Abstract
Aim
To evaluate the effect of diabetes mellitus type 2 (T2DM) on the outcomes after treatment of hepatocellular carcinoma (HCC).
Methods
PubMed and Cochrane Central Register of Controlled Trials Databases were systematically searched. Three HCC clinical outcomes were explored: death, progressive disease after locoregional therapies, and recurrence. Sub-analysis was performed according to the use of potentially curative (resection, transplantation, termo-ablation) or non-curative therapies. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to compare the pooled data between T2DM and non-T2DM groups.
Results
A total of 27 studies were analyzed. Overall, 85.2% of articles were from Asia. T2MD was associated with an increased risk of death (OR 3.60; 95%CI 2.18-5.95; P < 0.001), irrespective of the treatment approach: curative (OR 1.30 95%CI 1.09-1.54; P = 0.003) or non-curative (OR 1.05; 95%CI 1.00-1.10; P = 0.045), increased HCC recurrence (OR 1.30; 95%CI 1.03-1.63; P = 0.03), and increased disease progressiveness (OR 1.24; 95%CI 1.09-1.41; P = 0.001).
Conclusions
Current data provide strong evidence that T2DM unfavorably affects HCC progression and recurrence, and patients' survival after treatment, irrespective of the approach used.
The prevalence of hepatocellular carcinoma (HCC) associated with non-alcoholic fatty liver disease (NAFLD) is increasing (1,2) as the result of the globally increased prevalence of NALFD, which is estimated to be about 25% (3). NAFLD patients have a two- to three-fold increase in the risk of developing diabetes mellitus type 2 (T2DM), and the risk is even higher in those with more severe hepatic disease and fibrosis (4-6). On the other hand, patients with T2DM have a higher prevalence of non-alcoholic steatohepatitis (NASH), liver fibrosis, and end-stage liver disease (7).
Several studies have documented the relation between T2DM and the incidence of different cancer types, while the data on the relationship between T2DM and increased risk of incident HCC seem especially robust and clinically reliable (8-10). Observational studies suggest higher mortality of patients developing HCC in the presence of T2DM (11,12). On the other hand, data from meta-analyses suggest that both the risk and prognosis of patients with HCC and diabetes might be influenced by the type of anti-diabetic treatment, where metformin, unlike sulphonylurea, potentially protects against cancer and leads to better prognosis in case of cancer development (13,14).
The underlying mechanisms linking T2DM and HCC are still under scientific scrutiny. However, the interconnections between metabolic derangements characteristic for T2DM, obesity, and NAFLD suggest that insulin resistance on the hepatic and systemic level and the release of pro-inflammatory cytokines, vasoactive factors, and pro-oxidant molecules are potentially implicated in the development and progression of HCC.
With the intent to gain a better insight into this issue, we performed a meta-analysis to evaluate the effect of T2DM on poor outcomes after HCC treatment. To explore several different clinical settings, three outcomes of interest were investigated: death, progressive disease after locoregional therapies, and recurrence. Moreover, sub-analyses were performed according to the use of potentially curative (resection, transplantation, termo-ablation) or non-curative therapies.
MATERIALS AND METHODS
Search sources and study design
A systematic review of the published literature was undertaken focusing on the role of T2DM in the outcomes of HCC patients receiving any tumor therapy. The search strategy followed the Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) guidelines (15). In terms of inclusion criteria, data extraction, quality assessment, and statistical analysis, we used the same methodological approach as in our previous article (16).
The research question formulated in the present study included the following Patients, Intervention, Comparator, Outcome components:
Patient: patients with HCC and T2DM;
Intervention: any HCC therapy;
Comparison: patients with HCC without T2DM treated with the same approach;
Outcome: death, progressive disease, or recurrence.
We searched the PubMed and Cochrane Central Register of Controlled Trials databases using the following terms: (recurrence or death or survival) and (diabetes or T2DM) and (HCC or hepatocellular cancer or hepatocellular carcinoma or hepatoma). The search period was from 2000/01/01 to 2020/11/30.
The systematic qualitative review included only studies in English that involved human patients. Reports were excluded based on several criteria: a) data on animal models; b) studies that lacked enough clinical details; c) studies that had non-primary source data (eg, review articles, non-clinical studies, letters to the editor, expert opinions, and conference summaries). In the case of studies originating from the same center, the possible overlapping of clinical cases was examined, and the most informative study was considered eligible.
Data extraction and definitions
After a full-text review of the eligible studies, two independent authors (QL and FG) extracted the data and crosschecked all outcomes. During article selection and data extraction, potential discrepancies were resolved by a consensus with a third reviewer (AM). Collected data included the first author of the publication, year of publication, country, and the number of treated and recurred patients according to the different therapies adopted.
Quality assessment
Selected studies were systematically reviewed with the intent to identify potential sources of bias. The articles' quality was assessed by using the Risk of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool (17).
Statistical analysis
The results are expressed as odds ratio (OR) with 95% confidence intervals (CIs). The statistical heterogeneity was evaluated with the Higgins statistic squared (I2). I2 values of 0%-25% were considered as an index of low heterogeneity between studies, 26%-50%: moderate heterogeneity, and ≥51%: high heterogeneity. The fixed-effects model was used when low or moderate (0%-50%) heterogeneity was detected between studies, while the random effects model was used when high heterogeneity was present. The value P < 0.05 indicated statistical significance. The meta-analysis was performed by using OpenMetaAnalyst (http://www.cebm.brown.edu/openmeta/index.html).
RESULTS
Search results and study characteristics
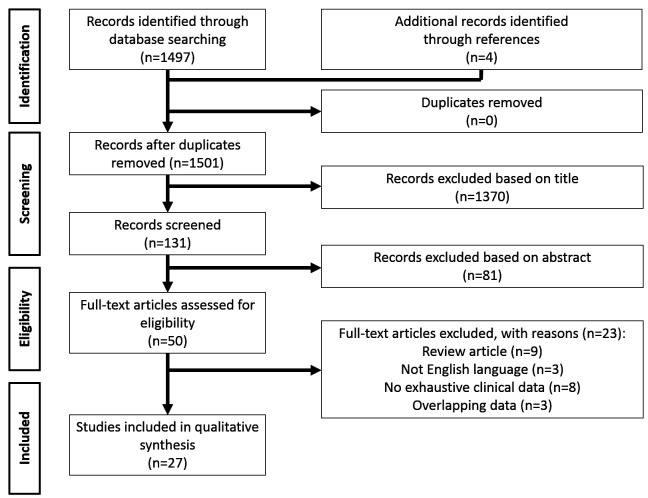
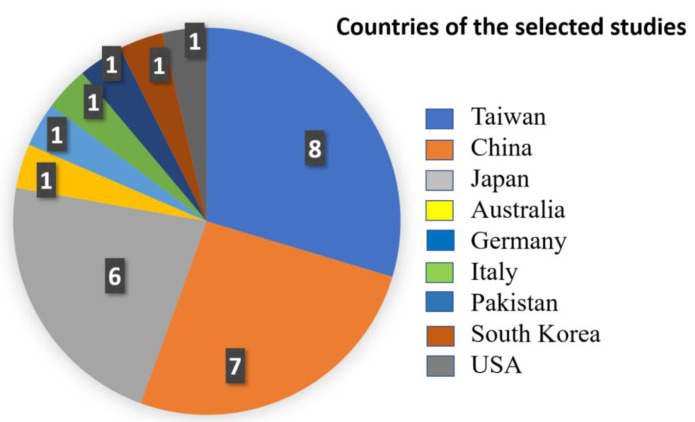
The PRISMA flow diagram schematically depicts the article selection process (Figure 1). Among the 1497 articles screened, 27 were included in this review (18-44). Of these, 8 (29.6%) were published from 2001 to 2009, and 19 (70.4%) during the last decade. Twenty-three articles (85.2%) were from Asia, of which 8 (29.6%) were from Taiwan, while 2 studies (7.4%) were from Europe, 1 (3.7%) was from North America, and 1 (3.7%) from Oceania (Figure 2).
Figure 1.
PRISMA diagram of the article selection process.
Figure 2.
Geographical distribution of the selected studies.
Qualitative assessment of the included studies
All the 27 selected articles were retrospective analyses. As for the ROBINS-I tool quality assessment, all the studies had a low risk of bias.
Review of the eligible studies
Details of the selected articles are reported in Tables 1-3. A total of 23 (85.2%) studies investigated the risk of death after any HCC treatment (Table 1) (18-27,29-37,40-42,44). Tumor recurrence was investigated in 14 (51.9%) studies (Table 2) (19,21,22,24,27,28,30,31,35,37,39-41,43), while the risk of progressive disease was reported in 7 (25.9%) studies (Table 3) (18,26,37,38,41,42,44). Overall, 5 (18.5%) studies had sample sizes of more than 1000 patients (25,34,37,41,44).
Table 1.
Characteristics of included studies that assessed the risk of death in patients with hepatocellular carcinoma*
| Ref. | First author | Year | City | Country | Study period | Design | N | Therapy | T2DM | N events | No T2DM | N events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17 |
Toyoda |
2001 |
Ogaki |
Japan |
Jan 1990-Jun 1999 |
Retro |
581 |
Mix |
92 |
39 |
489 |
209 |
| 18 |
Poon |
2002 |
Hong Kong |
China |
Jan 1989-Sep 1999 |
Retro |
525 |
HR |
62 |
29 |
463 |
229 |
| 19 |
Li |
2003 |
Beijing |
China |
Jan 1998-Dec 2001 |
Retro |
225 |
No surg |
28 |
10 |
197 |
63 |
| 20 |
Huo |
2004 |
Taipei |
Taiwan |
Apr 1996-March 2001 |
Retro |
255 |
HR |
41 |
23 |
214 |
85 |
| 312 |
No surg |
79 |
39 |
233 |
85 |
|||||||
| 21 |
Komura |
2007 |
Ishikawa |
Japan |
Jun 1987-May 2004 |
Retro |
90 |
HR |
30 |
17 |
60 |
14 |
| 22 |
Sumie |
2007 |
Fukuoka |
Japan |
Jan 1994-Dec 2000 |
Retro |
118 |
Mix |
39 |
22 |
79 |
34 |
| 23 |
Kawamura |
2008 |
Tokyo |
Japan |
Jan 1980-Dec 2006 |
Retro |
40 |
HR/RFA |
18 |
6 |
22 |
7 |
| 24 |
Huo |
2009 |
Taipei |
Taiwan |
Jan 2002-Dec 2008 |
Retro |
1713 |
Mix |
392 |
235 |
1321 |
674 |
| 25 |
Feng |
2010 |
Tainan City |
Taiwan |
Aug 2007-Jun 2008 |
Retro |
52 |
TACE |
14 |
2 |
38 |
6 |
| 26 |
Chen |
2011 |
Taichung |
Taiwan |
Jul 2003-Jun 2009 |
Retro |
135 |
RFA |
53 |
26 |
82 |
19 |
| 28 |
Shau |
2012 |
Taipei |
Taiwan |
Jan 2003-Dec 2004 |
Retro |
931 |
Mix |
185 |
96 |
746 |
248 |
| 29 |
Ting |
2012 |
Taipei |
Taiwan |
Jan 2000-Dec 2008 |
Retro |
389 |
HR |
117 |
59 |
272 |
106 |
| 30 |
Hosokawa |
2013 |
Tokyo |
Japan |
Jan 1999-Dec 2007 |
Retro |
344 |
RFA |
159 |
19 |
185 |
12 |
| 31 |
Bhat |
2014 |
Rochester |
USA |
Jan 2005-Jun 2011 |
Retro |
701 |
NA |
263 |
170 |
438 |
257 |
| 32 |
Masood |
2014 |
Lahore |
Pakistan |
Jun 2006-Dec 2012 |
Retro |
282 |
Mix |
97 |
50 |
185 |
95 |
| 33 |
Tsai |
2014 |
Taichung |
Taiwan |
Jan 2000- Dec 2010 |
Retro |
5924 |
HR |
2962 |
41 |
2962 |
35 |
| 34 |
Wang |
2014 |
Nanning |
China |
Jun 2003-Feb 2011 |
Retro |
505 |
HR |
134 |
1 |
371 |
8 |
| 35 |
Zhang |
2014 |
Wuhan |
China |
Mar2002-Aug 2012 |
Retro |
138 |
TACE |
34 |
34 |
104 |
102 |
| 36 |
Chan |
2016 |
Taipei |
Taiwan |
Jan 1995-Dec 2011 |
Retro |
26 267 |
HR |
6663 |
3929 |
19 604 |
10 183 |
| 91 482 |
No surg |
21 449 |
18 931 |
70 034 |
61 539 |
|||||||
| 39 |
Yoshida |
2017 |
Tokyo |
Japan |
Jan 2001-Dec 2013 |
Retro |
224 |
HR |
112 |
41 |
112 |
33 |
| 40 |
Li |
2018 |
Hangzhou |
China |
Jan 2008-Dec 2015 |
Retro |
11 048 |
LT |
3136 |
760 |
7912 |
1730 |
| 41 |
Liu |
2018 |
Beijing |
China |
Jan 2005-Dec 2012 |
Retro |
308 |
RFA |
64 |
34 |
244 |
172 |
| 43 |
Liu |
2020 |
Shanghai |
China |
Apr 2011-Jan 2017 |
Retro |
1052 |
TACE |
289 |
161 |
763 |
379 |
| Total | - | - | - | - | - | - | 144 566 | - | 36 689 | 24 815 | 107 877 | 25 957 |
*Abbreviations: Ref – reference; N – number; T2DM – type 2 diabetes mellitus; retro – retrospective; HR – hepatic resection; RFA – radiofrequency ablation; TACE – trans-arterial chemo-embolization; LT – liver transplantation.
Table 2.
Characteristics of included studies that assessed the risk of hepatocellular carcinoma recurrence*
| Ref. | First author | Year | City | Country | Study period | Design | N | Therapy | T2DM | N events | No T2DM | N events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18 |
Poon |
2002 |
Hong Kong |
China |
Jan 1989-Sep 1999 |
Retro |
525 |
HR |
62 |
35 |
463 |
275 |
| 20 |
Huo |
2004 |
Taipei |
Taiwan |
Apr 1996-March 2001 |
Retro |
255 |
HR |
41 |
18 |
214 |
104 |
| 21 |
Komura |
2007 |
Ishikawa |
Japan |
Jun 1987-May 2004 |
Retro |
90 |
HR |
30 |
22 |
60 |
27 |
| 23 |
Kawamura |
2008 |
Tokyo |
Japan |
Jan 1980-Dec 2006 |
Retro |
40 |
HR/RFA |
18 |
15 |
22 |
7 |
| 26 |
Chen |
2011 |
Taichung |
Taiwan |
Jul 2003-Jun 2009 |
Retro |
53 |
RFA |
53 |
30 |
82 |
19 |
| 27 |
Howell |
2011 |
Melbourne |
Australia |
Jan 2000-Aug 2007 |
Retro |
135 |
Mix |
58 |
17 |
77 |
19 |
| 29 |
Ting |
2012 |
Taipei |
Taiwan |
Jan 2000-Dec 2008 |
Retro |
389 |
HR |
117 |
63 |
272 |
116 |
| 30 |
Hosokawa |
2013 |
Tokyo |
Japan |
Jan 1999-Dec 2007 |
Retro |
344 |
RFA |
159 |
116 |
185 |
138 |
| 34 |
Wang |
2014 |
Nanning |
China |
Jun 2003-Feb 2011 |
Retro |
505 |
HR |
134 |
57 |
371 |
175 |
| 36 |
Chan |
2016 |
Taipei |
Taiwan |
Jan 1995-Dec 2011 |
Retro |
26 267 |
HR |
6663 |
2851 |
19 604 |
8380 |
| 38 |
Choi |
2017 |
Busan |
Korea |
Jan 2010-Sep 2014 |
Retro |
58 |
HR |
14 |
8 |
44 |
12 |
| 39 |
Yoshida |
2017 |
Tokyo |
Japan |
Jan 2001-Dec 2013 |
Retro |
224 |
HR |
112 |
69 |
112 |
74 |
| 40 |
Li |
2018 |
Hangzhou |
China |
Jan 2008-Dec 2015 |
Retro |
11 048 |
LT |
3136 |
282 |
7912 |
489 |
| 42 |
Billeter |
2020 |
Heidelberg |
Germany |
Oct 2001-Dec 2017 |
Retro |
88 |
HR |
44 |
26 |
44 |
25 |
| Total | - | - | - | - | - | - | 40 021 | - | 10 641 | 3609 | 29 462 | 9860 |
*Abbreviations: Ref – reference; N – number; T2DM – type 2 diabetes mellitus; retro – retrospective; HR – hepatic resection; RFA – radiofrequency ablation; LT – liver transplantation.
Table 3.
Characteristics of included studies that assessed the risk of hepatocellular carcinoma progressive disease*
| Ref. | Name | Year | City | Country | Study period | Design | N | Therapy | T2DM | N events | No T2DM | N events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17 |
Toyoda |
2001 |
Ogaki |
Japan |
Jan 1990-Jun 1999 |
Retro |
581 |
Mix |
92 |
12 |
489 |
55 |
| 25 |
Feng |
2010 |
Tainan City |
Taiwan |
Aug 2007-Jun 2008 |
Retro |
52 |
TACE |
14 |
6 |
38 |
6 |
| 36 |
Chan |
2016 |
Taipei |
Taiwan |
Jan 1995-Dec 2011 |
Retro |
91 482 |
No surg |
21 449 |
5314 |
70 033 |
14 685 |
| 37 |
Di Costanzo |
2016 |
Naples |
Italy |
Oct 2008-Jun 2014 |
Retro |
313 |
Sorafenib |
80 |
9 |
233 |
41 |
| 40 |
Li |
2018 |
Hangzhou |
China |
Jan 2008-Dec 2015 |
Retro |
15 776 |
Wait list |
4450 |
674 |
11 326 |
1557 |
| 41 |
Liu |
2018 |
Beijing |
China |
Jan 2005-Dec 2012 |
Retro |
308 |
RFA |
64 |
54 |
244 |
169 |
| 43 |
Liu |
2020 |
Shanghai |
China |
Apr 2011-Jan 2017 |
Retro |
1052 |
TACE |
289 |
201 |
763 |
463 |
| Total | - | - | - | - | - | - | 109 564 | - | 26 438 | 6270 | 83 126 | 16 976 |
*Abbreviations: Ref – reference; N – number; T2DM – type 2 diabetes mellitus; retro – retrospective; TACE – trans-arterial chemo-embolization; RFA – radiofrequency ablation.
Death in HCC patients with vs without T2DM
Twenty-three studies reported post-treatment death rates in HCC patients with vs without T2DM (Table 1). Two studies contextually reported both data on post-hepatic resection and post-non- curative approach cases (21,37).
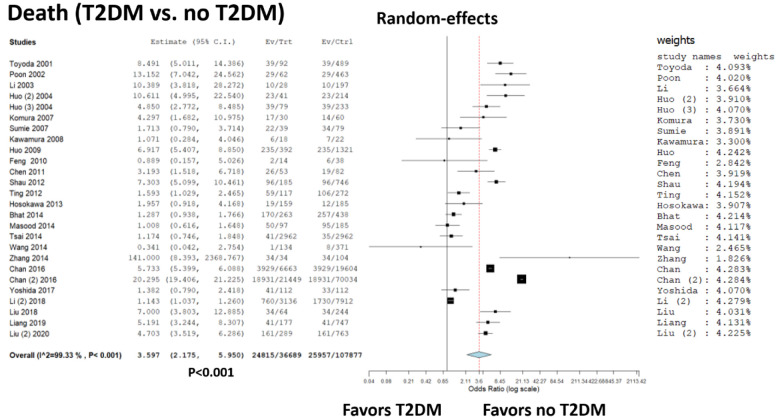
A total of 144 566 patients were considered, with 50 772 (35.1%) deaths. In detail, 24 815/36 689 (67.6%) and 25 957/107 877 (24.1%) deaths were observed in the T2DM and no-T2DM group, respectively. The selected studies showed great heterogeneity, with an I2 = 99.3% (P < 0.001). Most of the studies showed a lower risk of death in T2DM absence (Figure 3). The summary OR showed an increased risk of death in T2DM patients, being 3.60 (95%CI 2.18-5.95; P < 0.001).
Figure 3.
Forest plot and meta-analysis of a disease recurrence after any hepatocellular carcinoma treatment: type 2 diabetes mellitus (T2DM) vs no-T2DM patients.
Recurrence in HCC patients with vs without T2DM
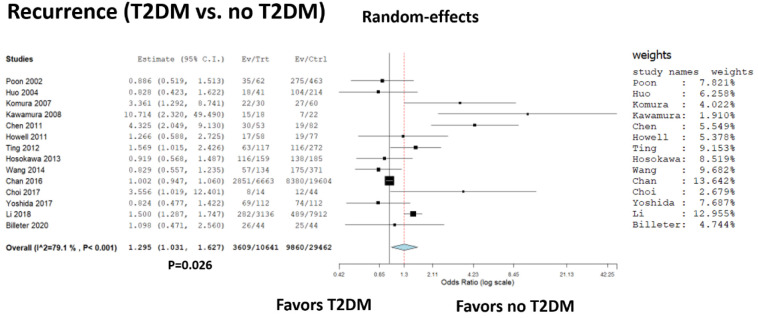
Fourteen studies reported post-treatment recurrence rates in HCC patients with vs without T2DM (Table 2). A total of 40 021 patients were considered, with 13 469 (33.7%) recurrences. In detail, 3609/10 641 (33.9%) and 9860/29 462 (33.5%) recurrences were observed in the T2DM and no-T2DM group, respectively. The selected studies showed great heterogeneity, with an I2 = 79.1% (P < 0.001). Most of the studies showed a lower risk of recurrence in T2DM absence (Figure 4). The summary OR showed an increased risk of recurrence in T2DM patients, being 1.30 (95%CI 1.03-1.63; P = 0.03).
Figure 4.
Forest plot and meta-analysis of death after any hepatocellular carcinoma treatment: type 2 diabetes mellitus (T2DM) vs no-T2DM patients.
Progressive disease in HCC patients with vs without T2DM
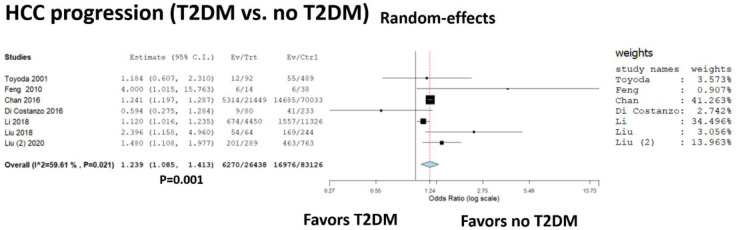
Seven studies reported post-treatment progressive disease rates in HCC patients with vs without T2DM (Table 3). A total of 109 564 patients were considered, with 23 246 (21.2%) progressive diseases. In detail, 6270/26 438 (23.7%) and 16 979/83 126 (20.4%) progressive diseases were observed in the T2DM and no-T2DM group, respectively. The selected studies showed heterogeneity, with an I2 = 59.6% (P = 0.02). Most of the studies showed a lower risk of progressive disease in T2DM absence (Figure 5). The summary OR showed an increased risk of progressive disease in T2DM patients, being 1.24 (95%CI = 1.09-1.41; P = 0.001).
Figure 5.
Forest plot and meta-analysis of progressive disease after any hepatocellular carcinoma treatment: type 2 diabetes mellitus (T2DM) vs no-T2DM patients.
Death in HCC patients with vs without T2DM after curative or non-curative treatments
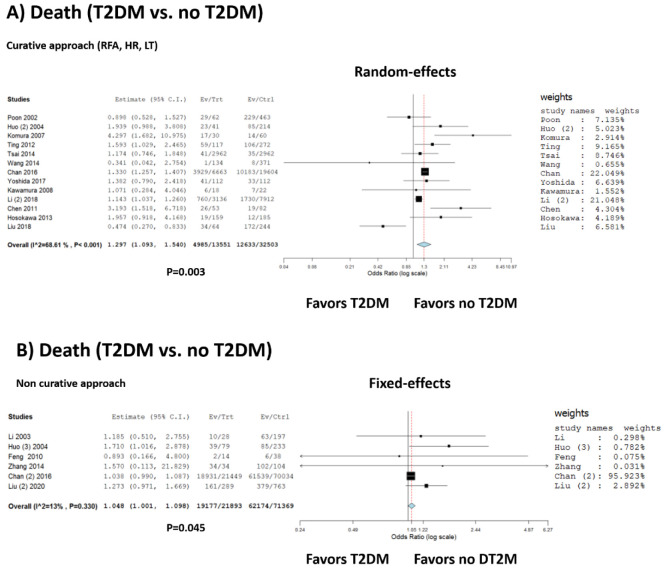
Thirteen studies reported death rates in HCC patients with vs without T2DM after a curative treatment, namely radiofrequency ablation, hepatic resection, and transplantation (Table 1). A total of 46 054 patients were considered, with 17 618 (38.3%) deaths. In detail, 4985/13 551 (36.8%) and 12 633/32 503 (38.9%) deaths were observed in the T2DM and no-T2DM group, respectively. The selected studies showed great heterogeneity, with an I2 = 68.6% (P < 0.001). Most of the studies showed a lower risk of death in T2DM absence (Figure 6A). The summary OR showed an increased risk of death in T2DM patients after curative approach, being 1.30 (95%CI 1.09-1.54; P = 0.003).
Figure 6.
Forest plot and meta-analysis of death after (A) curative hepatocellular carcinoma (HCC) treatments and (B) non-curative HCC treatments: type 2 diabetes mellitus (T2DM) vs no-T2DM patients.
Six studies reported death rates in HCC patients with vs without T2DM after a non-curative treatment, namely any therapy other than radiofrequency ablation, hepatic resection, and transplantation. A total of 93 262 patients were considered, with 81 351 (87.2%) deaths. In detail, 19 177/21 893 (87.6%) and 62 174/71 369 (87.1%) deaths were observed in the T2DM and no-T2DM group, respectively. The selected studies showed great homogeneity, with an I2 = 13% (P = 0.33). Most of the studies showed a lower risk of death in T2DM absence (Figure 6B). The summary OR showed an increased risk of death in T2DM patients after non-curative approach, being 1.05 (95%CI 1.00-1.10; P = 0.045).
Discussion
Our study found strong evidence that T2DM unfavorably affects HCC patients' outcomes in terms of progression and recurrence of the disease and patients' survival after treatment, irrespective of the approach used.
HCC is a common malignancy with still unfavorable prognosis (45). Among the growing risk factors for its development are metabolic derangements associated with NASH and T2DM, with insulin resistance as a common denominator (46). In addition, NAFLD-related HCC occurs both in tandem and in the absence of underlying cirrhosis, substantiating NAFLD’s tumorigenic effects (47,48). According to epidemiologic studies, T2DM can increase the incidence of HCC and unfavorably alter the prognosis of patients with HCC (49).
In reference to its multisystemic effects, T2DM, in some centers, is considered a relative or absolute contraindication for curative treatments such as liver transplantation due to poor outcomes and higher incidence of complications (50). Nevertheless, a recent meta-analysis showed that NAFLD-HCC patients could enjoy long-term survival benefits when treated with aggressive curative approaches (resection, transplantation, or thermo-ablation) with no difference in overall survival compared with non-NAFLD HCC patients (51). On the other hand, this meta-analysis provided solid evidence that T2DM patients compared with non-T2DM patients have an increased risk of death after curative HCC treatments, irrespective of the approach. Most out of 13 studies included in this meta-analysis showed a lower risk of death in T2DM absence. Furthermore, we demonstrated that the same T2DM impact on survival could also be expected after non-curative HCC approaches.
Most studies (85.2%) included in this meta-analysis were from Asia, where hepatitis B infection is still the leading cause of HCC. The incidence of alcohol- and HCV-related HCC in Asian countries is relatively stable and low, while NASH has become a growing epidemic with the prevalence of approximately 10%-20% (52,53). NASH is mainly accompanied by T2DM, dyslipidemia, and obesity, common factors leading to cardiovascular events. NASH is the fastest-growing cause of HCC in US liver transplant candidates (54), and the same is expected to occur in Asia due to changes in eating habits and sedentary lifestyles. NASH is strongly associated with HCC and liver-related mortality, yet the death in NAFLD is due mainly to cardiovascular diseases (55,56).
In addition, this meta-analysis shows that diabetes is inevitably associated with a greater risk of HCC progression. Only a study by Di Costanzo et al (38) showed longer time-to-progression (10 months vs 9 months) in 80 diabetic HCC patients treated with sorafenib compared with 233 non-diabetic HCC patients (38). Other six studies reporting data on HCC progression (17,25,36,40,41,43) showed an increased diabetes-associated risk (26 358 patients with vs 82 893 without diabetes) on HCC progression.
Overall, 14 included studies reported data on HCC recurrence (19,21,22,24,27,28,30,31,35,37,39-41,43). Robust evidence confirmed that diabetes increased HCC recurrence risk, irrespective of treatment. In the case of 508 out of 10 641 diabetic HCC patients included in the analysis, the risk of recurrence did not differ from that observed for non-diabetic HCC patients (19,21,31,35,40). Of note, the cause of HCC in the mentioned studies was mostly hepatitis virus-related.
In light of the observed results, it would be prudent to improve the prevention of T2DM by promoting a healthy diet and incorporating structured physical activity into everyday life (57). Moreover, an important role in the prevention of HCC can be played by interventions that lead to T2DM remission, such as those with glucagon-like peptide-1 receptor agonists (liraglutide and semaglutide) and bariatric surgery, which significantly reduce (>10%) and help maintain weight (58-61). The potential chemo-preventive effect of antidiabetic drugs such as metformin in HCC patients requires further clarification (62).
The present study has some limitations. First, a large part of the investigated population is from Asian countries. Therefore, the reported results should present some geographical peculiarities precluding their generalizability in Western populations. Further larger studies coming from North America and Europe are required. Another limitation relates to the great heterogeneity reported among different studies. Unfortunately, different aims and different approaches were reported in the examined studies, increasing the difficulty to obtain definitive results. We tried to minimize the heterogeneous effect by performing sub-analyses focused on specific classes of patients (ie, treated with curative vs non-curative therapies).
Further studies are required to better clarify the effect of T2DM in larger Western series and investigate the effect of anti-diabetic drugs in protecting against the risk of tumor evolution in HCC patients receiving curative or non-curative therapies.
Acknowledgments
Funding None.
Ethical approval Not required.
Declaration of authorship AM, MCB, and QL conceived and designed the study; FG and QL acquired the data; FG and QL analyzed and interpreted the data; AM and MCB drafted the manuscript; all authors critically revised the manuscript for important intellectual content; all authors gave approval of the version to be submitted; all authors agree to be accountable for all aspects of the work.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1. White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342–1359.e2. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17:748–755.e3. doi: 10.1016/j.cgh.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4. McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223–43. doi: 10.1016/j.cld.2011.03.006. . vii-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang H, Gao C, Fang L, Yao SK. Increased international normalized ratio level in hepatocellular carcinoma patients with diabetes mellitus. World J Gastroenterol. 2013;19:2395–403. doi: 10.3748/wjg.v19.i15.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Margini C, Dufour JF. The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int. 2016;36:317–24. doi: 10.1111/liv.13031. [DOI] [PubMed] [Google Scholar]
- 7. Noureddin M, Rinella ME. Nonalcoholic fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin Liver Dis. 2015;19:361–79. doi: 10.1016/j.cld.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207–21. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 9. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 10. Wainwright P, Scorletti E, Byrne CD. Type 2 diabetes and hepatocellular carcinoma: risk factors and pathogenesis. Curr Diab Rep. 2017;17:20. doi: 10.1007/s11892-017-0851-x. [DOI] [PubMed] [Google Scholar]
- 11. El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–80. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 12. Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28:109–22. doi: 10.1002/dmrr.1291. [DOI] [PubMed] [Google Scholar]
- 13. Miele L, Bosetti C, Turati F, Rapaccini G, Gasbarrini A, La Vecchia C, et al. Diabetes and insulin therapy, but not metformin, are related to hepatocellular cancer risk. Gastroenterol Res Pract. 2015;2015:570356. doi: 10.1155/2015/570356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klil-Drori AJ, Azoulay L, Pollak MN. Cancer, obesity, diabetes, and antidiabetic drugs: is the fog clearing? Nat Rev Clin Oncol. 2017;14:85–99. doi: 10.1038/nrclinonc.2016.120. [DOI] [PubMed] [Google Scholar]
- 15. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 16. Lai Q, Mennini G, Giovanardi F, Rossi M, Giannini EG. Immunoglobulin, nucleos(t)ide analogues and hepatitis B virus recurrence after liver transplant: A meta-analysis. Eur J Clin Invest. 2021:e13575. doi: 10.1111/eci.13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toyoda H, Kumada T, Nakano S, Takeda I, Sugiyama K, Kiriyama S, et al. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma. Cancer. 2001;91:957–63. doi: 10.1002/1097-0142(20010301)91:5<957::AID-CNCR1085>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 19. Poon RT, Fan ST, Wong J. Does diabetes mellitus influence the perioperative outcome or long term prognosis after resection of hepatocellular carcinoma? Am J Gastroenterol. 2002;97:1480–8. doi: 10.1111/j.1572-0241.2002.05792.x. [DOI] [PubMed] [Google Scholar]
- 20. Li XP, Chen Z, Meng ZQ, Huang WX, Liu LM. Concurrent hyperglycemia does not influence the long-term prognosis of unresectable hepatocellular carcinomas. World J Gastroenterol. 2003;9:1848–52. doi: 10.3748/wjg.v9.i8.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huo TI, Wu JC, Lui WY, Huang YH, Lee PC, Chiang JH, et al. Differential mechanism and prognostic impact of diabetes mellitus on patients with hepatocellular carcinoma undergoing surgical and nonsurgical treatment. Am J Gastroenterol. 2004;99:1479–87. doi: 10.1111/j.1572-0241.2004.30024.x. [DOI] [PubMed] [Google Scholar]
- 22. Komura T, Mizukoshi E, Kita Y, Sakurai M, Takata Y, Arai K, et al. Impact of diabetes on recurrence of hepatocellular carcinoma after surgical treatment in patients with viral hepatitis. Am J Gastroenterol. 2007;102:1939–46. doi: 10.1111/j.1572-0241.2007.01354.x. [DOI] [PubMed] [Google Scholar]
- 23. Sumie S, Kawaguchi T, Komuta M, Kuromatsu R, Itano S, Okuda K, et al. Significance of glucose intolerance and SHIP2 expression in hepatocellular carcinoma patients with HCV infection. Oncol Rep. 2007;18:545–52. doi: 10.3892/or.18.3.545. [DOI] [PubMed] [Google Scholar]
- 24. Kawamura Y, Ikeda K, Arase Y, Yatsuji H, Sezaki H, Hosaka T, et al. Diabetes mellitus worsens the recurrence rate after potentially curative therapy in patients with hepatocellular carcinoma associated with nonviral hepatitis. J Gastroenterol Hepatol. 2008;23:1739–46. doi: 10.1111/j.1440-1746.2008.05436.x. [DOI] [PubMed] [Google Scholar]
- 25. Huo TI, Hsu CY, Huang YH, Hsia CY, Lin HC, Lee PC, et al. Diabetes mellitus as an independent prognostic predictor and its association with renal dysfunction in patients with hepatocellular carcinoma. Liver Int. 2010;30:198–207. doi: 10.1111/j.1478-3231.2009.02143.x. [DOI] [PubMed] [Google Scholar]
- 26. Feng YH, Lin CY, Huang WT, Wu CL, Fang JL, Tsao CJ. Diabetes mellitus impairs the response to intra-arterial chemotherapy in hepatocellular carcinoma. Med Oncol. 2011;28:1080–8. doi: 10.1007/s12032-010-9650-9. [DOI] [PubMed] [Google Scholar]
- 27. Chen TM, Lin CC, Huang PT, Wen CF. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J Gastroenterol Hepatol. 2011;26:858–65. doi: 10.1111/j.1440-1746.2011.06664.x. [DOI] [PubMed] [Google Scholar]
- 28. Howell J, Yiu M, Gibson R, Thomson B, Stella D, Gorelik A, et al. Type 2 diabetes does not worsen prognosis in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2011;35:214–20. doi: 10.1016/j.clinre.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 29. Shau WY, Shao YY, Yeh YC, Lin ZZ, Kuo R, Hsu CH, et al. Diabetes mellitus is associated with increased mortality in patients receiving curative therapy for hepatocellular carcinoma. Oncologist. 2012;17:856–62. doi: 10.1634/theoncologist.2012-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ting CT, Chen RC, Chen CC, Liu MH, Chu D, Kuo NW. Diabetes worsens the surgical outcomes in cirrhotic patients with hepatocellular carcinoma. Tohoku J Exp Med. 2012;227:73–81. doi: 10.1620/tjem.227.73. [DOI] [PubMed] [Google Scholar]
- 31. Hosokawa T, Kurosaki M, Tsuchiya K, Matsuda S, Muraoka M, Suzuki Y, et al. Hyperglycemia is a significant prognostic factor of hepatocellular carcinoma after curative therapy. World J Gastroenterol. 2013;19:249–57. doi: 10.3748/wjg.v19.i2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bhat M, Chaiteerakij R, Harmsen WS, Schleck CD, Yang JD, Giama NH, et al. Metformin does not improve survival in patients with hepatocellular carcinoma. World J Gastroenterol. 2014;20:15750–5. doi: 10.3748/wjg.v20.i42.15750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Masood MA, Zafar W, Yusuf MA. Is diabetes mellitus a poor prognostic factor for hepatocellular carcinoma? J Gastrointest Cancer. 2014;45:448–51. doi: 10.1007/s12029-014-9631-x. [DOI] [PubMed] [Google Scholar]
- 34. Tsai MS, Lin CL, Chang SN, Lee PH, Kao CH. Diabetes mellitus and increased postoperative risk of acute renal failure after hepatectomy for hepatocellular carcinoma: a nationwide population-based study. Ann Surg Oncol. 2014;21:3810–6. doi: 10.1245/s10434-014-3777-4. [DOI] [PubMed] [Google Scholar]
- 35. Wang YY, Huang S, Zhong JH, Ke Y, Guo Z, Liu JQ, et al. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma after curative hepatectomy. PLoS One. 2014;9:e113858. doi: 10.1371/journal.pone.0113858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang J, Gong F, Li L, Zhao M, Song J. Diabetes mellitus and the neutrophil to lymphocyte ratio predict overall survival in non-viral hepatocellular carcinoma treated with transarterial chemoembolization. Oncol Lett. 2014;7:1704–10. doi: 10.3892/ol.2014.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chan KM, Kuo CF, Hsu JT, Chiou MJ, Wang YC, Wu TH, et al. Metformin confers risk reduction for developing hepatocellular carcinoma recurrence after liver resection. Liver Int. 2017;37:434–41. doi: 10.1111/liv.13280. [DOI] [PubMed] [Google Scholar]
- 38. Di Costanzo GG, Tortora R, Morisco F, Addario L, Guarino M, Cordone G, et al. Impact of diabetes on outcomes of sorafenib therapy for hepatocellular carcinoma. Target Oncol. 2017;12:61–7. doi: 10.1007/s11523-016-0454-5. [DOI] [PubMed] [Google Scholar]
- 39. Choi Y, Choi Y, Choi CS, Lee YH. Diabetes mellitus increases the risk of intrahepatic recurrence of hepatocellular carcinoma after surgical resection. Tumori. 2017;103:279–85. doi: 10.5301/tj.5000594. [DOI] [PubMed] [Google Scholar]
- 40. Yoshida N, Midorikawa Y, Higaki T, Nakayama H, Tsuji S, Matsuoka S, et al. Diabetes mellitus not an unfavorable factor on the prognosis of hepatitis C virus-related hepatocellular carcinoma. Hepatol Res. 2018;48:28–35. doi: 10.1111/hepr.12888. [DOI] [PubMed] [Google Scholar]
- 41. Li Z, Gao Z, Xiang J, Zhou J, Yan S, Hu Z. Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma: The impact of pre-existing diabetes mellitus. Liver Int. 2019;39:361–70. doi: 10.1111/liv.13982. [DOI] [PubMed] [Google Scholar]
- 42. Liu SR, Chao R, Liang P, Yu XL, Cheng ZG, Han ZY. Diabetes mellitus may worsen the prognosis in hepatocellular carcinoma patients undergoing curative microwave ablation. J BUON. 2018;23:958–64. [PubMed] [Google Scholar]
- 43. Billeter AT, Müller PC, Albrecht T, Roessler S, Löffler M, Lemekhova A, et al. Impact of type 2 diabetes on oncologic outcomes of hepatocellular carcinomas in non-cirrhotic, non-alcoholic steatohepatitis: a matched-pair analysis. J Gastrointest Surg. 2020 doi: 10.1007/s11605-020-04628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu G, Xia F, Fan G, Yu J, Bao L, Zhang C, et al. Type 2 diabetes mellitus worsens the prognosis of intermediate-stage hepatocellular carcinoma after transarterial chemoembolization. Diabetes Res Clin Pract. 2020;169:108375. doi: 10.1016/j.diabres.2020.108375. [DOI] [PubMed] [Google Scholar]
- 45. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 46. Jinjuvadia R, Patel S, Liangpunsakul S. The association between metabolic syndrome and hepatocellular carcinoma: systemic review and meta-analysis. J Clin Gastroenterol. 2014;48:172–7. doi: 10.1097/MCG.0b013e3182a030c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G, et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436–43. doi: 10.1002/ijc.25797. [DOI] [PubMed] [Google Scholar]
- 48. Perumpail RB, Wong RJ, Ahmed A, Harrison SA. Hepatocellular carcinoma in the setting of non-cirrhotic nonalcoholic fatty liver disease and the metabolic syndrome: US Experience. Dig Dis Sci. 2015;60:3142–8. doi: 10.1007/s10620-015-3821-7. [DOI] [PubMed] [Google Scholar]
- 49. Ali Kamkar MM, Ahmad R, Alsmadi O, Behbehani K. Insight into the impact of diabetes mellitus on the increased risk of hepatocellular carcinoma: mini-review. J Diabetes Metab Disord. 2014;13:57. doi: 10.1186/2251-6581-13-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thuluvath PJ. When is diabetes mellitus a relative or absolute contraindication to liver transplantation? Liver Transpl. 2005;(Suppl 2):S25–9. doi: 10.1002/lt.20606. [DOI] [PubMed] [Google Scholar]
- 51.Chin KM, Prieto M, Cheong CK, Di Martino M, Ielpo B, Goh BKP, et al. Outcomes after curative therapy for hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: a meta-analysis and review of current literature. HPB (Oxford). 2021:S1365-182X(21)00025-3. [DOI] [PubMed]
- 52. Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862–73. doi: 10.1016/j.jhep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 53. Sarin SK, Kumar M, Eslam M, George J, Al Mahtab M, Akbar SMF, et al. Liver diseases in the Asia-Pacific region: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2020;5:167–228. doi: 10.1016/S2468-1253(19)30342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17:748–755.e3. doi: 10.1016/j.cgh.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 55. Chiriac S, Stanciu C, Girleanu I, Cojocariu C, Sfarti C, Singeap AM, et al. Nonalcoholic fatty liver disease and cardiovascular diseases: the heart of the matter. Can J Gastroenterol Hepatol. 2021;2021:6696857. doi: 10.1155/2021/6696857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–12. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 57. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–44. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 58. Iepsen EW, Lundgren J, Dirksen C, Jensen JE, Pedersen O, Hansen T, et al. Treatment with a GLP-1 receptor agonist diminishes the decrease in free plasma leptin during maintenance of weight loss. Int J Obes. 2015;39:834–41. doi: 10.1038/ijo.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ahrén B, Atkin SL, Charpentier G, Warren ML, Wilding JPH, Birch S, et al. Semaglutide induces weight loss in subjects with type 2 diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials. Diabetes Obes Metab. 2018;20:2210–9. doi: 10.1111/dom.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397:971–84. doi: 10.1016/S0140-6736(21)00213-0. [DOI] [PubMed] [Google Scholar]
- 61. Park JY. Prediction of Type 2 diabetes remission after bariatric or metabolic surgery. J Obes Metab Syndr. 2018;27:213–22. doi: 10.7570/jomes.2018.27.4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cho WR, Wang CC, Tsai MY, Chou CK, Liu YW, Wu YJ, et al. Impact of metformin use on the recurrence of hepatocellular carcinoma after initial liver resection in diabetic patients. PLoS One. 2021;16:e0247231. doi: 10.1371/journal.pone.0247231. [DOI] [PMC free article] [PubMed] [Google Scholar]