Abstract
Vitamin D has been a focus of attention in liver cancer due to its direct and indirect antineoplastic effects. This review critically evaluates data from recently published basic and clinical studies investigating the role of vitamin D in liver cancer. Basic studies indicate that vitamin D plays an important role in liver cancer development by suppressing the activity of hepatic stellate cells and Kupffer cells. Furthermore, vitamin D has a direct anti-proliferative, anti-angiogenic, proapoptotic, and prodifferentiative effect on liver cancer cells. Recent investigation suggested several interesting mechanisms of these actions, such as inactivation of Notch signaling, p27 accumulation, and tyrosine-protein kinase Met/extracellular signal-regulated kinases inhibition. On the other hand, data from clinical observational studies, although promising, are still inconclusive. Unfortunately, studies on the effect of vitamin D supplementation were generally focused on short-term outcomes of chronic liver diseases (liver enzyme levels or elastographic finding); therefore, there are still no reliable data on the effect of vitamin D supplementation on liver cancer occurrence or survival.
Numerous observational studies emphasized a possible link between vitamin D deficiency and cancer risk. The association was first established for cancers with a greater incidence in high-latitude regions, where vitamin D deficiency is more prevalent, such as colon cancer. Although liver cancers, due to a strong association with known risk factors such as hepatitis B (HBV) and C virus (HCV) and alcohol abuse, do not follow this latitude-related pattern, recent studies have suggested that vitamin D may still have a role in liver cancer development. This review critically evaluates data from basic and clinical studies investigating the possible role of vitamin D in liver cancer.
Pathophysiological background
Principal liver cells, hepatocytes, usually express a very low level of vitamin D receptor (VDR) or none at all. However, VDR is highly expressed in nonparenchymal liver cells such as Kupffer cells, hepatic stellate cells, and sinusoidal endothelial cells, which play an important role in liver tumor development (1). The main effects of vitamin D on various liver cells are summarized in Table 1.
Table 1.
The main effects of vitamin D or its analogs on various liver cells*
| Cell type | Main effects |
|---|---|
| Hepatocytes |
A very low level of VDR expression
Expression is induced in NAFLD but decreased in NASH or chronic hepatitis C (8,9)
VDR activation might be associated with lipid accumulation and contribute to steatosis development (8,10) |
| Kupffer cells |
Abundant expression of VDR that exhibits anti-inflammatory effects upon activation:
VDR activation suppresses the LPS-induced inflammation and downregulates IL-6, TNF, and IL-1b expression (2)
VDR activation mitigates inflammatory response in macrophages following ER stress challenge (3) |
| Hepatic stellate cells |
Significant VDR expression
Vitamin D and its analogs exert inhibitory effects on primary murine hepatic cells or human cell lines, possibly through inactivation of TGF-β/Smad signaling (5-7) |
| Cholangiocytes |
High VDR expression with immunoregulatory functions
Ursodeoxycholic acid and vitamin D induce the expression of antimicrobial peptide cathelicidin through a VDR-dependent mechanism (12)
VDR deficiency promotes cholestatic liver injury through disruption of biliary epithelial cell junctions (13)
Vitamin D or its analog ameliorate liver injury through a VDR-independent pathway (16) |
| Liver cancer cells |
VDR is expressed in human liver cancer cell lines and specimens of human HCC (1,22,23)
KLF4 might play a pivotal role in the regulation of VDR expression in HCC (39)
Supplementation with vitamin D or its analogs inhibits the proliferation of cancer cell lines and induces apoptosis through several mechanisms:
disruption of HGF/c-met/ERK pathway due to downregulation of c-met and ERK (23)
increase in E-cadherin and decrease in Akt expression (28)
induction of cell cycle arrest through p27 accumulation (25)
decreased HDAC2 with increased p21 (WAF1/Cip1) expression and subsequent modulation of p53, Bax, DR5, caspase 8, and Bcl-2 protein expressions (26,27)
modulation of TLR7 expression and β-catenin activation (32)
stimulation of TXNIP expression, inactivation of Notch signaling and/or p27(kip1)-dependent suppression of proinflammatory cytokines secretion (34-36) |
| Cholangiocarcinoma | VDR expression in human cholangiocarcinoma tissue specimens (41-43) Treatment with vitamin D or analogs impairs proliferation and induces apoptosis in cultured cells. Proposed mechanisms include induction of cell cycle arrest through regulation of cyclin D1, cyclin D3, CDK4, CDK6, p21, and/or p27 (44-47) VDR dependent downregulation of LCN2 expression (46,47,49) |
*Abbreviations: CDK – cyclin dependent kinase; c-met – tyrosine-protein kinase Met; DR – death receptor; ER – endoplasmic reticulum; ERK – extracellular signal-regulated kinases; HCC –hepatocellular carcinoma; HDAC2 – histone deacetylase 2; HGF – hepatocyte growth factor; IL-6 – interleukin-6; KLF4 – Krüppel-like factor 4; LCN2 – lipocalin 2; LPS – lipopolysaccharide; NAFLD – nonalcoholic fatty liver disease; NASH – nonalcoholic steatohepatitis; Smad – mothers against decapentaplegic homologue; TGF-β – transforming growth factor beta; TLR – toll-like receptor; TNF – tumor necrosis factor; TXNIP – thioredoxin interacting protein; VDR – vitamin D receptor.
The most abundant expression of VDR in the liver was found in Kupffer cells. The effect of VDR activation in these cells is anti-inflammatory. The activation of VDR in Kupffer cells reduces the degree of lipopolysaccharide-induced activation by decreasing the secretion of interleukin (IL)-6, IL-1, and tumor necrosis factor alpha (TNF-alpha) (2). Similarly, VDR diminishes the induction of endoplasmatic reticulum stress by tunicamycin and subsequent inflammatory response (3). As pro-inflammatory milieu in the liver is associated with cancerogenesis, these mechanisms might explain one aspect of vitamin D anticancer properties.
Hepatic stellate cells, critical contributors to liver fibrosis, also express a significant amount of VDR. Various in vitro studies reported that inhibitory effect of VDR agonists on primary murine hepatic stellate cells (4) or human cell lines HSC-T6 and LX-2 (5-7) is mediated by a decrease in transforming growth factor-beta (TGF-β)/Smad signaling (7). These findings suggest that vitamin D may suppress liver fibrosis occurrence and progression, thereby decreasing liver cancer risk.
Although healthy hepatocytes do not express a significant amount of VDR, the expression may change in certain diseases. Hepatocyte expression of VDR was induced in non-alcoholic fatty liver disease (NAFLD) (8) and decreased in non-alcoholic steatohepatitis (NASH) or chronic hepatitis C (8,9). However, VDR activation in hepatocytes could promote lipid accumulation and contribute to steatosis development (8-10). Whether VDR effects on nonparenchymal cells override the potentially harmful effects of VDR stimulation in parenchymal cells is still controversial.
Cholangiocyte expression of VDR is high (1,11). VDR activation regulates the expression of the antimicrobial peptide cathelicidin in biliary epithelial cells, and ursodeoxycholic acid and vitamin D induce cathelicidin expression through a VDR-dependent mechanism (12). The absence of VDR aggravates cholestatic liver injury in mice through disruption of biliary epithelial cell junctions (13). VDR deficiency might also promote sustained inflammatory response in primary biliary cholangitis (14). Furthermore, the vitamin D/VDR pathway affected the extent of injury and fibrosis in a mouse model of sclerosing cholangitis (Abcb4 knockout mice) (15,16). Mice on a low-vitamin D diet exhibited a higher level of fibrosis (15). In contrast, VDR knockout mice had an increased cholestatic liver injury level and a significant lifespan reduction (16).
Liver cancer models and liver cancer cell lines
During the last decades, several vitamin D properties that may hamper cancer development and growth have emerged, such as anti-proliferative, anti-inflammatory, anti-angiogenic, proapoptotic, and prodifferentiative effect (17-19). However, in terms of liver cancer cells, the most prominent effect of vitamin D is the inhibition of proliferation.
Multiple in vitro and in vivo studies have shown that supplementation with either vitamin D or vitamin D analogs inhibits the proliferation of various liver cancer cell lines and reduces the size of the tumors in mice (20-28). The anti-proliferative effect could be ascribed to disruption of hepatocyte growth factor/tyrosine-protein kinase Met/extracellular signal-regulated kinases (HGF/c-met/ERK) signaling pathway by vitamin D-induced downregulation of c-met and ERK (23), modulation of E-cadherin, and Akt expression (28), and/or induction of cell cycle arrest presumably due to p27 protein accumulation (25). Furthermore, treatment with vitamin D decreases the expression of histone deacetylase 2 (HDAC2) and increases the expression of p21 (WAF1/Cip1) in HepG2 cells, resulting not only in decreased cell growth but also in the induction of apoptotic cell death (26). Apoptosis could be induced through extrinsic and intrinsic pathways, as vitamin D treatment upregulates death receptor 5 and Bax protein expressions along with Bcl-2 downregulation. These findings were further confirmed in vivo as vitamin D-treated mice exhibited suppressed growth of xenograft human hepatocellular carcinoma (HCC) with a large area of necrosis (27).
Vitamin D may also affect TGF-β signaling in the liver. TGF-β is a pleiotropic cytokine that exhibits opposite functions depending on the context: it acts as a tumor suppressor in normal hepatocytes and early stages of tumorigenesis, but it can also promote tumor development in later stages, and it is highly expressed in HCC tissue (29-31). Vitamin D deficiency increases the tumor burden in TGF-β/Smad3-deficient mice through modulation of toll-like receptor 7 expression and β-catenin activation. Additionally, vitamin D supplementation restored the Smad3 expression in cirrhosis and HCC patients and reduced β-catenin expression in liver tissue of HCC patients, providing a rationale for vitamin D treatment in specific patients with disrupted TGF-β signaling (32). On the other hand, in later stages of the disease, the downregulation of TGF-β signaling may be beneficial. In this context, the finding that vitamin D treatment significantly reduces TGF-β level and Smad3, Snail, and matrix metalloproteinase-2 gene expression in experimental HCC model in rats, along with improvement of a histopathological picture, sounds promising (33).
Several other mechanisms involving vitamin D may attenuate carcinogenesis in the liver. Vitamin D stimulates the expression of thioredoxin-interacting protein (34) and inactivates Notch signaling in liver cancer cell lines, leading to anti-proliferative, anti-invasive, and proapoptotic effects (35). Vitamin D also decreases the secretion of pro-inflammatory cytokines from immune cells in a p27(kip1)-dependent way, hence undermining HCC development (36). The anti-tumor effect also may be exerted through the protection of hepatic progenitor cells. Vitamin D suppresses the aflatoxin B1-induced proliferation and dedifferentiation of liver progenitor cells (37).
VDR is expressed in both human liver cancer cell lines, especially HepG2 cell lines and specimens of human HCC (1,22,23). However, in certain circumstances, VDR expression may be reduced in liver cancer tissue, providing an escape mechanism from vitamin D effects (38,39). Furthermore, the tumor suppressor Krüppel-like factor 4 (KLF4) plays a pivotal role in regulating VDR expression in HCC. While decreased or lost KLF4 expression correlates with decreased VDR expression, overexpression of KLF4 upregulates VDR and sensitizes the cells to the vitamin D effects (39). Therefore, the cancer cell response to vitamin D treatment also depends on the expression of VDR in these cells. The finding that VDR expression might be upregulated by antihistamines, such as astemizole, which leads to the synergistic effect of astemizole and vitamin D (40), provides novel insights and confers the conclusion that additional basic studies are still warranted in order to elucidate detailed mechanisms and provide new targets for HCC treatment.
VDR expression was also found in human cholangiocarcinoma (CCA) tissue specimens (41-43). As in HCC, the treatment with vitamin D and/or vitamin D analogs showed beneficial effects in CCA cell cultures and in vivo models of CCA (41-47). Vitamin D or its analogs significantly impaired proliferation (41,42) and induced apoptosis in cultured cells (44), suppressed cholangiocarcinogenesis (43,48) and significantly inhibited tumor growth and progression in murine models of CCA (43,44,47). Several studies examined the mechanisms behind these effects, suggesting that vitamin D induces cell cycle arrest through regulation of cyclin D1 (44,45), cyclin D3 (47), p21 (44), and p27 expression (47). Furthermore, vitamin D/VDR signaling is involved in regulating the expression of lipocalin 2 (LCN2), an oncogene highly expressed in human intrahepatic cholangiocarcinoma tissue. Vitamin D significantly downregulates the expression of LCN2, which attenuates proliferation. This was further confirmed in LCN2 knockdown settings, where the loss of LCN2 made the cells less responsive to vitamin D or its analog treatment (46,47,49).
Data from observational clinical studies
The relationship between vitamin D and predisposing diseases for liver cancer such as NAFLD, alcoholic liver diseases, or viral hepatitis has been extensively investigated, often with conflicting results (50,51). On the other hand, mounting data suggest that vitamin D deficiency reflects hepatic dysfunction, and as such, is associated with mortality in patients with liver cirrhosis, regardless of the underlying causes (52,53). In the context of liver cancer, higher VDR gene promoter methylation was detected in the HCC tissue (54). Data from vitamin D studies investigating liver cancer occurrence are summarized in Table 2.
Table 2.
Studies investigating vitamin D and liver cancer occurrence
| Incidence study | Number of patients | Key findings |
|---|---|---|
| Chinese Linxian Nutrition Intervention Trials (55) |
255 |
modest evidence for associations with incident liver cancer, which became significant only among participants with higher baseline serum calcium |
| Nested case-control study within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort (57) |
138 |
higher vitamin D levels were associated with a 49% reduction of HCC; the finding did not vary by time from enrolment to diagnosis, or changed after adjustment for biomarkers of preexisting liver damage or chronic HBV or HCV infection |
| Japan Public Health Center-based Prospective Study cohort (56) |
110 |
vitamin D concentration was inversely associated with liver cancer, with corresponding hazard ratios for trend of 0.45 (0.26 to 0.79) (P = 0.006) |
| Sir Run Shaw Hospital, China (59) | 100 | vitamin D level greater than 20 ng/mL increased HCC risk (odds ratio 7.56, 95% confidence interval 4.58–12.50) |
*Abbreviations: HCC – hepatocellular carcinoma; HBV – hepatitis B virus; HCV – hepatitis C virus.
The Chinese Linxian Nutrition Intervention Trials showed no significant associations between the risk of liver cancer occurrence and serum vitamin D levels. However, the risk estimates decreased across increasing quartiles of vitamin D concentrations (55). A large case-control study by Budhathoki et al (56) reported an inverse association between the pre-diagnostic vitamin D levels and liver cancer, which was independent of dietary factors or viral hepatitis infection. Interestingly, they found no association between vitamin D and other investigated cancers (gastric, rectal, colon, or lung cancer). Fedirko et al (57) previously reported similar findings in a nested case-control study within the EPIC cohort study. However, the EPIC cohort also demonstrated that a dairy-source vitamin D increased the risk of HCC. In contrast, a non-dairy source showed no association (58). Contrary to this, a recent study by Liu et al reported a higher vitamin D level among post-diagnostically sampled HCC patients (59). Except for the sampling time point, the studies further differed in many other important patient characteristics (diet, lifestyle, environmental exposures, and HCC risk factor profiles), which explains divergent results.
The prognostic value of vitamin D in liver cancer has been investigated in several trials (Table 3). A prospective German study showed that newly diagnosed HCC patients with serum total 25(OH)D≤10 ng/mL had significantly decreased overall survival compared with patients with 25(OH)D>10 ng/mL (60). A Finnish Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study demonstrated that liver cancer patients with higher total 25(OH)D levels (up to 28 years before cancer diagnosis) had a suggestive, although not significant, improvement in liver cancer-specific survival (61). In a recent Chinese study, Fang et al (62) showed that higher serum bioavailable vitamin D levels (calculated from measured free vitamin D, albumin, and affinity constant between 25(OH)D and albumin) rather than total vitamin D levels were independently associated with improved survival. Data on vitamin D role in CCA are scarce. We identified two studies that reported better survival of patients with higher VDR expression in resected tumor tissue (42,47).
Table 3.
Studies investigating vitamin D levels and liver cancer survival*
| Survival study | Number of patients | Key findings |
|---|---|---|
| German Prospective cohort study (60) |
200 |
low levels of vitamin D were associated with increased mortality risk from HCC independently of the MELD score and high AFP levels |
| Nested study form Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study in Finnish smoker population (61) |
206 |
higher levels of vitamin D were not significantly associated with better survival of liver cancer patients in a population of Finnish smokers |
| Guangdong Liver Cancer Cohort study (62) |
1031 |
higher bioavailable vitamin D levels were significantly associated with better survival, independent of Barcelona Clinic Liver Cancer stage, cancer treatment, and serum C-reactive protein
neither total nor free vitamin D levels were significantly associated with survival |
| *Abbreviations: AFP – alpha-fetoprotein; HCC – hepatocellular carcinoma; MELD – Model for End-Stage Liver Disease. | ||
The results of the studies on single nucleotide polymorphism further support the link between vitamin D and liver tumor development (Table 4). VDR polymorphism was associated with a risk of HCC occurrence in an alcoholic- (63), HCV- (64,65), and HBV- (66,67) related cirrhosis. There are still no published studies regarding the association between the VDR polymorphism and CCA.
Table 4.
Studies on vitamin D-related single nucleotide polymorphism and liver tumor development
| Reference | Etiology/ population/ N | VDR SNPs | Key points |
|---|---|---|---|
| Falleti et al (63) |
HCV, HBV, ALD/
Italian/80 HCC, 236 healthy controls |
VDR gene
FokI
BsmI
ApaI
TaqI |
Association with HCC was found for b/b genotype of BsmI, T/T genotype of TaqI, absence of the A-T-C protective allele of BAT, and carriage of the BAT A-T-C and G-T-T haplotypes |
| Hoan et al (66) |
HBV/Vietnamese/171 HCC,
183 CHB,
89 LC,
238 healthy controls |
VDR gene
FokI
BsmI
ApaI
TaqI |
ApaI CA genotype is less frequent, and APAL AA is more frequent in HCC vs CHB patients
No association between TaqI, FokI, and BsmI polymorphisms and any clinical outcome was found |
| Barooah et al (65) |
HCV/
Indian/
60 HCC,
167 CHC,
124 LC,
102 healthy controls |
VDR
BsmI
ApaI
TaqI |
ApaI CC genotype, ApaI C allele, and bAt haplotype were significantly associated with liver cancer
paI CC genotype and bAt haplotype were independent predictors of HCC development |
| Rafat Rowida et al (64) |
HCV/
Egyptian/
80 HCC,
80 LC,
80 healthy controls |
VDR gene
Apa1 |
Apa1 CC is associated with greater risk for HCC development. It is also associated with a more severe Child-Pugh score and MELD score (P < 0.05) |
| Peng et al (67) | HBV/ Chinese/ 184 HCC, 296 HBV non-HCC, 180 healthy controls | VDR gene Fok1 rs3782905 Cdx2 DBP gene rs7041 | Fok1 T allele and rs7041 G allele were associated with a significantly increased HBV-related HCC risk no significant effect of VDR rs11568820, and rs3782905 polymorphisms on HBV-related HCC risk |
*Abbreviations: ALD – alcoholic liver disease; CHB – chronic hepatitis B, CHC – chronic hepatitis C; HCC – hepatocellular carcinoma; HBV – hepatitis B virus; HCV – hepatitis C virus; LC– liver cirrhosis; MELD – Model for End-Stage Liver Disease; SNP – single nucleotide polymorphism; VBP– vitamin D binding protein; VDR – vitamin D receptor.
Data from clinical supplementation studies
In the last five years, numerous studies have investigated the effect of vitamin D supplementation on various chronic liver diseases. However, these studies were generally focused on short-term outcomes (liver enzyme levels or elastographic finding), and we found no reliable data on the effect of vitamin D supplementation on liver cancer occurrence or survival. The results are limited to one uncontrolled trial that suggested a weak effect of vitamin D analog, seocalcitol, in patients with inoperable HCC, and a pilot study that reported serious adverse effects in CCA patients treated with high-dose calcitriol in combination with chemotherapeutic drugs (68,69).
Conclusion
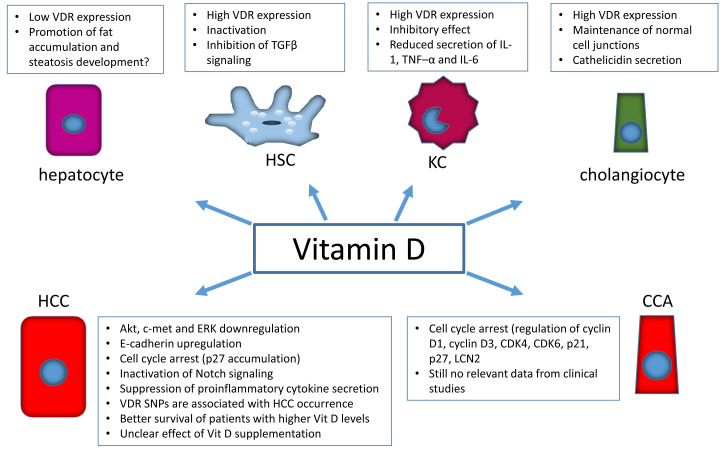
The main effects of vitamin D on processes involved in liver cancer development are summarized in Figure 1. After reviewing recently published studies, we conclude that basic studies conducted on cell lines or animals provided compelling evidence that vitamin D plays an important role in liver cancer development. On the other hand, data from clinical observational studies, although promising, are still inconclusive. Studies on the effect of vitamin D supplementation were generally focused on short-term outcomes of chronic liver diseases. There are still no reliable data on the effect of vitamin D supplementation on liver cancer occurrence or survival, and its role should be further investigated in clinical studies.
Figure 1.
Summary of main effects of vitamin D on processes involved in liver cancer development. CCA – cholangiocarcinoma; CDK – cyclin dependent kinase; c-met – tyrosine-protein kinase Met; ERK – extracellular signal-regulated kinases; HCC –hepatocellular carcinoma; HSC – hepatic stellate cell; IL-1 – interleukin-1, IL-6 –interleukin-6; KC – Kupffer cell; LCN2 – lipocalin 2; SNP – single nucleotide polymorphism; TGF-β – transforming growth factor beta; TNF – tumor necrosis factor; VDR – vitamin D receptor; vit D – vitamin D.
Acknowledgments
Funding None.
Ethical approval Not required.
Declaration of authorship AMa and TK conceived and designed the study; HM, HS, and AMr acquired the data; AMr analyzed and interpreted the data; AMa, TK, HM, and HS drafted the manuscript; AmA, TK, and AMr critically revised the manuscript for important intellectual content; all authors gave approval of the version to be submitted; all authors agree to be accountable for all aspects of the work.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1. Gascon-Barré M, Demers C, Mirshahi A, Néron S, Zalzal S, Nanci A. The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology. 2003;37:1034–42. doi: 10.1053/jhep.2003.50176. [DOI] [PubMed] [Google Scholar]
- 2. Dong B, Zhou Y, Wang W, Scott J, Kim K, Sun Z, et al. Vitamin D receptor activation in liver macrophages ameliorates hepatic inflammation, steatosis, and insulin resistance in mice. Hepatology. 2020;71:1559–74. doi: 10.1002/hep.30937. [DOI] [PubMed] [Google Scholar]
- 3. Zhou Y, Dong B, Kim KH, Choi S, Sun Z, Wu N, et al. Vitamin D receptor activation in liver macrophages protects against hepatic endoplasmic reticulum stress in mice. Hepatology. 2020;71:1453–66. doi: 10.1002/hep.30887. [DOI] [PubMed] [Google Scholar]
- 4. Reiter FP, Hohenester S, Nagel JM, Wimmer R, Artmann R, Wottke L, et al. 1,25-(OH)-vitamin D prevents activation of hepatic stellate cells in vitro and ameliorates inflammatory liver damage but not fibrosis in the Abcb4(−/−) model. Biochem Biophys Res Commun. 2015;459:227–33. doi: 10.1016/j.bbrc.2015.02.074. [DOI] [PubMed] [Google Scholar]
- 5. Wan LY, Zhang YQ, Li JM, Tang HQ, Chen MD, Ni YR, et al. Liganded vitamin d receptor through its interacting repressor inhibits the expression of type I collagen α1. DNA Cell Biol. 2016;35:498–505. doi: 10.1089/dna.2016.3367. [DOI] [PubMed] [Google Scholar]
- 6. Reiter FP, Ye L, Bösch F, Wimmer R, Artmann R, Ziesch A, et al. Antifibrotic effects of hypocalcemic vitamin D analogs in murine and human hepatic stellate cells and in the CCl4 mouse model. Lab Invest. 2019;99:1906–17. doi: 10.1038/s41374-019-0310-1. [DOI] [PubMed] [Google Scholar]
- 7. Lu W, Li X, Liu N, Zhang Y, Li Y, Pan Y, et al. Vitamin D alleviates liver fibrosis by inhibiting histidine-rich calcium binding protein (HRC). Chem Biol Interact. 2021;334:109355. doi: 10.1016/j.cbi.2020.109355. [DOI] [PubMed] [Google Scholar]
- 8. Bozic M, Guzmán C, Benet M, Sánchez-Campos S, García-Monzón C, Gari E, et al. Hepatocyte vitamin D receptor regulates lipid metabolism and mediates experimental diet-induced steatosis. J Hepatol. 2016;65:748–57. doi: 10.1016/j.jhep.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 9. Barchetta I, Carotti S, Labbadia G, Gentilucci UV, Muda AO, Angelico F, et al. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology. 2012;56:2180–7. doi: 10.1002/hep.25930. [DOI] [PubMed] [Google Scholar]
- 10. Martínez-Sena T, Soluyanova P, Guzmán C, Valdivielso JM, Castell JV, Jover R. The vitamin D receptor regulates glycerolipid and phospholipid metabolism in human hepatocytes. Biomolecules. 2020;10:493. doi: 10.3390/biom10030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vogel A, Strassburg CP, Manns MP. Genetic association of vitamin D receptor polymorphisms with primary biliary cirrhosis and autoimmune hepatitis. Hepatology. 2002;35:126–31. doi: 10.1053/jhep.2002.30084. [DOI] [PubMed] [Google Scholar]
- 12. D’Aldebert E, Biyeyeme Bi Mve MJ, Mergey M, Wendum D, Firrincieli D, Coilly A, et al. Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology. 2009;136:1435–43. doi: 10.1053/j.gastro.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 13. Firrincieli D, Zúñiga S, Rey C, Wendum D, Lasnier E, Rainteau D, et al. Vitamin D nuclear receptor deficiency promotes cholestatic liver injury by disruption of biliary epithelial cell junctions in mice. Hepatology. 2013;58:1401–12. doi: 10.1002/hep.26453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kempinska-Podhorodecka A, Milkiewicz M, Wasik U, Ligocka J, Zawadzki M, Krawczyk M, et al. decreased expression of vitamin D receptor affects an immune response in primary biliary cholangitis via the VDR-miRNA155-SOCS1 pathway. Int J Mol Sci. 2017;18:289. doi: 10.3390/ijms18020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hochrath K, Stokes CS, Geisel J, Pollheimer MJ, Fickert P, Dooley S, et al. Vitamin D modulates biliary fibrosis in ABCB4-deficient mice. Hepatol Int. 2014;8:443–52. doi: 10.1007/s12072-014-9548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gonzalez-Sanchez E, El Mourabit H, Jager M, Clavel M, Moog S, Vaquero J, et al. Cholangiopathy aggravation is caused by VDR ablation and alleviated by VDR-independent vitamin D signaling in ABCB4 knockout mice. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166067. doi: 10.1016/j.bbadis.2020.166067. [DOI] [PubMed] [Google Scholar]
- 17.El-Sharkawy A, Malki A. Vitamin D signaling in inflammation and cancer: molecular mechanisms and therapeutic implications. Molecules. 2020;25:3219. [DOI] [PMC free article] [PubMed]
- 18. Jeon SM, Shin EA. Exploring vitamin D metabolism and function in cancer. Exp Mol Med. 2018;50:1–14. doi: 10.1038/s12276-018-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Louka ML, Fawzy AM, Naiem AM, Elseknedy MF, Abdelhalim AE, Abdelghany MA. Vitamin D and K signaling pathways in hepatocellular carcinoma. Gene. 2017;629:108–16. doi: 10.1016/j.gene.2017.07.074. [DOI] [PubMed] [Google Scholar]
- 20. Pourgholami MH, Akhter J, Lu Y, Morris DL. In vitro and in vivo inhibition of liver cancer cells by 1,25-dihydroxyvitamin D3. Cancer Lett. 2000;151:97–102. doi: 10.1016/S0304-3835(99)00416-4. [DOI] [PubMed] [Google Scholar]
- 21. Akhter J, Lu Y, Finlay I, Pourgholami MH, Morris DL. 1alpha,25-Dihydroxyvitamin D3 and its analogues, EB1089 and CB1093, profoundly inhibit the in vitro proliferation of the human hepatoblastoma cell line HepG2. ANZ J Surg. 2001;71:414–7. doi: 10.1046/j.1440-1622.2001.02147.x. [DOI] [PubMed] [Google Scholar]
- 22. Caputo A, Pourgholami MH, Akhter J, Morris DL. 1,25-Dihydroxyvitamin D(3) induced cell cycle arrest in the human primary liver cancer cell line HepG2. Hepatol Res. 2003;26:34–9. doi: 10.1016/S1386-6346(02)00328-5. [DOI] [PubMed] [Google Scholar]
- 23. Wu FS, Zheng SS, Wu LJ, Teng LS, Ma ZM, Zhao WH, et al. Calcitriol inhibits the growth of MHCC97 heptocellular cell lines by down-modulating c-met and ERK expressions. Liver Int. 2007;27:700–7. doi: 10.1111/j.1478-3231.2007.01487.x. [DOI] [PubMed] [Google Scholar]
- 24. Ghous Z, Akhter J, Pourgholami MH, Morris DL. Inhibition of hepatocellular cancer by EB1089: in vitro and in vive study. Anticancer Res. 2008;28:3757–61. [PubMed] [Google Scholar]
- 25. Luo W, Chen Y, Liu M, Du K, Zheng G, Cai T, et al. EB1089 induces Skp2-dependent p27 accumulation, leading to cell growth inhibition and cell cycle G1 phase arrest in human hepatoma cells. Cancer Invest. 2009;27:29–37. doi: 10.1080/07357900802438569. [DOI] [PubMed] [Google Scholar]
- 26. Huang J, Yang G, Huang Y, Kong W, Zhang S. 1,25(OH)2D3 inhibits the progression of hepatocellular carcinoma via downregulating HDAC2 and upregulating P21(WAFI/CIP1). Mol Med Rep. 2016;13:1373–80. doi: 10.3892/mmr.2015.4676. [DOI] [PubMed] [Google Scholar]
- 27. Huang J, Yang G, Huang Y, Zhang S. 1,25(OH)2D3 induced apoptosis of human hepatocellular carcinoma cells in vitro and inhibited their growth in a nude mouse xenograft model by regulating histone deacetylase 2. Biochimie. 2018;146:28–34. doi: 10.1016/j.biochi.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 28. Ye L, Zhu L, Wang J, Li F. Inhibition of vitamin D analog eldecalcitol on hepatoma in vitro and in vivo. Open Med (Wars) 2020;15:663–71. doi: 10.1515/med-2020-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tu S, Huang W, Huang C, Luo Z, Yan X. Contextual regulation of TGF-β signaling in liver cancer. Cells. 2019;8:1235. doi: 10.3390/cells8101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dituri F, Mancarella S, Cigliano A, Chieti A, Giannelli G. TGF-β as multifaceted orchestrator in hcc progression: signaling, EMT, immune microenvironment, and novel therapeutic perspectives. Semin Liver Dis. 2019;39:53–69. doi: 10.1055/s-0038-1676121. [DOI] [PubMed] [Google Scholar]
- 31. Abou-Shady M, Baer HU, Friess H, Berberat P, Zimmermann A, Graber H, et al. Transforming growth factor betas and their signaling receptors in human hepatocellular carcinoma. Am J Surg. 1999;177:209–15. doi: 10.1016/S0002-9610(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 32. Chen J, Katz LH, Muñoz NM, Gu S, Shin JH, Jogunoori WS, et al. Vitamin D deficiency promotes liver tumor growth in transforming growth factor-β/Smad3-deficient mice through Wnt and toll-like receptor 7 pathway modulation. Sci Rep. 2016;6:30217. doi: 10.1038/srep30217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saad El-Din S, Fouad H, Rashed LA, Mahfouz S, Hussein RE. Impact of mesenchymal stem cells and vitamin d on transforming growth factor beta signaling pathway in hepatocellular carcinoma in rats. Asian Pac J Cancer Prev. 2018;19:905–12. doi: 10.22034/APJCP.2018.19.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hamilton JP, Potter JJ, Koganti L, Meltzer SJ, Mezey E. Effects of vitamin D3 stimulation of thioredoxin-interacting protein in hepatocellular carcinoma. Hepatol Res. 2014;44:1357–66. doi: 10.1111/hepr.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cai L, Luo L, Tang Z, Meng X. Combined antitumor effects of 1,25-dihydroxy vitamin D3 and Notch inhibitor in liver cancer. Oncol Rep. 2018;40:1515–24. doi: 10.3892/or.2018.6549. [DOI] [PubMed] [Google Scholar]
- 36. Guo J, Ma Z, Ma Q, Wu Z, Fan P, Zhou X, et al. 1, 25(OH)-D3 inhibits hepatocellular carcinoma development through reducing secretion of inflammatory cytokines from immunocytes. Curr Med Chem. 2013;20:4131–41. doi: 10.2174/09298673113209990248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang J, Chen Y, Mo PL, Wei YJ, Liu KC, Zhang ZG, et al. 1α,25-dihydroxyvitamin D3 inhibits aflatoxin B1-induced proliferation and dedifferentiation of hepatic progenitor cells by regulating PI3K/Akt and Hippo pathways. J Steroid Biochem Mol Biol. 2018;183:228–37. doi: 10.1016/j.jsbmb.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 38. Horváth E, Balla B, Kósa J, Lakatos PA, Lazáry Á, Németh D, et al. Vitamin D metabolism and signaling in human hepatocellular carcinoma and surrounding non-tumorous liver. Orv Hetil. 2016;157:1910–8. doi: 10.1556/650.2016.30592. [in Hungarian] [DOI] [PubMed] [Google Scholar]
- 39. Li Q, Gao Y, Jia Z, Mishra L, Guo K, Li Z, et al. Dysregulated Krüppel-like factor 4 and vitamin D receptor signaling contribute to progression of hepatocellular carcinoma. Gastroenterology. 2012;143:799–810. doi: 10.1053/j.gastro.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu J, Wang Y, Zhang Y, Dang S, He S. Astemizole promotes the anti-tumor effect of vitamin D through inhibiting miR-125a-5p-meidated regulation of VDR in HCC. Biomed Pharmacother. 2018;107:1682–91. doi: 10.1016/j.biopha.2018.08.153. [DOI] [PubMed] [Google Scholar]
- 41. Kennedy L, Baker K, Hodges K, Graf A, Venter J, Hargrove L, et al. Dysregulation of vitamin D3 synthesis leads to enhanced cholangiocarcinoma growth. Dig Liver Dis. 2013;45:316–22. doi: 10.1016/j.dld.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 42. Seubwai W, Wongkham C, Puapairoj A, Khuntikeo N, Wongkham S. Overexpression of vitamin D receptor indicates a good prognosis for cholangiocarcinoma: implications for therapeutics. Cancer. 2007;109:2497–505. doi: 10.1002/cncr.22716. [DOI] [PubMed] [Google Scholar]
- 43. Chiang KC, Yeh CN, Lin KJ, Su LJ, Yen TC, Pang JH, et al. Chemopreventive and chemotherapeutic effect of dietary supplementation of vitamin D on cholangiocarcinoma in a chemical-induced animal model. Oncotarget. 2014;5:3849–61. doi: 10.18632/oncotarget.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seubwai W, Wongkham C, Puapairoj A, Okada S, Wongkham S. 22-oxa-1,25-dihydroxyvitamin D3 efficiently inhibits tumor growth in inoculated mice and primary histoculture of cholangiocarcinoma. Cancer. 2010;116:5535–43. doi: 10.1002/cncr.25478. [DOI] [PubMed] [Google Scholar]
- 45. Baek S, Lee YS, Shim HE, Yoon S, Baek SY, Kim BS, et al. Vitamin D3 regulates cell viability in gastric cancer and cholangiocarcinoma. Anat Cell Biol. 2011;44:204–9. doi: 10.5115/acb.2011.44.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chiang KC, Yeh CN, Huang CC, Yeh TSS, Pang JH, Hsu JT, et al. 25(OH)D is effective to repress human cholangiocarcinoma cell growth through the conversion of 25(OH)D to 1α,25(OH)2D3. Int J Mol Sci. 2016;17:1326. doi: 10.3390/ijms17081326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chiang KC, Yeh TS, Huang CC, Chang YC, Juang HH, Cheng CT, et al. MART-10 represses cholangiocarcinoma cell growth and high vitamin D receptor expression indicates better prognosis for cholangiocarcinoma. Sci Rep. 2017;7:43773. doi: 10.1038/srep43773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kawaura A, Tanida N, Akiyama J, Nonaka K, Mizutani M, Sawada K, et al. Inhibitory effect of 1α-hydroxyvitamin D3 on N-nitrosobis(2-oxopropyl)amine-induced cholangiocarcinogenesis in Syrian hamsters. Acta Med Okayama. 2011;65:193–7. doi: 10.18926/AMO/46631. [DOI] [PubMed] [Google Scholar]
- 49. Chiang KC, Yeh CN, Lin KJ, Su LJ, Yen TC, Pang JH, et al. Chemopreventive and chemotherapeutic effect of dietary supplementation of vitamin D on cholangiocarcinoma in a chemical-induced animal model. Oncotarget. 2014;5:3849–61. doi: 10.18632/oncotarget.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hoan NX, Tong HV, Song LH, Meyer CG, Velavan TP. Vitamin D deficiency and hepatitis viruses-associated liver diseases: A literature review. World J Gastroenterol. 2018;24:445–60. doi: 10.3748/wjg.v24.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Iruzubieta P, Terán Á, Crespo J, Fábrega E. Vitamin D deficiency in chronic liver disease. World J Hepatol. 2014;6:901–15. doi: 10.4254/wjh.v6.i12.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stokes CS, Krawczyk M, Reichel C, Lammert F, Grünhage F. Vitamin D deficiency is associated with mortality in patients with advanced liver cirrhosis. Eur J Clin Invest. 2014;44:176–83. doi: 10.1111/eci.12205. [DOI] [PubMed] [Google Scholar]
- 53. Paternostro R, Wagner D, Reiberger T, Mandorfer M, Schwarzer R, Ferlitsch M, et al. Low 25-OH-vitamin D levels reflect hepatic dysfunction and are associated with mortality in patients with liver cirrhosis. Wien Klin Wochenschr. 2017;129:8–15. doi: 10.1007/s00508-016-1127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Abdalla M, Khairy E, Louka ML, Ali-Labib R, Ibrahim EA. Vitamin D receptor gene methylation in hepatocellular carcinoma. Gene. 2018;653:65–71. doi: 10.1016/j.gene.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 55. Wang JB, Abnet CC, Chen W, Dawsey SM, Fan JH, Yin LY, et al. Association between serum 25(OH) vitamin D, incident liver cancer and chronic liver disease mortality in the Linxian Nutrition Intervention Trials: a nested case-control study. Br J Cancer. 2013;109:1997–2004. doi: 10.1038/bjc.2013.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Budhathoki S, Hidaka A, Yamaji T, Sawada N, Tanaka-Mizuno S, Kuchiba A, et al. Plasma 25-hydroxyvitamin D concentration and subsequent risk of total and site specific cancers in Japanese population: large case-cohort study within Japan Public Health Center-based Prospective Study cohort. BMJ. 2018;360:k671. doi: 10.1136/bmj.k671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fedirko V, Duarte-Salles T, Bamia C, Trichopoulou A, Aleksandrova K, Trichopoulos D, et al. Prediagnostic circulating vitamin D levels and risk of hepatocellular carcinoma in European populations: a nested case-control study. Hepatology. 2014;60:1222–30. doi: 10.1002/hep.27079. [DOI] [PubMed] [Google Scholar]
- 58. Duarte-Salles T, Fedirko V, Stepien M, Trichopoulou A, Bamia C, Lagiou P, et al. Dairy products and risk of hepatocellular carcinoma: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2014;135:1662–72. doi: 10.1002/ijc.28812. [DOI] [PubMed] [Google Scholar]
- 59. Liu H, Jiang X, Qiao Q, Chen L, Matsuda K, Jiang G, et al. Association of circulating 25-Hydroxyvitamin D and its related genetic variations with hepatocellular carcinoma incidence and survival. Ann Transl Med. 2020;8:1080. doi: 10.21037/atm-20-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Finkelmeier F, Kronenberger B, Köberle V, Bojunga J, Zeuzem S, Trojan J, et al. Severe 25-hydroxyvitamin D deficiency identifies a poor prognosis in patients with hepatocellular carcinoma - a prospective cohort study. Aliment Pharmacol Ther. 2014;39:1204–12. doi: 10.1111/apt.12731. [DOI] [PubMed] [Google Scholar]
- 61. Weinstein SJ, Mondul AM, Yu K, Layne TM, Abnet CC, Freedman ND, et al. Circulating 25-hydroxyvitamin D up to 3 decades prior to diagnosis in relation to overall and organ-specific cancer survival. Eur J Epidemiol. 2018;33:1087–99. doi: 10.1007/s10654-018-0428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fang AP, Long JA, Zhang YJ, Liu ZY, Li QJ, Zhang DM, et al. Serum bioavailable, rather than total, 25-hydroxyvitamin D levels are associated with hepatocellular carcinoma survival. Hepatology. 2020;72:169–82. doi: 10.1002/hep.31013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Falleti E, Bitetto D, Fabris C, Cussigh A, Fontanini E, Fornasiere E, et al. Vitamin D receptor gene polymorphisms and hepatocellular carcinoma in alcoholic cirrhosis. World J Gastroenterol. 2010;16:3016–24. doi: 10.3748/wjg.v16.i24.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Raafat Rowida I, Eshra KA, El-Sharaby RM, Eissa R, Saied SM, Am I, et al. Apa1 (rs7975232) SNP in the vitamin D receptor is linked to hepatocellular carcinoma in hepatitis C virus cirrhosis. Br J Biomed Sci. 2020;77:53–7. doi: 10.1080/09674845.2019.1680166. [DOI] [PubMed] [Google Scholar]
- 65. Barooah P, Saikia S, Bharadwaj R, Sarmah P, Bhattacharyya M, Goswami B, et al. Role of VDR, GC, and CYP2R1 polymorphisms in the development of hepatocellular carcinoma in hepatitis C virus-infected patients. Genet Test Mol Biomarkers. 2019;23:325–31. doi: 10.1089/gtmb.2018.0170. [DOI] [PubMed] [Google Scholar]
- 66. Hoan NX, Khuyen N, Giang DP, Binh MT, Toan NL, Anh DT, et al. Vitamin D receptor ApaI polymorphism associated with progression of liver disease in Vietnamese patients chronically infected with hepatitis B virus. BMC Med Genet. 2019;20:201. doi: 10.1186/s12881-019-0903-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peng Q, Yang S, Lao X, Li R, Chen Z, Wang J, et al. Association of single nucleotide polymorphisms in VDR and DBP genes with HBV-related hepatocellular carcinoma risk in a Chinese population. PLoS One. 2014;9:e116026. doi: 10.1371/journal.pone.0116026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dalhoff K, Dancey J, Astrup L, Skovsgaard T, Hamberg KJ, Lofts FJ, et al. A phase II study of the vitamin D analogue Seocalcitol in patients with inoperable hepatocellular carcinoma. Br J Cancer. 2003;89:252–7. doi: 10.1038/sj.bjc.6601104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sookprasert A, Pugkhem A, Khuntikeo N, Chur-in S, Chamadol N, Prawan A, et al. Evaluation of efficacy, safety and tolerability of high dose-intermittent calcitriol supplementation to advanced intrahepatic cholangiocarcinoma patients–a pilot study. Asian Pac J Cancer Prev. 2012;13(Suppl):161–7. [PubMed] [Google Scholar]