Abstract
In this study, the in vitro and in vivo efficacies of free sodium stibogluconate (SSG) and a nonionic surfactant vesicular formulation of SSG (SSG-NIV) against a laboratory strain of Leishmania donovani (MHOM/ET/67:LV82) and different clinical isolates of L. donovani were determined. Treatment with SSG-NIV was more effective against intramacrophage amastigotes than treatment with SSG. In vivo murine studies showed that there was interstrain variability in the infectivity of the different L. donovani strains, with two of the strains (20001 and 20003) giving low parasite burdens. In addition, interstrain variability in the antileishmanial efficacy of SSG in a single dose containing 300 mg of Sb(V)/kg of body weight was observed. This dose of free drug either caused a >97% reduction in liver parasite burdens or had no significant effect on parasite burdens compared with the result with the respective control. In some instances, treatment with this free SSG dose also caused a significant reduction in spleen (strain 20006) or bone marrow (strains 20001 and 20009) parasite burdens. Treatment with SSG-NIV was more effective than that with SSG against all of the strains tested. In SSG-responsive strains, the reduction in liver parasite burdens by SSG-NIV treatment was similar to that caused by free SSG. In SSG-nonresponsive strains, SSG-NIV treatment caused at least a 95% reduction in liver parasite burdens. Overall, these results indicate that the use of a vesicular formulation of SSG is likely to increase its clinical efficacy against visceral leishmaniasis.
Visceral leishmaniasis (VL) caused by Leishmania donovani infection poses a serious health threat and is endemic in 82 countries. Once clinical illness develops, the disease progresses and patients die if untreated (9). Reactivation of latent Leishmania infections can also occur in human immunodeficiency virus-infected individuals. Studies in France, Italy, Spain, and Portugal have shown that between 1.5 and 9% of human immunodeficiency virus-positive patients develop VL (9). The pentavalent antimonials, such as sodium stibogluconate (SSG), are the main chemotherapeutic drugs used to treat the infection. The pentavalent antimonials have a number of clinical limitations; for example, in vivo pharmacokinetic characteristics of the antimonials necessitate the use of extended treatment regimens (1, 6). Therefore, effective alternative chemotherapies or improved formulations of existing drugs are required. In our previous studies, we have shown that the use of a nonionic surfactant vesicular formulation of SSG (SSG-NIV) can markedly improve the therapeutic efficacy of SSG (18). In a murine model, SSG-NIV treatment was more or as effective as lipid-based amphotericin B drug formulations which are now commercially available for VL treatment (16). The emergence of clinical resistance to the antimonials (12, 15) suggests that future development of this formulation may be inappropriate. However, it is possible that the NIV formulation may circumvent parasite resistance to SSG and extend the clinical life of this drug. In this study, the efficacies of free-SSG and SSG-NIV formulations against different clinical isolates of L. donovani were determined in vitro and in vivo.
MATERIALS AND METHODS
Materials.
SSG [31.1% (wt/wt) Sb (V)] was provided by Glaxo Wellcome Ltd. (Ware, United Kingdom). The nonionic surfactant tetraethylene glycol mono-n-hexadecylether was purchased from Chesham Chemicals Ltd., (Harrow, United Kingdom). Dicetyl phosphate and ash-free cholesterol were obtained from Sigma, and all other reagents were of analytical grade.
Animals and parasites.
Age-matched 8- to 10-week-old BALB/c mice (in-house-inbred males and females) were used in this study. In-house-bred or commercially obtained (B & K Universal, Hull, United Kingdom) golden Syrian hamsters (Mesocricetus auratus) were used for maintenance of L. donovani strains. A standard laboratory strain (strain MHOM/ET/67:MHOM/ET/67:LV82) and various clinically isolated strains obtained from India in 1996 (Food and Drug Administration [FDA] strains) or 2000 were used in this study. Clinical strains, from spleen aspirate isolates, were maintained in vitro using TC-100 insect medium (Sigma) containing 20% fetal calf serum (Gibco) and no antibiotics.
Hamsters were infected by intraperitoneal or intravenous injection of L. donovani promastigotes (stationary phase) obtained from in vitro cultures or amastigotes harvested from an infected hamster's spleen (3). Intravenous injections were carried out under anesthesia (4). The neck region of the anesthetized hamster was swabbed with antiseptic solution, and an incision was made to the right or left side of a central line. Parasites were injected into the exposed jugular vein. Pressure was applied to limit bleeding, and then the incision was closed up using 7.5- by 1.75-mm clips. The area was swabbed with antiseptic, and the animal was left to recover. Two to three weeks postinfection, any remaining clips were removed. Mice were infected (day 0 of the experiment) by intravenous injection (tail vein, with no anesthetic) with 1 × 107 to 2 × 107 L. donovani amastigotes (3) or 1 × 107 to 4 × 107 promastigotes from in vitro cultures.
Drug formulations.
One-hundred-fifty-micromole vesicle constituents, consisting of a 3:3:1 molar ratio of mono-n-hexadecyl ether tetraethylene glycol, cholesterol, and dicetyl phosphate, were melted by heating at 130°C for 5 min. The molten mixture was cooled to 70°C and hydrated with 5 ml of preheated (70°C) 100-mg/ml SSG solution and homogenized at 8,000 ± 100 rpm for 15 min at 70°C, using a model L4R SU mixer (Silverson Machines, Chesham, United Kingdom) fitted with a 5/8-in.-diameter tubular work head. Vesicle suspensions were stored at room temperature. SSG-NIV suspensions were sized by photon correlation spectroscopy using a Zetasizer 4 (Malvern Instruments Ltd., Malvern, United Kingdom).
Macrophage studies.
Five milliliters of complete medium (RPMI 1640 supplemented with 100 μg of penicillin-streptomycin/ml and l-glutamine per ml) was used to harvest the cells from the peritoneal cavity of each mouse. Cells from three to four mice were pooled and washed, and live cells were enumerated by trypan blue exclusion. Cell suspensions were adjusted to 1 × 106 to 2 × 106 cells/ml in complete medium (RPMI 1640 supplemented with 10% fetal calf serum, 100 μg of penicillin or streptomycin per ml, and l-glutamine), and 1-ml volumes were added to each well of a 24-well tissue culture plate, which contained round 13-mm-diameter glass coverslips. After 2 h of incubation at 37°C in 5% CO2, the medium was removed from each well and replaced with 0.9 ml of fresh medium. The removed supernatants were pooled, and the number of cells present was assessed so that the mean number of cells attached per well could be determined.
L. donovani promastigotes (in 0.1 ml of complete medium, stationary phase), obtained from in vitro cultures, were added to each well at various parasite-to-cell ratios, and incubation was continued for 1 to 4 h or overnight at 37°C in 5% CO2. The medium was then removed from each well and replaced with 1 ml of fresh medium (controls) or with 0.9 ml of fresh medium and 0.1 ml of one of the filter (0.2-μm pore size)-sterilized formulations (SSG drug solution [free drug], SSG-NIV, or phosphate-buffered saline [PBS]-loaded NIV [PBS-NIV]). In some cases, 25 or 50 μl of free SSG [58.9 μg of Sb(V)/ml] was added; medium was then added so that the final volume was 100 μl. The cells were then incubated at 37°C in 5% CO2 for up to 96 h, the contents of each well were removed, and the remaining coverslip was washed with PBS, fixed in methanol, and stained with 10% (vol/vol) Giemsa stain. Microscopic examination of 200 randomly chosen cells was used to determine the percentage of cells infected. The mean number of parasites per infected cell was determined from the first 20 infected cells.
Drug treatment.
Infected animals (n = 5) were treated intravenously via the tail vein on day 7 or 8 with either a single dose of PBS (controls) or a single dose of either SSG-NIV [31 to 300 mg of Sb(V)/kg of body weight], PBS-NIV, or SSG solution [266 to 300 mg of Sb(V)/kg]. Mice were sacrificed on day 14 or 15.
Determination of parasite numbers.
Parasite burdens in the livers, spleens, and bone marrows of control and drug-treated mice were determined (3). Leishman-Donovan units (LDU) were calculated per organ for the liver and spleen using the following formula: LDU = amastigote number per 1,000 host cell nuclei × organ weight in grams (modified from a previously used method [2] which used organ weight in milligrams to calculate LDU).
Presentation and statistical analysis of data.
Parasite suppression (mean percent ± standard error of the mean [SE]) was determined for a particular site by comparing each experimental parasite burden with the relevant mean control value. For each experiment, the mean control parasite burden (LDU per organ for spleen and liver and number of parasites per 1,000 host cell nuclei for the bone marrow) is shown. Parasite burdens were analyzed using Student's unpaired t test on the log10-transformed parasite burden data.
RESULTS
Macrophage studies.
There was intrastrain variability in the infection levels obtained for different strains (based on mean number of cells infected and mean number of parasites per host cell; data for three strains are shown in Table 1). Treatment with free SSG resulted in a dose-dependent antiparasitic effect for strains 20005 (based on the reduction in the percentage of cells infected) and 200013 (based on the reduction in the percentage of cells infected and the mean number of parasites per host cell). Intramacrophage survival of strain 200011 was not significantly inhibited by any of the drug doses used (Table 1). Treatment with PBS-NIV had no significant effect on the percentage of cells infected for all three strains but did result in a significant reduction in the mean number of parasites per host cell for strain 200013. SSG-NIV treatment was more effective than the corresponding free-drug dose [58.9 μg of Sb(V)/ml] against strains 20005 and 200013 (demonstrated as a greater suppression in the percentage of cells infected) but not 200011.
TABLE 1.
Comparison of the efficacies of different SSG formulations on the intracellular survival of L. donovani strainsa
| Strain and treatment | % of cells infected | % Suppressionb | No. of parasites/ host cell | % Suppressionc |
|---|---|---|---|---|
| 20005 | ||||
| Control | 75 ± 4 | 2.6 ± 0.4 | ||
| 58.9 μg of Sb(V)/ml | 43 ± 5** | 43 ± 7 | 1.9 ± 0.2 | 6 ± 8 |
| 29.5 μg of Sb(V)/ml | 48 ± 3** | 35 ± 3 | 2.5 ± 0.2 | 7 ± 4 |
| 14.73 μg of Sb(V)/ml | 60 ± 3.7 | 19 ± 4.9 | 2.4 ± 0.2 | 12 ± 7 |
| PBS-NIV | 60 ± 8.7 | 21 ± 10.8 | 2.7 ± 0.2 | 0 ± 0 |
| SSG-NIV | 19 ± 1.0** | 75 ± 1.5 | 2.1 ± 0.1 | 21 ± 2 |
| 200013 | ||||
| Control | 99 ± 1 | 11.0 ± 0.9 | ||
| 58.9 μg of Sb(V)/ml | 90 ± 2** | 10 ± 2 | 4.0 ± 0.3*** | 64 ± 3 |
| 29.5 μg of Sb(V)/ml | 89 ± 3** | 10 ± 3 | 5.9 ± 0.8** | 50 ± 7 |
| 14.73 μg of Sb(V)/ml | 99 ± 1 | 2 ± 1 | 8.5 ± 0.5** | 23 ± 5 |
| PBS-NIV | 100 ± 0 | 0 ± 0 | 8.6 ± 0.3** | 22 ± 3 |
| SSG-NIV | 76 ± 1*** | 23 ± 1 | 4.4 ± 0.6*** | 61 ± 5 |
| 200011 | ||||
| Control | 49 ± 1 | 2.1 ± 0.3 | ||
| 58.9 μg of Sb(V)/ml | 58 ± 8 | 0 ± 0 | 2 ± 0.5 | 14 ± 14 |
| 29.5 μg of Sb(V)/ml | 42 ± 2 | 13 ± 3 | 2.2 ± 0.2 | 3 ± 3 |
| 14.73 μg of Sb(V)/ml | 44 ± 10 | 20 ± 12 | 2.1 ± 0.2 | 10 ± 10 |
| PBS-NIV | 38 ± 6 | 23 ± 12 | 2.1 ± 0.5 | 12 ± 12 |
| SSG-NIV | 39 ± 9 | 20 ± 19 | 3.0 ± 0.3 | 0 ± 0 |
Macrophages (3.4 × 104/well) from female BALB/c mice were infected with L. donovani promastigotes at a parasite/cell ratio of 2:1. Cells were incubated for 96 h, and the mean percentage of cells infected and the mean number of parasites per host cell were determined. All values are means ± SEs. In comparison to the values for the relevant controls, the values in some cases were significantly different at a P of <0.0005 (***), <0.05 (**), and <0.05 (*).
Percent reduction in percent of cells infected was calculated relative to the mean control values.
Percent reduction in number of parasites per host cell was calculated relative to the mean control values.
In vivo studies.
Intraperitoneal infection of hamsters with all of the FDA strain promastigotes did not result in any obvious signs of VL infection. A few parasites were present in the spleens and livers of hamsters intravenously infected with all of the FDA strain promastigotes. However, parasites could not be cultured from the spleen or liver samples taken from these animals. Mice infected intravenously with promastigotes of six of the FDA strains had low parasite burdens (Table 2). Immunosuppression of the mice during infection did not improve infection rates (data not shown). Interestingly, the use of promastigotes to infect mice with the standard laboratory strain MHOM/ET/67:LV82 also failed to result in high parasite burdens in the spleen, liver, or bone marrow (Table 2).
TABLE 2.
Comparison of the infectivities of different L. donovani parasite strains
| Strain | Parasite burden (mean ± SE) (n = 3)a
|
||
|---|---|---|---|
| Spleen | Liver | Bone marrow | |
| FDA 9667 | 5 ± 0.6 | 23 ± 2 | 9 ± 5 |
| FDA 9668 | 9 ± 5 | 16 ± 7 | 6 ± 3 |
| FDA 9533 | 10 ± 4 | 76 ± 12 | 12 ± 2 |
| FDA 9535 | 32 ± 3 | 131 ± 17 | 12 ± 2 |
| FDA 9527 | 19 ± 4 | 35 ± 9 | 15 ± 3 |
| FDA 9630 | 16 ± 4 | 44 ± 13 | 16 ± 2 |
| MHOM/ET/67:LV82 | 18 ± 9 | 67 ± 33 | 16 ± 4 |
Parasite burdens on day 14 postinfection of mice infected with 1 × 107 to 4 × 107 L. donovani promastigotes from bulk cultures.
Intravenous infection of hamsters with promastigotes of strains obtained in Varanasi, India, in 2000 resulted in patent infections in most of the hamsters (8 out of 10 strains tested). Parasites were harvested from the spleens of hamsters when they showed signs of infection (usually a rapid weight loss). In most cases, more than 109 amastigote parasites were recovered from the spleens of infected animals. The length of time animals took to become ill varied between strains but was in the range of 4 to 7 months postinfection.
There was interstrain variability in the in vivo infectivities of the different L. donovani strains (Table 3). Two of the strains (20001 and 20003) gave quite low parasite burdens. There was interstrain variability in the antileishmanial efficacy of a single SSG dose containing 300 mg of Sb(V)/kg (Table 3). This dose of free drug either caused a >97% reduction in liver parasite burdens (strains 20001, 20006, 20009, 200016, and MHOM/ET/67:LV82) or had no significant effect on parasite burdens (strains 20003, 200011, and 200015) compared with the results for the respective controls. In some cases, treatment with this free-drug dose also caused a significant reduction in spleen (strain 20006) or bone marrow (strains 20001 and 20009) parasite burdens (Table 3). However, this occurred only in strains which also exhibited a significant reduction in liver parasite burdens.
TABLE 3.
Comparison of the effects of SSG formulations on the parasite burdens of different L. donovani strainsa
| Strain and treatment | % Suppression (mean ± SE)
|
||
|---|---|---|---|
| Spleen | Liver | Bone marrow | |
| 20001b | |||
| SSG | 25 ± 10 | 97 ± 2** | 86 ± 5** |
| PBS-NIV | 50 ± 0* | 7 ± 7 | 51 ± 11* |
| SSG-NIV | 63 ± 22 | 94 ± 4** | 95 ± 5** |
| 20003b | |||
| SSG | 13 ± 10 | 21 ± 18 | 24 ± 14 |
| PBS-NIV | ND | ND | ND |
| SSG-NIV | 33 ± 24 | 95 ± 1*** | 24 ± 14 |
| 20006 | |||
| SSG | 41 ± 12* | 97 ± 0.9*** | 46 ± 15 |
| PBS-NIV | 0.3 ± 0.3 | 8 ± 6 | 15 ± 9 |
| SSG-NIV | 99 ± 0.9*** | 99.6 ± 0.2*** | 98 ± 0.8*** |
| 20009 | |||
| SSG | 52 ± 13** | 98 ± 0.5*** | 43 ± 17 |
| PBS-NIV | 27 ± 17 | 9 ± 9 | 2 ± 2 |
| SSG-NIV | 98 ± 1*** | 99.8 ± 0.1*** | 97 ± 1*** |
| 200011 | |||
| SSG | 24 ± 17 | 18 ± 6 | 19 ± 15 |
| PBS-NIV | 15 ± 7 | 0.25 ± 0.25 | 7 ± 7 |
| SSG-NIV | 17 ± 10 | 92 ± 1*** | 47 ± 12*** |
| 200015 | |||
| SSG | 11 ± 11 | 18 ± 6 | 10 ± 10 |
| PBS-NIV | 6 ± 6 | 7 ± 2 | 9 ± 5 |
| SSG-NIV | 84 ± 14*** | 98 ± 0.5*** | 79 ± 13* |
| 200016 | |||
| SSG | 36 ± 19 | 99 ± 0.25 | 52 ± 28 |
| PBS-NIV | 41 ± 17 | 29 ± 3 | 1 ± 1 |
| SSG-NIV | 88 ± 4** | 99.7 ± 0.01*** | 77 ± 14** |
| MHOM/ET/67:LV82 | |||
| SSG | 29 ± 12 | 98 ± 1*** | 20 ± 20 |
| PBS-NIV | 14 ± 4 | 13 ± 9 | 42 ± 4 |
| SSG-NIV | 99 ± 1*** | 99.6 ± 0.15*** | 99 ± 0.8*** |
BALB/c mice were infected with different strains of L. donovani amastigotes and treated on day 7 postinfection with PBS (controls) (data not shown), SSG [300 mg of Sb(V)/kg], SSG-NIV [300 mg of Sb(V)/kg], or PBS-NIV (NIV control). On day 14 postinfection, parasite burdens in the liver, spleen, and bone marrow were determined. The mean reduction in parasite burdens was calculated relative to the mean control burdens for the relevant site. In comparison to the value for the relevant controls, the values in some cases were significantly different at a P of <0.001 (***), <0.01 (**), and <0.05 (*). ND, not determined.
Treated as described in footnote a, except animals were treated on day 8 postinfection and were sacrificed on day 15 postinfection.
Treatment with SSG-NIV was more effective than treatment with SSG against all of the strains tested (Table 3). In SSG-responsive strains, the reduction in liver parasite burdens by SSG-NIV treatment was similar to that caused by SSG. In nonresponsive strains, this formulation caused at least a 95% reduction in liver parasite burdens.
There were interorgan differences in the antileishmanial efficacy of SSG-NIV treatment (Table 3). In some cases this formulation was equally effective (based on a reduction in parasite burdens in all three sites compared to the respective control values) against the laboratory strain (MHOM/ET/67:LV82) and clinical strains (20006 and 20009). In others (strains 20001, 20003, 200011, 200015, and 200016), this treatment was less effective (based on its ability to cause a lower or insignificant reduction in parasite burdens in one site or more, compared to the respective control value).
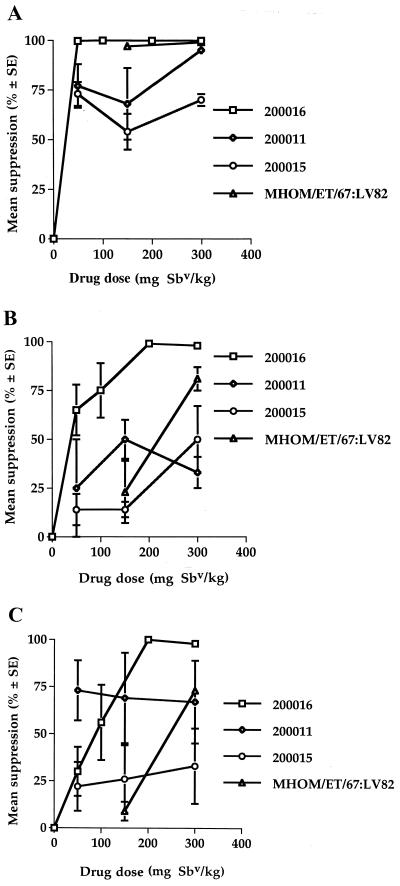
Dose-response studies showed that there was interstrain variability in the response of the L. donovani strains to SSG-NIV treatment (Fig 1). The strains tested could be ranked on the basis of their overall responses to drug treatment (based on data in Fig 1 and Table 3, MHOM/ET/67:LV82 > 200016 > 200015 > 200011).
FIG. 1.
Effects of SSG-NIV treatment on the parasite burdens in the spleens livers and bone marrows of mice infected with different L. donovani strains. BALB/c mice were infected with different strains of L. donovani amastigotes and treated on day 7 with PBS (controls) or different doses of SSG-NIV. On day 14 postinfection, parasite burdens in the spleen (A), liver (B), and bone marrow (C) were determined. The mean reduction in parasite burdens was calculated using the mean control burdens for the relevant site.
DISCUSSION
Macrophage experiments gave a good indication of a strain's in vivo susceptibility to SSG. Survival of strain 200011, which was obtained from a patient previously exposed to antimonial therapy, was unaffected by SSG treatment in macrophage studies and in the BALB/c mouse model. Primary resistance to Sb(V) occurs in 10 to 30% of cases in India (9). Results from this study gave an incidence of 67.7% [based on in vivo responses of the year 2000 strains to a single SSG dose containing 300 mg of Sb(V)/kg].
In the present study, strains were cultured in the absence of drug pressure; therefore, their resistance to SSG was presumably due to a stable change in the parasite's genome. Previous studies using axenic Leishmania amastigotes have also shown that antimony resistance is due to an intrinsic genomic change in the parasite (18, 19). Specific genetic changes (e.g., amplification in the ABC transporter gene psgpA [14], amplification of the orfSbV gene [10], or overexpression of the ornithine decarboxylase gene [11]) or phenotypic differences (e.g., elevated levels of trypanothione [16] or a deficiency in the ability to reduce Sb(V) in the amastigote [20]) have been characterized for different Leishmania strains. Studies are under way to characterize the relative amounts of glutathione and trypanothione levels in these strains.
The results of this study showed that the SSG-NIV was more effective than free SSG both in vitro and in vivo. In vitro studies showed that PBS-NIV did have antileishmanial properties, which confirms results of previous studies (21). However, treatment with PBS-NIV did not significantly suppress parasite burdens in vivo, a finding which was obtained in previous studies (22). It was shown that the in vivo efficacies of SSG-NIV formulations are dependent on the hydrating SSG concentration used when NIVs are formed, which controls intravesicular drug content (22). However, for a particular NIV formulation, there is a maximum tolerated hydrating drug dose. Above this dose, vesicles do not form. Studies have shown that factors such as solution pH and ionic strength strongly influence NIV formation (17). The greater efficacy of SSG-NIV in vivo presumably reflects its ability to modify its in vivo distribution and thus direct a great proportion of the drug dose to tissues (7, 8). For example, tissue antimony levels (spleen, liver, femur, and humerus) at 6 days posttreatment were similar in free-SSG- and SSG-NIV-treated animals, despite a 70-fold difference in the drug dose administered (8). There was interstrain variability in susceptibility to SSG-NIV. Host factors can influence the outcome of drug treatment (5), but since these studies were carried out using inbred BALB/c mice, any differences are likely to be due to differences between strains and their inherent susceptibilities to antimony. Studies are under way to determine whether an increased number of administered SSG-NIV doses can cure mice infected with the strains which are less responsive to SSG treatment.
The inability to infect hamsters with the FDA L. donovani strains may be related to the length of time these strains have been maintained in vitro. Infection of mice with FDA strain promastigotes or promastigotes of MHOM/ET/67:LV82, the standard L. donovani strain used in our laboratory, gave low parasite burdens. It is well known that other factors such as components in sand fly saliva can increase the infectivity of Leishmania promastigotes (13). However, this study did show that intravenous infection of hamsters with recently isolated Leishmania strains can result in high parasite burdens.
In summary, this study showed that the use of a carrier system increases the antileishmanial efficacy of SSG against Leishmania strains. These antimony-resistant strains offer a unique opportunity to evaluate the biological basis of antimony resistance and to determine the in vivo efficacies of alternate chemotherapeutic regimens in clinical practice, e.g., paromomycin or amphotericin B (Fungizone, AmBisome, Abelcet, and Amphocil), or in the advanced stages of clinical evaluation, e.g., miltefosine.
ACKNOWLEDGMENT
This study was supported by a grant awarded by Tenovus-Scotland.
REFERENCES
- 1.Berman J D. Human leishmaniasis: clinical, diagnostic and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 2.Bradley D J, Kirkley J. Regulation of Leishmania populations within the host. I. The variable course of Leishmania donovani infections in mice. Clin Exp Immunol. 1977;30:119–129. [PMC free article] [PubMed] [Google Scholar]
- 3.Carter K C, Baillie A J, Alexander J, Dolan T F. The therapeutic effect of sodium stibogluconate in the BALB/c mice infected with L. donovani is organ dependent. J Pharm Pharmacol. 1988;40:370–373. doi: 10.1111/j.2042-7158.1988.tb05271.x. [DOI] [PubMed] [Google Scholar]
- 4.Carter K C, O'Grady J, Dolan T F, Baillie A J, Alexander J, Keys J. A direct comparison of sodium stibogluconate treatment in two animal models of human visceral leishmaniasis, mouse and hamster. Int J Pharm. 1989;53:129–137. [Google Scholar]
- 5.Carter K C, Baillie A J, Alexander J. Genetic control of drug-induced recovery from murine visceral leishmaniasis. J Pharm Pharmacol. 1993;45:795–798. doi: 10.1111/j.2042-7158.1993.tb05687.x. [DOI] [PubMed] [Google Scholar]
- 6.Chulay J D, Bryceson A D. Quantitation of amastigotes of Leishmania donovani in smears of splenic aspirates from patients with visceral leishmaniasis. Am J Trop Med Hyg. 1983;32:475–479. doi: 10.4269/ajtmh.1983.32.475. [DOI] [PubMed] [Google Scholar]
- 7.Collins M, Baillie A J, Carter K C. Visceral leishmaniasis in the BALB/c mouse: sodium stibogluconate treatment during acute and chronic stages of infection. II. Changes in tissue drug distribution. Int J Pharm. 1992;83:251–256. [Google Scholar]
- 8.Collins M, Carter K C, Baillie A J, O'Grady J. The distribution of free and non-ionic vesicular sodium stibogluconate in the dog. J Drug Target. 1993;1:133–142. doi: 10.3109/10611869308996069. [DOI] [PubMed] [Google Scholar]
- 9.Davidson R N. Visceral leishmaniasis in clinical practice. J Infect Dis. 1999;39:112–116. doi: 10.1016/s0163-4453(99)90001-4. [DOI] [PubMed] [Google Scholar]
- 10.Haimeur A, Ouellette M. Gene amplification in Leishmania tarentaole selected for resistance to sodium stibogluconate. Antimicrob Agents Chemother. 1998;42:1689–1694. doi: 10.1128/aac.42.7.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haimeur A, Guimond C, Pilote S, Mukhopadhyay R, Rosen B P, Poulin R, Ouellette M. Elevated levels of polyamines and trypanothione resulting from overexpression of the ornithine decarboxylase gene in arsenite-resistant Leishmania. Mol Microbiol. 1999;34:726–735. doi: 10.1046/j.1365-2958.1999.01634.x. [DOI] [PubMed] [Google Scholar]
- 12.Jackson J E, Tally J D, Ellis W Y, Mebrahtu Y B, Lawyer P G, Were J B, Reed S G, Panisko D M, Limmer B L. Quantitative in vitro drug potency and drug susceptibility evaluation of Leishmania spp. from patients unresponsive to pentavalent antimony therapy. Am J Trop Med. 1990;43:464–480. doi: 10.4269/ajtmh.1990.43.464. [DOI] [PubMed] [Google Scholar]
- 13.Kamhawi S. The biological and immunological properties of sand fly saliva and its role in the establishment of Leishmania infections. Microbes Infect. 2000;2:1765–1773. doi: 10.1016/s1286-4579(00)01331-9. [DOI] [PubMed] [Google Scholar]
- 14.Legare D, Papadopoulou B, Roy G, Mukhopadhyay R, Haimeur A, Dey S, Grondin K, Brochu C, Rosen B P, Ouellette M. Efflux systems and increased trypanothionine levels in arsenite-resistant Leishmania. Exp Parasitol. 1997;87:275–282. doi: 10.1006/expr.1997.4222. [DOI] [PubMed] [Google Scholar]
- 15.Lira R, Sundar S, Makharia A, Kenney R, Gam A, Saraiva E, Sacks D. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J Infect Dis. 1999;180:564–567. doi: 10.1086/314896. [DOI] [PubMed] [Google Scholar]
- 16.Mullen A B, Baillie A J, Carter K C. Visceral leishmaniasis in the BALB/c mouse: a comparison of the efficacy of sodium stibogluconate with those of three proprietary formulations of amphotericin B. Antimicrob Agents Chemother. 1998;42:2722–2725. doi: 10.1128/aac.42.10.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullen A B, Baillie A J, Carter K C. Non-ionic surfactant vesicles for the treatment of visceral leishmaniasis. In: Uchegbu I F, editor. Synthetic surfactant vesicles: niosomes and other non-phospholipid vesicular systems. Amsterdam, The Netherlands: Harwood Academic Publishers; 2000. pp. 97–113. [Google Scholar]
- 18.Sereno D, Lemesre J L. Axenically cultured amastigote forms as an in vitro model for investigation of antileishmanial agents. Antimicrob Agents Chemother. 1997;41:972–976. doi: 10.1128/aac.41.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sereno D, Lemesre J L. In vitro life cycle of pentamidine-resistant amastigotes: stability of the chemoresistant phenotypes is dependent on the level of resistance induced. Antimicrob Agents Chemother. 1997;41:1898–1903. doi: 10.1128/aac.41.9.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaked-Mishen P, Ulrich N, Ephos M, Zilberstein D. Novel intracellular Sbv reducing activity correlates with antimony susceptibility in Leishmania donovani. J Biol Chem. 2001;276:3971–3976. doi: 10.1074/jbc.M005423200. [DOI] [PubMed] [Google Scholar]
- 21.Williams D, Mullen A B, Baillie A J, Carter K C. Comparison of the efficacy of free and non-ionic-surfactant vesicular formulation of paromomycin in a murine model of visceral leishmaniasis. J Pharm Pharmacol. 1998;50:1351–1356. doi: 10.1111/j.2042-7158.1998.tb03358.x. [DOI] [PubMed] [Google Scholar]
- 22.Williams D M, Carter K C, Baillie A J. Visceral leishmaniasis in the BALB/c mouse: a comparison of the in vivo activity of five non-ionic surfactant vesicle preparations of sodium stibogluconate. J Drug Target. 1995;3:1–7. doi: 10.3109/10611869509015926. [DOI] [PubMed] [Google Scholar]