Abstract
Significant variations in the patterns of care, incidence, and mortality rates of several common cancers have been noted. These disparities have been attributed to a complex interplay of factors, including genetic, environmental, and healthcare-related components. Within this review, primarily focusing on commonly occurring cancers (breast, lung, colorectal), we initially summarize the burden of these disparities with regard to incidence and screening patterns. We then explore the interaction between several proven genetic, epigenetic, and environmental influences that are known to contribute to these disparities.
Racial and ethnic disparities in healthcare are common and are a complex result of biologic, environmental, behavioral, and socioeconomic factors (Fig. 1). A large body of research has consistently demonstrated worse outcomes in minority patients. One of the most concerning components is that of increased incidence and mortality in Black Americans.1 Encouragingly, a growing awareness of this issue, combined with strong advocacy, has led to the implementation of targeted interventions resulting in noticeable progress, thereby narrowing the gap between Black and White Americans over the last 2 decades.2,3
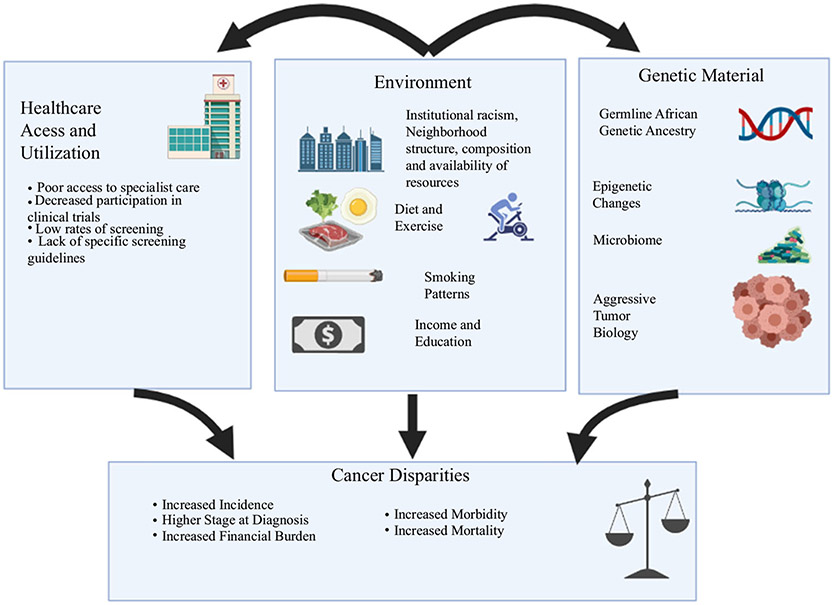
FIG. 1.
Outline of factors contributing to cancer health disparities, as well as the interconnected nature of these influences (created with BioRender.com)
While there are improvements in health outcomes for Black patients, there remains a concomitant lack of awareness, understanding, and common learned knowledge regarding those factors, which drive these disparities. This article serves as a foundational primer to provide a framework for understanding these disparities. The structure is formatted to highlight the layers and multilevel facilitators of disparities and identify areas in which clinicians may focus efforts to ameliorate worse outcomes. We will focus primarily on the disparities between Black and White Americans as the data on these populations are most robust, although there are differences that exist between other racial and ethnic groups.
DIFFERENCES IN BURDEN OF DISEASE (INCIDENCE, SURVIVAL, STAGE DISTRIBUTION, TUMOR HISTOLOGY/PHENOTYPE)
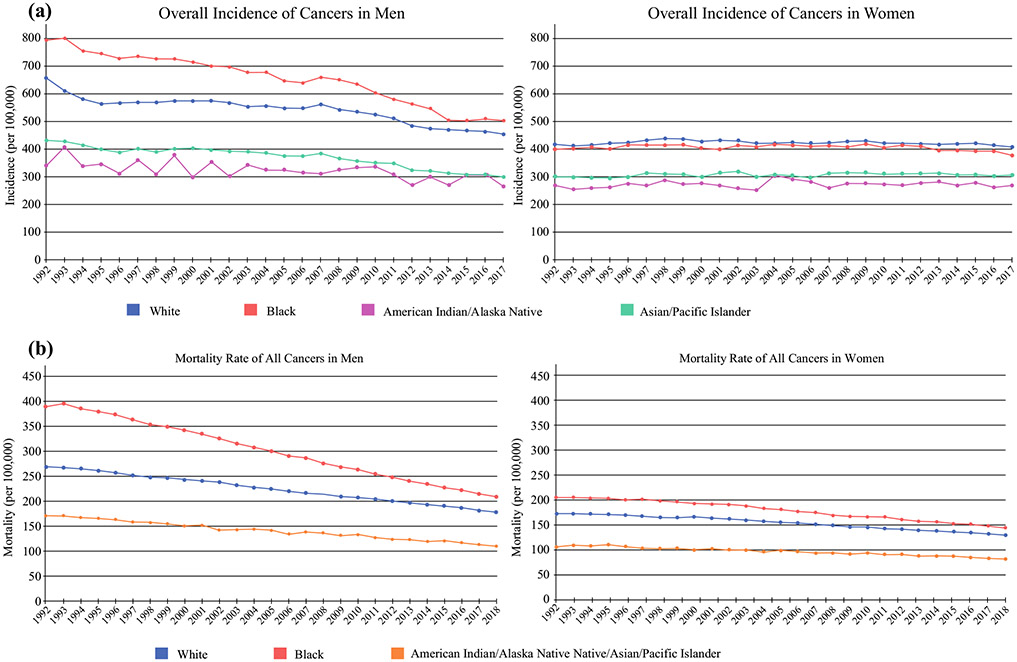
While the overall incidence of new cancers is similar between Black and White patients, variations based on cancer type exist. Notably, Black patients have a disproportionately high all-cancer mortality (Fig. 2).4 Multiple studies have repeatedly demonstrated that underrepresented minorities have an increased incidence of advanced-stage cancers at diagnosis, which is further compounded by the fact that they are less likely to receive treatment and have worse survival per stage. Such findings are confounded by discrepancies in access to care and socioeconomic status (SES). In addition, more recent data have demonstrated differences in tumor biology of certain cancer types between individuals of different races.5 This suggests that the observed discrepancy in outcomes is likely multifactorial, involving biology, cultural, social, and economic factors, and, additionally, requires consideration of individual factors and intersectionality when discussing such outcomes.6
FIG. 2.
Trend in the incidence of a all cancers in men and women, and b mortality from all cancers in men and women. Incidence data from SEER Research Data, 13 Registries, Nov 2019 Sub (1992–2017), and mortality data from Mortality-All COD, Aggregated with State, Total US (1969–2018). Rates are age-adjusted per 100,000. SEER Surveillance, Epidemiology, and End Results
Incidence
Historically, Black Americans have endured an increased overall cancer incidence compared with White Americans; however, recent trends demonstrate a similar overall incidence between these groups (Fig. 2).1,3 Surveillance, Epidemiology, and End Results (SEER) data demonstrate that the rate of new cancers for White Americans is 425.8 (per 100,000 persons), and 429.6 in Black Americans, and even lower in Hispanics, Asians, and American Indians;7 however, there is significant variability in cancer incidence depending on sex and cancer type (Fig. 3).7,8 When broken down by sex, Black men have the highest incidence of new cancers (501.9 per 100,000) and Black women (379.0 per 100,000) have the lowest, with White men and women falling in between (453.4 and 409.8 per 100,000, respectively). The lifetime probability of being diagnosed with cancer is higher for both White men and women compared with Black men and women.3,4
FIG. 3.
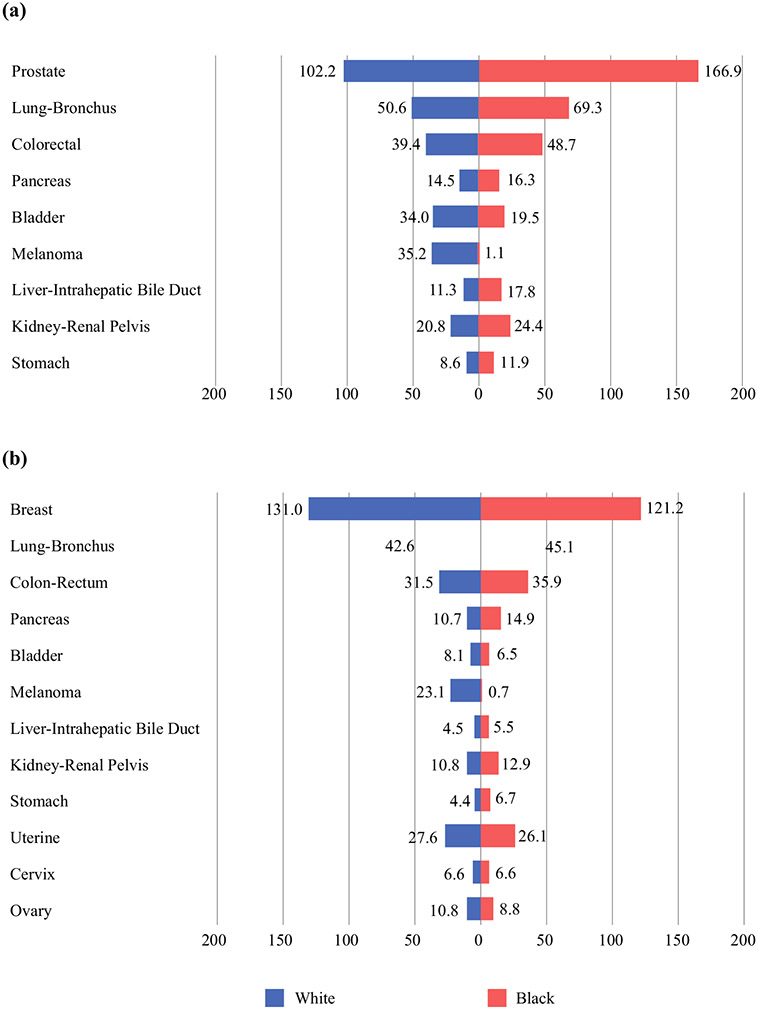
Incidence of the most common cancers of White and Black American a men and b women between 1992 and 2017. Data from SEER Research Data, 13 Registries, Nov 2019 Sub (1992–2017). Rates are age-adjusted per 100,000. SEER Surveillance, Epidemiology, and End Results
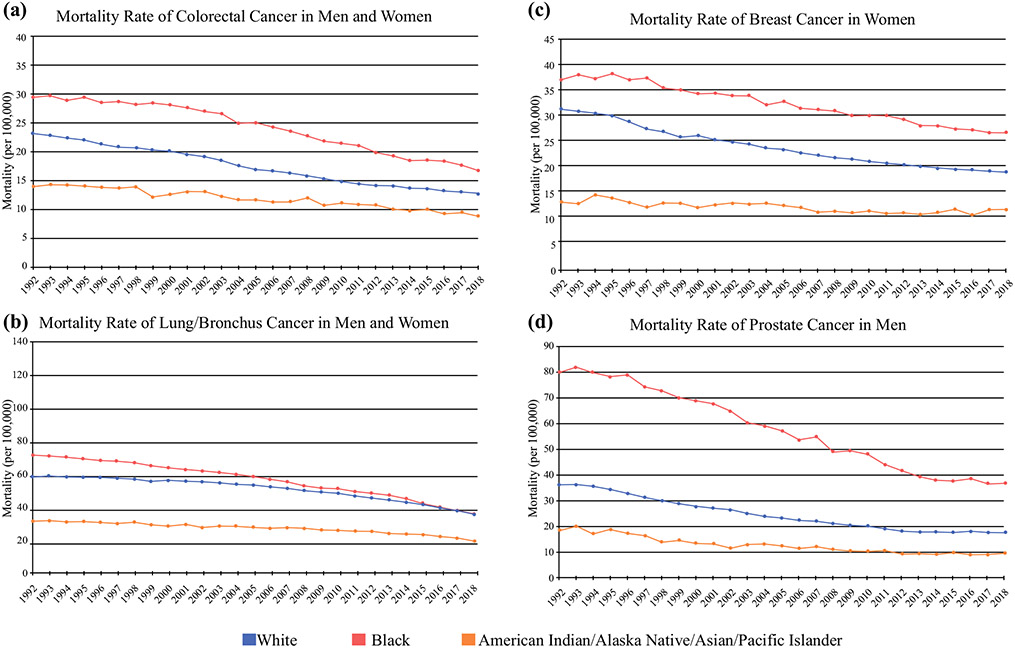
When focusing on the incidence of new cases of female breast cancer, the incidence rate is greater in White patients compared with Black patients. In contrast, a greater incidence of prostate, colorectal, and lung cancer was observed in Black male patients (Fig. 4).7 Notably, prostate cancer accounts for the largest discrepancy in incidence between races, with a significantly increased incidence in Black men.2,7 Lung cancer is unique in its incidence, as approximately 80% of cancers are related to tobacco use. Nevertheless, studies have shown that Black Americans have an increased incidence of lung cancer, even when controlling for pack-year smoking history while consuming a lower number of cigarettes per day,9 indicating that there are other factors associated with this discrepancy in incidence. Undoubtedly, disparities in incidence of any cancer type can be attributed to underlying differences in various areas, such as risk factors, routine cancer screening, or access to care. In the following sections, we will explore these issues in detail.
FIG. 4.
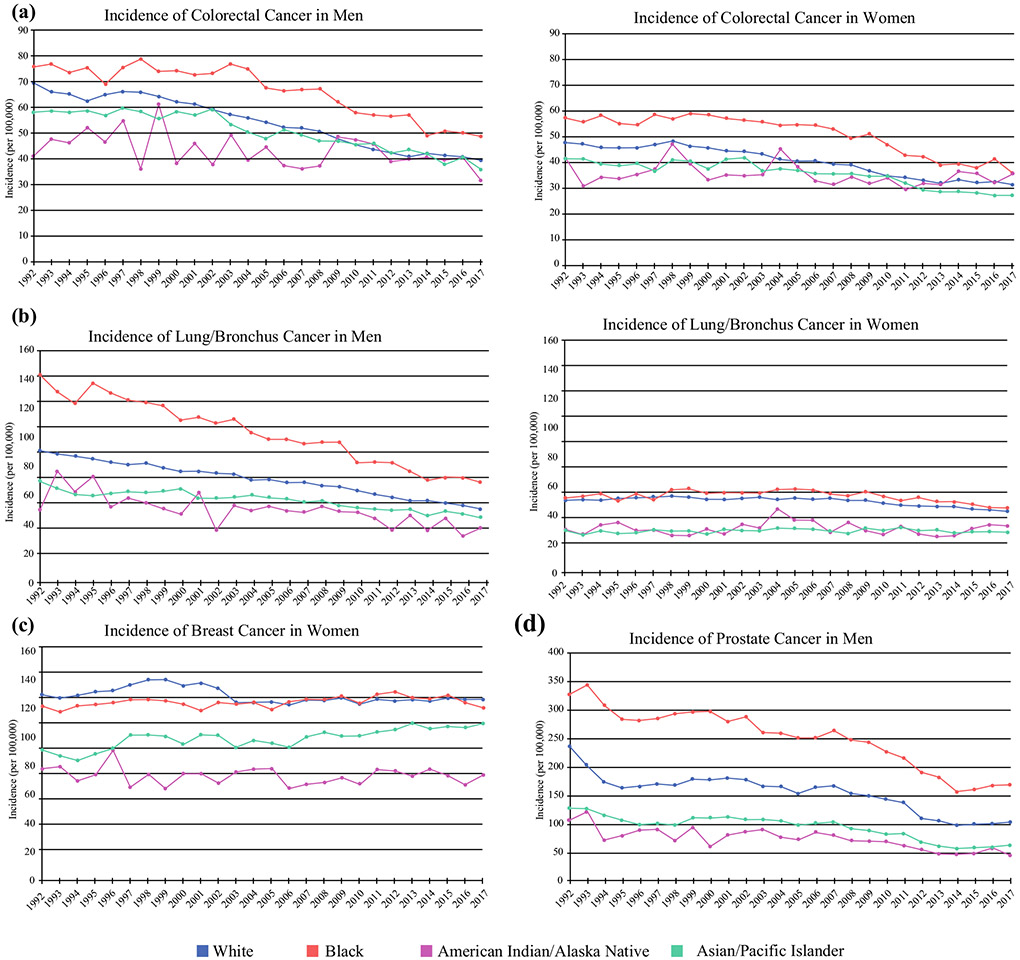
Trend in the incidence of a colorectal cancer, b lung/bronchus cancer, c breast cancer in women, and d prostate cancer in men. Data from SEER Research Data, 13 Registries, Nov 2019 Sub (1992–2017). Rates are age-adjusted per 100,000. SEER Surveillance, Epidemiology, and End Results
Survival
Black Americans have the highest death rate from all cancer sites combined; specifically, lung, colorectal, breast, and prostate cancers carry the highest mortality rates across all races (Fig. 5). Cancer mortality has been greater for Black patients compared with White patients since the 1960s, and although mortality rates have decreased over the last 2 decades in both groups, there remains a significant disparity in cancer-related mortality between Black and White Americans (Fig. 2). The age-adjusted all-cancer mortality rate is approximately 1.3 times higher in Black males compared with White males, and 1.2 times higher in Black females compared with White females.1,10 This trend is seen in all cancer types, with an increased relative risk of mortality ranging between 1.1 and 2.37 for colorectal, lung, gastric, liver/biliary, esophageal, breast, cervical, and prostate cancers.1
FIG. 5.
Trend in the mortality of a colorectal cancer, b lung/bronchus cancer, c breast cancer in women, and d prostate cancer in men. Mortality data from Mortality-All COD, Aggregated with State, Total US (1969–2018). Rates are age-adjusted per 100,000.
Many factors that have been shown to be associated with overall survival, including SES, stage at diagnosis, tumor biology, treatment, and access to healthcare. Some of these topics will be covered later in this review, in additional sections. In particular, patients belonging to a lower SES group have worse outcomes overall, regardless of race. However, Singh and Jemal demonstrated that across each of their five classified socioeconomic groups, Black American patients repeatedly demonstrated lower cancer-associated survival compared with non-Hispanic White (NHW) American patients.1 Furthermore, data have demonstrated that stage at diagnosis has the greatest impact on mortality in breast, prostate, and colorectal cancer (CRC), accounting for approximately 25% of differences.11
Breast cancer has been shown to have one of the greatest disparities in cancer-associated mortality rates. Mortality secondary to breast cancer ranges from 1.1- to 2-fold higher in Black women compared with White women.1,11 Even when statistical models adjust for confounding variables, breast cancer-associated mortality remains significantly greater in Black women.12-14 Individual studies have demonstrated that stage at diagnosis and individual tumor characteristics amplify such differences as the risk of mortality varies based on stage, hormone receptor status, and molecular subtype.11,12,15-18
Racial/ethnic disparities exist in colon cancer in both men and women. Multiple studies have demonstrated that Black race is associated with lower survival as well as an increased risk of recurrent colon cancer.19,20 A study by Lai et al. showed that in a cohort of Black and White patients with CRC, 5-year overall survival difference was 8.3% when matching for SES, 5% when further matching for stage at presentation, and 4.9% when matching for treatment.21 A second study confirmed such results, with a 5-year overall survival difference of 9.9% when matching for demographics, 4.9% when further matching for stage, and 4.3% when including treatment.22 Even in populations that are matched for basic patient and tumor characteristics, overall survival in Black Americans remains 4–5% lower than that of their White counterparts.
Stage at Diagnosis
Black American patients tend to be diagnosed at later stages than White American patients, in many solid organ tumor types. Consequently, a greater percentage of Black patients compared with White patients are diagnosed with metastatic versus localized disease in breast, prostate, colorectal, and lung cancers.2,11 Such findings are also true in other cancer types that are less common in the US, including gastric and liver/biliary cancers.2 This is thought to reflect tumor biology and differences in screening and access to health care, and has been shown to contribute to the increased cancer mortality rates seen in Black Americans.
In breast cancer, multiple studies have shown that Black women are more likely to be diagnosed with later stage disease compared with White, Hispanic, and Asian women.12-14 It is thought that 10–30% of survival differences in breast cancer between Black and White Americans can be attributed to stage at presentation.11,12,17 This is largely thought to be secondary to tumor biology, as prior data have demonstrated that Black women with tumors < 2 cm in size were more likely to have positive nodal disease and distant metastases than White women, as well as triple-negative breast cancers in addition to access to screening and care.13
Similarly, compared with White patients, Black patients are also diagnosed with CRC at a later stage. This finding is more pronounced in women compared with men, and in elderly patients.23,24 Studies have demonstrated that stage at presentation in patients with colon cancer accounted for 40–50% of the disparity in 5-year survival between Black and White Americans.21,22 Some of this is thought to be due to environmental and dietary factors, genetics, and also secondary to screening and access to care.
Phenotype/Histology
In many cases, differences regarding tumor phenotype closely mirror racial/ethnic disparities among stage at diagnosis. Tumor biology has been shown to be different among different races, which is secondary to multiple factors, including genetics, gene and protein expression, epigenetics, histology and the microbiome, and the complex interactions between them. Herein, we focus on the differences in phenotype between common cancers that contribute to disparities in outcomes.
For instance, hormone receptor status has been shown to account for 9–23% of excess mortality in Black women compared with White women.11,12 Compared with other ethnicities, Black women had the highest proportion of aggressive variants, such as triple-negative breast cancer,13,15 but there was some variation with age.25 The risk of overall mortality varies based on hormone receptor status and molecular subtype.12,15,16,18 The results of several studies have demonstrated that there were significant disparities in survival between patients with estrogen receptor-negative (ER−) or progesterone receptor-positive (PR?) tumors,12,15,18 but the results are mixed with regard to human epidermal growth factor receptor 2-positive (HER2?) tumors.12,15 Most studies did not demonstrate differences in survival between racial/ethnic groups in patients with triple-negative breast cancer.12,15,16 Other studies have shown that receptor status impacts outcome differently for Black versus White Americans, but this is dependent on the stage of disease at diagnosis.26
In colon cancer, multiple differences have been described in tumor biology between Black and White patients. Distinct differences in genetic, epigenetic, microRNA and gene expression have been identified.27,28 Such topics will be covered in greater detail in further sections. Additionally, differences in immunophenotype and the role of inflammation have been noted between Black and White Americans. Recent data also suggest that there are differences between inflammation-related genes and tumor immune cell infiltrates in Black and White Americans, yet further validation of these findings is warranted.29-31
The importance of identifying differences in tumor biology between race/ethnicity is critical in decreasing the disparities in outcomes. As we gain a deeper understanding of the processes underlying tumor development and progression, we will be better able to address how such differences impact disparities in outcomes. For example, in breast cancer, Black Americans experience worse survival outcomes than White Americans with the luminal A subtype (hormone receptor-positive [HR+]/HER2−), but less so in triple-negative cancers. This subtype has an overall good prognosis, suggesting that differences in treatment, access to healthcare, or other behavioral factors account for worse outcomes. Similarly, as we learn more about tumor initiation and progression in colon cancer, we can develop a better understanding of why Black Americans are diagnosed at a younger age. Ultimately, these new insights can hopefully aid in the identification of novel risk factors, facilitate improved screening regimens, and allow for the development of uniquely tailored treatment modalities that can be utilized to improve patient outcomes.
RACIAL DISPARITIES IN SCREENING AND TREATMENT AMONG BREAST, COLORECTAL, AND LUNG CANCER PATIENTS
Black patients with breast, colorectal, and lung cancer face significant barriers and challenges in accessing healthcare across the cancer continuum. Racial disparities in screening and treatment continue to portend higher mortality rates for Black patients.32,33 Specifically, Black patients are more likely to present with advanced disease stages at younger ages than their White counterparts.34 Explanations for racial disparities in screening and treatment include underlying tumor characteristics in conjunction with a myriad of social determinants of health, such as insurance, geography, finances, and healthcare access.1,35,36 This section provides an overview of disparities in screening and treatment among Black and White patients, with a focus on breast, colorectal, and lung cancers.
Breast
Screening
Recent screening mammography utilization estimates suggest non-Hispanic Black (NHB) women undergo screening at equivalent rates to NHW women.37 Unfortunately, increased screening has not improved mortality from breast cancer for Black women.38 Compared with their White counterparts, Black women are more likely to present with advanced stages of disease and more aggressive subtypes (i.e. triple-negative breast cancer).3,38 Notably, the majority of age-based screening guidelines do not consider the aforementioned racial disparities, nor do they include race or ancestry in their recommendations. The lack of race or ancestry-based screening recommendations is problematic as studies indicate racial differences in age distribution patterns at diagnosis.39 Specifically, White breast cancer patients peak in their 60s compared with non-White patients, who peak in their 40s.39 To date, the American College of Radiology screening guidelines, released in 2018, are one of the first to provide screening guidelines that mention race explicitly.40 In order to narrow the stage and mortality disparity between Black and White women, future screening guidelines may need to consider the implications of race and ancestry on presentation and clinical outcomes (i.e. recurrence and mortality) among Black patients with breast cancer.
Treatment
Advancements in multimodal treatments for breast cancer have translated into increased survival among White patients but not their Black counterparts.38 Black women face barriers in accessing high-quality and guideline-concordant surgical care, systemic treatment, and radiation therapy.41 Compared with White women, Black women are more likely to have surgery omitted, face delays in time to surgery, and have lower post-mastectomy reconstruction rates.42-44 Moreover, a recent study showed Black women were less likely to undergo sentinel lymph node biopsy after axillary downstaging post neoadjuvant chemotherapy than White women.45 Disparities in locoregional management extend beyond surgery to include radiation therapy. For example, Black breast cancer patients undergoing breast conservation therapy (breast conservation surgery and radiation) are less likely to receive radiation therapy.46 Possible contributors to disparities in radiation therapy and surgical management include geography, SES, and specialists’ availability.47-49
Racial differences in chemotherapy initiation and modification (i.e. dosing, frequency) continue to adversely contribute to poor outcomes for Black women.50 Delays in chemotherapeutic treatment initiation, lower rates of adherence to guideline-concordant regimens by clinicians, and reductions in dosing and frequency are primary drivers of racial disparities in the receipt and administration of chemotherapeutic agents.51,52 Of note, developing data suggest African ancestry may play a role in increased susceptibility to adverse effects, e.g. peripheral neuropathy from taxanes.53 Regrettably, racial disparities in chemotherapy are mirrored in the utilization of targeted therapies such as trastuzumab.54
Hormone-positive breast cancers are the most common breast cancer subtype and have the highest survival rates.38 Substantial improvements in survival and reductions in recurrence are attributed to the utilization of selective estrogen receptor modulators and aromatase inhibitors;55,56 however, lower initiation and adherence to endocrine therapies may be preventing Black women from fully benefitting from these treatment modalities.57 The reasons for racial differences in endocrine therapy and its implications on clinical outcomes are significant areas of research interest.58-60 Emerging data suggest that higher mortality rates from hormone-positive cancers in Black women could be mostly driven by tumor biology rather than hormone therapy adherence.61 Furthermore, it appears recurrence scores based on the genomic test Oncotype DX may be less prognostic in Black women.61 Continued research is needed in this area to understand the intersection of adherence, ancestry, and mortality.
Colorectal
Screening
Due to higher rates of advanced disease at diagnosis and a younger age of onset, American College of Gastroenterology guidelines recommend CRC screening for Black patients, starting at age 45 years.62 Nevertheless, despite improvements in CRC screening rates among both Black and White patients, Black men and women continue to lag behind their White counterparts.37 Estimates from the National Health Interview survey from 2000 to 2015 show increasing CRC screening rates of 32–62% for Black patients aged 50 years and older, compared with 40–65% among NHW patients.63,64 These racial disparities in screening rates persist among patients 45 years and older.63 Explanations for the differences in screening rates are a multifactorial interplay between social determinations of health, including SES, insurance, low health literacy, and medical knowledge.65,66 Moreover, other factors such as poor doctor–patient relationships mired by mistrust and inadequate communication further contribute to disparities in screening.67 Avenues to reduce racial inequalities in CRC screening include increased telehealth utilization, a clear description of the screening process, and training providers to effectively communicate with patients centering health literacy and cultural competency needs.68,69
Treatment
Black patients with CRC cancer are less likely to undergo surgery, chemotherapy, or radiation therapy than White patients.70,71 Additionally, when Black patients do undergo treatment, they incur higher cost compared with White patients, even after controlling for potentially confounding variables such as stage, SES, geographic region, comorbidities, and treatment type.70 Racial disparities in CRC treatment are most likely secondary to issues with access to care, quality of care, and patient-related characteristics.72 Studies suggest institutional drivers of racial disparities in treatment include Black patients receiving care at facilities with lower volumes of CRCs, fewer oncologic specialists, and lower rates of providing guideline-concordant care.72,73 Breslin et al.’s examination of the implications of hospital-level factors on CRC mortality indicate they account for almost half the excess late mortality risk.73
The impact of equitable access to care on clinical outcomes such as survival and recurrence among CRC patients is unclear. An evaluation of care in the Military Health Systems by Eaglehouse et al. indicated that Black and White patients experienced similar delays in time to treatment in this equal-access setting;74 however, Eaglehouse and colleagues did not explore the implications of delays in time to treatment on survival. Examinations of integrated health systems in California suggest Black patients receiving treatment at these facilities were more likely to receive guideline-concordant care and had similar survival rates to White patients.75 Furthermore, Laryea et al. noted that prior disparities in treatment and survival at their institution were eliminated after administering equal treatment to both White and Black patients.76 Conversely, a recent study by Snyder et al. suggests Black patients with locoregional disease receiving guideline-concordant care similar to White patients still had higher rates of recurrence and mortality.19 These inconsistent results across studies warrant further exploration and clarification; however, they do not negate the need or impetus for improving access and quality of care for Black CRC patients.
Lung
Screening
Lung cancer is the leading cause of cancer-related mortality among Black men and women.37 Initial 2011 screening recommendations by the US Preventative Service Task Force (USPSTF) were based on the seminal National Lung Cancer Screening Trial (NLST). The NLST reported a 20% relative reduction in lung cancer mortality among heavy smokers randomized to undergo annual chest low-dose computed tomography compared with a chest X-ray.77 Based on this trial, the USPSTF screening recommendations focused on individuals aged 55–80 years with a 30 pack-year smoking history who quit within 15 years, or current smokers. This recommendation was problematic as it reduced the number of eligible high-risk Black smokers.78 Black smokers have fewer pack-years and are intermittent or light smokers compared with White smokers.9 Furthermore, Black patients with lung cancer have earlier ages of diagnosis, with age-adjusted incidence rates higher at ages 50–54 years than White patients.79 Nevertheless, uptake of screening recommendations is low across all racial and ethnic groups, with only 2% of the 7.6 million eligible smokers undergoing screening in 2016. Recent screening guidelines by the USPSTF address prior criteria, excluding high-risk Black smokers. Current USPSTF guidelines recommend annual low-dose computed tomography screening for individuals aged 50–80 years, with a reasonable life expectancy, and with a 20 pack-year smoking history who quit smoking within 15 years or who are current smokers.80 Nonetheless, additional efforts to educate patients and providers are needed to increase awareness and participation across all racial and ethnic groups.
Treatment
An evaluation of the SEER–Medicare program showed Black patients with lung cancer aged ≥ 65 years were more likely to have treatment omitted than White patients.81 In the review of SEER patients aged ≤ 64 years by Taioli and Flores, early-stage (stage I) Black patients with lung cancer received radiation therapy more often than surgery.82 Moreover, patients treated surgically were less likely to undergo guideline-recommended mediastinal lymph node evaluation.83 In the study by Fang et al., among stage I non-small cell lung cancer (NSCLC) patients, 60% of Black patients versus 75% of White patients underwent treatments with curative intent, and for stage III patients, 36% of Black patients versus 41% of White patients also underwent treatments with curative intent.84 Curative intent for stage I patients was defined as stereotactic body radiation or surgery, and, for stage III patients, curative intent was defined as radiation therapy or surgery plus chemotherapy.84 These studies highlight the difficulties Black patients with lung cancer face in accessing and receiving guideline-concordant care across all ages and stages. The review by Lin et al. of cultural factors associated with racial disparities in lung cancer care indicate Black patients with lung cancer have higher rates of mistrust, fatalism, and negative treatment beliefs.85 To address these issues, it is important to examine how patient–institution and patient–physician relationships influence these cultural beliefs and practices.
RACIAL DISPARITIES IN CANCER INCIDENCE: AFRICAN GENETIC ANCESTRY, EPIGENETICS, ALLOSTATIC LOAD, AND THE MICROBIOME
The disparities in cancer incidence that are outlined in earlier sections of this review illustrate the immense challenges experienced by disadvantaged communities. In order to address these disparities appropriately, there is a critical need to understand the myriad of factors and the complex nature with which they interplay. Undoubtedly, genetics and baseline characteristics may contribute to a certain degree; however, it is increasingly clear that environmental and societal cultural influences, social determinants of health, can in turn adversely affect the inherited traits of individuals, especially epigenetics, and this may ultimately lead to cancer. In these following sections, we will explore data examining the role of genetics in explaining disparities, with a focus on germline African American ancestry and epigenetics. We will discuss the concept of allostatic load and its known associations with race-related disparities, with a focus on disparities relevant to cancer incidence. Lastly, we will consider the role of the microbiome and associated changes that pertain to these common cancers.
Germline African Genetic Ancestry, Epigenetics, and Allostatic Load
When we begin to unravel racial/ethnic disparities, a natural starting point is the consideration of baseline differences in their respective inherited genomes, and the possibility of germline mutations. Germline mutations refer to inheritance of specific alleles across generations that may predispose individuals to certain types of genetic variants, clinical phenotypes, and the subsequent development of cancer.86 Germline mutations, such as those observed with PALB2,87 as well as MTCL188 and GNB5,88 may partially explain the occurrence of racial disparities in breast cancer between Black and White women. Similarly, a number of inherited germline mutations have been implicated in the development of lung89-92 and CRCs.93,94 Conversely, African heritage may be protective against certain cancers. For instance, a genome-wide analysis of 54 Black Americans with esophageal cancer or Barretts esophagus found no associations of genetic regions with excess African ancestry and disease. In fact, patients with malignancy included those with a rich European ancestry.95 As noted by Palmer et al., there is also a considerable overlap of susceptibility genes between White and Black American women who develop breast cancer.86 This is not surprising, as the ‘melting pot’ nature of American society where many people possess a rich and diverse ancestral background has led to a considerable level of shared genetic information. A recent large-scale genetic study supports this stance. In this study, individuals who claimed African American heritage, on average, possessed up to one-quarter of their genes from traditionally European DNA.96 This would suggest that while inherited genetics may partially contribute to the observed racial disparities in cancer incidence, as a singular cause, it cannot fully explain the wide gap observed between White and Black patients.
A more careful investigation of the cause of racial disparities therefore requires a closer examination of the complex interplay between environmental exposures and genetics. A possible link between these two elements may involve epigenetic changes and the role of allostatic load.97-102 A growing consensus has emerged suggesting that an individual with repetitive or low-level chronic exposure to both chemical and non-chemical stressors over a life course may gradually incorporate traces of those stressors within their genetic material in the form of epigenetic changes.103,104 Epigenetic changes involve alterations of gene expression that are mediated by the raveling and unravelling of DNA at the histone level, without any alteration within the actual DNA sequence.105 These changes may even be passed on across generations, in a heritable fashion.106 Thus, the health outcomes observed today may be a direct consequence of repetitive, chronic stress experienced by prior generations. Several recent studies have demonstrated that Black patients develop distinct epigenetic profiles in a variety of cancers.107-112 For instance, within a cohort of breast cancer patients derived from The Cancer Genome Atlas, as many as 142 genes were found to be differentially expressed in Black patients compared with White patients.113 Similar differences were also noted in a genome-wide methylation analysis, thus suggesting that there are separate race-specific mutations producing cancers, dependent on specific oncogenic pathways.109 A number of comprehensive reviews examining epigenetic alterations and its associations with race have explored this topic in detail.5,97,98,114,115
A multitude of environmental and societal factors may precede the development of epigenetic changes. These include, for instance, diet,116 pollutants,117 maternal stress,118 and residence in low-income households.119 Recognizing the genomic impact of these factors is critical for many reasons. First, they provide a potential avenue for widespread, impactful risk-modifying interventions at a community level. Second, they can be addressed in a targeted fashion as potential therapeutic modalities.120 The latter approach may become increasingly relevant as the drive towards adopting ‘precision medicine’ continues to gain momentum with a narrow, focused, and effective targeted approach towards disease. Recognizing and acknowledging the fundamental differences in tumor biology is therefore increasingly paramount. This highlights the importance of critically assessing therapeutic outcomes of clinical trials in the US, particularly given the woefully low recruitment and participation of Black populations within clinical trials.121
Similar to these environmentally mediated epigenetic alterations, chronic psychosocial stress has also been postulated as a mediator of health-related racial and ethnic disparities. This notion was first introduced by McEwen and Stellar, who, in their landmark paper, introduced the term ‘allostatic load’.122 This concept highlighted the underrecognized contribution of repetitive, chronic stress towards disease processes. Central to this framework is the dysregulation of the hypothalamic-pituitary-adrenal axis, thereby impairing the body’s ability to achieve homeostasis appropriately.123 This can then be further quantified into a numerical estimate by measuring biomarkers such as cortisol, epinephrine, DHEA-S, etc.124 Several studies have examined the relationship between allostatic load and cancer and were recently consolidated into a meta-analysis.125 Notably, the authors found that a one-unit increase in allostatic load dramatically elevated the risk of cancer-specific mortality by 9%. A race-specific examination found that elevated allostatic load was associated with a history of breast cancer in Black women, a relationship not seen in White women, suggesting that Black women experienced a greater biological toll.126 However, a larger, more recent prospective study suggested that the association of allostatic load with cancer-specific mortality was similar across both races.127 These confounding results indicate the need for further investigations.
Microbiome
The microbiome refers to the collective community of microscopic organisms that reside within our bodies.128 This collection of various bacteria, fungi, and viruses are known residents of our skin and various hollow viscus organs, most notably the gastrointestinal tract.129 Here, they largely function in a symbiotic fashion, aiding the immune and digestive systems.130 The microbiome can be influenced by a number of external factors,131 and recently, changes in the microbiome have been noted to influence the development and progression of cancer.132 Given the unique heritage and rich nature of Black American culture, this raises the possibility of ethnicity-specific microbiome signatures that may influence the development of certain cancers.
This relationship is perhaps easiest to explore within the gastrointestinal tract, a system that is known to be heavily populated with a rich and diverse microbial community. This microbiome is known to be influenced by environmental influences such as diet. An initial pilot study that examined the relationship of microbiome constituents across various ethnicities found that distinct differences with regard to the proportion of various species are present.133 Furthermore, these authors also noted a decrease in various substrates known to be influenced by bacterial degradation, such as butyrate. Previous work has illustrated butyrate as a potential factor for the prevention of colon cancer.134,135 Similar findings demonstrating the presence of proinflammatory bacteria such as Fusobacterium and Enterobacter were also seen in a larger, more recent study.136 Fusobacterium, in particular, has emerged as a key point of interest, with recent investigations pointing to an associated poorer prognosis, potentially mediated via enhanced chemoresistance.137-139
Similarly, there may be an underrecognized contribution of the microbiome in breast cancer where Black women are known to be disproportionately affected by aggressive variants such as triple-negative breast cancer. An observational analysis of the microbiome of these tumors in comparison with adjacent normal tissue demonstrated that Black women had markedly less microbial diversity in tumor samples,140 which was in contrast to the findings observed in White patients. A similar investigation also demonstrated that the microbiome profile of Black American individuals significantly differed from their NHW peers.141 These findings provide a strong foundation for future work to investigate microbial dysbiosis as a potential contributor towards cancer disparities. Unravelling this link could permit the development of novel biomarkers and potential therapeutic targets in an effort to reduce race-related disparities.
THE ROLE OF LIFESTYLE IN EXPLAINING DISPARITIES
In this final section, we discuss in further detail the associations between various lifestyle traits and cancer disparities. It is well-recognized that behavioral choices regarding diet, tobacco use, physical exercise, etc., as well as an individual’s external environment, contribute to the risk of cancer development. These factors may be responsible for up to 90% of all cancers.142 Importantly, many of these influences are modifiable, therefore an improved understanding of these factors would permit the potential implementation of targeted, cost-effective interventions that could reduce the burden of disparities by primary prevention.143-145
Diet
Differences in nutritional intake are increasingly recognized as important contributors to cancer risk.146,147 A more thorough understanding of nutritional elements has identified certain dietary components that are carcinogenic and inflammatory in nature, and others that aid in immunity and may potentially thereby decrease the risk of cancer.148 By virtue of cultural and socioeconomic influences, dietary differences across ethnicities are well known.149-151 Various authors152-154 have examined the historical context of this variability. Similarly, the social duress and economic hardship currently faced by Black communities undoubtedly limits their purchasing power, thereby artificially restricting their ability to access healthier options.154 Consequently, as noted by Hargreaves et al., despite indicated preferences towards healthy eating, grocery shopping patterns in Black households are simply overwhelmed by the powerful forces of ‘access, traditions, social influences, habits and price’.155 It is therefore possible, to a certain degree, that these dietary differences may play a role in contributing towards cancer disparities.
With regard to colon and prostate cancer, for instance, the consumption of processed meat has recently been implicated as a potential carcinogen.156 Black men have been noted to have higher rates of red meat intake.157 This intake, and in particular the presence of cooked, processed meat such as sausages and bacon, was noted to disproportionately increase the risk of prostate cancer in Black men compared with their White counterparts.157 Similarly, for colon cancer, Yazici et al. noted that in addition to greater daily servings of meat, their Black participants consumed diets with higher fat and protein ratios. They also linked these dietary changes to an increased proportion of sulfidogenic bacteria in biopsy samples. These organisms have been implicated in the downstream development of colon cancer.158 Conversely, Black adults have been noted to have a lower consumption of dietary fiber.150,159 The protective effects of fiber are becoming increasingly highlighted. A recent prospective screening trial demonstrated that elevated dietary fiber intake was associated with a lower risk of distal polyp and colon cancer development.160 Moreover, diets in Black households have been noted to be lacking in folate and calcium intake, two other elements that also have a protective effect against colon cancer.148,161,162 A similar dietary association was noted by Boggs et al. in their study of breast cancer risk in Black women. These authors noted an inverse association of breast cancer risk with increased intake of both carrots and cruciferous vegetables.163
Obesity and Exercise
Obesity and obesity-associated conditions account for approximately 20% of all cancers.164 The resultant chronic proinflammatory state and metabolic alterations caused by obesity can significantly alter host immune responses, thereby promoting the development of different cancers.165,166 Likewise, obesity may attenuate the effect of chemotherapy and radiation, thereby further worsening outcomes for patients.164 Recent estimates from the population-based National Health and Nutrition Examination Survey have placed the prevalence of obesity in the general population at approximately 44%.167 Among these participants, Blacks subjects were noted to have higher rates compared with White participants (49.6% vs. 42.2%), with a markedly larger difference in women (56.9% vs. 39.8%).167 These universally high rates of obesity appear to predispose individuals to cancer, regardless of race.168 However, the higher rates of obesity may predispose Black patients to the development of certain malignancies. A comparison of male US veterans found that Black veterans were at a significantly elevated risk for cancers of the colon, prostate, thyroid, extrahepatic bile duct, and various hematologic malignancies.168 Similarly, in women, obesity has been linked to the development of triple-negative breast cancer, a subtype well-documented to disproportionately affect Black women.169 An analysis of the National Surgical Adjuvant Breast and Bowel Project data demonstrated that compared with their White peers, Black women with ER− breast cancer had a less favorable prognosis with poorer disease-free survival and a higher risk of non-breast cancer death.170 Surprisingly, a protective relationship between BMI and survival in Black patients with NSCLC has been observed in contrast to their White peers. The authors of this study hypothesized that this may relate to the higher lean mass observed in their cohort of Black patients, in contrast to the higher rates of sarcopenic obesity seen in White patients.171
Closely tied to the issue of obesity is the role of physical exercise. Several studies have conclusively demonstrated the protective benefits of physical activity against cancer development.172-174 Unfortunately, Black individuals have been noted to inconsistently engage in leisurely physical activity.175 There are several barriers that lead to these low rates of activity, including intrapersonal barriers such as lack of time, access to healthy outdoor spaces, access and cost of gym memberships, as well as interpersonal barriers such as caregiver responsibilities and lack of social support.176 Additionally, several environmental barriers, including safety concerns and lack of facilities, prevent the appropriate engagement of disenfranchised communities in such activities.176 For instance, neighborhoods with higher percentages of Black subjects have less green space coverage and larger distance to parks, thereby hindering community engagement.177
Alcohol and Smoking
While alcohol is an established risk factor for several malignancies, it has generally not been considered a significant contributor to healthcare disparities. However, recently, a statement from the American Society of Clinical Oncology stated that ‘alcohol drinking may be a contributing factor to cancer disparities’ and supported the conduct of further research into this area.178 Future studies will be required to further delineate this relationship. Conversely, smoking is a well-known lifestyle determinant of many cancers. Approximately 21% of all cancer deaths globally can be linked to smoking.179 Several well-described mechanisms link smoking to various carcinogenic pathways.180 Although smoking prevalence appears to be similar across ethnicities,181 variations in smoking patterns may still influence cancer disparities.182 For instance, Black patients are less likely to receive counseling for smoking cessation,183 and are also more often excluded from community interventions targeted towards smoking cessation.184 Additionally, Black individuals have been noted to have a higher intake of nicotine per cigarette (and potentially tobacco carcinogens), suggesting that they may be more susceptible to the harmful effects of smoking despite consuming a lower number of cigarettes per day.9,185 As a result, smoking significantly contributes to the Black–White gap in life expectancy above the age of 50 years.186 Additionally, Black workers are far more likely to experience environmental or workplace exposure to secondhand smoke.187 Approximately 7/10 children between the ages of 3 and 11 years in Black households also face exposure to secondhand smoke.188 This early exposure can precipitate a cascade of health-related issues; therefore, tobacco control and reforms aimed at reducing exposure represent a critical avenue for combatting cancer disparities.
Environment and Neighborhood
Lastly, we considered the influence of local environments and residential neighborhoods on cancer disparities. The connections between where we live and the significant influences these portend on our health are becoming increasingly recognized. Several of the causative agents that were outlined earlier are directly affected by an individual’s residence. For instance, dietary factors can be traced to the quality and availability of local groceries. Similarly, the ability to engage in physical exercise ties directly with the presence or absence of recreational facilities. A review of the California Cancer Registry showed that neighborhood SES was a crucial determinant for all types of cancer and in particular for minorities such as Black subjects.11 A similar review performed in Ohio found that estimates of neighborhood conditions derived from census data strongly influenced the development of lung cancer, despite controlling for individual variables.189
De la Roca et al. noted that despite the decline in ethnic segregation, many neighborhoods continue to experience high levels of separation, with Black subjects continuing to reside in more disadvantaged neighborhoods.190 This results in a clear barrier to healthcare access, leading to a variety of healthcare-related disparities. For instance, lower rates of CRC screening are evident in segregated communities.191. Similarly, individuals in Florida, residing in more segregated communities, were less likely to receive surgery for treatment of lung cancer.192 A systematic review by Landrine et al. examining this association in breast cancer found that of the 17 articles that met their inclusion criteria, 70% indicated in their analyses that neighborhood segregation contributed to cancer and racial cancer disparities.193 This new understanding has led to the development of a “place-based approach towards health, where community needs are prioritized thereby focusing on ‘upstream drivers of health outcomes’”.194 Such strategies will be vital in addressing cancer disparities.
CONCLUSION
Despite significant advancements in the screening and treatment of breast, colorectal, and lung cancers, Black patients continue to present with advanced stages of cancer and face challenges in accessing treatment. Much research has been conducted in the last decade to increase the understanding of the multifactorial nature of disparities in cancer care, and we have learned that discrepancies in incidence, diagnosis, and outcomes result from a complex interplay of individual behavior, SES, access to healthcare, and tumor biology. As health equity moves to the forefront of national discourse, future studies should utilize multidimensional frameworks that integrate genetic ancestry, social determinants of health, behavior, healthcare access, structural inequalities and systemic racism to better define and address these persistent disparities.
DISCLOSURES
Samilia Obeng-Gyasi is funded by the Paul Calabresi Career Development Award (K12 CA133250). Wasay Nizam, Heather L. Yeo, Malcolm V. Brock, and Fabian M. Johnston have no disclosures to declare.
REFERENCES
- 1.Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211–33. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute. SEER cancer stat facts: cancer disparities [cited 13 Mar 2021]. https://seer.cancer.gov/statfacts/html/disparities.html.
- 5.Smith CJ, Minas TZ, Ambs S. Analysis of tumor biology to advance cancer health disparity research. Am J Pathol. 2018;188(2):304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zavala VA, Bracci PM, Carethers JM, Carvajal-Carmona L, Coggins NB, Cruz-Correa MR, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021;124(2):315–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Cancer Institute. SEER cancer facts SEER.
- 8.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 9.Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333–42. [DOI] [PubMed] [Google Scholar]
- 10.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78–93. [DOI] [PubMed] [Google Scholar]
- 11.Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong Y-N, Edge SB, et al. Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol. 2015;33(20):2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. 2015;313(2):165–73. [DOI] [PubMed] [Google Scholar]
- 14.Wu AH, Gomez SL, Vigen C, Kwan ML, Keegan TH, Lu Y, et al. The California Breast Cancer Survivorship Consortium (CBCSC): prognostic factors associated with racial/ethnic differences in breast cancer survival. Cancer Causes Control. 2013;24(10):1821–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao F, Copley B, Niu Q, Liu F, Johnson JA, Sutton T, et al. Racial disparities in survival outcomes among breast cancer patients by molecular subtypes. Breast Cancer Res Treat. 2021;185(3):841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma H, Lu Y, Malone KE, Marchbanks PA, Deapen DM, Spirtas R, et al. Mortality risk of black women and white women with invasive breast cancer by hormone receptors, HER2, and p53 status. BMC Cancer. 2013;13(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silber JH, Rosenbaum PR, Clark AS, Giantonio BJ, Ross RN, Teng Y, et al. Characteristics associated with differences in survival among Black and White women with breast cancer. JAMA. 2013;310(4):389–97. [DOI] [PubMed] [Google Scholar]
- 18.Rauscher GH, Silva A, Pauls H, Frasor J, Bonini MG, Hoskins K. Racial disparity in survival from estrogen and progesterone receptor-positive breast cancer: implications for reducing breast cancer mortality disparities. Breast Cancer Res Treat. 2017;163(2):321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder RA, Hu C-Y, Zafar SN, Francescatti A, Chang GJ. Racial disparities in recurrence and overall survival in patients with locoregional colorectal cancer. J Natl Cancer Inst. 2021;113(6):770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White A, Joseph D, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001–2009): findings from the CONCORD-2 study. Cancer. 2017;123:5014–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai Y, Wang C, Civan JM, Palazzo JP, Ye Z, Hyslop T, et al. Effects of cancer stage and treatment differences on racial disparities in survival from colon cancer: a United States population-based study. Gastroenterology. 2016;150(5):1135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silber JH, Rosenbaum PR, Ross RN, Niknam BA, Ludwig JM, Wang W, et al. Racial disparities in colon cancer survival: a matched cohort study. Ann Intern Med. 2014;161(12):845–54. [DOI] [PubMed] [Google Scholar]
- 23.Valeri L, Chen JT, Garcia-Albeniz X, Krieger N, VanderWeele TJ, Coull BA. The role of stage at diagnosis in colorectal cancer black–white survival disparities: a counterfactual causal inference approach. Cancer Epidemiol Prev Biomark. 2016;25(1):83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan C, Lopez A, Castaneda G, Bhuket T, Liu B, Yee S, et al. Black patients with colorectal cancer have more advanced cancer stage at time of diagnosis: a community-based safety-net hospital experience. J Commun Health. 2017;42(4):724–9. [DOI] [PubMed] [Google Scholar]
- 25.Parise CA, Bauer KR, Caggiano V. Variation in breast cancer subtypes with age and race/ethnicity. Crit Rev Oncol Hematol. 2010;76(1):44–52. [DOI] [PubMed] [Google Scholar]
- 26.Tao L, Gomez SL, Keegan TH, Kurian AW, Clarke CA. Breast cancer mortality in African-American and non-Hispanic white women by molecular subtype and stage at diagnosis: a population-based study. Cancer Epidemiol Prev Biomark.2015;24(7):1039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guda K, Veigl ML, Varadan V, Nosrati A, Ravi L, Lutterbaugh J, et al. Novel recurrently mutated genes in African American colon cancers. Proc Natl Acad Sci USA. 2015;112(4):1149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li E, Ji P, Ouyang N, Zhang Y, Wang XY, Rubin DC, et al. Differential expression of miRNAs in colon cancer between African and Caucasian Americans: implications for cancer racial health disparities. Int J Oncol. 2014;45(2):587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curran T, Sun Z, Gerry B, Findlay VJ, Wallace K, Li Z, et al. Differential immune signatures in the tumor microenvironment are associated with colon cancer racial disparities. Cancer Med. 2021;10(5):1805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace K, Lewin DN, Sun S, Spiceland CM, Rockey DC, Alekseyenko AV, et al. Tumor-infiltrating lymphocytes and colorectal cancer survival in African American and Caucasian patients. Cancer Epidemiol Prev Biomark. 2018;27(7):755–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jovov B, Araujo-Perez F, Sigel CS, Stratford JK, McCoy AN, Yeh JJ, et al. Differential gene expression between African American and European American colorectal cancer patients. PLoS ONE. 2012;7(1):e30168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C, Zhang C, Wang Q, Li Z, Lin J, Wang H. Differences in stage of cancer at diagnosis, treatment, and survival by race and ethnicity among leading cancer types. JAMA Netw Open. 2020;3(4):e202950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robbins HA, Engels EA, Pfeiffer RM, Shiels MS. Age at cancer diagnosis for blacks compared with whites in the United States. J Natl Cancer Inst. 2015;107(3):diu489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aizer AA, Wilhite TJ, Chen M-H, Graham PL, Choueiri TK, Hoffman KE, et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120(10):1532–9. [DOI] [PubMed] [Google Scholar]
- 35.Obeng-Gyasi S, Timsina L, Bhattacharyya O, Fisher CS, Haggstrom DA. Breast cancer presentation, surgical management and mortality across the rural–urban continuum in the national cancer database. Ann Surg Oncol. 2020;27(6):1805–15. [DOI] [PubMed] [Google Scholar]
- 36.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222–31. [DOI] [PubMed] [Google Scholar]
- 37.America Cancer Society. Cancer facts & figures for African-Americans 2019–2021. American Cancer Society; 2019. [Google Scholar]
- 38.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–51. [DOI] [PubMed] [Google Scholar]
- 39.Stapleton SM, Oseni TO, Bababekov YJ, Hung Y-C, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153(6):594–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15(3):408–14. [DOI] [PubMed] [Google Scholar]
- 41.Daly B, Olopade OI. A perfect storm: how tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65(3):221–38. [DOI] [PubMed] [Google Scholar]
- 42.Jackson DK, Li Y, Eskander MF, Tsung A, Oppong BA, Bhattacharyya O, et al. Racial disparities in low-value surgical care and time to surgery in high-volume hospitals. J Surg Oncol. 2021;123(2):676–86. [DOI] [PubMed] [Google Scholar]
- 43.Bleicher RJ, Ruth K, Sigurdson ER, Beck JR, Ross E, Wong Y-N, et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2(3):330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Restrepo DJ, Boczar D, Huayllani MT, Sisti A, Gabriel E, McLaughlin SA, et al. Influence of race, income, insurance, and education on the rate of breast reconstruction. Anticancer Res. 2019;39(6):2969–73. [DOI] [PubMed] [Google Scholar]
- 45.Relation T, Obeng-Gyasi S, Bhattacharyya O, Li Y, Eskander MF, Tsung A, et al. Racial differences in response to neoadjuvant chemotherapy: impact on breast and axillary surgical management. Ann Surg Oncol. 2021. 10.1245/s10434-021-09657-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du XL, Gor BJ. Racial disparities and trends in radiation therapy after breast-conserving surgery for early-stage breast cancer in women, 1992 to 2002. Ethn Dis. 2007;17(1):122. [PMC free article] [PubMed] [Google Scholar]
- 47.Parise CA, Bauer KR, Caggiano V. Disparities in receipt of adjuvant radiation therapy after breast-conserving surgery among the cancer-reporting regions of California. Cancer. 2012;118(9):2516–24. [DOI] [PubMed] [Google Scholar]
- 48.Wheeler SB, Carpenter WR, Peppercorn J, Schenck AP, Weinberger M, Biddle AK. Structural/organizational characteristics of health services partly explain racial variation in timeliness of radiation therapy among elderly breast cancer patients. Breast Cancer Res Treat. 2012;133(1):333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butler PD, Familusi O, Serletti JM, Fox JP. Influence of race, insurance status, and geographic access to plastic surgeons on immediate breast reconstruction rates. Am J Surg. 2018;215(6):987–94. [DOI] [PubMed] [Google Scholar]
- 50.Reeder A, Attar M, Nazario L, Bathula C, Zhang A, Hochbaum D, et al. Stress hormones reduce the efficacy of paclitaxel in triple negative breast cancer through induction of DNA damage. BrJ Cancer. 2015;112(9):1461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol. 2016;2(3):322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Griggs JJ, Sorbero ME, Stark AT, Heininger SE, Dick AW. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003;81(1):21–31. [DOI] [PubMed] [Google Scholar]
- 53.Schneider BP, Li L, Radovich M, Shen F, Miller KD, Flockhart DA, et al. Genome-wide association studies for taxane-induced peripheral neuropathy in ECOG-5103 and ECOG-1199. Clin Cancer Res. 2015;21(22):5082–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reeder-Hayes K, Hinton SP, Meng K, Carey LA, Dusetzina SB. Disparities in use of human epidermal growth hormone receptor 2-targeted therapy for early-stage breast cancer. J Clin Oncol. 2016;34(17):2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11(12):1135–41. [DOI] [PubMed] [Google Scholar]
- 56.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339(22):1609–18. [DOI] [PubMed] [Google Scholar]
- 57.Roberts MC, Wheeler SB, Reeder-Hayes K. Racial/ethnic and socioeconomic disparities in endocrine therapy adherence in breast cancer: a systematic review. Am J Public Health. 2015;105(Suppl 3):e4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Albain KS, Gray RJ, Makower DF, Faghih A, Hayes DF, Geyer CE, et al. Race, ethnicity, and clinical outcomes in hormone receptor-positive, HER2-negative, node-negative breast cancer in the randomized TAILORx trial. J Natl Cancer Inst. 2021;113(4):390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sadigh G, Gray RJ, Sparano JA, Yanez B, Garcia SF, Timsina LR, et al. Breast cancer patients’ insurance status and residence zip code correlate with early discontinuation of endocrine therapy: an analysis of the ECOG-ACRIN TAILORx trial. Cancer. 2021. 10.1002/cncr.33527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Makower D, Lin J, Xue X, Sparano JA. Lymphovascular invasion, race, and the 21-gene recurrence score in early estrogen receptor-positive breast cancer. NPJ Breast Cancer. 2021;7(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoskins KF, Danciu OC, Ko NY, Calip GS. Association of race/ethnicity and the 21-gene recurrence score with breast cancer-specific mortality among US women. JAMA Oncol. 2021;7(3):370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2008. Am J Gasterenterol. 2009;104(3):739–50. [DOI] [PubMed] [Google Scholar]
- 63.National Center for Health Statistics. National Health Survery, 2015. Public-use data file and documentation. 2015. [cited 20 Mar 2021]. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fnchs%2Fnhis%2Fquest_data_related_1997_forward.html.
- 64.National Center for Health Statistics. National health interview survery, 2000 [cited 20 Mar 2021]. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fnchs%2Fnhis%2Fquest_data_related_1997_forward.htm.
- 65.Jackson CS, Oman M, Patel AM, Vega KJ. Health disparities in colorectal cancer among racial and ethnic minorities in the United States. J Gastrointest Oncol. 2016;7(Suppl 1):S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andersen SW, Blot WJ, Lipworth L, Steinwandel M, Murff HJ, Zheng W. Association of race and socioeconomic status with colorectal cancer screening, colorectal cancer risk, and mortality in southern US adults. JAMA Netw Open. 2019;2(12):e1917995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halbert CH, Armstrong K, Gandy OH, Shaker L. Racial differences in trust in health care providers. Arch Intern Med. 2006;166(8):896–901. [DOI] [PubMed] [Google Scholar]
- 68.Khankari K, Eder M, Osborn CY, Makoul G, Clayman M, Skripkauskas S, et al. Improving colorectal cancer screening among the medically underserved: a pilot study within a federally qualified health center. J Gen Intern Med. 2007;22(10):1410–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naylor K, Ward J, Polite BN. Interventions to improve care related to colorectal cancer among racial and ethnic minorities: a systematic review. J Gen Intern Med. 2012;27(8):1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tramontano AC, Chen Y, Watson TR, Eckel A, Hur C, Kong CY. Racial/ethnic disparities in colorectal cancer treatment utilization and phase-specific costs, 2000–2014. PLoS ONE. 2020;15(4):e0231599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sineshaw HM, Ng K, Flanders WD, Brawley OW, Jemal A. Factors that contribute to differences in survival of black vs white patients with colorectal cancer. Gastroenterology. 2018;154(4):906–915.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gill AA, Enewold L, Zahm SH, Shriver CD, Stojadinovic A, McGlynn KA, et al. Colon cancer treatment: are there racial disparities in an equal-access healthcare system? Dis Colon Rectum. 2014;57(9):1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Breslin TM, Morris AM, Gu N, Wong SL, Finlayson EV, Banerjee M, et al. Hospital factors and racial disparities in mortality after surgery for breast and colon cancer. J Clin Oncol. 2009;27(24):3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eaglehouse YL, Georg MW, Shriver CD, Zhu K. Racial comparisons in timeliness of colon cancer treatment in an equal-access health system. J Natl Cancer Inst. 2020;112(4):410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rhoads KF, Patel MI, Ma Y, Schmidt LA. How do integrated health care systems address racial and ethnic disparities in colon cancer? J Clin Oncol. 2015;33(8):854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laryea JA, Siegel E, Klimberg S. Racial disparity in colorectal cancer: the role of equal treatment. Dis Colon Rectum. 2014;57(3):295–302. [DOI] [PubMed] [Google Scholar]
- 77.National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haddad DN, Sandler KL, Henderson LM, Rivera MP, Aldrich MC. Disparities in lung cancer screening: a review. Ann Am Thorac Soc. 2020;17(4):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Annangi S, Nutalapati S, Foreman MG, Pillai R, Flenaugh EL. Potential racial disparities using current lung cancer screening guidelines. J Racial Ethn Health Disparities. 2019;6(1):22–6. [DOI] [PubMed] [Google Scholar]
- 80.Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, et al. Screening for lung cancer: US preventive services task force recommendation statement. JAMA. 2021;325(10):962–70. [DOI] [PubMed] [Google Scholar]
- 81.Wolf A, Alpert N, Tran BV, Liu B, Flores R, Taioli E. Persistence of racial disparities in early-stage lung cancer treatment. J Thorac Cardiovasc Surg. 2019;157(4):1670–1679.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341(16):1198–205. [DOI] [PubMed] [Google Scholar]
- 83.Taioli E, Flores R. Appropriateness of surgical approach in black patients with lung cancer—15 years later, little has changed. J Thorac Oncol. 2017;12(3):573–7. [DOI] [PubMed] [Google Scholar]
- 84.Fang P, He W, Gomez D, Hoffman KE, Smith BD, Giordano SH, et al. Racial disparities in guideline-concordant cancer care and mortality in the United States. Adv Radiat Oncol. 2018;3(3):221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin JJ, Mhango G, Wall MM, Lurslurchachai L, Bond KT, Nelson JE, et al. Cultural factors associated with racial disparities in lung cancer care. Ann Am Thorac Soc. 2014;11(4):489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palmer JR, Polley EC, Hu C, John EM, Haiman C, Hart SN, et al. Contribution of germline predisposition gene mutations to breast cancer risk in African American women. JNCI J Natl Cancer Inst. 2020;112(12):1213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng Y, Zhang J, Niu Q, Huo D, Olopade OI. Novel germline PALB2 truncating mutations in African American breast cancer patients. Cancer. 2012;118(5):1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang S, Pitt JJ, Zheng Y, Yoshimatsu TF, Gao G, Sanni A, et al. Germline variants and somatic mutation signatures of breast cancer across populations of African and European ancestry in the US and Nigeria. Int J Cancer. 2019;145(12):3321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jones CC, Bush WS, Crawford DC, Wenzlaff AS, Schwartz AG, Wiencke JK, et al. Germline genetic variants and lung cancer survival in African Americans. Cancer Epidemiol Biomark Prev. 2017;26(8):1288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schenk A, López S, Kschischo M, McGranahan N. Germline ancestry influences the evolutionary disease course in lung adenocarcinomas. Evol Appl. 2020;13(7):1550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Campbell JD, Lathan C, Sholl L, Ducar M, Vega M, Sunkavalli A, et al. Comparison of prevalence and types of mutations in lung cancers among Black and White populations. JAMA Oncol. 2017;3(6):801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hebbring SJ, Adjei AA, Baer JL, Jenkins GD, Zhang J, Cunningham JM, et al. Human SULT1A1 gene: copy number differences and functional implications. Hum Mol Genet. 2007;16(5):463–70. [DOI] [PubMed] [Google Scholar]
- 93.Weber TK, Chin H-M, Rodriguez-Bigas M, Keitz B, Gilligan R, O’Malley L, et al. Novel hMLH1 and hMSH2 germline mutations in African Americans with colorectal cancer. JAMA. 1999;281(24):2316–20. [DOI] [PubMed] [Google Scholar]
- 94.Guindalini RSC, Win AK, Gulden C, Lindor NM, Newcomb PA, Haile RW, et al. Mutation spectrum and risk of colorectal cancer in African American families with Lynch syndrome. Gastroenterology. 2015;149(6):1446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun X, Chandar AK, Canto MI, Thota PN, Brock M, Shaheen NJ, et al. Genomic regions associated with susceptibility to Barrett’s esophagus and esophageal adenocarcinoma in African Americans: the cross BETRNet admixture study. PloS One. 2017;12(10):e0184962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bryc K, Durand EY, Macpherson JM, Reich D, Mountain JL. The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet. 2015;96(1):37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mohammed SI, Springfield S, Das R. Role of epigenetics in cancer health disparities. Methods Mol Biol. 2012;863:395–410. [DOI] [PubMed] [Google Scholar]
- 98.Ahmad A, Azim S, Zubair H, Khan MA, Singh S, Carter JE, et al. Epigenetic basis of cancer health disparities: looking beyond genetic differences. Biochim Biophys Acta-Rev Cancer. 2017;1868(1):16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lerner L, Winn R, Hulbert A. Lung cancer early detection and health disparities: the intersection of epigenetics and ethnicity. J Thorac Dis. 2018;10(4):2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thayer ZM, Kuzawa CW. Biological memories of past environments: epigenetic pathways to health disparities. Epigenetics. 2011;6(7):798–803. [DOI] [PubMed] [Google Scholar]
- 101.Carlson ED, Chamberlain RM. Allostatic load and health disparities: a theoretical orientation. Res Nurs Health. 2005;28(4):306–15. [DOI] [PubMed] [Google Scholar]
- 102.Rodriquez EJ, Kim EN, Sumner AE, Nápoles AM, Pérez-Stable EJ. Allostatic load: Importance, markers, and score determination in minority and disparity populations. J Urban Health. 2019;96(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Olden K, Lin Y-S, Bussard D. Epigenome: a biomarker or screening tool to evaluate health impact of cumulative exposure to chemical and non-chemical stressors. Biosensors. 2016;6(2):12. [Google Scholar]
- 104.Cunliffe VT. The epigenetic impacts of social stress: how does social adversity become biologically embedded? Epigenomics. 2016;8(12):1653–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–28. [DOI] [PubMed] [Google Scholar]
- 106.Grønbaek K, Hother C, Jones PA. Epigenetic changes in cancer. Apmis. 2007;115(10):1039–59. [DOI] [PubMed] [Google Scholar]
- 107.Lara OD, Wang Y, Asare A, Xu T, Chiu H-S, Liu Y, et al. Pan-cancer clinical and molecular analysis of racial disparities. Cancer. 2020;126(4):800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nesset KA, Perri AM, Mueller CR. Frequent promoter hypermethylation and expression reduction of the glucocorticoid receptor gene in breast tumors. Epigenetics. 2014;9(6):851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ambrosone CB, Young AC, Sucheston LE, Wang D, Li Y, Liu S, et al. Genome-wide methylation patterns provide insight into differences in breast tumor biology between American women of African and European ancestry. Oncotarget. 2014;5(1):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mehrotra A, Liu H, Adams JL, Wang MC, Lave JR, Thygeson NM, et al. Comparing costs and quality of care at retail clinics with that of other medical settings for 3 common illnesses. Ann Intern Med. 2009;151(5):321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mokarram P, Kumar K, Brim H, Naghibalhossaini F, Saberifiroozi M, Nouraie M, et al. Distinct high-profile methylated genes in colorectal cancer. PLoS ONE. 2009;4(9):e7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kumar K, Brim H, Giardiello F, Smoot DT, Nouraie M, Lee EL, et al. Distinct BRAF (V600E) and KRAS mutations in high microsatellite instability sporadic colorectal cancer in African Americans. Clin Cancer Res. 2009;15(4):1155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huo D, Hu H, Rhie SK, Gamazon ER, Cherniack AD, Liu J, et al. Comparison of breast cancer molecular features and survival by African and European Ancestry in The Cancer Genome Atlas. JAMA Oncol. 2017;3(12):1654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Charan M, Verma AK, Hussain S, Misri S, Mishra S, Majumder S, et al. Molecular and cellular factors associated with racial disparity in breast cancer. Int J Mol Sci. 2020;21(16):5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saini G, Ogden A, McCullough LE, Torres M, Rida P, Aneja R. Disadvantaged neighborhoods and racial disparity in breast cancer outcomes: the biological link. Cancer Causes Control. 2019;30(7):677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hardy TM, Tollefsbol TO. Epigenetic diet: impact on the epigenome and cancer. Epigenomics. 2011;3(4):503–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sharavanan VJ, Sivaramakrishnan M, Sivarajasekar N, Senthilrani N, Kothandan R, Dhakal N, et al. Pollutants inducing epigenetic changes and diseases. Environ Chem Lett. 2020;18(2):325–43. [Google Scholar]
- 118.Vidal AC, Neelon SEB, Liu Y, Tuli AM, Fuemmeler BF, Hoyo C, et al. Maternal stress, preterm birth, and DNA methylation at imprint regulatory sequences in humans. Genet Epigenetics. 2014;6:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoare J, Stein DJ, Heany SJ, Fouche J-P, Phillips N, Er S, et al. Accelerated epigenetic aging in adolescents from low-income households is associated with altered development of brain structures. Metab Brain Dis. 2020;35(8):1287–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pasculli B, Barbano R, Parrella P. Epigenetics of breast cancer: biology and clinical implication in the era of precision medicine. Semin Cancer Biol. 2018;51:22–35. [DOI] [PubMed] [Google Scholar]
- 121.Al Hadidi S, Mims M, Miller-Chism CN, Kamble R. Participation of African American persons in clinical trials supporting US food and drug administration approval of cancer drugs. Ann Intern Med. 2020;173(4):320–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153(18):2093–101. [PubMed] [Google Scholar]
- 123.Kinlein SA, Wilson CD, Karatsoreos IN. Dysregulated hypothalamic–pituitary–adrenal axis function contributes to altered endocrine and neurobehavioral responses to acute stress. Front Psychiatry. 2015;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation—allostatic load and its health consequences: MacArthur studies of successful aging. Arch Intern Med. 1997;157(19):2259–68. [PubMed] [Google Scholar]
- 125.Mathew A, Doorenbos AZ, Li H, Jang MK, Park CG, Bronas UG. Allostatic load in cancer: a systematic review and mini meta-analysis. Biol Res Nurs. 2020. 10.1177/1099800420969898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Parente V, Hale L, Palermo T. Association between breast cancer and allostatic load by race: National Health and Nutrition Examination Survey 1999–2008. Psychooncology. 2013;22(3):621–8. [DOI] [PubMed] [Google Scholar]
- 127.Akinyemiju T, Wilson LE, Deveaux A, Aslibekyan S, Cushman M, Gilchrist S, et al. Association of allostatic load with all-cause and cancer mortality by race and body mass index in theREGARDS cohort. Cancers (Basel). 2020;12(6):1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13(11):800–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morgan XC, Huttenhower C. Human microbiome analysis. PLoS Comput Biol. 2012;8(12):e1002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Malard F, Dore J, Gaugler B, Mohty M. Introduction to host microbiome symbiosis in health and disease. Mucosal Immunol. 2021;14(3):547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med. 2018;24(4):392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bultman SJ. Emerging roles of the microbiome in cancer. Carcinogenesis. 2014;35(2):249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hester CM, Jala VR, Langille MG, Umar S, Greiner KA, Haribabu B. Fecal microbes, short chain fatty acids, and colorectal cancer across racial/ethnic groups. World J Gastroenterol. 2015;21(9):2759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sengupta S, Muir JG, Gibson PR. Does butyrate protect from colorectal cancer? J Gastroenterol Hepatol. 2006;21(1):209–18. [DOI] [PubMed] [Google Scholar]
- 135.Gonçalves P, Martel F. Butyrate and colorectal cancer: the role of butyrate transport. Curr Drug Metab. 2013;14(9):994–1008. [DOI] [PubMed] [Google Scholar]
- 136.Farhana L, Antaki F, Murshed F, Mahmud H, Judd SL, Nangia-Makker P, et al. Gut microbiome profiling and colorectal cancer in African Americans and Caucasian Americans. World J Gastrointest Pathophysiol. 2018;9(2):47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shang F-M, Liu H-L. Fusobacterium nucleatum and colorectal cancer: a review. World J Gastrointest Oncol. 2018;10(3):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170(3):548–563.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65(12):1973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thyagarajan S, Zhang Y, Thapa S, Allen MS, Phillips N, Chaudhary P, et al. Comparative analysis of racial differences in breast tumor microbiome. Sci Rep. 2020;10(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Smith A, Pierre JF, Makowski L, Tolley E, Lyn-Cook B, Lu L, et al. Distinct microbial communities that differ by race, stage, or breast-tumor subtype in breast tissues of non-Hispanic Black and non-Hispanic White women. Sci Rep. 2019;9(1):11940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu S, Powers S, Zhu W, Hannun YA. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529(7584):43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Spring B, King AC, Pagoto SL, Van Horn L, Fisher JD. Fostering multiple healthy lifestyle behaviors for primary prevention of cancer. Am Psychol. 2015;70(2):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138(6):2029–2043.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bellanger M, Barry K, Rana J, Regnaux J-P. Cost-effectiveness of lifestyle-related interventions for the primary prevention of breast cancer: a rapid review. Front Med. 2020;6:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gonzalez CA. Nutrition and cancer: the current epidemiological evidence. Br J Nutr. 2006;96(Suppl 1):S42–5. [DOI] [PubMed] [Google Scholar]
- 147.Donaldson MS. Nutrition and cancer: a review of the evidence for an anti-cancer diet. Nutr J. 2004;3(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Key TJ, Bradbury KE, Perez-Cornago A, Sinha R, Tsilidis KK, Tsugane S. Diet, nutrition, and cancer risk: what do we know and what is the way forward? BMJ. 2020;368:m511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bahr PR. Race and nutrition: an investigation of Black-White differences in health-related nutritional behaviours. Sociol Health Illn. 2007;29(6):831–56. [DOI] [PubMed] [Google Scholar]
- 150.Thompson TL, Singleton CR, Springfield SE, Thorpe RJ Jr, Odoms-Young A. Differences in nutrient intake and diet quality between non-Hispanic black and non-Hispanic white men in the United States. Public Health Rep. 2020;135(3):334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bartley K, Jung M, Yi S. Diet and blood pressure: differences among whites, blacks and Hispanics in New York City 2010. Ethn Dis. 2014;24(2):175–81. [PubMed] [Google Scholar]
- 152.Airhihenbuwa CO, Kumanyika S, Agurs TD, Lowe A, Saunders D, Morssink CB. Cultural aspects of African American eating patterns. Ethn Health. 1996;1(3):245–60. [DOI] [PubMed] [Google Scholar]
- 153.Semmes CE. Racism, health, and post-industrialism: a theory of African-American health. 1996. [Google Scholar]
- 154.Vance KE. Culture, food, and racism: the effects on African American health. 2018. [Google Scholar]
- 155.Hargreaves MK, Schlundt DG, Buchowski MS. Contextual factors influencing the eating behaviours of African American women: a focus group investigation. Ethn Health. 2002;7(3):133–47. [DOI] [PubMed] [Google Scholar]
- 156.Santarelli RL, Pierre F, Corpet DE. Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr Cancer. 2008;60(2):131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rodriguez C, McCullough ML, Mondul AM, Jacobs EJ, Chao A, Patel AV, et al. Meat consumption among Black and White men and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Prev Biomark. 2006;15(2):211–6. [DOI] [PubMed] [Google Scholar]
- 158.Yazici C, Wolf PG, Kim H, Cross T-WL, Vermillion K, Carroll T, et al. Race-dependent association of sulfidogenic bacteria with colorectal cancer. Gut. 2017;66(11):1983–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Storey M, Anderson P. Income and race/ethnicity influence dietary fiber intake and vegetable consumption. Nutr Res. 2014;34(10):844–50. [DOI] [PubMed] [Google Scholar]
- 160.Kunzmann AT, Coleman HG, Huang W-Y, Kitahara CM, Cantwell MM, Berndt SI. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Clin Nutr. 2015;102(4):881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Heaney RP. Low calcium intake among African Americans: effects on bones and body weight. J Nutr. 2006;136(4):1095–8. [DOI] [PubMed] [Google Scholar]
- 162.Yang Q-H, Carter HK, Mulinare J, Berry RJ, Friedman JM, Erickson JD. Race-ethnicity differences in folic acid intake in women of childbearing age in the United States after folic acid fortification: findings from the National Health and Nutrition Examination Survey, 2001–2002. Am J Clin Nutr. 2007;85(5):1409–16. [DOI] [PubMed] [Google Scholar]
- 163.Boggs DA, Palmer JR, Wise LA, Spiegelman D, Stampfer MJ, Adams-Campbell LL, et al. Fruit and vegetable intake in relation to risk of breast cancer in the Black Women’s Health Study. Am J Epidemiol. 2010;172(11):1268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15(6):556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23(38):6365–78. [DOI] [PubMed] [Google Scholar]
- 166.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3(9):565–74. [DOI] [PubMed] [Google Scholar]
- 167.Liu B, Du Y, Wu Y, Snetselaar LG, Wallace RB, Bao W. Trends in obesity and adiposity measures by race or ethnicity among adults in the United States 2011–18: population based study. BMJ. 2021;372:n365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Samanic C, Gridley G, Chow W-H, Lubin J, Hoover RN, Fraumeni JFJ. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15(1):35–43. [DOI] [PubMed] [Google Scholar]
- 169.Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;137(1):307–14. [DOI] [PubMed] [Google Scholar]
- 170.Dignam JJ, Wieand K, Johnson KA, Raich P, Anderson SJ, Somkin C, et al. Effects of obesity and race on prognosis in lymph node-negative, estrogen receptor-negative breast cancer. Breast Cancer Res Treat. 2006;97(3):245–54. [DOI] [PubMed] [Google Scholar]
- 171.Jiang M, Fares AF, Shepshelovich D, Yang P, Christiani D, Zhang J, et al. The relationship between body-mass index and overall survival in non-small cell lung cancer by sex, smoking status, and race: A pooled analysis of 20,937 International lung Cancer consortium (ILCCO) patients. Lung Cancer. 2021;152:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Westerlind KC. Physical activity and cancer prevention—mechanisms. Med Sci Sports Exerc. 2003;35(11):1834–40. [DOI] [PubMed] [Google Scholar]
- 173.Rogers CJ, Colbert LH, Greiner JW, Perkins SN, Hursting SD. Physical activity and cancer prevention. Sports Med. 2008;38(4):271–96. [DOI] [PubMed] [Google Scholar]
- 174.Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 2018;27(1):10–21. [DOI] [PubMed] [Google Scholar]
- 175.August KJ, Sorkin DH. Racial/ethnic disparities in exercise and dietary behaviors of middle-aged and older adults. J Gen Intern Med. 2011;26(3):245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Joseph RP, Ainsworth BE, Keller C, Dodgson JE. Barriers to Physical activity among African American women: an integrative review of the literature. Women Health. 2015;55(6):679–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wen M, Zhang X, Harris CD, Holt JB, Croft JB. Spatial disparities in the distribution of parks and green spaces in the USA. Ann Behav Med. 2013;45(Suppl 1):S18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.LoConte NK, Brewster AM, Kaur JS, Merrill JK, Alberg AJ. Alcohol and cancer: a statement of the American Society of Clinical Oncology. J Clin Oncol. 2018;36(1):83–93. [DOI] [PubMed] [Google Scholar]
- 179.Ezzati M, Henley SJ, Lopez AD, Thun MJ. Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int J Cancer. 2005;116(6):963–71. [DOI] [PubMed] [Google Scholar]
- 180.Hecht SS. Cigarette smoking: cancer risks, carcinogens, and mechanisms. Langenbecks Arch Surg. 2006;391(6):603–13. [DOI] [PubMed] [Google Scholar]
- 181.Agaku IT, King BA, Dube SR. Current cigarette smoking among adults—United States, 2005–2012. MMWR Morb Mortal Wkly Rep. 2014;63(2):29–34. [PMC free article] [PubMed] [Google Scholar]
- 182.Holford TR, Levy DT, Meza R. Comparison of Smoking History Patterns Among African American and White Cohorts in the United States Born 1890 to 1990. Nicotine Tob Res. 2016;18(Suppl 1):S16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hooks-Anderson DR, Salas J, Secrest S, Skiöld-Hanlin S, Scherrer JF. Association between race and receipt of counselling or medication for smoking cessation in primary care. Fam Pract. 2018;35(2):160–5. [DOI] [PubMed] [Google Scholar]
- 184.Hooper MW, Asfar T, Unrod M, Dorsey A, Correa JB, Brandon KO, et al. Reasons for exclusion from a smoking cessation trial: an analysis by race/ethnicity. Ethn Dis. 2019;29(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Perez-Stable EJ, Herrera B, Jacob P III, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280(2):152–6. [DOI] [PubMed] [Google Scholar]
- 186.Ho JY, Elo IT. The contribution of smoking to black-white differences in U.S. mortality. Demography. 2013;50(2):545–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Assari S, Bazargan M. Unequal effects of educational attainment on workplace exposure to second-hand smoke by race and ethnicity; minorities’ diminished returns in the National Health Interview Survey (NHIS). J Med Res Innov. 2019;3(2):e000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Homa DM, Neff LJ, King BA, Caraballo RS, Bunnell RE, Babb SD, et al. Vital signs: disparities in nonsmokers’ exposure to secondhand smoke—United States, 1999–2012. MMWR Morb Mortal Wkly Rep. 2015;64(4):103–8. [PMC free article] [PubMed] [Google Scholar]
- 189.Adie Y, Kats DJ, Tlimat A, Perzynski A, Dalton J, Gunzler D, et al. Neighborhood disadvantage and lung cancer incidence in ever-smokers at a safety net health-care system: a retrospective study. Chest. 2020;157(4):1021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.De la Roca J, Ellen IG, O’Regan KM. Race and neighborhoods in the 21st century: what does segregation mean today? Reg Sci Urban Econ. 2014;47:138–51. [Google Scholar]
- 191.Buehler JW, Castro JC, Cohen S, Zhao Y, Melly S, Moore K. Personal and neighborhood attributes associated with cervical and colorectal cancer screening in an urban African American population. Prev Chronic Dis. 2019;29(16):E118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Johnson AM, Johnson A, Hines RB, Mohammadi R. Neighborhood context and non-small cell lung cancer outcomes in Florida non-elderly patients by race/ethnicity. Lung Cancer. 2020;142:20–7. [DOI] [PubMed] [Google Scholar]
- 193.Landrine H, Corral I, Lee JGL, Efird JT, Hall MB, Bess JJ. Residential segregation and racial cancer disparities: a systematic review. J Racial Ethn Health Disparities. 2017;4(6):1195–205. [DOI] [PubMed] [Google Scholar]
- 194.Amobi A, Plescia M, Alexander-Scott N. The business case for investing in place-based public health initiatives. J Public Health Manag Pract. 2019;25(6):612–5. [DOI] [PubMed] [Google Scholar]