ω5-type gliadin.134 The allergic antigens are often cereal proteins such as globulins, α-amylase and trypsin and their inhibitors, and albumins. Allergic symptoms to cereal proteins include hay fever, nettle rash, and asthmatic symptoms in the case of baker’s asthma.135 The prevalence of food challenge–proven wheat allergy is approximately 0.2%–1%.136
Allergic responses are mostly treated with anti-histamines to reduce symptoms. For many years, the only treatment for celiac disease was to avoid wheat, barley, and rye intake. Oats can be part of a gluten-free diet, but care must be taken to avoid products contaminated with wheat, which is an issue caused by, eg, shared milling facilities or product lines. Processing solutions to degrade gluten have been explored. Partial sourdough fermentation, as in commercial breads, does not affect TG2-binding motifs,137 but the complete degradation of gluten by fermentation and the addition of proteases can be used to produce a gluten-free product.138
FUTURE PROSPECTS
Replacing animal proteins with cereal proteins – a scenario
To illustrate how a transition from animal to plant protein using cereal grains could translate into improved aspects of environmental sustainability and health, a scenario for Europe, one of the continents with a need for dietary change, was sketched. If 20% of the current daily European animal protein intake of 58 g/person (Table 4) were to be replaced one-for-one with protein from plant sources, 11.6 g/day of plant protein would need to be added to the diet. If half this amount, ie, 5.8 g/day, originated from cereals, the current daily cereal protein intake would need to increase by 19%. Such per capita consumption would create a need for an additional 3 million tons of plant protein for foods (based on 700 million inhabitants in Europe), of which 1.5 million tons should come from cereals. With an average protein content in grains of 10%, this would mean an additional need for 15 million tons of grains, corresponding to only 5% of the current European grain production of 295 million tons.139 Since only about one third of European grain production is currently used for food,139 use of grains for food would increase by 15%, leaving the majority of grains for feed use. However, there would be nutritional consequences from such a transition.
As discussed above, the dietary quality of cereal proteins is not as high as that of animal proteins, and careful processing and product design would be needed to reach an optimal protein balance in the human diet, ie, proteins with a well-balanced amino acid profile and high bioavailability. Different foods could be consumed to achieve the added daily cereal grain protein intake outlined in the scenario above, eg, 84 g of rye whole-grain bread, which corresponds to about three slices, or 290 g (large portion) of oat porridge would meet the hypothetical cereal protein target of 5.8 g. Depending on the food sources, the intake of dietary fiber, nutrients, and phytochemicals would be affected to different degrees. In whole-grain foods, the dietary fiber content is typically of the same order as the protein content, meaning that the required cereal grain protein intake would also provide an additional amount of dietary fiber corresponding to about 20% of the recommended daily intake of dietary fiber. On the other hand, consumption of bread and porridge also brings a lot of carbohydrate, another major macronutrient in the diet. Even if many protein sources are typically prepared for food or consumed with carbohydrate-containing meal items (potato, rice, or pasta), a change towards use of more cereal protein will need to be considered at a diet level to ensure that the additional carbohydrates that come along will fit into an overall diet.
Regional adaptations in cereal production and consumption
As shown in the scenario for Europe, additional cereal protein intake could come through many different foods. Regional adaptations that take account of traditions and culture, important for consumer acceptance, and environmental benefits and constraints, will play a key role in transition towards healthier and sustainable diets.6 In the Nordic countries, oats and rye are common grains, in addition to wheat, and both have a strong cultural tradition and excellent conditions for cultivation. These grains are rich in dietary fiber and phytochemicals in addition to protein and starch, contributing to healthy and sustainable consumption. Moreover, oats are an excellent vector for food innovations, including pulled oats (a meat substitute made of oats and pulses), oat protein concentrates, and dairy product alternatives, providing new ways of consuming cereal protein. Rye is typically used for traditional foods, such as breads, but it has unlocked innovation potential.
To further accelerate the development of new cereal ingredients and products that could contribute to increased plant protein consumption, new raw materials need to be adapted to meet new quality criteria. This will require active breeding to produce new varieties with suitable traits at high yields and low costs, but also high protein yield and high content of the essential amino acids.64 Malnutrition – too little or too much energy and too little nutrients – remains a significant public health issue in many parts of the world. The use of refined wheat products, cooked white rice, and cooked maize, devoid of minerals, fiber, vitamins, and phytochemicals, may contribute to this.
Rice is one of the main cereals world-wide and the most consumed in many regions in Asia. As rice production uses considerable freshwater resources and causes relatively intense nutrient leakage (Table 1), there is an urgent global need to develop novel ways to modify, produce, and consume their proteins, in order to improve environmental sustainability. Overall, cereal crop species and cultivars that can adapt to harsh environmental conditions, especially drought, and still produce satisfactory yields may have an advantage in future agricultural systems.39 Some of the most adaptable cereal crops, such as sorghum and millet, have poor nutritional quality in terms of amino acid availability,39 so the amino acid and protein composition of diets including these cereals should be a target in dietary interventions and in biotechnology research. High nitrogen efficiency is another desirable trait in cereal crops, in order to reduce the environmental burden caused by fertilizers.140 Co-cultivation of other crops, such as legumes, could increase the sustainability of cereal grain production by reducing the need for artificial fertilizer, and could also help balance the amino acid supply in cereal-based diets.39
Development of new cereal protein–rich crops
There are some concerns regarding the nutritional quality and adequacy of cereal proteins (lack of some essential dietary amino acids) in the diet. Adjusting plant breeding strategies to balance the amino acid and protein content in cereal grains could be a useful approach to improve amino acid composition. Some of the annual increase in wheat production world-wide can be explained by the success of effective cereal breeding programs to improve yields and wheat applicability in animal feeds.141 However, these breeding programs, in combination with future climate change, lead to a reduction in protein content and quality.142 In order to maintain or improve wheat protein content and quality in changing environments while maintaining yield levels, delayed flowering and increased grain filling should be targeted in breeding.142 Breeding can also be used to improve protein digestibility, eg, by altering the location of sorghum prolamins in the grain.143 Compared with traditional breeding methods, modern genetic engineering tools have greater efficiency in and more potential for modifying the amino acid profile of crops.144
New cereal protein–enriched foods
Cereal foods in many product categories are consumed in large quantities, offering good potential to increase dietary protein intake by boosting cereal protein content. Some protein-enriched products (bread, pasta, etc.) are already available, and many more could be developed. Under European Union legislation, foods can be labeled a “protein source” when protein provides 12% of the energy content, and “high in protein” when protein provides 20% of the energy content.145 Increased availability and use of such foods could decrease the intake of animal protein from other food categories.
Another way of providing more cereal protein in the diet would be to replace dairy and meat foods with alternative products made of cereal protein concentrates. Addition of 5.8 g cereal protein in the European scenario presented above would allow about 200 mL milk, or 150 mL yoghurt, or 20 g meat, or a small egg to be removed from a diet, if only protein content is considered. The 5.8 g/day protein target could be met by, eg, 400–500 mL oat-based yoghurt alternative or 500 mL oat-based milk alternative, or 29 g of oat-based meat alternative.
Although new types of foods based on cereal proteins could facilitate a protein shift from animal-based products, it is important to note that many of the meat and dairy alternatives currently available have a non-optimal nutrient profile, since dietary fiber and embedded bioactive phytochemicals may have been removed, and ingredients such as hydrogenated oils and fats, sugars, refined carbohydrates, etc. may have been added.146 Products with well-balanced nutritional composition should be encouraged as part of a healthy diet with a low environmental footprint.
Increased cereal consumption can also increase the risk of immune reactions, leading to celiac disease and allergy. However, research has led to progress in the mitigation of immune reactions and can provide potential solutions for the future, such as the use of enzymes that can cleave gluten peptides147 and interfere with TG2 binding sites on gluten peptides, without affecting the structural properties of gluten.148 In a broader context, the health implications of various plant-based protein source alternatives should be scrutinized in future food and nutrition research. However, the current concept of health and environmental sustainability needs to be expanded to include additional dimensions of sustainability (eg, economic, social, cultural) and to develop new food concepts for cereals, the globally most abundant food and feed crops.
CONCLUSIONS
In order to improve environmental sustainability and human health, there is a clear global need to replace animal-based foods with plant-based foods. In the Western world, such a transition could be made partly by shifting the use of grains from feed to human consumption, and an added benefit would be a reduced environmental footprint. Increasing protein intake from cereal foods and reducing protein intake from animal products will result in an increased intake of starch and energy, but also of dietary fiber, vitamins, minerals and bioactive compounds if the grains are consumed as whole grains. Grains also offer an array of options for new protein-rich ingredients, which could leave starch for other industrial uses. It is important that the shift is made with a focus on the total dietary composition, rather than on selected nutrients. A high intake of cereal protein would need complementation with proteins from legumes to ensure adequate intake of all the essential amino acids. Evolving food technologies will bring about versatile technological functionalities and food concepts, making cereal protein available in new food forms. The effects of processing on the digestibility and bioavailability of cereal protein remain an important issue. Consumer access to a diversity of tasty, affordable cereal protein–based foods is a prerequisite for increased use. This offers great opportunities for the food industry, for the long-term benefits of consumers, society, and the planet.
Author contributions. A.K.E., D.P.J., E.N., I.M.M., K.J.H., K.S.P., Ne.S., M.K., and R.L. conceptualized the review. All authors contributed to the writing process of the original draft and the revision of the manuscript. K.S.P. and R.L. coordinated the writing process. A.O.K., K.S.P., and R.L. edited the final manuscript. All authors have read and accepted the final version of the manuscript.
Funding. This review was supported by the Nordic Rye Forum (www.nordicryeforum.info), a pre-competitive platform for rye and health research by academia, institutes, and food industries in the Nordic countries, and by Healthgrain Forum (https://healthgrain.org/), an association founded as the follow-up organization of the 6th EU Framework Programme Integrated Project HEALTHGRAIN – “Exploiting Bioactivity of European Cereal Grains for Improved Nutrition and Health Benefits” with the mission to promote science-based concepts fully unlocking the health-promoting potential in the entire grain food production chain to obtain healthy, convenient, and appealing foods. Nordic Rye Forum is funded through membership fees from companies in the food industry and is also supported by The Nordic Joint Committee for Agricultural and Food Research (NKJ). HealthGrain Forum is funded by member-fees from academia and industry. None of the companies supporting Nordic Rye Forum or HealthGrainForum had any role in or impact on the planning, execution, or interpretation in this review. None of the authors received salary, remuneration, or any other financial support from the Nordic Rye Forum or Healthgrain Forum.
Declaration of interest. K.S.P.: board member of companies Leipurin and High Metal. R.L.: project leader and budget administrator for the Nordic Rye Forum, for which funding is provided by industrial partners and NKJ; principal investigator in research projects funded by Lantmännen and Barilla; no remuneration, salary, or any other financial recompense from the food industry. Na.M.S.: principal investigator in research funded by Lantmännen. The remaining authors have no relevant interests to declare.
Acknowledgments
A.O.K., C.G.-G., and M.K. would like to acknowledge the regional Food Valley project (A73605) for supporting and building up the field of knowledge with respect to composition and bioavailability of food proteins. We thank Mary McAfee, ScanText (Wiltshire, England), for skillful and efficient proofreading of the paper.
Contributor Information
Kaisa S Poutanen, VTT Technical Research Centre of Finland, Espoo, Finland.
Anna O Kårlund, Faculty of Health Sciences, Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Kuopio, Finland.
Carlos Gómez-Gallego, Faculty of Health Sciences, Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Kuopio, Finland.
Daniel P Johansson, Department of Molecular Sciences, Swedish University of Agricultural Sciences, Uppsala, Sweden.
Nathalie M Scheers, Division of Food and Nutrition Science, Department of Biology and Biological Engineering, Chalmers University of Technology, Gothenburg, Sweden.
Ingela M Marklinder, Department of Food Studies, Nutrition and Dietetics, Uppsala University, Uppsala, Sweden.
Anne K Eriksen, Unit of Diet, Genes and Environment, Danish Cancer Society Research Center, Copenhagen, Denmark.
Pia C Silventoinen, VTT Technical Research Centre of Finland, Espoo, Finland.
Emilia Nordlund, VTT Technical Research Centre of Finland, Espoo, Finland.
Nesli Sozer, VTT Technical Research Centre of Finland, Espoo, Finland.
Kati J Hanhineva, Faculty of Health Sciences, Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Kuopio, Finland; Division of Food and Nutrition Science, Department of Biology and Biological Engineering, Chalmers University of Technology, Gothenburg, Sweden; Food Chemistry and Food Development Unit, Department of Biochemistry, University of Turku, Turku, Finland.
Marjukka Kolehmainen, Faculty of Health Sciences, Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Kuopio, Finland.
Rikard Landberg, Division of Food and Nutrition Science, Department of Biology and Biological Engineering, Chalmers University of Technology, Gothenburg, Sweden.
References
- 1. Afshin A, Sur PJ, Fay KA, et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393:1958–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization, Food and Agriculture Organization of the United Nations, eds. Sustainable healthy diets: guiding principles. 2019. Available at: https://www.who.int/publications/i/item/9789241516648. Accessed October 28, 2020.
- 3. Wood A, Gordon L, Röös E, et al. Nordic food systems for improved health and sustainability. Stockholm Resilience Centre Report; 2019. Available at: https://ec.europa.eu/info/sites/info/files/food-farming-fisheries/plants_and_plant_products/documents/report-plant-proteins-com2018-757-final_en.pdf. Accessed October 27, 2020.
- 4. Monteiro CA, Cannon G, Moubarac JC, et al. Dietary guidelines to nourish humanity and the planet in the twenty-first century. A blueprint from Brazil. Public Health Nutr. 2015;18:2311–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meltzer HM, Brantsæter AL, Trolle E, et al. Environmental sustainability perspectives of the Nordic diet. Nutrients. 2019;11:2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Willett W, Rockström J, Loken B, et al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393:447–492. [DOI] [PubMed] [Google Scholar]
- 7. Alexandratos N, Bruinsma J. World agriculture towards 2030/2050: the 2012 revision. ESA Working paper No. 12-03. Rome: FAO; 2012.
- 8. Poore J, Nemecek T. Reducing food’s environmental impacts through producers and consumers. Science. 2018;360:987–992. [DOI] [PubMed] [Google Scholar]
- 9. Ritchie H, Roser M. Environmental impacts of food production. Our World in Data. 2020. Available at: https://ourworldindata.org/environmental-impacts-of-food. Accessed August 11, 2020.
- 10. McKevith B. Nutritional aspects of cereals. Nutr Bulletin. 2004;29:111–142. [Google Scholar]
- 11. Imadi SR, Shazadi K, Gul A. Sustainable crop production system. In: Hakeem KR, Akhtar MS, Abdullah SNA, eds. Plant, Soil, and Microbes. Cham, Switzerland: Springer International Publishing; 2016:103–116. [Google Scholar]
- 12. Davis KF, Chhatre A, Rao ND, et al. Assessing the sustainability of post-Green Revolution cereals in India. Proc Natl Acad Sci USA. 2019;116:25034–25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kramer GFH, Martinez EV, Espinoza-Orias ND, et al. Comparing the performance of bread and breakfast cereals, dairy, and meat in nutritionally balanced and sustainable diets. Front Nutr. 2018;5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SAI Platform. Principles and practices for the sustainable production of cereals; 2006. Available at: https://saiplatform.org/wp-content/uploads/2006/06/sai_platform_principles_practices_cereals.pdf. Accessed March 26, 2021.
- 15. Miller KB. Review of whole grain and dietary fiber recommendations and intake levels in different countries. Nutr Rev. 2020;78:29–36. [DOI] [PubMed] [Google Scholar]
- 16. Herforth A, Arimond M, Álvarez-Sánchez C, et al. A global review of food-based dietary guidelines. Adv Nutr. 2019;10:590–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FAOSTAT. Data. FAO. Available at: http://www.fao.org/faostat/en/#data. Accessed March 26, 2021.
- 18. Schader C, Muller A, El-Hage Scialabba N, et al. Impacts of feeding less food-competing feedstuffs to livestock on global food system sustainability. J R Soc Interface. 2015;12:20150891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davies RW. Nutrients separating the wheat from the chaff: nutritional value of plant proteins and their potential contribution to human health. Nutrients. 2020;12:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mogensen L, Heusale H, Sinkko T, et al. Potential to reduce GHG emissions and land use by substituting animal-based proteins by foods containing oat protein concentrate. J Clean Prod. 2020;274:122914. [Google Scholar]
- 21.World Health Organization, Food and Agriculture Organization of the United Nations. Diet, nutrition and the prevention of chronic diseases. Report of a joint WHO/FAO expert consultation. WHO Technical Report Series 916. Geneva, Switzerland: WHO; 2003. Available at: http://www.fao.org/3/ac911e/ac911e00.htm. Accessed October 28, 2020.
- 22. Mathews R, Chu YF. Global review of whole grain definitions and health claims. Nutr Rev. 2020;78:98–106. [DOI] [PubMed] [Google Scholar]
- 23.U.S.D.A. (U.S. Department of Agriculture). Wheat flour, whole-grain, soft wheat. FoodData Central; 2019. Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/168944/nutrients. Accessed October 29, 2020.
- 24. Micha R, Khatibzadeh S, Shi P, et al. ; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NutriCoDE). Global, regional and national consumption of major food groups in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys worldwide. BMJ Open. 2015;5:e008705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Valsta L, Kaartinen N, Tapanainen H, et al. , eds. Nutrition in Finland – the National FinDiet 2017 Survey [in Finnish, abstract and figure and table titles and vocabulary in English]. Finland Institute for Health and Welfare, Report 12/2018. Helsinki; 2018. Available at http://urn.fi/URN:ISBN:978-952-343-238-3. Accessed September 18, 2021.
- 26. Kårlund A, Gómez-Gallego C, Korhonen J, et al. Harnessing microbes for sustainable development: food fermentation as a tool for improving the nutritional quality of alternative protein sources. Nutrients. 2020;12:1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown IJ, Tzoulaki I, Candeias V, et al. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009;38:791–813. [DOI] [PubMed] [Google Scholar]
- 28. Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) and nonallergic food intolerance: FODMAPs or food chemicals? Therap Adv Gastroenterol. 2012;5:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lionetti E, Pulvirenti A, Vallorani M, et al. Re-challenge studies in non-celiac gluten sensitivity: a systematic review and meta-analysis. Front Physiol. 2017;8:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barabási A-L, Menichetti G, Loscalzo J. The unmapped chemical complexity of our diet. Nat Food. 2020;1:33–37. [Google Scholar]
- 31. Zhu Y, Sang S. Phytochemicals in whole grain wheat and their health-promoting effects. Mol Nutr Food Res. 2017;61:1600852. [DOI] [PubMed] [Google Scholar]
- 32. Koistinen VM, Hanhineva K. Microbial and endogenous metabolic conversions of rye phytochemicals. Mol Nutr Food Res. 2017;61:1600627. [DOI] [PubMed] [Google Scholar]
- 33. Pihlava JM, Hellström J, Kurtelius T, et al. Flavonoids, anthocyanins, phenolamides, benzoxazinoids, lignans and alkylresorcinols in rye (Secale cereale) and some rye products. J Cereal Sci. 2018;79:183–192. [Google Scholar]
- 34. Sang S, Chu Y. Whole grain oats, more than just a fiber: role of unique phytochemicals. Mol Nutr Food Res. 2017;61:1600715. [DOI] [PubMed] [Google Scholar]
- 35. Cui S, Liu RH. Health benefits of oat phytochemicals. In: Chu, Y, ed. Oats Nutrition and Technology. Chichester, United Kingdom: John Wiley & Sons Ltd; 2013:171–194. [Google Scholar]
- 36. Sookwong P, Mahatheeranont S. Some strategies for utilization of rice bran functional lipids and phytochemicals. J Oleo Sci. 2018;67:669–678. [DOI] [PubMed] [Google Scholar]
- 37. Saleh ASM, Wang P, Wang N, et al. Technologies for enhancement of bioactive components and potential health benefits of cereal and cereal-based foods: research advances and application challenges. Crit Rev Food Sci Nutr. 2019;59:207–227. [DOI] [PubMed] [Google Scholar]
- 38. Mäkinen OE, Sozer N, Ercili-Cura D, et al. Protein from oat: structure, processes, functionality, and nutrition. In: Nadathur S, Wanasundara JPD, Scanlin L, eds. Sustainable Protein Sources. San Diego, CA: Academic Press, Elsevier Inc; 2016:105–119. [Google Scholar]
- 39. Taylor JRN, Taylor J. Proteins from sorghum and millets. In: Nadathur S, Wanasundara JPD, Scanlin L, eds. Sustainable Protein Sources. San Diego, CA: Academic Press, Elsevier Inc; 2007:79–104. [Google Scholar]
- 40. Han F, Han F, Wang Y, et al. Digestible indispensable amino acid scores of nine cooked cereal grains. Br J Nutr. 2019;121:30–41. [DOI] [PubMed] [Google Scholar]
- 41. Boye J, Zare F, Pletch A. Pulse proteins: processing, characterization, functional properties and applications in food and feed. Food Res Int. 2010;43:414–431. [Google Scholar]
- 42. Mathai JK, Liu Y, Stein HH. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br J Nutr. 2017;117:490–499. [DOI] [PubMed] [Google Scholar]
- 43. Yu TY, Morton JD, Clerens S, et al. Cooking-induced protein modifications in meat. Compr Rev Food Sci Food Saf. 2017;16:141–159. [DOI] [PubMed] [Google Scholar]
- 44. Bär C, Mathis D, Neuhaus P, et al. Protein profile of dairy products: simultaneous quantification of twenty bovine milk proteins. Int Dairy J. 2019;97:167–175. [Google Scholar]
- 45. Bailey HM, Mathai JK, Berg EP, et al. Most meat products have digestible indispensable amino acid scores that are greater than 100, but processing may increase or reduce protein quality. Br J Nutr. 2020;124:14–22. [DOI] [PubMed] [Google Scholar]
- 46. Loveday SM. Food proteins: technological, nutritional, and sustainability attributes of traditional and emerging proteins. Annu Rev Food Sci Technol. 2019;10:311–339. [DOI] [PubMed] [Google Scholar]
- 47. Cervantes-Pahm SK, Liu Y, Stein HH. Digestible indispensable amino acid score and digestible amino acids in eight cereal grains. Br J Nutr. 2014;111:1663–1672. [DOI] [PubMed] [Google Scholar]
- 48. Shewry PR, Halford NG. Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot. 2002;53:947–958. [DOI] [PubMed] [Google Scholar]
- 49. Han SW, Chee KM, Cho SJ. Nutritional quality of rice bran protein in comparison to animal and vegetable protein. Food Chem. 2015;172:766–769. [DOI] [PubMed] [Google Scholar]
- 50. De Brier N, Gomand SV, Celus I, et al. Extractability and chromatographic characterization of wheat (Triticum aestivum L.) bran protein. J Food Sci. 2015;80:C967–C974. [DOI] [PubMed] [Google Scholar]
- 51. Rombouts I, Lamberts L, Celus I, et al. Wheat gluten amino acid composition analysis by high-performance anion-exchange chromatography with integrated pulsed amperometric detection. J Chromatogr A. 2009;1216:5557–5562. [DOI] [PubMed] [Google Scholar]
- 52. Li S, Tian Y, Wu K, et al. Modulating plant growth—metabolism coordination for sustainable agriculture. Nature. 2018;560:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yu Z, Islam S, She M, et al. Wheat grain protein accumulation and polymerization mechanisms driven by nitrogen fertilization. Plant J. 2018;96:1160–1177. [DOI] [PubMed] [Google Scholar]
- 54. Mosleth EF, Wan Y, Lysenko A, et al. A novel approach to identify genes that determine grain protein deviation in cereals. Plant Biotechnol J. 2015;13:625–635. [DOI] [PubMed] [Google Scholar]
- 55.Food and Agriculture Organization of the United Nations. Dietary protein quality evaluation in human nutrition. Report of an FAO Expert Consultation. FAO Food and Nutrition Paper 92; 2013. Available at http://www.fao.org/documents/card/en/c/ab5c9fca-dd15-58e0-93a8-d71e028c8282/. Accessed September 22, 2020. [PubMed]
- 56. Prolla IRD, Rafii M, Courtney-Martin G, et al. Lysine from cooked white rice consumed by healthy young men is highly metabolically available when assessed using the Indicator Amino Acid Oxidation Technique. J Nutr. 2013;143:302–306. [DOI] [PubMed] [Google Scholar]
- 57. Rafii M, Elango R, Ball RO, et al. Metabolic availability of the limiting amino acids lysine and tryptophan in cooked white African cornmeal assessed in healthy young men using the indicator amino acid oxidation technique. J Nutr. 2018;148:917–924. [DOI] [PubMed] [Google Scholar]
- 58.Food and Agriculture Organization of the United Nations. World Health Organization, the United Nations University. Protein digestibility and absorption: effects of fibre, and the extent of individual variation. Joint FAO/WHO/UNU expert consultation on energy and protein requirements; 1981. Available at: http://www.fao.org/3/M2836e/M2836e00.htm. Accessed November 16, 2020.
- 59. Uraipong C, Zhao J. In vitro digestion of rice bran proteins produces peptides with potent inhibitory effects on α-glucosidase and angiotensin I converting enzyme. J Sci Food Agric. 2018;98:758–766. [DOI] [PubMed] [Google Scholar]
- 60. Guo X, Yao H, Chen Z. Effect of heat, rutin and disulfide bond reduction on in vitro pepsin digestibility of Chinese tartary buckwheat protein fractions. Food Chem. 2007;102:118–122. [Google Scholar]
- 61. Zhang P, Ma G, Wang C, et al. Effect of irrigation and nitrogen application on grain amino acid composition and protein quality in winter wheat. PLoS One. 2017;12:e0178494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Punia D, Khetarpaul N. Physico-chemical characteristics, nutrient composition and consumer acceptability of wheat varieties grown under organic and inorganic farming conditions. Int J Food Sci Nutr. 2008;59:224–245. [DOI] [PubMed] [Google Scholar]
- 63. Day L. Proteins from land plants – potential resources for human nutrition and food security. Trends Food Sci Technol. 2013;32:25–42. [Google Scholar]
- 64. Shewry PR. Improving the protein content and composition of cereal grain. J Cereal Sci. 2007;46:239–250. [Google Scholar]
- 65. de Boer J, Aiking H. Prospects for pro-environmental protein consumption in Europe: cultural, culinary, economic and psychological factors. Appetite. 2018;121:29–40. [DOI] [PubMed] [Google Scholar]
- 66. Vainio A, Niva M, Jallinoja P, et al. From beef to beans: eating motives and the replacement of animal proteins with plant proteins among Finnish consumers. Appetite. 2016;106:92–100. [DOI] [PubMed] [Google Scholar]
- 67. Pettigrew S. Pleasure: an under-utilised ‘P’ in social marketing for healthy eating. Appetite. 2016;104:60–69. [DOI] [PubMed] [Google Scholar]
- 68. Heiniö RL, Noort MWJ, Katina K, et al. Sensory characteristics of wholegrain and bran-rich cereal foods – a review. Trends Food Sci Technol. 2016;47:25–38. [Google Scholar]
- 69. Keast DR, Rosen RA, Arndt EA, et al. Dietary modeling shows that substitution of whole-grain for refined-grain ingredients of foods commonly consumed by US children and teens can increase intake of whole grains. J Am Diet Assoc. 2011;111:1322–1328. [DOI] [PubMed] [Google Scholar]
- 70. Sandvik P, Nydahl M, Kihlberg I, et al. Consumers’ health-related perceptions of bread – implications for labeling and health communication. Appetite. 2018;121:285–293. [DOI] [PubMed] [Google Scholar]
- 71. Prakash J, Ramanatham G. Effect of stabilization of rice bran on the extractability and recovery of proteins. Nahrung. 1994;38:87–95. [Google Scholar]
- 72. Coda R, Rizzello CG, Curiel JA, et al. Effect of bioprocessing and particle size on the nutritional properties of wheat bran fractions. Innov Food Sci Emerg Technol. 2014;25:19–27. [Google Scholar]
- 73. Drakos A, Malindretou K, Mandala I, et al. Protein isolation from jet milled rye flours differing in particle size. Food Bioprod Process. 2017;104:13–18. [Google Scholar]
- 74. Schutyser MAI, van der Goot AJ. The potential of dry fractionation processes for sustainable plant protein production. Trends Food Sci Technol. 2011;22:154–164. [Google Scholar]
- 75. Assatory A, Vitelli M, Rajabzadeh AR, Legge RL. Dry fractionation methods for plant protein, starch and fiber enrichment: a review. Trends Food Sci Technol. 2019;86:340–351. [Google Scholar]
- 76. Silventoinen P, Rommi K, Holopainen-Mantila U, et al. Biochemical and techno-functional properties of protein- and fibre-rich hybrid ingredients produced by dry fractionation from rice bran. Food Bioprocess Technol. 2019;12:1487–1499. [Google Scholar]
- 77. De Bondt Y, Rosa-Sibakov N, Liberloo I, et al. Study into the effect of microfluidisation processing parameters on the physicochemical properties of wheat (Triticum aestivum L.) bran. Food Chem. 2020;305:125436. [DOI] [PubMed] [Google Scholar]
- 78. Ren X, Wei X, Ma H, et al. Effects of a dual-frequency frequency-sweeping ultrasound treatment on the properties and structure of the zein protein. Cereal Chem J. 2015;92:193–197. [Google Scholar]
- 79. Li S, Yang X, Zhang Y, et al. Effects of ultrasound and ultrasound assisted alkaline pretreatments on the enzymolysis and structural characteristics of rice protein. Ultrason Sonochem. 2016;31:20–28. [DOI] [PubMed] [Google Scholar]
- 80. Nazari B, Mohammadifar MA, Shojaee-Aliabadi S, et al. Effect of ultrasound treatments on functional properties and structure of millet protein concentrate. Ultrason Sonochem. 2018;41:382–388. [DOI] [PubMed] [Google Scholar]
- 81. Silventoinen P, Sozer N. Impact of ultrasound treatment and pH-shifting on physicochemical properties of protein-enriched barley fraction and barley protein isolate. Foods. 2020;9:1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhang H, Chen G, Liu M, et al. Effects of multi-frequency ultrasound on physicochemical properties, structural characteristics of gluten protein and the quality of noodle. Ultrason Sonochem. 2020;67:105135. [DOI] [PubMed] [Google Scholar]
- 83. Poutanen K, Flander L, Katina K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009;26:693–699. [DOI] [PubMed] [Google Scholar]
- 84. Nikinmaa M, Kajala I, Liu X, et al. The role of rye bran acidification and in situ dextran formation on structure and texture of high fibre extrudates. Food Res Int. 2020;137:109438. [DOI] [PubMed] [Google Scholar]
- 85. Nikinmaa M, Alam SA, Raulio M, et al. Bioprocessing of bran with exopolysaccharide producing microorganisms as a tool to improve expansion and textural properties of extruded cereal foams with high dietary fibre content. LWT – Food Sci Technol. 2017;77:170–177. [Google Scholar]
- 86. Wu T, Taylor C, Nebl T, et al. Effects of chemical composition and baking on in vitro digestibility of proteins in breads made from selected gluten-containing and gluten-free flours. Food Chem. 2017;233:514–524. [DOI] [PubMed] [Google Scholar]
- 87. Lemmens E, Moroni AV, Pagand J, et al. Impact of cereal seed sprouting on its nutritional and technological properties: a critical review. Compr Rev Food Sci Food Saf. 2019;18:305–328. [DOI] [PubMed] [Google Scholar]
- 88. Benincasa P, Falcinelli B, Lutts S, et al. Sprouted grains: a comprehensive review. Nutrients. 2019;11:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nkhata SG, Ayua E, Kamau EH, et al. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci Nutr. 2018;6:2446–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Flander L, Rouau X, Morel E, et al. Effects of laccase and xylanase on the chemical and rheological properties of oat and wheat doughs. J Agric Food Chem. 2008;56:5732–5742. [DOI] [PubMed] [Google Scholar]
- 91. Xu X, Liu W, Liu C, et al. Effect of limited enzymatic hydrolysis on structure and emulsifying properties of rice glutelin. Food Hydrocoll. 2016;61:251–260. [Google Scholar]
- 92. Nisov A, Ercili-Cura D, Nordlund E. Limited hydrolysis of rice endosperm protein for improved techno-functional properties. Food Chem. 2020;302:125274. [DOI] [PubMed] [Google Scholar]
- 93. Kortekangas A, Silventoinen P, Nordlund E, et al. Phytase treatment of a protein-enriched rice bran fraction improves heat-induced gelation properties at alkaline conditions. Food Hydrocoll. 2020;105:105787. [Google Scholar]
- 94. Nordlund E, Katina K, Aura AM, et al. Changes in bran structure by bioprocessing with enzymes and yeast modifies the in vitro digestibility and fermentability of bran protein and dietary fibre complex. J Cereal Sci. 2013;58:200–208. [Google Scholar]
- 95. Gomes Almeida Sá A, Maria Franco Moreno Y, Augusto Mattar Carciofi B, et al. Food processing for the improvement of plant proteins digestibility. Crit Rev Food Sci Nutr. 2020;60:3367–3386. [DOI] [PubMed] [Google Scholar]
- 96.MarketsandMarkets. Dairy alternatives market by source (soy, almond, coconut, oats, rice, hemp), application (milk, yogurt, ice creams, cheese, creamers), distribution channel (supermarkets, health food stores, pharmacies), formulation, and region – global forecast to 2026. Impact of COVID-19 on Dairy Alternatives Market; 2021. Available at: https://www.marketsandmarkets.com/Market-Reports/dairy-alternative-plant-milk-beverages-market-677.html. Accessed March 26, 2021.
- 97.MarketsandMarkets. Meat substitutes market by source (soy protein, wheat protein, pea protein, and other sources), product (tofu, tempeh, seitan, quorn, and other products), type (textured, concentrates, and isolates), form, category, and region global forecast to 2027. Impact of COVID-19 on meat substitutes market; 2021. Available at: https://www.marketsandmarkets.com/Market-Reports/meat-substitutes-market-979.html. Accessed March 26, 2021.
- 98. Eklund-Jonsson C, Sandberg A-S, Larsson Alminger M. Reduction of phytate content while preserving minerals during whole grain cereal tempe fermentation. J Cereal Sci. 2006;44:154–160. [Google Scholar]
- 99. Feng XM, Passoth V, Eklund-Jonsson C, et al. Rhizopus oligosporus and yeast co-cultivation during barley tempeh fermentation – nutritional impact and real-time PCR quantification of fungal growth dynamics. Food Microbiol. 2007;24:393–402. [DOI] [PubMed] [Google Scholar]
- 100. Hachmeister KA, Fung DYC. Tempeh: a mold-modified indigenous fermented food made from soybeans and/or cereal grains. Crit Rev Microbiol. 1993;19:137–188. [DOI] [PubMed] [Google Scholar]
- 101. Dekkers BL, Boom RM, van der Goot AJ. Structuring processes for meat analogues. Trends Food Sci Technol. 2018;81:25–36. [Google Scholar]
- 102. Sha L, Xiong YL. Plant protein-based alternatives of reconstructed meat: science, technology, and challenges. Trends Food Sci Technol. 2020;102:51–61. [Google Scholar]
- 103. Sethi S, Tyagi SK, Anurag RK. Plant-based milk alternatives an emerging segment of functional beverages: a review. J Food Sci Technol. 2016;53:3408–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mäkinen OE, Wanhalinna V, Zannini E, et al. Foods for special dietary needs: non-dairy plant-based milk substitutes and fermented dairy-type products. Crit Rev Food Sci Nutr. 2016;56:339–349. [DOI] [PubMed] [Google Scholar]
- 105. Tangyu M, Muller J, Bolten CJ, et al. Fermentation of plant-based milk alternatives for improved flavour and nutritional value. Appl Microbiol Biotechnol. 2019;103:9263–9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Brückner-Gühmann M, Banovic M, Drusch S. Towards an increased plant protein intake: rheological properties, sensory perception and consumer acceptability of lactic acid fermented, oat-based gels. Food Hydrocoll. 2019;96:201–208. [Google Scholar]
- 107. Brückner‐Gühmann M, Vasil’eva E, Culetu A, et al. Oat protein concentrate as alternative ingredient for non‐dairy yoghurt‐type product. J Sci Food Agric. 2019;99:5852–5857. [DOI] [PubMed] [Google Scholar]
- 108. Song M, Fung TT, Hu FB, et al. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med. 2016;176:1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Qi XX, Shen P. Associations of dietary protein intake with all-cause, cardiovascular disease, and cancer mortality: a systematic review and meta-analysis of cohort studies. Nutr Metab Cardiovasc Dis. 2020;30:1094–1105. [DOI] [PubMed] [Google Scholar]
- 110. Budhathoki S, Sawada N, Yamaji T, et al. ; Japan Public Health Center–based Prospective Study Group. Association of animal and plant protein intake with all-cause and cause-specific mortality in a Japanese cohort. JAMA Intern Med. 2019;179:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tharrey M, Mariotti F, Mashchak A, et al. Patterns of plant and animal protein intake are strongly associated with cardiovascular mortality: the Adventist Health Study-2 cohort. Int J Epidemiol. 2018;47:1603–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kwok CS, Gulati M, Michos ED, et al. Dietary components and risk of cardiovascular disease and all-cause mortality: a review of evidence from meta-analyses. Eur J Prev Cardiol. 2019;26:1415–1429. [DOI] [PubMed] [Google Scholar]
- 113. Reynolds A, Mann J, Cummings J, et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393:434–445. [DOI] [PubMed] [Google Scholar]
- 114. Marventano S, Vetrani C, Vitale M, et al. Whole grain intake and glycaemic control in healthy subjects: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017;9:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hajihashemi P, Haghighatdoost F. Effects of whole-grain consumption on selected biomarkers of systematic inflammation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Nutr. 2019;38:275–285. [DOI] [PubMed] [Google Scholar]
- 116. Hui S, Liu K, Lang H, et al. Comparative effects of different whole grains and brans on blood lipid: a network meta-analysis. Eur J Nutr. 2019;58:2779–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hollænder PL, Ross AB, Kristensen M. Whole-grain and blood lipid changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr. 2015;102:556–572. [DOI] [PubMed] [Google Scholar]
- 118. Hansen LBS, Roager HM, Søndertoft NB, et al. A low-gluten diet induces changes in the intestinal microbiome of healthy Danish adults. Nat Commun. 2018;9:4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Canfora EE, R Meex RC, Venema K, et al. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15:261–273. [DOI] [PubMed] [Google Scholar]
- 120. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55–71. [DOI] [PubMed] [Google Scholar]
- 121. Goverse G, Molenaar R, Macia L, et al. Diet-derived short chain fatty acids stimulate intestinal epithelial cells to induce mucosal tolerogenic dendritic cells. J Immunol. 2017;198:2172–2181. [DOI] [PubMed] [Google Scholar]
- 122. Donohoe DR, Collins LB, Wali A, et al. The Warburg effect dictates the mechanism of butyrate mediated histone acetylation and cell proliferation. Mol Cell. 2012;48:612–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Reigstad CS, Salmonson CE, Rainey JF, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Dinan TG, Cryan JF. The microbiome–gut–brain axis in health and disease. Gastroenterol Clin North Am. 2017;46:77–89. [DOI] [PubMed] [Google Scholar]
- 125. De Mello VD, Paananen J, Lindström J, et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci Rep. 2017;7:46337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Martin AM, Yabut JM, Choo JM, et al. The gut microbiome regulates host glucose homeostasis via peripheral serotonin. Proc Natl Acad Sci USA. 2019;116:19802–19804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kundi ZM, Lee JC, Pihlajamäki J, et al. Dietary fiber from oat and rye brans ameliorate Western diet–induced body weight gain and hepatic inflammation by the modulation of short‐chain fatty acids, bile acids, and tryptophan metabolism. Mol Nutr Food Res. 2021;65:e1900580. [DOI] [PubMed] [Google Scholar]
- 128. Kárpáti S, Sárdy M, Németh K, et al. Transglutaminases in autoimmune and inherited skin diseases: the phenomena of epitope spreading and functional compensation. Exp Dermatol. 2018;27:807–814. [DOI] [PubMed] [Google Scholar]
- 129. Pinkas DM, Strop P, Brunger AT, et al. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007;5:e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Walker MM, Murray JA, Ronkainen J, et al. Detection of celiac disease and lymphocytic enteropathy by parallel serology and histopathology in a population-based study. Gastroenterology. 2010;139:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mäki M, Mustalahti K, Kokkonen J, et al. Prevalence of celiac disease among children in Finland. N Engl J Med. 2003;348:2517–2524. [DOI] [PubMed] [Google Scholar]
- 132. Wierdsma NJ, Van Bokhorst-De Van Der Schueren MAE, Berkenpas M, et al. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients. 2013;5:3975–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Andrén Aronsson C, Lee HS, Hård Af Segerstad EM, TEDDY Study Group, et al. Association of gluten intake during the first 5 years of life with incidence of celiac disease autoimmunity and celiac disease among children at increased risk. JAMA – J Am Med Assoc. 2019;322:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Morita E, Matsuo H, Chinuki Y, et al. Food-dependent exercise-induced anaphylaxis – importance of omega-5 gliadin and HMW-glutenin as causative antigens for wheat-dependent exercise-induced anaphylaxis. Allergol Int. 2009;58:493–498. [DOI] [PubMed] [Google Scholar]
- 135. Garcia-Casado G, Armentia A, Sanchez-Monge R, et al. Rye flour allergens associated with baker’s asthma. Correlation between in vivo and in vitro activities and comparison with their wheat and barley homologues. Clin Exp Allergy. 1996;26:428–435. [PubMed] [Google Scholar]
- 136. Cianferoni A. Wheat allergy: diagnosis and management. J Asthma Allergy. 2016;9:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Engström N, Sandberg AS, Scheers N. Sourdough fermentation of wheat flour does not prevent the interaction of transglutaminase 2 with α2-gliadin or gluten. Nutrients. 2015;7:2134–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Di Cagno R, Barbato M, Di Camillo C, et al. Gluten-free sourdough wheat baked goods appear safe for young celiac patients: a pilot study. J Pediatr Gastroenterol Nutr. 2010;51:777–783. [DOI] [PubMed] [Google Scholar]
- 139.European Comission. Cereals, oilseeds, protein crops and rice. Available at: https://ec.europa.eu/info/food-farming-fisheries/plants-and-plant-products/plant-products/cereals_en. Accessed November 16, 2020.
- 140. Flambeau M, Redl A, Respondek F. Proteins from wheat: sustainable production and new developments in nutrition-based and functional applications. In: Nadathur S, Wanasundara JPD, Scanlin L, eds. Sustainable Protein Sources. San Diego, CA: Academic Press, Elsevier Inc; 2016:67–78. [Google Scholar]
- 141. Evans J, Lawson T. From green to gold: agricultural revolution for food security. J Exp Bot. 2020;71:2211–2215. [DOI] [PubMed] [Google Scholar]
- 142. Asseng S, Martre P, Maiorano A, et al. Climate change impact and adaptation for wheat protein. Glob Chang Biol. 2019;25:155–173. [DOI] [PubMed] [Google Scholar]
- 143. Oria MP, Hamaker BR, Axtell JD, et al. A highly digestible sorghum mutant cultivar exhibits a unique folded structure of endosperm protein bodies. Proc Natl Acad Sci USA. 2000;97:5065–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Wang G, Xu M, Wang W, et al. Fortifying horticultural crops with essential amino acids: a review. Int J Mol Sci 2017;18:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.European Comission. Nutrition claims. Available at: https://ec.europa.eu/food/safety/labelling_nutrition/claims/nutrition_claims_en. Accessed November 17, 2020.
- 146. Bohrer BM. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci Hum Wellness. 2019;8:320–329. [Google Scholar]
- 147. Cavaletti L, Taravella A, Carrano L, et al. E40, a novel microbial protease efficiently detoxifying gluten proteins, for the dietary management of gluten intolerance. Sci Rep. 2019;9:13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Engstrom N, Saenz-Méndez P, Scheers J, et al. Towards celiac-safe foods: decreasing the affinity of transglutaminase 2 for gliadin by addition of ascorbyl palmitate and ZnCl2 as detoxifiers. Sci Rep. 2017;7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]


