Abstract
We found earlier that deferoxamine (DFO), a drug used for treatment of iron overload, is active against a rat model of Pneumocystis carinii pneumonia (PCP). We had assumed a mode of action by deprivation of nutritional iron; however, data here show that DFO penetrates P. carinii, causing irreversible damage, thus indicating a different mode of action. Penetration was demonstrated by showing DFO uptake by high-pressure liquid chromatography analysis. By using calcein-AM as an indicator, exposure to DFO was shown to cause a reduction in P. carinii cytoplasmic free iron. Exposure to ≥100 μM DFO for ≥8 h in vitro caused growth to cease and cell numbers to decline over several days. This direct and irreversible damage to P. carinii led to the prediction that infrequent delivery of DFO to the lungs via an aerosol would be an effective treatment in the animal model of PCP. This prediction was confirmed by demonstrating that a once-a-week aerosol treatment of rats was 100% effective both as a prophylactic and as a curative treatment in a rat model of PCP.
Pneumocystis carinii is an opportunistic fungus that causes P. carinii pneumonia (PCP), a life-threatening disease in persons with compromised immune systems due to human immunodeficiency virus (HIV) infection or malnourishment as well as treatments for cancer, rheumatic disease, and prevention of transplant rejection. Although there are several drugs and drug combinations available for PCP, all have shortcomings of toxicity, lack of efficacy, or both (30), so that introduction of a new highly effective and well-tolerated anti-P. carinii agent would be a welcome development. We and others previously reported that the iron chelator deferoxamine (DFO), currently used to treat iron overload, is active in animal models of PCP (3, 15, 16, 29). Significantly, the plasma DFO concentration effective in a rat model of PCP is only one-third of that for β-thalassemia patients administered a daily, lifelong DFO dosage to reduce the iron overload resulting from the transfusions required to treat this disease (15).
Our working hypothesis regarding the mechanism of DFO activity against P. carinii had been that DFO deprived P. carinii of the iron required for growth. This hypothesis was supported by the fact that nearly all cells require iron for growth, the only known exceptions being Lactobacillus spp. and, as very recently reported, Borrelia burgdorferi (22). A nonspecific mammalian host response to infection takes advantage of this; during infection, concentrations of the iron binding proteins transferrin and lactoferrin in plasma increase markedly, thus reducing the free iron available to any infectious agent within the host (10). The concept of reducing iron availability to pathogens has been described previously as “nutritional immunity” and was proposed as an approach to discovery of anti-infective drugs (9). Lactoferrin has direct anti-P. carinii activity in vitro when used either as a single agent or in combination with compounds with known anti-PCP activity (2). These authors state that their results support the concept of using iron-chelating agents for therapy of P. carinii infections, in agreement with what had been our working hypothesis for the mechanism of action of DFO against P. carinii. As HIV disease progresses, plasma lactoferrin concentrations decline, and this has been suggested elsewhere as a mechanism underlying susceptibility to opportunistic infections in addition to the immunosuppression caused by loss of CD4 cells (6). These observations were extended in another study that found the lactoferrin decline to be significantly more marked in patients with symptomatic HIV infections than in patients with nonsymptomatic infections, although both were significantly reduced compared to healthy controls (28); these authors reported that oral candidiasis always coincided with low salivary lactoferrin. Analysis of bronchial alveolar fluid by others showed that total iron was six- to sevenfold higher in patients with PCP than in healthy controls and that most of this increase was in non-transferrin-bound iron, i.e., available iron (14); these authors also presented their results as supporting the concept of using iron chelators to treat PCP based on the concept that DFO acts against P. carinii by depriving it of iron. However, none of these correlations proves that DFO acts by restricting iron needed for growth. Even the activity of lactoferrin could be due to other than its iron-chelating ability, as was demonstrated elsewhere for the gut parasite Giardia lamblia (27).
Using an axenic system for culture of P. carinii (18), we tested our hypothesis by exposing P. carinii to DFO for relatively short periods. The reasoning was that transient nutrient deprivation would be expected to slow growth but not to kill cultured P. carinii, so that on relief of the deprivation growth would resume. The results were opposite to our expectation, i.e., upon removal of DFO and restoration of conditions that should allow growth, growth did not resume. This led to a further examination of the interaction of DFO and P. carinii and then to a markedly improved method of DFO administration to an animal model of PCP, resulting in increased efficacy with a reduced dosage.
MATERIALS AND METHODS
Animal model.
The model used for production of P. carinii for in vitro experiments, for inoculation of culture, and for testing of DFO in vivo was as previously detailed (16). Briefly, specific-pathogen-free SD rats from Taconic Farms (Germantown, N.Y.) were housed in a barrier colony, treated with a single injection of penicillin G plus procaine, and maintained on amoxicillin with clavulanic acid. One week after initiation of antibiotic treatment, treatment with 1.5 mg of dexamethasone liter of drinking water−1 began. After an additional week, the rats were inoculated intratracheally with lung homogenate prepared from rats heavily infected with P. carinii. The inoculation was repeated 3 days later. Signs of PCP became apparent 3 to 5 weeks after inoculation. Evaluation of the degree of infection was by microscopic examination of Giemsa-stained smears of lung homogenates, as we have described previously (25). The care and use of all animals used to collect the data reported here were approved by the New York University School of Medicine Institutional Animal Care and Use Committee and monitored by staff veterinarians. Animal use complied with all relevant local and national animal care and use guidelines.
P. carinii.
P. carinii isolated from infected rats (17) was cultured in an axenic system as previously described (18). The isolate used for the in vitro portions of the work had been in culture for over 3 months before the experiments described here were performed. Evaluation of the number of P. carinii organisms in culture depended on a fluorescence assay that we previously described (18).
Analysis of DFO in tissues.
Tissue collection and processing as well as high-pressure liquid chromatography (HPLC) analysis of DFO were performed as previously reported (15). Briefly summarized, this involved collection and homogenization of tissues, addition of ferric chloride to the homogenate to convert DFO to feroxamine (FO, the relatively stable DFO-ferric ion conjugate), and partial purification of FO by solid-phase extraction. For HPLC analysis, a Waters system was used with a 625MS pump, a 996 photodiode array detector, a 715 autosampler, a C8 reverse-phase column (Rainin Instrument Corp., Woburn, Mass.), and an aqueous phosphate buffer-methanol mobile phase. FO detection was based on the absorbance peak of 427.5 nm. Waters Millennium software was used for data collection and analysis. Although DFO is supplied as the mesylate salt, all results are reported in terms of DFO alone.
DFO uptake by P. carinii.
Uptake studies were performed with cultured cells exposed to 100 μM DFO added to the culture medium. At the indicated times, aliquots of the cell suspension were collected, rapidly microcentrifuged, and homogenized by sonication for 10 min (Heat Systems-Ultrasonics, Farmingdale, N.Y.; model W380 at full output and 40% duty cycle). Analysis of the DFO content of the homogenate was performed as for the tissues described above.
Demonstration of changed cytoplasmic iron concentration.
The technique used was based on work by Epsztejn et al. (7) and is dependent on the ability of iron to quench the fluorescence of the vital dye calcein. Cultured cells at a density of 5 × 107 cells ml−1 were washed with NKP buffer (2.68 mM KCl, 1.47 mM KH2PO4, 51.1 mM Na2HPO4, 7.43 mM NaH2PO4, and 62 mM NaCl) and resuspended in the same buffer. The cells were loaded by incubation with 2 μM calcein-AM (acetoxymethyl ester of calcein; Molecular Probes, Eugene, Oreg.) at 37°C for 15 min. Fluorescence was measured by autosampler injection of 70 μl of cell suspension directly into a Waters Model 470 detector using 486 nm for excitation and reading emission at 517 nm. Data were collected with Waters Millennium software.
Aerosol delivery of DFO to rats infected with P. carinii.
An exposure chamber 38 cm (15 in.) long, 25 cm (10 in.) wide, and 13 cm (5 in.) deep was constructed of 64-mm (1/4-in.) acrylic and polycarbonate plastic. Ten individual restraining cages were constructed within the chamber by using plastic guides to hold walls made of hardware cloth, a commonly available type of stiff wire screening with 64-mm (1/4-in.) openings. The tops and sides of the individual restraining cages were of wire screening, and there was a 5-cm space above the tops of the restraining cages and the top of the chamber so that the interior of the exposure chamber was very open, thus avoiding any impediment to the aerosol's penetration of all spaces within the chamber. Aerosol containing drug was generated by a nebulizer and routed to the chamber using standard respiratory tubing which fit tightly over the output tube of the nebulizer and tightly over one of two 5-cm-long, 2.22-cm (7/8-in.)-diameter acrylic tubes mounted into the cover at points centered from the sides and 5 cm from either end. Another length of respiratory tubing was fitted to the other 2.22-cm acrylic tube in the cover to route waste aerosol to a venting system which removed drug residue from the treatment area.
RESULTS
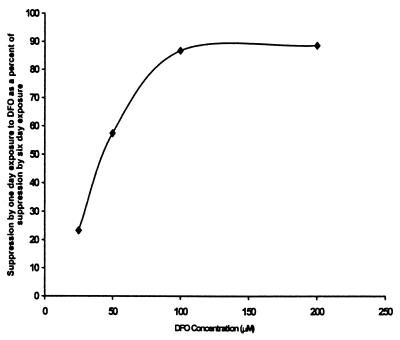
Six-day cultures of P. carinii were performed with DFO included either for the full 6 days or only for the first 24 h. Duplicate cultures were used. At the end of the 6 days, the control cultures contained 2.44 × 107 and 2.74 × 107 nuclei while cultures which included 25, 50, 100, and 200 μM DFO for the full 6 days contained 2.12 × 107 and 1.42 × 107, 1.29 × 107 and 1.20 × 107, 0.78 × 107 and 1.10 × 107, and 0.88 × 107 and 0.80 × 107 nuclei, respectively. Parallel cultures exposed to the same range of DFO concentrations but only for the first 24 h contained 2.20 × 107 and 2.61 × 107, 1.76 × 107 and 1.88 × 107, 1.21 × 107 and 1.11 × 107, and 0.84 × 107 and 1.24 × 107 nuclei, respectively. Figure 1 presents the effect of exposure to DFO for the first 24 h relative to the effect of exposure for the full 6-day culture period for each DFO concentration tested. At the higher concentrations of DFO, 24 h of exposure was nearly equal to 6 days of continuous exposure. In a repeat of this experiment but using a single DFO concentration of 100 μM DFO, shorter exposure periods were tested. At the end of the 6-day period, the control cultures contained 10.01 × 107, 9.96 × 107, and 10.08 × 107 nuclei and cultures exposed for the full 6 days contained 1.75 × 107 and 1.66 × 107 nuclei. Those exposed for only the first 3 h contained 9.50 × 107 and 9.66 × 107 nuclei, and those exposed for 8 h contained 1.79 × 107 and 1.79 × 107 nuclei, respectively; thus, the 3- and 6-h exposures produced 18 and 96% of the suppression, respectively, caused by exposure to the same DFO concentration for the full 6 days. These data suggest that, at an adequate concentration of DFO, short-term treatment causes irreversible damage to P. carinii.
FIG. 1.
Exposure to DFO and growth of P. carinii in culture. Cultures of P. carinii were grown for 6 days with the indicated concentrations of DFO present either for the first 24 h or for the full 6 days. The ordinate scale presents the reduction achieved by a 24-h exposure on the first day of the 6-day culture expressed as a percentage of the reduction achieved by exposure for the full 6 days. At an adequate concentration, exposure to DFO for 24 h was nearly as effective as was exposure for 6 days.
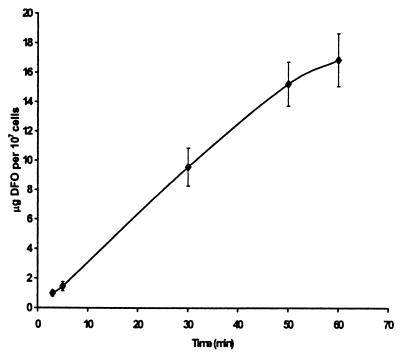
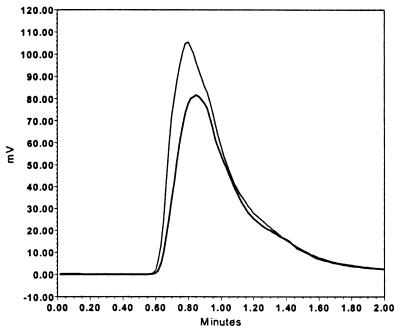
This effect of short-term exposure was unexpected and suggested that, despite being charged and having a mass of 656 Da, DFO might penetrate and act on an internal target in P. carinii. We investigated this possibility by measuring the uptake of DFO by cells exposed to 100 μM DFO for up to 1 h. The results in Fig. 2 show that DFO is transported into P. carinii. Confirmation of transport was obtained by demonstration of iron chelation in the cytoplasm using calcein-AM. This method is based on work by Epsztejn et al. (7) and is dependent on the ability of uncharged and nonfluorescent calcein-AM to penetrate the cell membrane and to be cleaved by cytoplasmic esterase activity, producing charged, fluorescent calcein which is trapped in the cytoplasm. Because calcein fluorescence is quenched by iron, the fluorescence of cells exposed to calcein-AM is inversely related to the concentration of free iron of the cytoplasm. Figure 3 shows the results of one experiment in which cells were treated with 100 μM DFO and then exposed to 2 μM calcein-AM. Control cells were treated the same, except that they were not exposed to DFO. The shape of the curves is due to use of a fluorescence detector-equipped HPLC system without a column and the pump set to produce a flow rate of 1.0 ml min−1; the elution times simply represent the transit time in the plumbing of the instrument. Cells exposed to DFO produced a greater peak than did control cells, thus indicating a reduced concentration of free iron in the cytoplasm. In four independent experiments, integration of the area under the curve produced values of 1.63 × 106 ± 0.18 × 106 for cells pretreated with DFO and 0.95 × 106 ± 0.22 × 106 for control cells. These results confirm that DFO penetrates P. carinii and demonstrate that DFO functions as an iron chelator in the cytoplasm.
FIG. 2.
Uptake of DFO by P. carinii incubated with 100 μM DFO. P. carinii cells were exposed to 100 μM DFO for the indicated times, washed, and analyzed for DFO as described in Materials and Methods. The vertical bars represent standard deviations derived from three independent experiments.
FIG. 3.
Indication of decreased chelatable iron in P. carinii cytoplasm after DFO exposure. This is an example of curves generated from the output of an HPLC fluorescence detector after injection of a suspension of P. carinii cells which had been incubated with the vital dye calcein-AM (see Materials and Methods). The lower tracing was collected from cells exposed only to calcein-AM, and the upper tracing was collected from cells exposed to 100 μM DFO as well as calcein-AM. The increase in internalized calcein fluorescence by DFO pretreatment indicates a reduction in chelatable iron in the cytoplasm of the cells, since ferric ions partially quench calcein fluorescence.
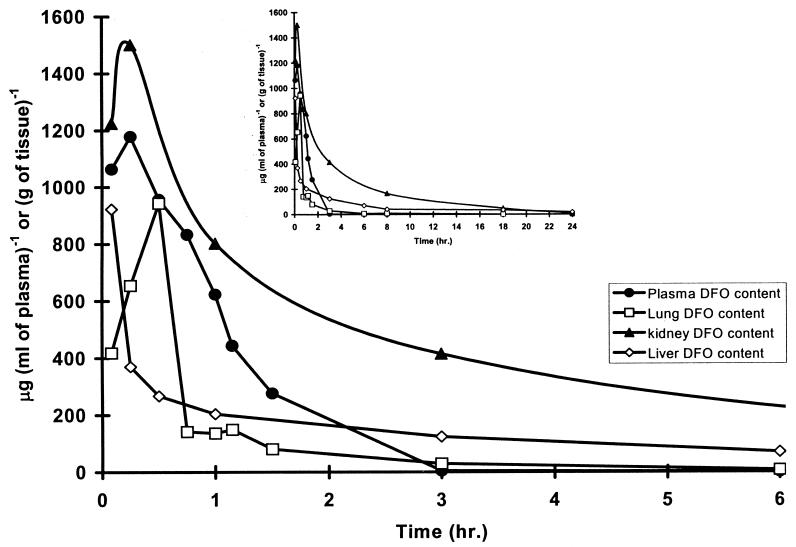
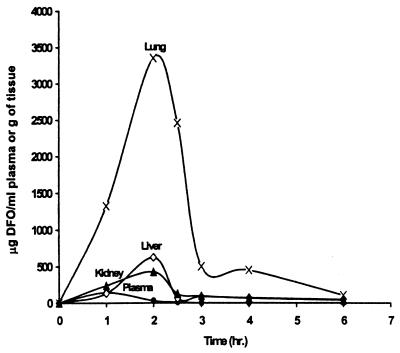
Because DFO has a shorter half-life in rats, 0.21 h (15), than in humans, 3.05 ± 1.30 h (12), we had earlier attempted to improve the dose response in a rat model of PCP by administering DFO by implanted infusion pump (16). The rationale was that, if DFO were operating by depriving P. carinii of the iron required for growth, then a low but constant drug concentration would be much more effective than the high peak and prolonged trough produced by the daily bolus dosage that we used initially. Delivery by infusion did improve the dose response but not to the degree expected. Among the plausible explanations for the unexpected relative effectiveness of bolus compared to continuous infusion dosage was the possibility of a significant difference between the distribution and pharmacokinetics of DFO in lungs and those in plasma. Because we could find no published data on DFO in the lungs, we studied the pharmacokinetics of DFO after bolus injections of 1,000 mg of DFO kg of body weight−1, a dose and route of administration that we had found effective in treating a rat model of PCP when given daily for 21 days (3, 16). We also analyzed plasma, kidney, and liver. The results are shown in Fig. 4. There were rapid rises in all tissues followed by rapid declines, with the drug remaining longest in the kidney and being cleared most rapidly from the plasma and lungs. Thus, neither selective distribution to the lungs nor slower removal from the lungs could account for the relative similarity in dose response for the once-per-day bolus administration and the continuous infusion administration.
FIG. 4.
Kinetics of DFO in tissues after an i.p. bolus injection of 1,000 mg kg−1. Tissue samples were collected at the indicated times after administration of a single dose of DFO. Tissue analysis was performed as described in Materials and Methods. The data in the two sets of curves are the same, with the inset covering the full observation period. One animal was used for each time point.
Considering both the in vitro effect of DFO on P. carinii and the data showing the distribution and elimination of DFO from rats, we thought that the dose response could be improved by direct delivery to the lungs, provided that an adequate drug concentration could be obtained. Furthermore, the in vitro data suggested to us that this need not be done on a daily basis. An AeroTech II nebulizer (CIS-US, Bedford, Mass.) was evaluated for suitability for generation of an aerosol to deliver DFO. Particle sizes generated by this nebulizer were analyzed with a Mercer cascade impactor (Intox Products, Albuquerque, N.Mex.). The nebulizer was loaded with a solution prepared from lyophilized DFO reconstituted with water following the directions of the manufacturer and driven by compressed breathing air regulated at 50 lb/in2. Under these conditions, the nebulizer delivered particles with a mass median aerodynamic diameter of 1.7 μm and a geometric standard deviation of 1.2. Approximately 22% of the aerosol, by mass, was in particles with a diameter less than 0.47 μm, a size judged suitable for alveolar drug deposition in rats. These conditions were used to deliver aerosol to an exposure chamber that we built which held rats in separate, small, wire-enclosed compartments with a large space above the compartments to ensure even distribution of aerosol to all compartments. In order to confirm lung deposition and to select a treatment exposure time, aerosolized DFO was first administered to uninfected rats for times ranging up to 2 h. At selected times, animals were sacrificed and the lungs were harvested for measurement of the delivered dose (Fig. 5). These data show that a 1-h exposure produces a concentration in the lung roughly equal to the maximum concentration in the lung reached after treatment with 1,000 mg kg−1 intraperitoneally (i.p.) (Fig. 4 and 5), an effective dose as noted above. Therefore, we chose a 1-h aerosol exposure for treatment. We also chose to treat only once per week because the effect of DFO on P. carinii was long lasting in the in vitro experiments described above.
FIG. 5.
Kinetics of DFO in tissues of rats treated with aerosolized DFO. Uninfected, nonimmunosuppressed rats were exposed to aerosolized DFO for up to 2 h. The techniques used for generation of the DFO-containing aerosol and exposure of rats are described in the text. Concentrations of DFO in all tissues rose rapidly during exposure and then dropped when exposure ended at 2 h. After a 1-h exposure, the concentration in the lung was approximately equal to the peak concentration in the lung after a bolus i.p. injection of 1,000 mg of DFO kg−1. One animal was used for each time point.
Table 1 shows the results of using this aerosolized DFO protocol as a prophylaxis against PCP and as a treatment for established PCP. Note that all groups initially contained five animals but one control animal died due to PCP. DFO prophylaxis was begun the day after intratracheal inoculation of immunosuppressed rats with P. carinii and was administered once per week for the following 7 weeks. DFO treatment was begun after development of frank signs of PCP (4 weeks after inoculation) and was administered once per week for 3 weeks. The control group was treated the same as the prophylaxis group, except that the nebulizer was filled with 0.85% saline. At the end of the 7- and 3-week treatment periods, the animals were given a final treatment and then sacrificed approximately 24 h later. Thus, the prophylactic treatment group was treated eight times and the treatment group was treated four times. In our experience with this model of PCP, rats of the size used for these experiments and without pneumonia have lung weights very close to 1.0 g whereas animals with PCP have elevated lung weights. Thus, lung weights reported in Table 1 provide an independent confirmation of the lack of pneumonia in the treated groups and demonstrate the similarity in the conditions of the rats in the prophylaxis and treatment groups. Since absolutely no P. carinii organisms could be found in the lungs of rats treated either prophylactically or after disease had developed, both groups had the lung weights of uninfected animals, and Giemsa-stained smears of lung homogenates of both groups were totally free of evidence of the inflammatory reaction to P. carinii, we consider this aerosol regimen to be highly effective.
TABLE 1.
Treatment of a rat model of PCP with aerosolized DFO
| Treatment group | n | Mean lung wt (g) ± SD | No. of P. carinii organisms (1010)/lung (± SD) |
|---|---|---|---|
| Control (no anti-P. carinii treatment) | 4 | 1.56 ± 0.1 | 2.45 ± 0.11 |
| DFO prophylaxis | 5 | 0.97 ± 0.03 | Nondetectable |
| DFO treatment | 5 | 1.03 ± 0.05 | Nondetectable |
DISCUSSION
We began the work reported here with the operating hypothesis that DFO treated an animal model of PCP by chelating iron within the body of the host, thus depriving P. carinii of the iron that it required for growth. This was a logical hypothesis supported by considerable general scientific evidence as described in the introduction. We examined this hypothesis by exposing P. carinii in a continuous axenic culture system to DFO over the relatively short intervals of 3, 8, and 24 h. After the exposure period, the drug was removed and P. carinii was expected to resume growth because the putative deficit in nutritional iron would have been corrected by supplying fresh, drug-free growth medium. The results were contrary to expectations in that growth did not recover if the exposure to DFO was ≥100 μM for ≥8 h. Since an irreversible effect suggested that DFO penetrates P. carinii, we examined this possibility and were able to demonstrate penetration by measuring increasing amounts of DFO in P. carinii with increasing time of exposure to the drug. Experiments with the fluorescent vital dye calcein-AM confirmed the ability of DFO to enter P. carinii cytoplasm; treatment with DFO caused an increase in the fluorescence of cells incubated with calcein-AM, demonstrating transport and indicating that DFO is active as an iron chelator within the cytoplasm of P. carinii.
Although DFO is a large hydrophilic molecule, the ability of this compound to penetrate and damage cells is not limited to P. carinii. DFO is also active against the malaria parasite Plasmodium falciparum, an activity that requires the chelator to penetrate both the host erythrocyte and the parasite membrane (26). Other than the action of DFO being internal, we have no information on the underlying molecular basis of the interaction of DFO and P. carinii, although it most likely is very different from that against the malaria parasite, since continuous exposure to low concentrations of DFO is effective against Plasmodium whereas high intermittent doses of DFO are not effective (23, 24); this is opposite to what we find with P. carinii.
The observed irreversible effect of DFO in our culture system led to the prediction that infrequent administration of an adequate dosage would be effective in vivo. Data in an abstract showing that weekly administration of DFO was effective in a mouse model of PCP (J. R. Boelaert, V. C. Jan, V. G. Frans, and F. Jan, presented at the 9th International Conference on AIDS, Berlin, Germany, 1993) supported this prediction, although, at the time that this appeared, we found it difficult to understand. We examined the efficacy of weekly aerosol delivery of DFO to the lungs of P. carinii-infected rats using an experimental design intended to deliver an amount of DFO to the lungs equivalent to that delivered by an i.p. injection of 1,000 mg kg−1, a dose that we had previously shown to be effective if given every day for 21 days (3, 16). The result was that this once-weekly treatment was completely effective as a prophylactic treatment and was also completely effective as a curative treatment for animals with fully developed PCP at the time of treatment initiation.
Although the number of animals in the treatment and control groups is small, when we found absolutely no P. carinii organisms in the lungs of treated animals and found the lungs to be of normal weight and smears to contain no evidence of the inflammatory cells and exudates characteristic of PCP, we considered this a very clear and conclusive result. In all the studies that we have performed to test the activity of compounds in our rat model of PCP, this is the only study that resulted in finding no P. carinii organisms in the lungs of animals after treatment (1, 3, 4, 16, 19). In control groups treated with the combination of trimethoprim plus sulfamethoxazole, the most effective of current anti-PCP treatments, the number of P. carinii cells in the lungs at the end of treatment was reduced by up to 99% or more compared to the controls, but residual organisms were always easily found, as was some residual evidence of inflammation and exudate.
Inclusion of steroids with specific anti-P. carinii medication for the initial treatment of PCP clearly improves treatment and is now the recommended protocol except for mild cases (11). In spite of HIV-related immunosuppression, the mechanism is thought to be prevention of inflammation, which would otherwise occur in response to antigens released by P. carinii killed by the anti-P. carinii agent. Iron chelation has been reported elsewhere to inhibit inflammation (8, 13, 20, 21), and a specific anti-P. carinii agent which also has anti-inflammatory activity may be particularly useful. Although we have not made a formal study of DFO and inflammation associated with treatment of the rat model of PCP, from our earliest work we have always observed a more rapid resolution of the symptoms of pneumonia in animals treated with DFO than in animals treated with the control treatment, a combination of trimethoprim with sulfamethoxazole. Because clinical trials of DFO as an anti-HIV agent have shown that this drug is tolerated at a relatively high dosage in AIDS patients (5), it is conceivable that low and infrequent dosage would be free of adverse clinical reactions and yet be effective for the treatment of PCP.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants R01 AI38825 (A.B.C.), R01 AI41947 (S.M.), and R01 AI43757 (A.B.C.) from the National Institute of Allergy and Infectious Diseases
REFERENCES
- 1.Chin K, Merali S, Saric M, Clarkson A B., Jr Continuous infusion of dl-α-difluoromethylornithine and improved efficacy against a rat model of Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1996;40:2318–2320. doi: 10.1128/aac.40.10.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cirioni O, Giacometti A, Barchiesi F, Scalise G. Inhibition of growth of Pneumocystis carinii by lactoferrins alone and in combination with pyrimethamine, clarithromycin and minocycline. J Antimicrob Chemother. 2000;46:577–582. doi: 10.1093/jac/46.4.577. [DOI] [PubMed] [Google Scholar]
- 3.Clarkson A B, Jr, Saric M, Grady R W. Deferoxamine and eflornithine (dl-α-difluoromethylornithine) in a rat model of Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1990;34:1833–1835. doi: 10.1128/aac.34.9.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarkson A B, Jr, Williams D E, Rosenberg C. Efficacy of dl-α-difluoromethylornithine in a rat model of Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1988;32:1158–1163. doi: 10.1128/aac.32.8.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costagliola D G, de Montalembert M, Lefrere J J, Briand C, Rebulla P, Baruchel S, Dessi C, Fondu P, Karagiorga M, Perrimond H, et al. Dose of desferrioxamine and evolution of HIV-1 infection in thalassaemic patients. Br J Haematol. 1994;87:849–852. doi: 10.1111/j.1365-2141.1994.tb06750.x. [DOI] [PubMed] [Google Scholar]
- 6.Defer M C, Dugas B, Picard O, Damais C. Impairment of circulating lactoferrin in HIV-1 infection. Cell Mol Biol (Noisy-le-Grand) 1995;41:417–421. [PubMed] [Google Scholar]
- 7.Epsztejn S, Kakhlon O, Glickstein H, Breuer W, Cabantchik I. Fluorescence analysis of the labile iron pool of mammalian cells. Anal Biochem. 1997;248:31–40. doi: 10.1006/abio.1997.2126. [DOI] [PubMed] [Google Scholar]
- 8.Hallaway P E, Hedlund B E. Therapeutic strategies to inhibit iron-catalyzed tissue damage. In: Lauffeu R B, editor. Iron and human disease. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 477–508. [Google Scholar]
- 9.Hershko C. Control of disease by selective iron depletion: a novel therapeutic strategy utilizing iron chelators. Bailliere's Clin Haematol. 1994;7:965–1000. doi: 10.1016/s0950-3536(05)80133-7. [DOI] [PubMed] [Google Scholar]
- 10.Jurado R L. Iron, infections, and anemia of inflammation. Clin Infect Dis. 1997;25:888–895. doi: 10.1086/515549. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs J A, Masur H. Are corticosteroids beneficial as adjunctive therapy for Pneumocystis pneumonia in AIDS? Ann Intern Med. 1990;113:1–3. doi: 10.7326/0003-4819-113-1-1. [DOI] [PubMed] [Google Scholar]
- 12.Lee P, Mohammed N, Marshall L, Abeysinghe R D, Hider R C, Porter J B, Singh S. Intravenous infusion pharmacokinetics of desferrioxamine in thalassaemic patients. Drug Metab Dispos. 1993;21:640–644. [PubMed] [Google Scholar]
- 13.Martelius T, Scholz M, Krogerus L, Hockerstedt K, Loginov R, Bruggeman C, Cinatl J, Doerr H, Lautenschlager I. Antiviral and immunomodulatory effects of desferrioxamine in cytomegalovirus-infected rat liver allografts with rejection. Transplantation. 1999;68:1753–1761. doi: 10.1097/00007890-199912150-00020. [DOI] [PubMed] [Google Scholar]
- 14.Mateos F, Gonzalez C, Dominguez C, Losa J E, Jimenez A, Perez-Arellano J L. Elevated non-transferrin bound iron in the lungs of patients with Pneumocystis carinii pneumonia. J Infect. 1999;38:18–21. doi: 10.1016/s0163-4453(99)90022-1. [DOI] [PubMed] [Google Scholar]
- 15.Merali S, Chin K, Del Angel L, Grady R W, Armstrong M, Clarkson A B., Jr Clinically achievable plasma deferoxamine concentrations are therapeutic in a rat model of Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1995;39:2023–2026. doi: 10.1128/aac.39.9.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merali S, Chin K, Grady R W, Weissberger L, Clarkson A B., Jr Response of rat model of Pneumocystis carinii pneumonia to continuous infusion of deferoxamine. Antimicrob Agents Chemother. 1995;39:1442–1444. doi: 10.1128/aac.39.7.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merali S, Clarkson A B., Jr Polyamine content of Pneumocystis carinii and response to the ornithine decarboxylase inhibitor dl-α-difluoromethylornithine. Antimicrob Agents Chemother. 1996;40:973–978. doi: 10.1128/aac.40.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merali S, Frevert U, Williams J H, Chin K, Bryan R, Clarkson A B. Continuous axenic cultivation of Pneumocystis carinii. Proc Natl Acad Sci USA. 1999;96:2402–2407. doi: 10.1073/pnas.96.5.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merali S, Saric M, Chin K, Clarkson A B., Jr Effect of a bis-benzyl polyamine analogue on Pneumocystis carinii. Antimicrob Agents Chemother. 2000;44:337–343. doi: 10.1128/aac.44.2.337-343.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Brien-Ladner A R, Blumer B M, Wesselius L J. Differential regulation of human alveolar macrophage-derived interleukin-1beta and tumor necrosis factor-alpha by iron. J Lab Clin Med. 1998;132:497–506. doi: 10.1016/s0022-2143(98)90128-7. [DOI] [PubMed] [Google Scholar]
- 21.O'Brien-Ladner A R, Nelson S R, Murphy W J, Blumer B M, Wesselius L J. Iron is a regulatory component of human IL-1beta production. Support for regional variability in the lung. Am J Respir Cell Mol Biol. 2000;23:112–119. doi: 10.1165/ajrcmb.23.1.3736. [DOI] [PubMed] [Google Scholar]
- 22.Posey J E, Gherardini F C. Lack of a role for iron in the Lyme disease pathogen. Science. 2000;288:1651–1653. doi: 10.1126/science.288.5471.1651. [DOI] [PubMed] [Google Scholar]
- 23.Postma N S, Boerman O C, Oyen W J, Zuidema J, Storm G. Absorption and biodistribution of 111indium-labelled desferrioxamine (111In-DFO) after subcutaneous injection of 111In-DFO liposomes. J Control Release. 1999;58:51–60. doi: 10.1016/s0168-3659(98)00139-4. [DOI] [PubMed] [Google Scholar]
- 24.Postma N S, Hermsen C C, Zuidema J, Eling W M. Plasmodium vinckei: optimization of desferrioxamine B delivery in the treatment of murine malaria. Exp Parasitol. 1998;89:323–330. doi: 10.1006/expr.1998.4282. [DOI] [PubMed] [Google Scholar]
- 25.Saric M, Clarkson A B., Jr Ornithine decarboxylase in Pneumocystis carinii and implications for therapy. Antimicrob Agents Chemother. 1994;38:2545–2552. doi: 10.1128/aac.38.11.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott M D, Ranz A, Kuypers F A, Lubin B H, Meshnick S R. Parasite uptake of desferroxamine: a prerequisite for antimalarial activity. Br J Haematol. 1990;75:598–602. doi: 10.1111/j.1365-2141.1990.tb07805.x. [DOI] [PubMed] [Google Scholar]
- 27.Turchany J M, Aley S B, Gillin F D. Giardicidal activity of lactoferrin and N-terminal peptides. Infect Immun. 1995;63:4550–4552. doi: 10.1128/iai.63.11.4550-4552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Strate B W, Harmsen M C, The T H, Sprenger H G, de Vries H, Eikelboom M C, Kuipers M E, Meijer D K, Swart P J. Plasma lactoferrin levels are decreased in end-stage AIDS patients. Viral Immunol. 1999;12:197–203. doi: 10.1089/vim.1999.12.197. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg G A. Iron chelators as therapeutic agents against Pneumocystis carinii. Antimicrob Agents Chemother. 1994;38:997–1003. doi: 10.1128/aac.38.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkin A, Feinberg J. Pneumocystis carinii pneumonia: a clinical review. Am Fam Physician. 1999;60:1699–1708. , 1713–1714. [PubMed] [Google Scholar]