Abstract
Context
Hibiscus sabdariffa (hibiscus) has been proposed to affect cardiovascular risk factors.
Objective
To review the evidence for the effectiveness of hibiscus in modulating cardiovascular disease risk markers, compared with pharmacologic, nutritional, or placebo treatments.
Data Sources
A systematic search of the Web of Science, Cochrane, Ovid (MEDLINE, Embase, AMED), and Scopus databases identified reports published up to June 2021 on randomized controlled trials using hibiscus as an intervention for lipid profiles, blood pressure (BP), and fasting plasma glucose levels in adult populations.
Data Extraction
Seventeen chronic trials were included. Quantitative data were examined using a random effects meta-analysis and meta-regression with trial sequential analysis to account for type I and type II errors.
Data Analysis
Hibiscus exerted stronger effects on systolic BP (−7.10 mmHg [95%CI, −13.00, −1.20]; I2 = 95%; P = 0.02) than placebo, with the magnitude of reduction greatest in those with elevated BP at baseline. Hibiscus induced reductions to BP similar to that resulting from medication (systolic BP reduction, 2.13 mmHg [95%CI, −2.81, 7.06], I2 = 91%, P = 0.40; diastolic BP reduction, 1.10 mmHg [95%CI, −1.55, 3.74], I2 = 91%, P = 0.42). Hibiscus also significantly lowered levels of low-density lipoprotein compared with other teas and placebo (−6.76 mg/dL [95%CI, −13.45, −0.07]; I2 = 64%; P = 0.05).
Conclusions
Regular consumption of hibiscus could confer reduced cardiovascular disease risk. More studies are warranted to establish an effective dose response and treatment duration.
Systematic Review Registration
PROSPERO registration no. CRD42020167295
Keywords: blood pressure, cardiovascular disease, Hibiscus sabdariffa, lipidemia, meta-analysis, roselle
INTRODUCTION
High blood pressure (BP) is one of the strongest predictors of cardiovascular diseases (CVDs), neurovascular conditions, and ischemic events.1 Dyslipidemia, insulin resistance,2 inflammation,3 and oxidative stress4 are all factors contributing to CVD development that may be caused by a combination of genetics, lifestyle factors, and/or diet quality.5 According to the World Health Organization, CVD is the number 1 cause of death worldwide, with an estimated 17.7 million people dying of CVD and its related conditions each year.6,7 Lowering of blood cholesterol levels and improvement of endothelial function (eg, through reducing BP) are important to reduce the risk of systemic hypertension and CVD.8 Although pharmaceuticals are effective, many produce considerable side effects, such as altered electrolyte balance, dizziness, and chronic fatigue. It is well documented that lifestyle and dietary changes can be adopted as effective therapies to lower BP and cholesterol.9 However, adherence to lifestyle recommendations, such as change of diet type and reduction of alcohol consumption and smoking, is poor.10–12 Focused interventions such as increasing the intake of anthocyanins, mainly present in fruit and vegetables, is associated with a decreased risk of all-cause mortality,13 with recent evidence demonstrating their effectiveness in reducing high BP, hypertension and lipids.14,15
Hibiscus sabdariffa (hibiscus), also known as roselle or sour tea, is an annual bushy plant in the Malvaceae family. It is widely grown in many African and southeast Asian countries, where it is typically consumed as a tea beverage or used in traditional medicine.16 Tea is the second most consumed beverage across the globe and consumption continues to rise, with health benefits being a key driver of intake.17 The global hibiscus flower market was expected to reach $119.4 million in 2020. Hibiscus is a source of bioactive compounds such as polyphenols, carotenoids, ascorbic acid, and tannins; however, the composition varies depending on the part of the plant used, climatic factors, maturity of the plant, and differences in genotypes.18 The calyces of hibiscus are a rich source of anthocyanins, mainly delphinidin 3-sambubioside and cyanidin 3-sambubioside, with delphinidin 3-glucoside and cyanidin 3-glucoside reported as minor anthocyanins.19 It is thought that the bioactive compounds in hibiscus exert antioxidant and anti-inflammatory effects that contribute to the reduction of CVD risk markers.20
Mechanistic explanations of hibiscus-induced reduction of BP are mainly derived from animal models. Some studies propose these effects are due to improved vasodilation21,22 through inhibiting calcium influx into vascular smooth muscle cells23,24 or by acting as a diuretic through increased excretion of sodium and chloride and increased kidney filtration.25 In tandem with these previous studies, delphinidin 3-sambubioside and cyanidin 3-sambubioside have been shown to compete with the substrate at the active site of the angiotensin-converting enzyme (ACE) in an in vitro enzyme inhibition model,26 suggesting that hibiscus may act as a competitive inhibitor of ACE, preventing the production of angiotensin II. Moreover, antihypertensive effects of the anthocyanins cyanidin and delphinidin have been shown via direct inhibition of the renin-angiotensin system in vitro,27 through upregulation of endothelial nitric oxide synthase28 and inhibition of inflammation,29 all contributing toward a multifaceted approach to BP management.
Data from human trials support the use of hibiscus on moderating blood lipids. In a randomized crossover study of 42 patients, Lin et al30 demonstrated that an aqueous extract of hibiscus consumed as a capsule for 1 month reduced total cholesterol (TC) and low-density lipoprotein (LDL) levels in patients with hypercholesterolemia. The authors also demonstrated that serum cholesterol levels were reduced by 8.3%–14.4% in volunteers consuming 2 capsules (1000 mg) of hibiscus, 3 times a day with a meal, compared with patients consuming 0.5 g/day or 1.5 g/day. Sabzghabaee et al31 reported modest beneficial effects of hibiscus on levels of TC, serum triglycerides (TGs), and LDL in obese adolescents. Several mechanisms that could explain the cholesterol- and lipid-modulating effects of hibiscus have been proposed, for example, inhibition of HMG-CoA reductase32 or inhibition of triacylglycerol synthesis by hibiscus acid racemization.33
The antidiabetic effect of hibiscus is a lesser studied topic. Oral administration of hibiscus at doses of 72 mg/200 g body weight and 288 mg/200 g body weight for 21 days decreased blood glucose levels in rats with chronic diabetes.34 Because insulin has a key role in regulating blood glucose levels, it is hypothesized that the antihyperglycemic effects of hibiscus may be attributed to increases in insulin excretion.35
How the vehicle of administration influences the absorption and peak concentration of bioactives derived from hibiscus is currently unknown. Data from in vivo studies demonstrate that the vehicle of administration may be important to determine pharmacokinetics of tea flavanols. Henning et al36 delivered the same quantity of epigallocatechin-3-galate in either green tea, black tea, or a green tea supplement to healthy participants. Flavanol concentration and antioxidant activity were highest after consumption of the green tea supplement. Additional evidence from Schär et al37 supports the hypothesis that bioactives and their metabolites are readily available in a beverage. Consumption of orange juice significantly increased mean plasma concentration of 8 flavanone and 15 phenolic metabolites, compared with a supplement control. However, this increase in phenolic concentration was in the absence of any improvements in CVD risk markers. More empirical research needs to be conducted to determine the magnitude of effect of vehicle of administration on concentrations of bioactive compounds.
Previous systematic reviews and meta-analyses have analyzed the effects of hibiscus preparations on cardiometabolic markers.38–42 However, these do not permit clear conclusions, because of the limited numbers of comparable studies, erroneous data inclusion and extraction,38 and inconsistent disaggregation of subgroups, which limits comparison of the outcomes. The purpose of the present systematic review and meta-analysis is to provide an up-to-date, critical evaluation of current evidence with a robust assessment of hibiscus supplementation on BP, blood lipids, and blood glucose CVD risk biomarkers.
METHODS
The Recommendations of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was adopted to conduct this systematic review,43 as documented in the PRISMA checklist (see Table S1 in the Supporting Information online). The protocol was registered at PROSPERO (registration no. CRD42020167295).
Databases and search terms
An electronic search of articles published up to June 2021 was conducted using the Ovid (Embase, MEDLINE, AMED), Web of Science, Scopus (natural sciences), and Cochrane Library databases. Search terms were (Hibiscus sabdariffa OR roselle OR sour tea) AND (hyperten$OR blood pressure) OR (lipid$) OR (glucose OR sugar). Search criteria were limited to randomized controlled trials of adults >18 years of age. Filters were applied to only include human studies published in English. A manual search was conducted by reviewing reference lists of included studies.
Inclusion and exclusion criteria
After the database searches, results were exported into EndNote X9, duplicates removed, and remaining studies evaluated in a stepwise manner. Full-text papers were included after review of their eligibility by 2 authors separately if they satisfied the criteria listed in Table 1. Eligible studies were included if data pre- and postintervention were included. Primary outcomes of interest were the following CVD risk factors: systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse pressure, LDL, high-density lipoprotein (HDL), TC, TGs, and FPG levels. Eligible study populations included healthy adults as well as patients diagnosed with comorbid conditions such as stage I hypertension.
Table 1.
PICOS criteria for inclusion of studies
| Parameter | Description |
|---|---|
| Population | Adult participants >18 years old (healthy or otherwise) |
| Intervention | Hibiscus sabdariffa as a stand-alone intervention |
| Comparator | Placebo, diet, other tea beverage, pharmaceutical medication |
| Outcomes | Outcomes regarding both systolic blood pressure and diastolic blood pressure (and pulse pressure, where included) and/or the following: levels of low-density lipoprotein, high-density lipoprotein, total cholesterol, triglycerides, fasting plasma glucose |
| Study design | Randomized controlled trial with parallel design |
Any participants receiving medication were ineligible for inclusion (unless this medication was included as the control group treatment). Animal or cell-culture studies were excluded, as were studies that were narrative reviews, case reports, or retrospective studies. There were no restrictions on the dates of publication, with any study conducted prior to the search dates eligible for inclusion. In studies in which interventions were used in different arms (eg, 2 different health conditions compared within 1 study), relevant data were included if the inclusion criteria were met.
Data extraction
Data were extracted into a Microsoft Excel document and categorized using the following criteria: first author and year of publication; journal; participant characteristics (sample size, sex, age, health status); and methodology (study design, duration, dose of hibiscus, and controls). Outcomes of interest we extracted were baseline and postintervention mean values and standard deviations (SDs) of the following: SBP, DBP, pulse pressure, FPG, LDL, HDL, TC, and TG. Mean change from baseline and standard deviation (SD) were also extracted where included, and if SD values were missing, they were computed using the following correlation coefficient (corr) formula44:
Assessment of bias
The Cochrane risk-of-bias tool for randomized controlled trials was used to detect the potential risk of bias for the included studies.44 The following methodological assessment criteria were considered: adequacy of sequence generation, allocation concealment, blinding, drop-out rates (incomplete outcome data), addressing incomplete outcome data, selective outcome reporting, and other potential sources of bias. Risk of bias was coded as “yellow,” indicating an unclear risk; “red,” indicating a high risk of bias; or “green,” indicating a low risk of bias.
Statistical analysis
Data were analyzed using Review Manager, version 5.3.5 (Cochrane Collaboration). Statistical significance was conferred when P < 0.05. The effect size in the meta-analysis was recorded as mean difference and 95%CI. Effect size was computed by pooling mean change from baseline and SDs of results. Heterogeneity of effect sizes was assessed primarily with the I2 statistic45 and τ2.46 Subgroup analyses were conducted according to the control group of the included studies, dosage of hibiscus, and by duration of study to explore heterogeneity. Publication bias was evaluated visually using a funnel plot and quantitatively by Egger’s linear regression test and Begg’s correlation rank.47,48 A traditional sensitivity analysis was also conducted using the leave=one=out method to determine the impact of each study on the overall effects.45
Meta-regression
Meta-regression was conducted using Comprehensive Meta-Analysis software (Biostat Inc) to evaluate if magnitude of effect size by hibiscus treatment was predicted by the baseline levels of the outcome being assessed.
Trial sequential analysis
Meta-analyses may result in type I and type II errors due to heterogeneity within the included studies and to overestimation of an effect size based on low participant numbers in the included studies.49 The risk of overestimation of effect size decreases exponentially with the number of participants and outcomes. Trial sequential analysis (TSA) is a method of analysis that aims to address this by calculating the required information size (RIS), which in terms of the meta-analysis is the sample size. Reaching the RIS and the corresponding number of trials ensures control of type I and type II errors. Furthermore, reaching the RIS can provide a good level of protection against overestimation. In TSA, the sample size, required for a single randomized clinical trial to have conclusive evidence for an intervention effect, is adjusted by measurement of statistical heterogeneity in the meta-analysis in order to reach the RIS. The z values are shown on the y-axis and represent the statistical summary of the accrued data. TSA is based on the O’Brien-Fleming B-spending function, which displays a cumulative Z-curve graph using a type I error of 5% and a type II error of 20% (80% power). TSA was carried out using TSA, version 0.9, beta software.50
RESULTS
As shown in the PRISMA flow diagram (Figure 1), in total, 648 references were found. After removal of duplicates and exclusion of irrelevant papers on the basis of titles and abstracts, 33 articles were retained for full-text review. Sixteen articles were excluded after full-text review with the reasons for removal of these studies detailed in Figure 1. Finally, 17 randomized controlled studies with 18 arms were included in the review.
Figure 1.
PRISMA flow diagram of the literature search process
Study characteristics
Characteristics of all included studies are outlined in Table 2.51–67 Data on SBP and DBP were reported by 12 randomized controlled trials,51–61 data on LDL, HDL, TC, and TG were reported by 9 studies54,58,62–67 and data on FPG were reported by 6 studies.54,56,58,63–65 The populations in the studies by Mozaffari et al53,62 were the same; however, the authors separated the results to be published in 2 separate papers. For BP outcomes across all studies, a total of 415 participants were allocated to the hibiscus group and 404 participants were allocated to the control group. For lipid outcomes across all studies, cumulatively, a total of 246 participants were randomized to hibiscus and 238 to control treatments. For studies in which FPG was assessed, a total of 197 participants were randomized to hibiscus and 207 to control treatments. In the study by Izadi et al,67 the reported BP values are implausible and likely erroneous; therefore, only the lipid values from that study were included in our analyses.
Table 2.
Characteristics of included randomized clinical trials evaluating the effect of hibiscus supplementation on cardiovascular risk factors
| Study | Design | Participants’ health condition and number by arm | Frequency and duration | Intervention and dose | Outcome parameters |
|---|---|---|---|---|---|
| Herrera-Arrelano et al 200451 | RCT |
|
Twice daily for 4 wk | 10 g HS calyx capsule (9.62 mg AC) vs 25 mg captopril | SBP, DBP, PP |
| Herrera-Arrelano et al 200652 | RCT, DB |
|
Once daily for 4 wk | HS calyx (250 mg AC) vs 10 mg lisinopril | SBP, DBP |
| Mozaffari-Khosravi et al 200953 | RCT, DB |
|
Twice daily for 4 wk | 2 g HS as tea vs 2 g black tea | SBP, DBP, PP |
| Gurrola-Diaz et al 201054 | RCT, 2 arms: A = healthy, B = MeSy |
|
Once daily for 4 wk | 100 mg HS calyx capsule vs a preventive diet | SBP, DBP, LDL, HDL, TC, TG, FPG |
| McKay et al 201055 | RCT, DB, PC |
|
3 cups a d for 6 wk | 1.25 g HS calyx as tea vs PC (artificial hibiscus-flavored water) | SBP, DBP |
| Mozaffari-Khosravi et al 201356 | RCT |
|
3 times a d for 4 wk | 3 g HS tea vs 3 g green tea | SBP, DBP, PP, FPG |
| Nwachukwu et al 201557 | RCT, comparative |
|
Daily for 4 wk and 1-wk withdrawal | HS calyx capsule (150 mg/kg) vs HCTZ (25 mg) vs placebo | SBP, DBP |
| Asgary et al 201658 | RCT, DB, PC |
|
Once daily for 4 wk | 500 mg HS calyx capsule vs maltodextrin PC | SBP, DBP, LDL, HDL, TC, TG, FPG. |
| Kafeshani et al 201759 | RCT, DB, PC |
|
Once daily for 6 wk | 450 mg HS capsule or 450 mg green capsule | SBP, DBP, LDL, HDL, TC, TG |
| Seck et al 201760 | RCT |
|
Once daily for 4 wk | 320 mg HS calyx vs 5 mg ramipril | SBP, DBP |
| Jalalyazdi et al 201961 | RCT, PC |
|
Twice daily for 4 wk | 1.25 g HS tea vs DASH diet | SBP, DBP |
| Mozaffari-Khosravi et al 200962 | RCT. DB |
|
Twice daily for 4 wk | 2 g HS as tea vs 2 g black tea | LDL, HDL, TC, TG |
| Kuriyan et al 201063 | DB, RCT, PC |
|
Once daily for 90 d | 1 g HS leaves capsule vs maltodextrin placebo | LDL, HDL, TC, TG, FPG |
| Mohagheghi et al 201164 | RCT |
|
Once daily for 15 d | 15 mg of calyx HS as tea vs black tea | LDL, HDL, TC, TG, FPG |
| Chang et al 201465 | DB, RCT, PC |
|
Once daily for 12 wk | 250 mg HS with 50 mg starch vs a 500 mg starch PC | LDL, HDL, TC, TG, FPG |
| Hajifaraji et al 201866 | RCT |
|
Twice daily for 12 wk | 2 g HS tea vs 2 g black tea | LDL, HDL, TC, TG |
| Izadi et al 202167 | RCT, DB, PC |
|
Once daily for 8 wk | 450 mg HS capsule vs placebo | LDL, HDL, TC, TG |
Abbreviations: AC, anthocyanin; C, control; DASH, Dietary Approaches to Stop Hypertension; DB, double blind; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HCTZ, hydrochlorothiazide; HDL, high-density lipoprotein; HS, hibiscus; I, intervention; LDL, low-density lipoprotein; MeSy, metabolic syndrome; NAFLD, nonalcoholic fatty liver disease; PC, placebo controlled; PP, pulse pressure; RCT, randomized controlled trial; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
In summary, across all outcomes, 6 studies compared effects of hibiscus with those of a placebo,55,57,58,63,65,67 2 studies compared hibiscus with a preventive diet,54,61 5 compared hibiscus with another tea beverage,53,56,59,64,66 and 4 compared hibiscus with a known pharmaceutical medication.51,52,57,60 Participant health conditions were different across the studies. In total, 6 studies enrolled participants with hypertension (n = 522), 3 included healthy adults (n = 119), 2 assessed patients with metabolic syndrome (MeSy; n = 90), 1 recruited obese adults with fatty liver (n = 36), 2 recruited patients with type 2 diabetes (n = 147), 1 recruited adults with nonalcoholic fatty liver disease (n = 61), and 1 study enrolled patients with polygenic dyslipidemia (n = 43). Of the studies, 10 administered hibiscus as a capsule and 7 as a tea beverage. Doses of hibiscus ranged from 15 mg to 9 g/day, and duration of studies ranged from 15 to 90 days. In total, polyphenol and/or anthocyanin amount was quantified in 9 studies.51,52,54,55,57–59,65,67 Anthocyanin content ranged from 3 mg to 250 mg/day.
Bias assessment
All the studies included in this review examined participants randomized to treatment, but the methodology surrounding allocation concealment was only provided in 6 studies.52,57–61,68 In 9 of the 17 studies, researchers used a clearly specified blinding method of the participants and/or personnel.52,53,55,57–59,63,65,67,68 All trials were defined as having a low risk of selective outcome reporting, as specified in the Methods section. The bias assessment risk score can be found in Figure S1 in the Supporting Information online.
Quantitative data analysis
Overall outcomes of each cardiometabolic risk marker were pooled to calculate an effect size. Trials with the same control were grouped together for subgroup analysis. Control groups compared hibiscus with: 1) a known pharmaceutical medication, 2) another tea beverage (black tea or green tea), 3) a placebo, or 4) a prescribed diet.
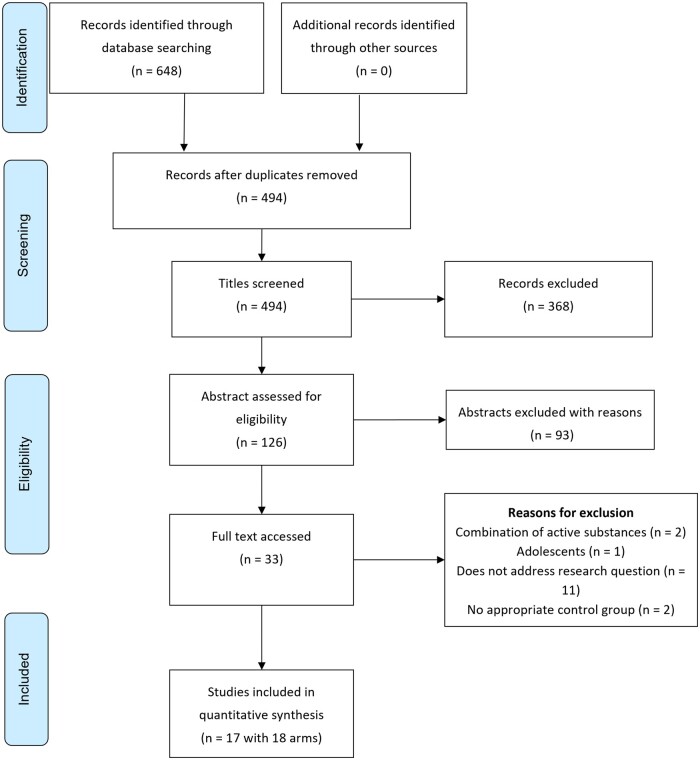
Figure 2 summarizes the results of the meta-analysis using a random effects model, which compared hibiscus with another tea beverage, a placebo, or a prescribed diet.53–56,58,59,61,69 Hibiscus resulted in a significant reduction of SBP (−7.10 mmHg; 95%CI, −13.00, −1.20; I2 = 95%; P = 0.02). The results for DBP favored hibiscus but did not reach statistical significance (−3.26 mmHg; 95%CI, −7.05, 0.52; I2 = 94%; P = 0.09). There was a significant effect of hibiscus, compared with placebo, on SBP (−10.05 mmHg; 95%CI, −17.58, −2.51; I2 = 81%; P = 0.009). However, no significant effect was observed comparing hibiscus with other tea beverages (−13.17 mmHg; 95%CI, −31.39, 5.05; I2 = 96%; P = 0.16) or with a dietary intervention (1.10 mmHg; 95%CI, −7.31, 9.51; I2 = 90%; P = 0.80) in SBP subgroup analysis.
Figure 2.
Meta-analysis of the effects of hibiscus supplementation on A) systolic blood pressure and B) diastolic blood pressure. Superscript denotes population of the Gurrola-Diaz study (a, healthy; b, metabolic syndrome) and the Nwachukwu study comparing hibiscus and placebo
Compared to other tea beverages, hibiscus exerted a statistically significant effect on DBP (−2.89 mmHg; 95%CI, −5.71, −0.06; I2 = 0%; P = 0.05). DBP was nonsignificantly lowered in the hibiscus group compared with participants receiving placebo interventions (−5.60 mmHg; 95%CI, −13.05, 1.85; I2 = 94%; P = 0.14) or compared with dietary interventions (−0.92 mmHg; 95%CI, −4.00, 2.17; I2 = 62%; P = 0.56). No significant difference was detected for pulse pressure (−7.59 mmHg; 95%CI, −21.46, 6.28; I2 = 96%; P = 0.28).
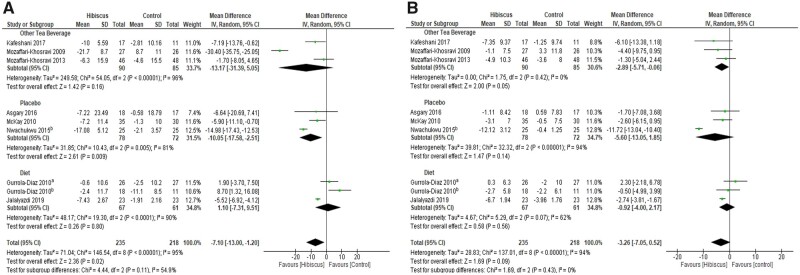
In terms of lipids, only a significant reduction of LDL was observed after hibiscus consumption (−6.76 mg/dL; 95%CI, −13.45, −0.07; I2 = 64%; P = 0.05). No significant effect was found for TC (−7.08 mg/dL; 95%CI, −15.62, 1.47; I2 = 59%; P = 0.10), for TG (0.21 mg/dL; 95%CI, −6.87, 7.30; I2 = 0%; P = 0.95), or for HDL (0.21 mg/dL; 95%CI, –1.55, 1.97; I2 = 49%; P = 0.82). Subgroup analysis revealed that hibiscus had a significant TC-lowering effect compared with other tea beverages (−11.07 mg/dL; 95%CI, −20.29, −1.85; I2 = 39%; P = 0.02). For HDL, subgroup analyses demonstrated a significant effect favoring other tea beverages (−1.92 mg/dL; 95%CI, −3.23, −0.62; I2 = 11%; P = 0.004). Forest plots summarizing the effect of hibiscus on lipid profiles can be seen in Figure 3.54,58,59,62–67
Figure 3.
Meta-analysis on the effects of hibiscus supplementation on lipid profiles. A) low-density lipoprotein; B) high-density lipoprotein (HDL); C) total cholesterol; and D) triglycerides. Superscript denotes population of the Gurrola-Diaz study (a, healthy; b, metabolic syndrome). Note in A, C and D the left side of the figure shows effects which favour hibiscus and are beneficial for health. In 3B the right side of the figure favours hibiscus because an increase in HDL is deemed beneficial.
Results of the meta-analysis on FPG did not reveal any significant effect of hibiscus intervention (−1.48 mg/dL; 95%CI, −4.21, 1.25; I2 = 0%; P = 0.29). No significant effects were observed during subgroup analysis (Figure S2 in the Supporting Information online).
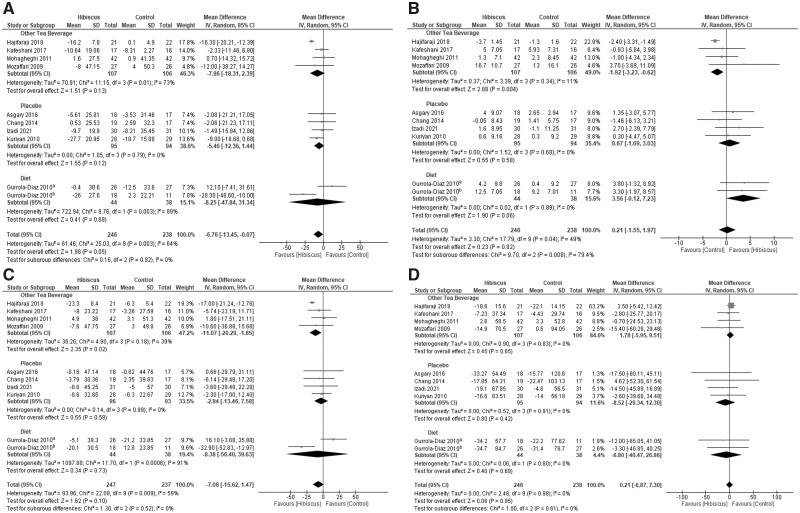
Forest plots summarizing the effect of hibiscus compared with a pharmaceutical intervention are depicted in Figure 4.51,52,57,60 Hibiscus had similar BP-lowering effects as pharmaceutical intervention, with a nonsignificant difference observed between the 2 treatments: SBP (2.13 mmHg; 95%CI, −2.81, 7.06; I2 = 91%; P = 0.40) and DBP (1.10 mmHg; 95%CI, −1.55, 3.74; I2 = 91%; P = 0.42).
Figure 4.
Meta-analysis on the effects of hibiscus supplementation on A) systolic blood pressure and B) diastolic blood pressure compared with pharmaceutical medications
Dose and duration effects
Subgroup analyses were performed to investigate the impact of hibiscus dose and study duration on BP, lipid profiles, and FPG levels. Studies in which the effects of hibiscus were compared with those of a pharmaceutical medication were omitted from this analysis.
Studies in which SBP and DBP were examined and that lasted > 4 weeks showed a significant lowering effect of hibiscus (P = 0.0001 and P = 0.04, respectively). Within the dose-response subgroup, ingestion of hibiscus with a dose ≤1 g/day was not significant for either SBP (P = 0.96) or DBP (P = 0.63).
Hibiscus supplementation markedly decreased TC level when the duration of supplementation was >4 weeks or when dose was > 500 mg (P = 0.008 and P = 0.02, respectively). Hibiscus significantly lowered LDL level when duration of supplementation was > 4 weeks and when dose was > 500mg (P = 0.04 and P = 0.0001, respectively). No effect of duration or dose was detected for FPG level. The findings of subgroup analyses for dose and duration of BP, lipids, and FPG levels are shown in Tables S2–S4 in the Supporting Information online. However, the small number of studies included limits the conclusions that can be drawn, such that it is not possible to propose a dose or duration for hibiscus treatment to affect lipids, BP, or FPG.
Meta-regression
Meta-regression was conducted to evaluate whether BP, lipid profiles, and FPG levels were related to baseline (ie, pretreatment values). As shown in Figure S3 in the Supporting Information online, random-effects meta-regression indicated a significant inverse association between baseline SBP and SBP reduction (coefficient = −0.11; P = 0.03) and baseline DBP and DBP reduction (coefficient = −0.13; P = 0.007). Conversely, lipid parameter results were independent of baseline values (LDL coefficient = −0.06, P = 0.69; HDL coefficient = −0.14, P = 0.49; TC coefficient = −0.27, P = 0.20; TG coefficient = 0.07, P = 0.75). The effect of hibiscus supplementation on FPG was independent of baseline values (coefficient = −0.08; P = 0.54).
Trial sequential analysis
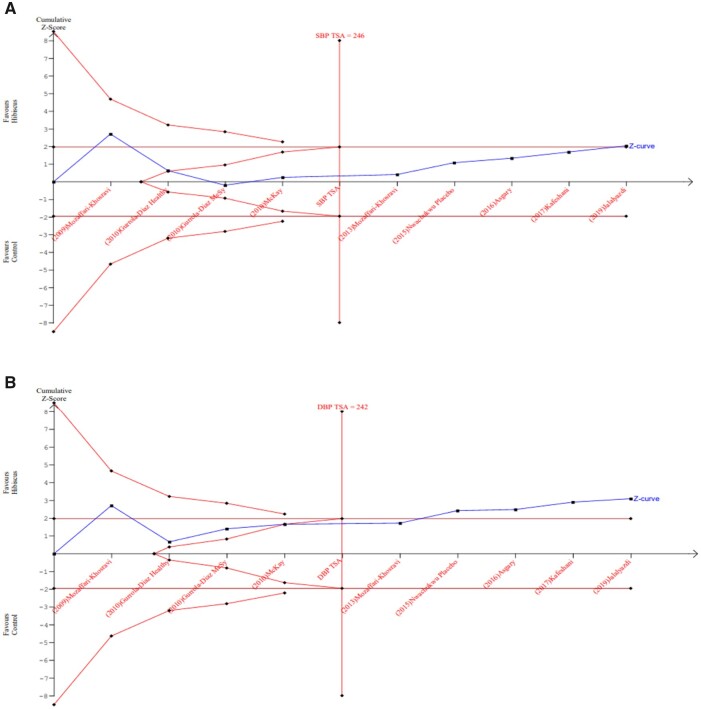
Comparisons with pharmaceutical groups were omitted and only studies with a diet, placebo, or other tea control group were included in TSA. Results indicated that only SBP and DBP (Figure 5) reached the RIS of 246 and 242, respectively, and passed the conventional test boundary of P < 0.05. However, SBP did not reach the TSA monitoring boundary. Within lipid analyses, the results for LDL crossed the conventional boundary for significance; however, none of the outcomes crossed the RIS boundaries. Within FPG analysis, the cumulative Z curve did not reach the RIS of 2212 nor did it cross the conventional test boundary for significance. For many of these analyses (Figures S4 and S5 in the Supporting Information online), TSA shows that the RIS was not reached to detect or reject the anticipated effects with certainty.
Figure 5.
Outcomes from random effects trial sequential analysis of A) systolic blood pressure (SBP) and B) diastolic blood pressure (DBP). Both SBP and DBP achieved the required information size (246 and 242) and crossed the conventional test boundary for significance. TSA, trial sequential analysis
Publication bias
Visual inspection of funnel plots showed no evidence of publication bias for any parameter (Figure S6 in the Supporting Information online). Furthermore, no evidence of publication bias was deducted using quantitative evaluation for the parameters, as follows (reported as Egger’s linear regression test and Begg’s correlation rank values, respectively): SBP (0.68; 0.70), DBP (0.85; 0.47), LDL (0.78; 0.47), HDL (0.41; 0.65), TC (0.06; 0.92), TG (0.37; 0.40), and FPG (0.27; 0.65).
Sensitivity analysis
Sensitivity analysis was performed by removing the data from each study one at a time and examining the impact of removal on the overall effect. With respect to lipid analyses, removing the MeSy patient arm of the study by Gurrola-Diaz et al54 rendered the effect of hibiscus on LDL nonsignificant (P = 0.15). Excluding the healthy participant arm of the same study resulted in a significant effect of hibiscus on TC (P = 0.005). The study by Hajifaraji et al66 was a source of uncertainty. Removal of this study reduced the I2 of TC from 59% to 38% and of HDL from 49% to 0%, and resulted in a nonsignificant effect of hibiscus on LDL (P = 0.13). With respect to BP outcomes, removing the data from the Nwachukwu et al57 study, which compared hibiscus with a placebo, reduced the I2 of the DBP analysis from 96% to 3% and modified the result to a significant effect (P < 0.001).
DISCUSSION
The present systematic review and meta-analysis of 17 studies suggests that consumption of H. sabdariffa may prevent or alleviate individual risk factors for CVD. There is evidence from the chronic studies in the review that high doses (>1 g/day) affect BP and doses between 500 and 1000 mg/day affect lipids. Typically, clinical studies have examined the effects of hibiscus on 1 or 2 biomarkers, with few studies considering multiple CVD markers simultaneously. Overall, hibiscus exerts stronger BP-lowering effects in individuals who have higher BP at baseline.
Effectiveness of hibiscus on BP
Our meta-analysis indicated that hibiscus significantly lowered SBP (−7.92% from baseline) and showed a nonsignificant trend to lower DBP (−6.84% from baseline). These findings are in line with animal trials that have consistently demonstrated the beneficial effects of hibiscus administration on both SBP and DBP. In the only acute study published to date, Abubakar et al68 provided 7.5 g of hibiscus to healthy adults and found no significant effect on SBP or DBP up to 4 hours after consumption. In contrast, Bell and Williams70 reported significantly lower DBP 1.5 hours postprandially after Haskap berry ingestion. The difference in results of these acute studies may be correlated with the different anthocyanin profile present in different foods.
There is limited evidence of a dose- or time-dependent response to hibiscus supplementation based on the studies included in this meta-analysis. Early evidence using rat models of hypertension indicate more positive effects of hibiscus at lower doses24 but not at high doses.71 Nwachukwu et al69 showed that 150 mg/kg hibiscus was more effective in reducing BP than 300 mg/kg for both SBP (150 mg/kg: −17.08 ± 4.25 (SEM) mmHg; 300 mg/kg: −10.56 ± 3.39 mmHg) and DBP (150 mg/kg −11.13 ± 3.92 mmHg; 300 mg/kg −7.36 ± 2.54 mmHg), although the researchers did not include a control group and, therefore, did not meet our inclusion criteria. Meta-regression results indicated that participants with higher baseline BP had a greater response to hibiscus treatment, offering the possibility that hibiscus may confer antihypertensive benefits. A decrease of 2 mmHg or 5 mmHg in SBP has been estimated to reduce mortality risk of coronary heart disease by 4% and 9%, respectively.72 At a population level, the average reduction in SBP of 8.8 mmHg observed in this meta-analysis by hibiscus could substantially reduce CVD risk.
Effectiveness of hibiscus on lipid parameters
Evidence from the 8 included studies in which lipid parameters were assessed indicated that hibiscus significantly lowered LDL (−6.9% from baseline). Although hibiscus ingestion lowered TC (−3.5% from baseline) and TG (−10.31% from baseline) levels and increased HDL (+11.14% from baseline) level, these effects were not significant.
The effects of other tea beverages compared with hibiscus were comparable in their ability to lower LDL and TG levels. However, an increase in HDL was more apparent in studies using green or black tea. Both green and black tea have been reported in previous studies to reduce TC and impact HDL in adults with mild hypercholesterolemia.73,74 Catechins present in green tea constitute 80%–90% of the total flavonoids, whereas black tea may only contain 20%–30% catechins,75 and catechins in hibiscus amount to only 3%–4% of the total flavonoid content.76 Animal data suggest that catechins can inhibit cholesterol absorption77 and reduce liver cholesterol concentrations by inhibiting the activity of squalene epoxidase (an enzyme important in sterol biosynthesis).78 Thus, if catechins exert a favorable effect on cholesterol and lipids, this may explain the results of the subgroup analyses of HDL whereby “other tea” controls showed stronger effects than hibiscus. Consistent with animal studies, epidemiological data indicate that tea consumption is associated with a decrease in rate of HDL lowering with age and a decrease in LDL independent of age.79
Despite no observed dose response in subgroup analysis, interestingly, a low dose (100 mg hibiscus, 19.24 mg anthocyanins54) of hibiscus had a greater effect on lipid parameters including HDL than a high dose (500 mg hibiscus, 83 mg anthocyanins58) in participants with MeSy. HDL is a protective factor for heart disease; therefore, the potential for hibiscus to increase HDL, as seen in patients with MeSy and to reduce LDL could indicate a particularly beneficial impact in those with elevated CVD risk or MeSy.
Effectiveness of hibiscus on blood glucose
The current meta-analysis of chronic trials showed no significant effect of hibiscus on FPG level (−1.48 mg/dL; P = 0.29). This contrasts with the findings of a recent meta-analysis by Bule et al40 in which the authors reported a significant reduction (−3.96 mg/dL; P = 0.001) in FPG with hibiscus consumption. However, the Bule et al40 analysis included both acute and chronic trials. Combining acute and chronic studies could obscure potentially different temporal mechanisms of action. In the chronic studies considered in our meta-analysis, there did not seem to be a clear dose-response or treatment-duration effect. Rodent studies in which high-fat feeding regimens80 or induced diabetes81 were used have shown hibiscus consumption to reduce blood glucose level. Furthermore, the acute inhibitory effect of hibiscus on starch digestion and postprandial blood glucose response has recently been demonstrated in our laboratory (unpublished work). Whether this is relevant to longer-term effects on glucose metabolism remains to be established.
Effect of hibiscus compared with dietary interventions
Only 2 intervention diets were compared with hibiscus. The Dietary Approaches to Stop Hypertension (DASH) diet is supported by substantial evidence in terms of its efficacy to lower BP.82 It is recommended for individuals with MeSy to follow a low-cholesterol diet according to guidelines from the National Cholesterol Education Program ATP-III reports.83
Results from this meta-analysis demonstrated a more favorable effect on LDL, TC, TG, and HDL levels of participants who consumed hibiscus compared with those following the National Cholesterol Education Program ATP-III diet alone.54 Interestingly, in BP analysis, a combination of hibiscus and the DASH diet was more effective than the DASH diet alone in lowering both SBP and DBP.61 A major limitation of dietary intervention studies is lack of adherence to the prescribed diet.84,85 Therefore, hibiscus could provide adjuvant therapy to well-studied diets for both lipid and BP management.
Effectiveness of hibiscus vs pharmaceuticals
Importantly, the effects of hibiscus on BP were not discernible from those of commonly used pharmaceuticals used to lower BP in participants with stage 1 hypertension (mean change from baseline: hibiscus SBP, −11.39%, combined pharmaceuticals SBP, −10.46%; hibiscus DBP, −11.66%, combined pharmaceuticals DBP, −10.75%). Individuals who consumed 10 g of hibiscus had similar reductions in SBP and DBP as taking 50 mg of captopril.51 However, 250 mg of hibiscus was not as effective as 10 mg of lisinopril.52 In patients with noncomplicated hypertension, 640 mg of hibiscus was less effective than 5 mg of ramipril for SBP, although the effects on DBP were similar.60 Notably, 150 mg/kg hibiscus had superior BP-lowering effects compared with 25 mg of hydrochlorothiazide.57 Bourqui et al86 recently reported hibiscus to be as effective as captopril in a 6-month study of patients with hypertension, a finding consistent with the results of our meta-analysis. However, Bourqui et al86 combined the hibiscus tea or capsule treatments with the corresponding kinkeliba (Combretum micranthum) treatment if either treatment did not produce a clinical response, rendering this study ineligible for inclusion in this meta-analysis.
The effect of hibiscus compared with other cardiometabolic pharmaceuticals has mainly been studied in animals. Hibiscus was equally effective as gilbenclamide (a drug therapy for type 2 diabetes) in reducing blood glucose levels in rats with induced diabetes.87 In addition, hibiscus mitigated increases in cholesterol and LDL levels in alloxan-induced diabetes in mice by 29% and 40%, respectively, which was comparable to the effects of the cholesterol-lowering drug lovastatin.88 The effect of hibiscus on HDL was similar to that of pravastatin in the only human study to have examined effects in adults with hypercholesterolemia.
Thus, hibiscus treatment of BP could be considered a viable alternative to pharmaceutical treatment of hypertension with diuretics or ACE inhibitors, though the effects on lipids and blood glucose require additional research.
Adverse reactions
Hibiscus is considered safe for consumption, with no evidence of toxicity in rodent studies up to 5000 mg/kg in an acute dose89 or chronic feeding up to 200 mg/kg over 3 months.90 In the studies included in this meta-analysis, authors of 1 study reported mild gastrointestinal symptoms using 1 g of hibiscus extract after the first week of supplementation; however, these symptoms subsided within 1 week.63 No other adverse effects of hibiscus were reported across the included studies in this analysis at doses up to 10 g/day.
Another important factor to consider is the possibility of herb–drug interactions. Concomitant intake of hibiscus (20–40 mg/kg body weight) with the diuretic drug hydrochlorothiazide (10 mg/kg) significantly increased urine volume in experimental rats over a 24-hour sampling period, which increases risk of dehydration.90 However, the dose of the drug used in that study does not translate to a physiological dose for human consumption. The interaction of hibiscus with ACE inhibitors (eg, ramipril) needs to be established because hibiscus has been identified as an ACE inhibitor.26 Thus, additional investigations are warranted on the safety of hibiscus–drug interactions.
Attrition and confounding effects
Overall, in studies that provided participant dropout rates, compliance was measured or calculated at ≥86% except for in the study by Herrera et al,51 in which 26% of patients withdrew from the hibiscus experimental group because they did not like the bitter taste of the beverage. Confounding factors influence the validity of inferences made about cause and effect, particularly in food-based research.91 Modest reductions in body weight of 5%–10% can confer improvements in CVD risk factors.92 In this meta-analysis, few studies reported any confounding effects that could have influenced the outcome of the study. Body weight and BMI were reduced over the course of some studies, but these reductions were independent of any changes to outcomes measured.56,63,65
Strengths and limitations
To date, to our knowledge, this is the most comprehensive review of the available literature that includes studies assessing the effect of hibiscus on lipid parameters, BP, and FPG, and that used TSA to account for type I and type II errors in the included studies. Results from TSA support the significant effect of hibiscus on SBP and DBP. Subgroup analysis and meta-regression were unable to explain heterogeneity among the limited number of included studies.
In this review, we identified several limitations of the available literature on nutritional interventions. Overall, the included studies have a moderate risk of bias, particularly with regard to allocation concealment and blinding. There is evidence that inadequate allocation concealment can lead to overestimation or underestimation of treatment effects.93 Ineffective blinding also influences participant response but is particularly challenging in nutritional intervention studies. Double blinding reduces the risk of bias within intervention studies. In some of the tea intervention studies in this meta-analysis, double blinding was achieved in various ways, for example, by adding flavor and color to match the hibiscus tea or providing treatments in blinded packaging.
Finally, lacking or inconsistent characterization of hibiscus intervention products renders comparisons between studies difficult.94 Variations in the content and composition of bioactive compounds in different extracts may account for some of the disparity in the effects observed and contribute to heterogeneity in the results of the meta-analysis reported. More research is required to characterize the active constituent of this plant responsible for the various beneficial effects on CVD risk markers.
CONCLUSION
Th findings from this systematic review and meta-analysis, summarizing the most recent evidence from randomized intervention studies, suggest hibiscus consumption could affect CVD risk markers beneficially. Individuals should be encouraged to consume hibiscus when they have chronically elevated BP. Overall, hibiscus is considered safe and well tolerated, and can be consumed regularly as part of a balanced diet. In view of the discussed limitations, the findings of this meta-analysis emphasize the need for more well-designed and controlled randomized controlled trials, especially with duration >8 weeks, to confirm these findings and elucidate a dose and duration response of hibiscus consumption on CVD risk biomarkers.
Supplementary Material
Acknowledgments
Author contributions. L.R.E., C.B., L.M., and L.D. designed the study; L.R.E. conducted the systematic review and S.Z. duplicated part of the screening; L.R.E. conducted the meta-analysis, supported by M.H.; L.R.E. drafted the manuscript; main revisions were made by C.B. and L.D.; all authors read and approved the manuscript.
Funding. L.R.E. is supported by the Emma and Lesley Reid Scholarship, University of Leeds. The project received partial support through a Diet and Health Seeding Award to C.B. from Biotechnology and Biological Sciences Research Council (BBSRC).
None of the funders had a role in conception, design, performance, or approval of the work.
Declaration of interest. Authors declare no conflict of interest.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Table S1 PRISMA checklist
Table S2 Subgroup analyses of the effects of dose and duration of hibiscus consumption on blood pressure in humans
Table S3 Subgroup analyses of the effects of dose and duration of hibiscus consumption on lipids in humans
Table S4 Subgroup analyses of the effects of dose and duration of hibiscus consumption on fasting plasma glucose in humans
Figure S1 Risk-of-bias assessment of the included studies. Yellow circle, unclear or unknown risk of bias; red circle, high risk of bias; green circle, low risk of bias
Figure S2 Meta-analysis on the effects of hibiscus supplementation on fasting plasma glucose. Superscript denotes population of the Gurrola-Diaz study (a = healthy, b = MeSy)
Figure S3 Random effect meta-regression showing the effects of baseline values on A) SBP and B) DBP. Each circle represents a study, telescoped by its weight in the analysis. The decreasing line suggests that individuals responded better to hibiscus when their baseline BP was higher
Figure S4 Outcomes from trial sequential analysis on lipids which did not meet the required information size (RIS)
Figure S5 Outcome from trial sequential analysis on fasting plasma glucose, which did not meet the required information size (RIS)
Figure S6 Funnel plots outlining no evidence of publication bias of the included studies: A) SBP, B) DBP, C) LDL, D) HDL, E) TC, F) TG, H) FPG
Contributor Information
Lucy R Ellis, School of Psychology, Faculty of Medicine and Health, University of Leeds, Leeds, United Kingdom.
Sadia Zulfiqar, School of Food Science and Nutrition, Faculty of Environment, University of Leeds, United Kingdom.
Mel Holmes, School of Food Science and Nutrition, Faculty of Environment, University of Leeds, United Kingdom.
Lisa Marshall, School of Food Science and Nutrition, Faculty of Environment, University of Leeds, United Kingdom.
Louise Dye, School of Psychology, Faculty of Medicine and Health, University of Leeds, Leeds, United Kingdom.
Christine Boesch, School of Food Science and Nutrition, Faculty of Environment, University of Leeds, United Kingdom.
References
- 1. Wu C, Hu H, Chou Y, et al. High blood pressure and all-cause and cardiovascular disease mortalities in community-dwelling older adults. Medicine (Baltimore). 2015;94:e2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dinh Q, Drummon G, Sobey C, et al. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int. 2014;2014:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dhingra R, Vasan R.. Biomarkers in cardiovascular disease: statistical assessment and section on key novel heart failure biomarkers. Trends Cardiovasc Med. 2017;27:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Savica V, Bellinghieri G, Kopple J.. The effect of nutrition on blood pressure. Annu Rev Nutr. 2010;30:365–401. [DOI] [PubMed] [Google Scholar]
- 5. Patel R, Masi S, Taddei S.. Understanding the role of genetics in hypertension. Eur Heart J. 2017;38:2309–2312. [DOI] [PubMed] [Google Scholar]
- 6. Sacks FM, Lichtenstein AH, Wu JHY, et al. ; American Heart Association. Dietary fats and cardiovascular disease: a Presidential Advisory from the American Heart Association. Circulation. 2017;136:e1–e23. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. NCD risk factors: blood pressure. July 2017. Available at: https://www.who.int/gho/ncd/risk_factors/blood_pressure_prevalence_text/en/. Accessed March 15, 2021.
- 8. Ettehad D, Emdin C, Kiran A, et al. Blood pressure lowering effect for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 9. Afolayan A, Wintola O.. Dietary supplements in the management of hypertension and diabetes - a review. Afr J Tradit Complement Altern Med. 2014;11:248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Laan D, Elders P, Boons C, et al. Factors associated with antihypertensive medication non-adherence: a systematic review. J Hum Hypertens. 2017;31:687–694. [DOI] [PubMed] [Google Scholar]
- 11. Serour M, Alqhenaei H, Al-Saqabi S, et al. Cultural factors and patients’ adherence to lifestyle measures. Br J Clin Pract. 2007;57:291–295. [PMC free article] [PubMed] [Google Scholar]
- 12. Lee Y, Kim R, Lee H, et al. Relationships among medication adherence, lifestyle modification, and health-related quality of life in patients with acute myocardial infarction: a cross-sectional study. Health Qual Life Outcomes. 2018;16:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aune D, Giovannucci E, Boffetta P, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46:1029–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fairlie-Jones L, Davison K, Fromentin E, et al. The effect of anthocyanin-rich foods or extracts on vascular function in adults: a systematic review and meta-analysis of randomised controlled trials. Nutrients. 2017;9:908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shah K, Shah P.. Effect of anthocyanin supplementations on lipid profile and inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Cholesterol. 2018;2018:8450793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riaz G, Chopra R.. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed Pharmacother. 2018;102:575–586. [DOI] [PubMed] [Google Scholar]
- 17. Vora J, Patwardhan A, Srinivasan P.. An insight into stimulated product development: hibiscus tea. J Biotech Biochem. 2016;2:36–44. [Google Scholar]
- 18. Christian K, Jackson J.. Changes in total phenolic and monomeric anthocyanin composition and antioxidant activity of three varieties of sorrel (Hibiscus sabdariffa) during maturity. J Food Compost Anal. 2009;22:663–667. [Google Scholar]
- 19. Sindi H, Marshall L, Morgan M.. Comparative chemical and biochemical analysis of extracts of Hibiscus sabdariffa. Food Chem. 2014;164:23–29. [DOI] [PubMed] [Google Scholar]
- 20. Ali B, Al Wabel N, Blunden G.. Phytochemical, pharmacological and toxicological aspects of Hibiscus sabdariffa L: a review. Phytother Res. 2005;19:369–375. [DOI] [PubMed] [Google Scholar]
- 21. Odigie I, Ettarh R, Adigun S.. Chronic administration of aqueous extract of Hibiscus sabdariffa attenuates hypertension and reserves cardiac hypertrophy in 2k-1C hypertensive rats. J Ethnopharmacol. 2003;86:181–185. [DOI] [PubMed] [Google Scholar]
- 22. Joven J, March I, Espinel E, et al. Hibiscus sabdariffa extract lowers blood pressure and improves endothelial function. Mol Nutr Food Res. 2014;58:1374–1378. [DOI] [PubMed] [Google Scholar]
- 23. Ajay M, Chai HJ, Mustafa A, et al. Mechanisms of the anti-hypertensive effect of Hibiscus sabdariffa L. calyces. J Ethnopharmacol. 2007;109:388–393. [DOI] [PubMed] [Google Scholar]
- 24. Mojiminiyi F, Dikko M, Muhammad B, et al. Antihypertensive effect of an aqueous extract of the calyx of Hibiscus sabdariffa. Fitoterapia. 2007;78:292–297. [DOI] [PubMed] [Google Scholar]
- 25. Alarcon-Alonso J, Zamilpa A, Alarcon-Aquilar F, et al. Pharmacological characterization of the diuretic effect of Hibiscus Sabdariffa Linn (Malvaceae) extract. J Ethnopharmacol. 2012;139:751–756. [DOI] [PubMed] [Google Scholar]
- 26. Ojeda D, Jimenez-Ferrer E, Zamilpa A, et al. Inhibition of angiotensin converting enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J Ethnopharmacol. 2010;127:7–10. [DOI] [PubMed] [Google Scholar]
- 27. Parichatikanond W, Pinthong D, Mangmool S.. Blockade of the renin-angiotensin system with delphinidin, cyanin, and quercetin. Planta Med. 2012;78:1626–1632. [DOI] [PubMed] [Google Scholar]
- 28. Xu JW, Ikeda K, Yamori Y.. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension. 2004;44:217–222. [DOI] [PubMed] [Google Scholar]
- 29. Min S-W, Ryu SN, Kim H.. “Anti-inflammatory effects of black rice, cyanidin3-O-β-d-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int Immunopharmacol. 2010;10:959–966. [DOI] [PubMed] [Google Scholar]
- 30. Lin TL, Lin HH, Chen C, et al. Hibiscus sabdariffa extract reduces serum cholesterol in men and women. Nutr Res. 2007;27:140–145. [Google Scholar]
- 31. Sabzghabaee A, Ataei E, Kelishadi R, et al. Effect of Hibiscus sabdariffa calices on dyslipidemia in obese adolescents: a triple-masked randomized controlled trial. Mater Sociomed. 2013;25:76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang M, Peng C, Chan K, et al. The hypolipidemic effect of Hibiscus sabdariffa polyphenols via inhibiting lipogenesis and promoting hepatic lipid clearance. J Agric Food Chem. 2010;58:850–859. [DOI] [PubMed] [Google Scholar]
- 33. Carvajal-Zarrabal O, Waliszewski SM, Barradas-Dermitz DM, et al. The consumption of Hibiscus sabdariffa dried calyx ethanolic extract reduced lipid profile in rats. Plant Foods Hum Nutr. 2005;60:(153–159. [DOI] [PubMed] [Google Scholar]
- 34. Mardiah F, Zakaria FR, Prangdimurti E, et al. The effect of roselle extract (Hibiscus sabdariffa Linn.) on blood glucose level and total antioxidant level on diabetic rat induced by streptozotocin. J Pharm. 2014;4:8–16. [Google Scholar]
- 35. Wisetmuen E, Pannangpetch P, Kongyingyoes B, et al. Insulin secretion enhancing activity of roselle calyx extract in normal and streptozotocin-induced diabetic rats. Pharmacognosy Res. 2013;5:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Henning S, Niu Y, Lee N, et al. Bioavailability and antioxidant activity of tea flavonols after consumption of green tea, black tea or a green tea extract supplement. Am J Clin Nutr. 2004;80:1558–1564. [DOI] [PubMed] [Google Scholar]
- 37. Schär M, Curtis P, Hazim S, et al. Orange juice–derived flavanone and phenolic metabolites do not acutely affect cardiovascular risk biomarkers: a randomized, placebo-controlled, crossover trial in men at moderate risk of cardiovascular disease. Am J Clin Nutr. 2015;101:931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sahebkar A, Serban C, Dragan S, et al. Effect of sour tea (Hibiscus sabdariffa L.) on arterial hypertension: a systematic review and meta-analysis of randomized controlled trials. Atherosclerosis. 2015;241:e190–e191. [DOI] [PubMed] [Google Scholar]
- 39. Aziz Z, Wong S, Chong N.. Effects of Hibiscus sabdariffa L. on serum lipids: a systematic review and meta-analysis. J Ethnopharmacol. 2013;150:442–450. [DOI] [PubMed] [Google Scholar]
- 40. Bule M, Albelbeisi A, Nikfar S, et al. The antidiabetic and antilipidemic effects of Hibiscus sabdariffa: a systematic review and meta-analysis of randomized clinical trials. Food Res Int. 2020;130. doi: 10.1016/j.foodres.2020.108980. [DOI] [PubMed] [Google Scholar]
- 41. Wahabi H, Alansary L, Al-Sabban A, et al. The effectiveness of Hibiscus sabdariffa in the treatment of hypertension. A systematic review. Phytomedicine. 2010;17:83–86. [DOI] [PubMed] [Google Scholar]
- 42. Boushehri S, Karimbeiki R, Ghasempour S, et al. The efficacy of sour tea (Hibiscus sabdariffa L.) on selected cardiovascular disease risk factors: a systematic review and meta‐analysis of randomized clinical trials. Phytotherapy Research. 2020;34:329–339. [DOI] [PubMed] [Google Scholar]
- 43. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLos Med. 2009;6:e1000097.( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sterne JC, Savovic J, Page MJ, et al. A revised tool for assessing risk of bias in randomized trials. BMJ. 2019;366:14989. [DOI] [PubMed] [Google Scholar]
- 45. Higgins J, Green S. Cochrane handbook for systematic reviews of interventions (version 5.2.0). Cochrane Collaboration. 2017. Available at: https://training.cochrane.org/handbook. Accessed May 2021.
- 46. Borenstein M, Higgins J, Hedges L, et al. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8:5–18. [DOI] [PubMed] [Google Scholar]
- 47. Begg C, Mazumdar M.. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 48. Egger M, Davey-Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Imberger G, Thorlund K, Gluud C, et al. False-positive findings in Cochrane meta-analyses with and without application of trial sequential analysis: an empirical review. BMJ Open. 2016;6:e011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thorlund K, Engstrom J, Wettersley J, et al. User manual for trial sequential analysis (TSA). 2011. Available at: www.ctu.dk/tsa. Accessed October 2020.
- 51. Herrera-Arellano A, Flores-Romero S, Chavez-Soto M, et al. Effectiveness and tolerability of a standardized extract from Hibiscus sabdariffa in patients with mild to moderate hypertension: a controlled and randomized clinical trial. Phytomedicine. 2004;11:375–382. [DOI] [PubMed] [Google Scholar]
- 52. Herrera-Arellano A, Miranda-Sánchez J, Avila-Castro P, et al. Clinical effects produced by a standarized herbal medicinal product of Hibiscus sabdariffa on patients with hypertension. A randomized, double-blind, lisinopril-controlled clinical trial. Planta Med. 2006;73:6–12. [DOI] [PubMed] [Google Scholar]
- 53. Mozaffari-Khosravi H, Jalali-Khanabadi B, Afkhami-Ardekani M, et al. The effects of sour tea (Hibiscus sabdariffa) on hypertension in patients with type II diabetes. J Hum Hypertens. 2009;23:48–54. [DOI] [PubMed] [Google Scholar]
- 54. Gurrola-Diaz C, Garcia-Lopez P, Sanchez-Enriquez S, et al. Effects of Hibiscus sabdariffa extract powder and preventive treatment (diet) on the lipid profiles of patients with metabolic syndrome (MeSy). Phytomedicine. 2010;17:500–505. [DOI] [PubMed] [Google Scholar]
- 55. McKay D, Chen O, Saltzman E, et al. Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in pre-hypertensive and mildly hypertensive adults. J Nutr. 2010;140:298–303. [DOI] [PubMed] [Google Scholar]
- 56. Mozaffari-Khosravi H, Ahadi Z, Barzegar K.. The effect of green tea and sour tea on blood pressure of patients with type 2 diabetes: a randomized clinical trial. J Diet suppl. 2013;10:105–115. [DOI] [PubMed] [Google Scholar]
- 57. Nwachukwu D, Aneke E, Nwachukwu N, et al. Effect of Hibiscus sabdariffa on blood pressure and electrolyte profile of mild to moderately hypertensive Nigerians: a comparative study with hydrochlorothiazide. Niger J Clin Pract. 2015;18:762–770. [DOI] [PubMed] [Google Scholar]
- 58. Asgary S, Soltani R, Zolghadr M, et al. Evaluation of the effects of roselle (Hibiscus sabdariffa L.) on oxidative stress and serum levels of lipids, insulin and hs-CRP in adult patients with metabolic syndrome: a double-blind placebo-controlled clinical trial. J Complement Integr Med. 2016;13:175–180. [DOI] [PubMed] [Google Scholar]
- 59. Kafeshani M, Entezari MH, Karimian J, et al. A comparative study of the effect of green tea and sour tea on blood pressure and lipid profile in healthy adult men. ARYA Atheroscler. 2017;13:109–116. [PMC free article] [PubMed] [Google Scholar]
- 60. Seck S, Doupa D, Dia D, et al. Clinical efficacy of African traditional medicines in hypertension: a randomized controlled trial with Combretum micranthum and Hibiscus sabdariffa. J Hum Hypertens. 2017;32:75–81. [DOI] [PubMed] [Google Scholar]
- 61. Jalalyazdi M, Ramezani J, Izadi-Moud A, et al. Effect of Hibiscus sabdariffa on blood pressure in patients with stage 1 hypertension. J Adv Pharm Technol Res. 2019;10:107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mozaffari-Khosravi H, Jalali-Khanabadi A, Afkhami-Ardekani M, et al. Effects of sour tea (Hibiscus sabdariffa) on lipid profile and lipoproteins in patients with type II diabetes. J Altern Complement Med. 2009;15:899–903. [DOI] [PubMed] [Google Scholar]
- 63. Kuriyan R, Kumar D, Rajendran R, et al. An evaluation of the hypolipidemic effect of an extract of Hibiscus sabdariffa leaves in hyperlipidemic Indians: a double blind, placebo-controlled trial. BMC Complement Altern Med. 2010;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mohagheghi A, Maghsoud S, Khashayar P, et al. The effect of Hibiscus sabdariffa on lipid profile, creatinine, serum electrolytes: a randomised clinical trial. ISRN Gastroenterol. 2011;2011:976019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chang H, Peng C, Yeh D, et al. Hibiscus sabdariffa extract inhibits obesity and fat accumulation, and improves liver steatosis in humans. Food Funct. 2014;5:734–739. [DOI] [PubMed] [Google Scholar]
- 66. Hajifaraji M, Matlabi M, Ahmadzadeh-Sani F, et al. Effects of aqueous extracts of dried calyx of sour tea (Hibiscus sabdariffa L.) on polygenic dyslipidemia: a randomized clinical trial. Avicenna J Phytomed. 2018;8:24–32. [PMC free article] [PubMed] [Google Scholar]
- 67. Izadi F, Farrokhzad A, Tamizifar B, et al. Effect of sour tea supplementation on liver enzymes, lipid profile, blood pressure, and antioxidant status in patients with non-alcoholic fatty liver disease: a double-blind randomized controlled clinical trial. Phytother Res. 2021;35:477–485. [DOI] [PubMed] [Google Scholar]
- 68. Abubakar SM, Ukeyima MT, Spencer JPE, et al. Acute effects of Hibiscus sabdariffa calyces on postprandial blood pressure, vascular function, blood lipids, biomarkers of insulin resistance and inflammation in humans. Nutrients. 2019;11:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nwachukwu D, Aneke E, Obika L, et al. Investigation of the antihypertensive effectiveness and tolerability of Hibiscus sabdariffa in mild to moderate hypertensive subjects in Enugu, South-East Nigeria. Am J Phytomed Clin Ther 2015b;3:339–345. [Google Scholar]
- 70. Bell L, Williams C.. A pilot dose-response study of the acute effects of Haskap berry extract (Lonicera caerulea) on cognition, mood and blood pressure in older adults. Eur J Nutr. 2019;58:3325–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Onyenekwe PC, Ajani EO, Ameh DA, et al. Antihypertensive effect of roselle (Hibiscus sabdariffa) calyx infusion in spontaneously hypertensive rats and a comparison of its toxicity with that in Wistar rats. Cell Biochem Funct. 1999;17:199–206. [DOI] [PubMed] [Google Scholar]
- 72. James P, Oparil S, Carter B, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 73. Kim A, Chiu A, Barone M, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011;111:1720–1729. [DOI] [PubMed] [Google Scholar]
- 74. Davies M, Judd J, Baer D, et al. Black tea consumption reduces total and LDL cholesterol in mildly hypercholesterolemic adults. J Nutr. 2003;133:3298S–3302S. [DOI] [PubMed] [Google Scholar]
- 75. Stangl V, Lorenz M, Stangl K.. The role of tea and tea flavonoids in cardiovascular health. Mol Nutr Food Res. 2006;50:218–228. [DOI] [PubMed] [Google Scholar]
- 76. Da-Costa-Rocha I, Bonnlaender B, Sievers H, et al. Hibiscus sabdariffa L. – A phytochemical and pharmacological review. Food Chem. 2014;165:424–443. [DOI] [PubMed] [Google Scholar]
- 77. Chan P, Fong W, Cheung Y, et al. Jasmine green tea epicatechins are hypolipidemic in hamsters (Mesocricetus auratus) fed a high fat diet. J Nutr. 1999;129:1094–1100. [DOI] [PubMed] [Google Scholar]
- 78. Abe I, Seki T, Umehara K, et al. Green tea polyphenols: novel and potent inhibitors of squalene epoxidase. Biochem Biophys Res Commun. 2000;268:767–771. [DOI] [PubMed] [Google Scholar]
- 79. Huang S, Li J, Wu Y, et al. Tea consumption and longitudinal change in high-density lipoprotein cholesterol concentrations in Chinese adults. J Am Heart Assoc. 2018;7:e008814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Janson B, Prasomthong J, Malakul W, et al. Hibiscus sabdariffa L. calyx extract prevents the adipogenesis of 3T3-L1 adipocytes, and obesity-related insulin resistance in high-fat diet-induced obese rats. Biomed Pharmacother. 2021;138:(111438. [DOI] [PubMed] [Google Scholar]
- 81. Peng C, Chyau C, Chan K, et al. Hibiscus sabdariffa polyphenolic extract inhibits hyperglycemia, hyperlipidemia, and glycation-oxidative stress which improving insulin resistance. J Agric Food Chem. 2011;59:9901–9909. [DOI] [PubMed] [Google Scholar]
- 82. Challa H, Ameer M, Uppaluri K, DASH diet to stop hypertension. 2020. https://www.ncbi.nlm.nih.gov/books/NBK482514/. Accessed August 2, 2021. [PubMed]
- 83. National Cholesterol Education Program. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 84. Epstein D, Sherwood A, Smith P, et al. Determinants and consequences of adherence to the Dietary Approaches to Stop Hypertension diet in African-American and white adults with high blood pressure. Results from the ENCORE trial. J Acad Nutr Diet. 2012;112:1763–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Soltani S, Arablou T, Jayedi A, et al. Adherence to the Dietary Approaches to Stop Hypertension (DASH) diet in relation to all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Nutr J. 2020;19:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bourqui A, Niang E, Graz B, et al. Hypertension treatment with Combretum mircanthum or Hibiscus sabdariffa, as decoction or tablet: a randomised clinical trial. J Hum Hypertens. 2021;35:800–808. [DOI] [PubMed] [Google Scholar]
- 87. Aba PE, Nwaigwe CU, Okwuagwu FO, et al. Effect of aqueous extract of Hibiscus sabdariffa on some biochemical parameters in alloxan-induced diabetic rats. Comp Clin Pathol. 2014;23:1675–1680. [Google Scholar]
- 88. Farombi E, Ige O.. Hypolipidemic and antioxidant effects of ethanolic extract from dried calyx of Hibiscus sabdariffa in alloxan-induced diabetic rats. Fundam Clin Pharmacol. 2007;21:601–609. [DOI] [PubMed] [Google Scholar]
- 89. Fakeye T, Pal A, Bawankule D, et al. Toxic effects of oral administration of extracts of dried calyx of Hibiscus sabdariffa Linn. (Malvaceae). Phytother Res. 2009;23:412–416. [DOI] [PubMed] [Google Scholar]
- 90. Ndu O, Nworu C, Ehiemere C, et al. Herb–drug interaction between the extract of Hibiscus sabdariffa L. and hydrochlorothiazide in experimental animals. J Med Food. 2011;14:640–644. [DOI] [PubMed] [Google Scholar]
- 91. Schwingshackl L, Schünemann H, Meerpohl J.. Improving the trustworthiness of findings from nutrition evidence syntheses: assessing risk of bias and rating the certainty of evidence. Eur J Nutr. 2021;60:2893–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Brown J, Buscemi J, Milsom V, et al. Effects on cardiovascular risk factors of weight losses limited to 5-10%. Transl Behav Med. 2016;6:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Paludan-Müller A, Laursen D, Hróbjartsson A.. Mechanisms and direction of allocation bias in randomised clinical trials. BMC Med Res Methodol. 2016;16:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fracassetti D, Del Bo C, Simonetti P, et al. Effect of time and storage temperature on anthocyanin decay and antioxidant activity in wild blueberry (Vaccinium angustifolium) powder. J Agric Food Chem. 2013;61:2999–3005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.