Key Points
Question
Have modifiable risk factors associated with Alzheimer disease and related dementias (ADRDs) changed in the US over the past decade, and do they differ by sex or race and ethnicity?
Findings
In this cross-sectional study, the modifiable risk factors most prominently associated with ADRDs were midlife obesity, physical inactivity, and low education. The proportion of ADRD cases associated with modifiable risk factors was higher in men and in American Indian and Alaska Native individuals, Black individuals, and Hispanic individuals (any race) compared with Asian individuals and White individuals.
Meaning
The findings suggest that the most prominently modifiable risk factors associated with ADRDs changed over the past decade and differed based on sex and race and ethnicity; Alzheimer risk reduction strategies may be more effective if they target higher-risk groups and higher-prevalence risk factors.
This cross-sectional study evaluates modifiable risk factors associated with Alzheimer disease and related dementias in the US and assesses risk differences by sex and race and ethnicity.
Abstract
Importance
Previous estimates suggested that 1 in 3 cases of Alzheimer disease and related dementia (ADRDs) in the US are associated with modifiable risk factors, the most prominent being physical inactivity, depression, and smoking. However, these estimates do not account for changes in risk factor prevalence over the past decade and do not consider potential differences by sex or race and ethnicity.
Objective
To update estimates of the proportion of ADRDs in the US that are associated with modifiable risk factors and to assess for differences by sex and race and ethnicity.
Design, Setting, and Participants
For this cross-sectional study, risk factor prevalence and communality were obtained from the nationally representative US Behavioral Risk Factor Surveillance Survey data from January 2018 to December 2018, and relative risks for each risk factor were extracted from meta-analyses. Data were analyzed from December 2020 to August 2021. Respondents included 378 615 noninstitutionalized adults older than 18 years. The number before exclusion was 402 410. Approximately 23 795 (~6%) had missing values on at least 1 of the variables of interest.
Exposures
Physical inactivity, current smoking, depression, low education, diabetes, midlife obesity, midlife hypertension, and hearing loss.
Main Outcomes and Measures
Individual and combined population-attributable risks (PARs) associated with ADRDs, accounting for nonindependence between risk factors.
Results
Among 378 615 individuals, 171 161 (weighted 48.7%) were male, and 134 693 (weighted 21.1%) were 65 years and older. Race and ethnicity data were self-reported and defined by the US Behavioral Risk Factor Surveillance System Data; 6671 participants (weighted 0.9%) were American Indian and Alaska Native, 8043 (weighted 5.1%) were Asian, 29 956 (weighted 11.7%) were Black, 28 042 (weighted 16.0%) were Hispanic (any race), and 294 394 (weighted 64.3%) were White. Approximately 1 in 3 of ADRD cases (36.9%) in the US were associated with 8 modifiable risk factors, the most prominent of which were midlife obesity (17.7%; 95% CI, 17.5-18.0), physical inactivity (11.8%; 95% CI, 11.7-11.9), and low educational attainment (11.7%; 95% CI, 11.5-12.0). Combined PARs were higher in men (35.9%) than women (30.1%) and differed by race and ethnicity: American Indian and Alaska Native individuals, 39%; Asian individuals, 16%; Black individuals, 40%; Hispanic individuals (any race), 34%; and White individuals, 29%. The most prominent modifiable risk factors regardless of sex were midlife obesity for American Indian and Alaska Native individuals, Black individuals, and White individuals; low education for Hispanic individuals; and physical inactivity for Asian individuals.
Conclusions and Relevance
The findings suggest that risk factors associated with ADRDs have changed over the past decade and differ based on sex and race and ethnicity. Alzheimer risk reduction strategies may be more effective if they target higher-risk groups and consider current risk factor profiles.
Introduction
As the aging of the population increases, there will likely be an accompanying increase in cases of dementia, which represents the primary cause of disability in older adults.1 Alzheimer disease is the most common form of dementia, accounting for 60% to 80% of cases. Alzheimer disease and related dementias (ADRDs) lead to a progressive decline in daily activities and independent living. ADRDs represent a substantial burden to individuals, families, and society and are among the most expensive diseases to manage, costing approximately $305 billion in the US in 2020.2 There is no curative treatment for dementia, and many people discontinue medication because of adverse effects.3 In the absence of disease-modifying treatments, ADRD risk reduction provides an important public health and prevention opportunity for addressing the projected increase in prevalence.
In 2011, we estimated that up to one-third of ADRD cases in the US were associated with 7 modifiable risk factors: physical inactivity, smoking, depression, low education, diabetes, midlife obesity, and midlife hypertension.4,5 These estimates have been confirmed and extended by several consensus groups and serve as the foundation for international initiatives to reduce risk of Alzheimer disease.6,7
It is important to update these estimates to account for changes in risk factor prevalence that may have occurred over the past decade. In addition, our prior study and others did not examine differences in dementia risk factors based on sex or race and ethnicity. This is especially relevant given differences by sex and race and ethnicity in the prevalence of ADRDs and ADRD-associated risk factors. Almost two-thirds of US adults with ADRDs are women, and compared with older White individuals, older Black individuals are twice as likely to have ADRDs and older Hispanic individuals are 1.5 times as likely.3 In addition, the prevalence of smoking is higher among men than women,8 and the proportion of low educational attainment is higher among Hispanic individuals compared with other racial and ethnic groups.9 These differences illustrate the importance of having separate estimates for specific subpopulations to inform optimal prevention efforts. Few studies to date have examined the unique ADRD risk factor profiles of individuals in the US by sex or race and ethnicity.
The primary goal of this study was to estimate the proportion of ADRD cases that are associated with modifiable risk factors in the US overall and within sex and racial and ethnic subpopulations using the most current data available. Attenuating these risk factors could potentially play a role in reducing the ADRD burden in the US. We provide estimates of the individual population-attributable risks (PARs; ie, the proportion of cases of a disease in a population that may be attributed to a given risk factor) for each of the 7 modifiable risk factors originally studied4,5 as well as hearing loss, selected because of growing evidence of its association with ADRDs10 and the combined PAR (accounting for nonindependence between factors) for the US overall and by sex and racial and ethnic subpopulations.
Methods
This study used publicly available deidentified data and as such did not qualify as human subject research; therefore, institutional review board approval was not required. Verbal informed consent was obtained prior to the survey being administrated.
Data Sources
We determined risk factor prevalence for 8 previously found modifiable ADRD risk factors using data from the nationally representative US Behavioral Risk Factor Surveillance System Data (BRFSS). The BRFSS is an annual survey and publicly available data system of health-related telephone surveys that collect state data on US residents regarding their health-related risk behaviors, chronic health conditions, and use of preventive services. These data include information for all 50 states as well as the District of Columbia and 3 US territories.9
We obtained relative risk estimates for the associations between each modifiable risk factor and ADRDs by searching the Cochrane Database of Systematic Reviews and PubMed to identify English-language systematic reviews and meta-analyses examining effects or associations between each risk factor and ADRDs in terms of the relative risk (ie, risk ratio or odds ratio).4,5 The search strategy details can be found in the eMethods in the Supplement. We selected the most recent and comprehensive meta-analyses available. Relative risk estimates for Alzheimer disease were used when available; otherwise, estimates for all-cause dementia were used.
Variables
In the BRFSS, participants provide sociodemographic information, including age, sex, and race and ethnicity. We categorized race and ethnicity from respondents’ self-reports into mutually exclusive categories of non-Hispanic American Indian and Alaska Native, non-Hispanic Asian, non-Hispanic Black, Hispanic (any race), and Non-Hispanic White. In addition, the 8 modifiable factors (physical inactivity, smoking, depression, low education, diabetes, midlife obesity, midlife hypertension, and hearing loss) were assessed using self-reported behavioral, physiologic, and clinical information. Details on how each risk factor was assessed and coded can be found in eTable 1 in the Supplement.
Statistical Analysis
Various analytical steps were undertaken to estimate the individual and combined PAR between the 8 risk factors and ADRDs. PAR estimates take into account the strength of the association between the risk factor and the outcome as well as the prevalence of the risk factor.11
Estimation of the Weighted Prevalence
We estimated the weighted prevalence of the modifiable risk factors from the 2018 BRFSS data. Because information on hypertension was not recorded in the 2018 survey, we used the 2017 BRFSS to obtain such information. We used R software version 3.6.3, SVYR package (R Foundation), as well the sampling unit, stratification, and weight information from the data to estimate the weighted prevalence.
Estimation of the Unique Variance Associated With Risk Factors
To incorporate the unique association of each factor with the PAR, we first used steps similar to that of the weighted prevalence to obtain the weighted association between each of the modifiable risk factors using data from the 2018 BRFSS. Next, using the weighted correlation for each risk factor, we extracted the principal components of the inter–risk factor tetrachoric correlation matrix4 and the corresponding communality among the different risk factors. As done elsewhere,4 the number of components to extract was determined by the Kaiser method.12 In this case, 2 components were then extracted. Communality is the explained variance shared with the other factors. From this metric we estimated the uniqueness—ie, the unique association of each factor with ADRD risk not explained by the other factors (ie, uniqueness was calculated as 1 minus the communality). We estimated the uniqueness for each factor in the entire sample as well as by subgroups defined by sex and race and ethnicity (sex-specific and race and ethnicity–specific uniqueness are shown in eTable 2 in the Supplement). Uniqueness was then used as a weight in the estimation of the PAR.
Estimation of the PAR of ADRDs Associated With Selected Modifiable Risk Factors Using the Levin PAR Formula
The individual unadjusted PAR for each individual risk factor can be calculated as follows:
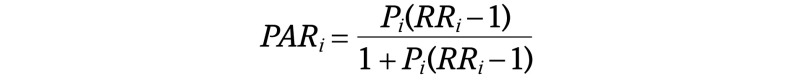 |
where Pi is the weighted prevalence of the given risk factor and RRi the relative risk (ie, risk ratio or odds ratio) for the association between the given risk factor and ADRDs.
Because the individual unadjusted PAR assumes independence of risk factors, we also calculated a combined PAR that accounts for the nonindependence of the risk factor as follows and as used elsewhere4:
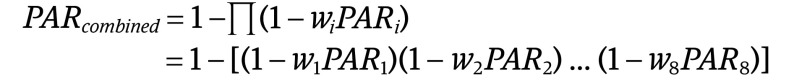 |
where w is the unique contribution of each factor as previously calculated. The adjusted combined PAR is calculated as 1 minus the product of 1 minus the individual weighted unadjusted PAR for each factor. The combined adjusted PAR as calculated here is adjusted for the 8 modifiable risk factors—ie, one that does not double count the contribution of each risk factor to ADRD risk by removing the shared variance of each risk factor as explained above. All analyses were conducted in R version 3.6.3 (R Foundation).
Results
Sample Characteristics
Among 378 615 included individuals, 171 161 (weighted 48.7%) were male, and 134 693 (weighted 21.1%) were 65 years and older; 6671 participants (weighted 0.9%) were American Indian and Alaska Native, 8043 (weighted 5.1%) were Asian, 29 956 (weighted 11.7%) were Black, 28 042 (weighted 16.0%) were Hispanic (any race), and 294 394 (weighted 64.3%) were White (Table 1). The most prominent risk factors in the overall population were midlife obesity (56 499 [36.0%]), midlife hypertension (67 430 [37.1%]), and physical inactivity (93 743 [23.9%]) (Table 2).
Table 1. Weighted Sample Characteristics, US Behavioral Risk Factor Surveillance System (BRFSS) Data, 2018.
| Characteristic | No. of respondents | US proportion, weighted % (95% CI) |
|---|---|---|
| Age 65 y and older | 134 693 | 21.1 (20.9-21.3) |
| Male sex | 171 161 | 48.7 (48.3-49.0) |
| Race and ethnicitya | ||
| Hispanic, any race | 28 042 | 16.0 (15.6-16.3) |
| Non-Hispanic | ||
| American Indian and Alaska Native | 6671 | 0.9 (0.9-1.0) |
| Asian | 8043 | 5.1 (4.9-5.3) |
| Black | 29 956 | 11.7 (11.5-12.0) |
| White | 294 394 | 64.3 (63.9-64.6) |
| Otherb | 11 509 | 2.0 (1.9-2.1) |
Race and ethnicity data were self-reported by participants according to BRFSS. Ethnicity was assessed using the question, “Are you Hispanic, Latino/a, or Spanish origin? [One or more categories may be selected.]” and race was assessed using a multiple choice question: “Which one or more of the following would you say is your race? (If 40 [Asian] or 50 [Pacific Islander] is selected read and code subcategories underneath major heading.) [Select all that apply.]”
Other included Native Hawaiian or Other Pacific Islander and multiracial groups, consolidated because numbers were small and therefore unstable. Unknown and unreported were considered missing and, as with other variables, were excluded.
Table 2. Sex-Specific and Race and Ethnicity–Specific Weighted Prevalence for Each Modifiable Risk Factor Using the US Behavioral Risk Factor Surveillance System (BRFSS) Data, 2018.
| Risk factor | No. (weighted %) | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Female | Male | American Indian and Alaska Nativea | Asiana | Blacka | Hispanica | Whitea | |
| Hearing loss | 34 374 (6.5) | 14 789 (5.1) | 19 585 (8.0) | 890 (11.8) | 307 (3.3) | 1726 (4.6) | 1541 (4.9) | 28 740 (7.4) |
| Diabetes | 52 566 (11.4) | 27 702 (11.0) | 24 864 (11.7) | 1472 (17.1) | 882 (9.1) | 6222 (15.1) | 3626 (11.3) | 38 647 (10.8) |
| Low education | 27 210 (12.8) | 14 250 (12.1) | 12 960 (13.5) | 966 (20.4) | 251 (4.8) | 3251 (13.7) | 7635 (34.6) | 14 229 (7.8) |
| Current smoking | 55 743 (15.7) | 28 140 (13.8) | 27 603 (17.7) | 1959 (29.2) | 700 (8.0) | 5031 | 3746 (12.7) | 41 852 (16.3) |
| Depression | 72 312 (18.7) | 48 301 (23.5) | 24 011 (13.6) | 1597 (25.4) | 687 (7.6) | 4860 (16.3) | 4679 (14.9) | 57 745 (20.6) |
| Physical inactivity | 93 743 (23.9) | 55 760 (26.3) | 37 983 (21.4) | 2047 (29.0) | 1591 (18.7) | 9458 (28.3) | 8230 (29.6) | 69 681 (22.1) |
| Midlife hypertensionb | 67 430 (37.1) | 33 922 (33.4) | 33 508 (40.9) | 1836 (47.4) | 1006 (25.9) | 8402 (51.9) | 4543 (32.7) | 49 690 (35.8) |
| Midlife obesity | 56 499 (36.0) | 29 130 (35.4) | 27 369 (36.5) | 1426 (42.5) | 441 (13.2) | 6529 (46.2) | 4200 (38.7) | 42 028 (34.9) |
Race and ethnicity data were self-reported by participants according to BRFSS. Ethnicity was assessed using the question, “Are you Hispanic, Latino/a, or Spanish origin? [One or more categories may be selected.]” and race was assessed using a multiple choice question: “Which one or more of the following would you say is your race? (If 40 [Asian] or 50 [Pacific Islander] is selected read and code subcategories underneath major heading.) [Select all that apply.]” Other data are not reported in this table owing to small numbers.
Midlife hypertension data were collected in 2017 because information on hypertension was not recorded in the 2018 survey.
The prevalence of risk factors varied by race and ethnicity (Table 2). American Indian and Alaska Native individuals, Black individuals, and Hispanic individuals had the highest prevalence of midlife obesity: 1426 (42.5%), 6529 (46.2%), and 4200 (38.7%), respectively. In addition, the prevalence of low educational attainment was the highest among Hispanic individuals (7635 [34.6%]) followed by American Indian and Alaska Native individuals (966 [20.4%]), Black individuals (3251 [13.7%]), White individuals (14 229 [7.8%]), and Asian individuals (251 [4.8%]). Overall, the prevalence of all risk factors was lowest among Asian individuals and highest among American Indian and Alaska Native individuals. For instance, the prevalence of smoking was 1959 (29.2%); midlife obesity, 1426 (42.5%); and midlife hypertension, 1836 (47.4%) for American Indian and Alaska Native individuals, the highest of all race and ethnicity subgroups, and 700 (8.0%), 441 (13.2%), and 1006 (25.9%), respectively, for Asian individuals, the lowest of all race and ethnicity subgroups.
There was also some variability in dementia risk factor prevalence by sex (Table 2). In particular, the prevalence of depression was higher in women (48 301 [23.5%]) than in men (24 011 [13.6%]), while midlife hypertension was more prevalent in men (33 508 [40.9%]) than in women (33 922 [33.4%]).
PAR
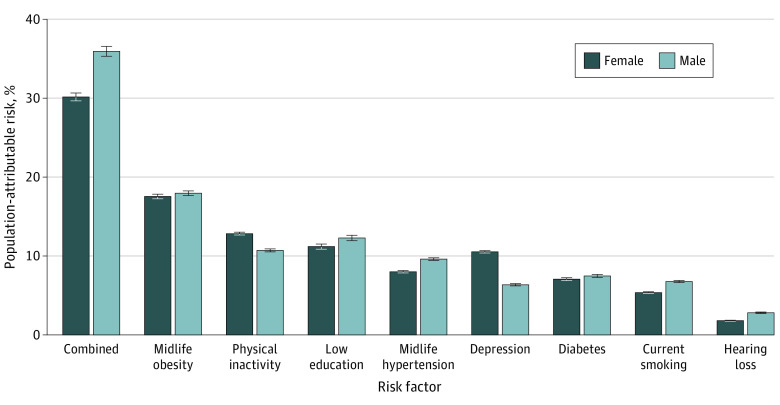
In the US, about one-third of ADRD cases (PAR, 36.9%; 95% CI, 36.5-37.3) were associated with the combination of modifiable risk factors evaluated in this study, with the most prominent being midlife obesity (17.7%; 95% CI, 17.5-18.0), physical inactivity (11.8%; 95% CI, 11.7-11.9), and low educational attainment (11.7%; 95% CI, 11.5-12.0) (Table 3). The combined PAR was higher in men (35.9%; 95% CI, 35.3-36.5) than women (30.1%; 95% CI, 29.6-30.6) with the strongest association with ADRD being midlife obesity in both men (18.0%; 95% CI, 17.7-18.2) and women (17.5%; 95% CI, 17.2-17.8) (Figure 1; eTable 3 in the Supplement). The PAR associated with depression was higher in women (10.5%; 95% CI, 10.4-10.7) than men (6.4%; 95% CI, 6.2-6.5). The PAR associated with other risk factors was relatively similar across sex.
Table 3. Unadjusted and Combined Population-Attributable Risk for 8 Modifiable Dementia Risk Factors Overall in the US, 2018.
| Risk factor | Relative risk (95% CI) | Uniqueness, % | Population-attributable risk, % (95% CI) |
|---|---|---|---|
| Hearing loss | 1.4 (1.1-1.7)13 | 86.7 | 2.3 (2.2-2.3) |
| Current smoking | 1.4 (1.2-1.6)14 | 46.9 | 6.0 (5.9-6.1) |
| Midlife hypertensiona | 1.3 (1.1-1.4)14 | 22.1 | 8.8 (8.7-8.9) |
| Diabetes | 1.7 (1.5-1.9)14 | 43.2 | 7.3 (7.1-7.4) |
| Depression | 1.5 (1.1-2.0)14 | 74.1 | 8.5 (8.4-8.6) |
| Low education | 2.0 (1.6-2.6)14 | 70.8 | 11.7 (11.5-12.0) |
| Physical inactivity | 1.6 (1.3-1.8)14 | 67.5 | 11.8 (11.7-11.9) |
| Midlife obesity | 1.6 (1.1-2.3)14 | 66.2 | 17.7 (17.5-18.0) |
| Combined | NA | NA | 36.9 (36.5-37.3) |
Midlife hypertension data were collected in 2017 because information on hypertension was not recorded in the 2018 survey.
Figure 1. Unadjusted and Combined Population-Attributable Risk for 8 Modifiable Dementia Risk Factors by Sex in the US, 2018.
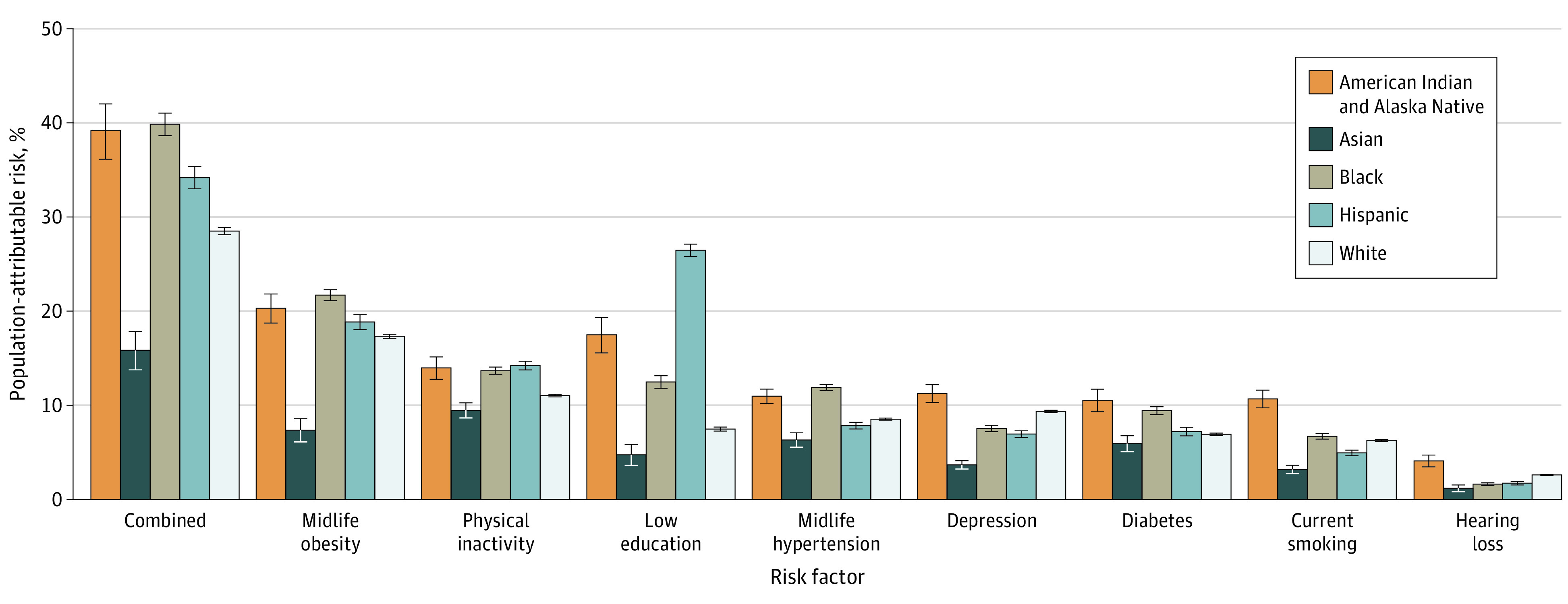
The combined PAR was higher in Black individuals (39.8%; 95% CI, 38.6-41.0), American Indian and Alaska Native individuals (39.2%; 95% CI, 36.1-42.0), and Hispanic individuals (34.2%; 95% CI, 33.0-35.3) compared with White individuals (28.5%; 95% CI, 28.1-28.9) and Asian individuals (15.8%; 95% CI, 13.8-17.8). The strongest association with ADRD risk also differed by race and ethnicity and was midlife obesity in Black individuals (21.7%; 95% CI, 21.1-22.3), American Indian and Alaska Native individuals (20.3%; 95% CI, 18.7-21.8), and White individuals (17.3%; 95% CI, 17.1-17.5); low educational attainment in Hispanic individuals (26.5%; 95% CI, 25.8-27.1); and physical inactivity in Asian individuals (9.5%; 95% CI, 8.7-10.3).
In addition, the PAR associated with different risk factors among Asian individuals was lowest compared with other racial and ethnic subgroups. The PAR associated with low education was highly variable across all racial subgroups: 26.5% for Hispanic individuals, 17.5% for American Indian and Alaska Native individuals, 12.5% for Black individuals, 7.5% for White individuals, and 4.7% for Asian individuals (Figure 2; eTable 4 in the Supplement). There was no evidence of an interaction between sex and race and ethnicity on the combined PAR. Within a given racial and ethnic group, there appeared to be no large sex differences. For instance, the PAR among American Indian and Alaska Native women (36.3%; 95% CI, 32.4-39.8) was similar in magnitude to that of American Indian and Alaska Native men (35.6%; 95% CI, 31.5-39.3) (eFigure in the Supplement).
Figure 2. Unadjusted and Combined Population-Attributable Risk for 8 Modifiable Dementia Risk Factors by Race and Ethnicity in the US, 2018.
Discussion
In this cross-sectional study, we sought to update our previous estimates of the proportion of ADRD cases that are associated with modifiable risk factors in the US using current risk factor prevalence data and to assess differences across sex and racial and ethnic subpopulations. Similar to our previous estimates, we found that overall, one-third of ADRD cases were associated with a combination of the 8 modifiable risk factors evaluated in this study, which included physical inactivity, depression, smoking, low education, diabetes, hearing loss, midlife obesity, and midlife hypertension. However, the most prominent modifiable risk factors associated with ADRDs in the US appear to have changed over the past decade. In 2011, the most prominent risk factors were physical inactivity, depression, and smoking.5 In contrast, the modifiable risk factors associated with the largest proportion of ADRD in the US in the present study were midlife obesity, physical inactivity, and low education. Our study also found that the proportion of ADRDs associated with these 8 modifiable risk factors was relatively higher among men vs women and among Black individuals, American Indian and Alaska Native individuals, and Hispanic individuals compared with Asian individuals and White individuals.
The findings suggest that the PAR of ADRDs associated with modifiable risk factors varied based on sex and race and ethnicity, likely owing to the fact that some risk factors are more prevalent in certain subpopulations. For instance, the prevalence of midlife obesity was highest among Black individuals and American Indian and Alaska Native individuals and that of low educational attainment was highest among Hispanic individuals, and these risk factors represented the most prominent associations with ADRDs in these subpopulations.
Our results are consistent with studies showing changes in dementia risk factors in the US over time. A study by Chen and Zissimopoulos15 found an increase in diseases associated with dementia (eg, hypertension, diabetes) across all ethnic and racial groups over a 12-year period. A corollary of this is that aiming to reduce disparities in ADRDs could involve striving to reduce disparities at the risk factor level, an observation also made by Chen and Zissimopoulos.15 Another explanation for these results could be that certain subpopulations may experience difficulty in accessing adequate health care to treat the risk factor (eg, hypertension) or any downstream conditions prior to Alzheimer disease diagnosis. Disparities in access to hypertension control,16 weight management,17 and diabetes control18,19 have been well documented. In addition, certain racial and ethnic subpopulations, such as Black individuals and Hispanic individuals, receive delayed diagnoses and delayed or inadequate health care services for dementia,20,21,22 all of which can exacerbate the disparities seen in ADRDs. It is also interesting to note that while the combined PAR was higher in men, the prevalence of ADRDs was higher in women.3 This could be because of the fact that women live longer than men on average, and older age is one of the most prominent risk factors for developing ADRDs.3,23
Our combined adjusted PAR estimates in the US were slightly higher than those reported by Norton et al4 in 2014. This higher PAR could be the result of several factors: the addition of hearing loss in our pool of modifiable risk factors, the difference in prevalence in some factors (eg, midlife hypertension: 14.0% in 2010 vs 37.1% in 2018), the difference in relative risks for some factors (eg, low education: 1.6 in Norton et al4 vs 2.0 in this study; hypertension: 1.6 in Norton et al4 vs 1.3 in this study), or differences in how some risk factors were defined (eg, physical inactivity). In addition, the top 3 modifiable risk factors associated with ADRD in the US in both 20115 and 20144 were physical inactivity (PAR in both 2011 and 2014: 21.0%), followed by depression (PAR 2011: 14.7%; 2014: 11.1%) and smoking (PARE in both 2011 and 2014: 10.8%). These top 3 risk factors differed from those found in our current study: midlife obesity (17.7%), physical inactivity (11.8%), and low educational attainment (11.7%). These differences are likely explained by changing trends in associated risk factors from almost a decade ago. In fact, the prevalence of midlife obesity in the US more than doubled from 13.1% in 2010 to 36% in 2018. Conversely, the prevalence of several other risk factors declined: physical inactivity was 32.5% in 2010 vs 23.9% in 2018, and smoking was 20.6% in 2010 vs 18.7% in 2018. Notably, the change in midlife obesity prevalence appeared to be the largest compared with other factors evaluated in this study, which potentially propelled midlife obesity to become the most prominent risk factor associated with ADRDs nearly a decade later.
Of the 8 modifiable risk factors studied here, low education followed by physical inactivity have consistently ranked as the most prominent risk factors associated with ADRDs across countries. In fact, the top 3 risk factors associated with ADRDs across the globe have been reported as follows: Europe (physical inactivity, 20%; low education, 14%; and smoking, 14%),24 Latin America (low education, 29%; midlife hypertension, 25%; midlife obesity, 21%),25 Australia (physical inactivity, 20%; midlife obesity, 17%; low education, 15%),26 China (low education, 31%; physical inactivity, 23%; midlife hypertension, 19%),25 and Mozambique (physical inactivity, 31%; low education, 21%; smoking, 11%).27 Midlife obesity, the risk factor that was associated with the largest proportion of ADRD in the US in our study, was not in the top 3 in most other countries with the exception of Australia and Latin America.
Limitations
Our study has several limitations. First, the study used self-reported data to estimate the prevalence of the risk factors. Some studies have suggested that study participants tend to slightly overreport their height and slightly underreport their weight and that body mass index calculated from self-reported data is typically lower than that from measured data for both men and women.28,29 However, these studies have also noted that misclassification resulting from self-reported data appears to be minimal.28,29 Second, in the estimation of the PAR, our study relied on meta-analyses of observational studies. For instance, given that depression may be a prodromal symptom of ADRDs in some cases rather than a true risk factor,30 the relative risk reported in the literature may not represent the true association and, as such, its use in our study could affect the true magnitude of the PAR estimated. Similarly, the relative risk for education does not take into account factors, such as language or education quality, that may differ based on race or ethnicity. Rigorous causal inference analyses are needed to better understand and quantify the contribution of these risk factors to the development of ADRDs. Nonetheless, using associational estimates to estimate PAR may represent the best evidence in the absence of randomized clinical trials. Furthermore, striving to target these modifiable risk factors (eg, physical inactivity, smoking, and obesity) could lead to overall better health outcomes in general.31,32 Third, our PAR estimates do not account for the competing risk of death. Therefore, these estimates should be interpreted as the excess caseload owing to the presence of these risk factors as opposed to their removal.33 To interpret them as the proportion of cases that could be prevented if one were to remove the modifiable factor of interest requires one to additionally assume that removal of the modifiable factors would not affect other competing events, such as death from other causes. This latter assumption may not always be reasonable. To assess how many cases could be prevented by removing such modifiable factors, randomized clinical trials or long-term observational studies that account for competing risk and other sources of biases are needed. Fourth, individual PARs are unadjusted and do not account for correlations with other factors, unlike the combined PAR. As in our original study, we applied adjusted relative risks instead of unadjusted relative risks to the Levin PAR formula to estimate the individual PAR, which can potentially lead to bias toward the null.11,34 However, by incorporating the nonindependence of risk factors in the combined PAR, it is reasonable to believe that the latter measure would be less prone to bias. Fifth, our study assumes that the relative risks associating modifiable risk factors with ADRDs were homogenous across sex and race and ethnicity. Therefore, our sex-specific and race and ethnicity–specific PAR could be underestimated if the sex-specific and race and ethnicity–specific differences in relative risk were in the same direction as the sex-specific and race and ethnicity–specific differences in risk factor prevalence and overestimated otherwise. Further studies should seek to incorporate sex-specific and race and ethnicity–specific relative risks in estimating PAR by sex and race and ethnicity. Sixth, our analysis did not explore regional or state-specific differences in the estimation of the PAR in the US. Given that cardiovascular risk factors in the US are known to be geographically distributed across states and regions in the US,35 future studies should investigate potential heterogeneity in PAR by region and state.
Conclusions
Midlife obesity, physical inactivity, and low education represented the top 3 modifiable risk factors associated with ADRDs in the US in this study. Together with depression, smoking, diabetes, hearing loss, and midlife hypertension, they were associated with one-third of ADRDs in the US overall. In addition, the proportion of ADRDs associated with these 8 modifiable risk factors differed by sex and race and ethnicity, with a relatively higher proportion of associated cases of ADRDs among men and American Indian and Alaska Native individuals, Black individuals, and Hispanic individuals. Understanding the sex-specific and race and ethnicity–specific associations with ADRDs burden may guide public health strategies. Policymakers and clinicians should also consider increasing efforts to prevent ADRDs by focusing on midlife obesity, physical inactivity, and low education, especially in higher-risk groups.
eMethods. Search strategy
eTable 1. Variables, questions and values in the 2018 BRFSS
eTable 2. Sex- and race/ethnicity-specific uniqueness for each modifiable risk factor using the 2018 BRFSS, U.S.
eTable 3. Unadjusted and combined Population Attributable Risk (PAR) for 8 Modifiable dementia risk factors by sex, 2018, U.S.
eTable 4. Unadjusted and combined Population Attributable Risk (PAR) for 8 Modifiable dementia risk factors by race/ethnicity, 2018, U.S.
eFigure. Unadjusted and combined Population Attributable Risk (PAR) for 8 Modifiable dementia risk factors by sex and race/ethnicity, 2018, U.S.
References
- 1.Prince MJ, Wimo A, Guerchet M, Ali G-C, Wu Y-T, Prina M; Alzheimer’s Disease International . World Alzheimer report 2015. the global impact of dementia: an analysis of prevalence, incidence, cost and trends. Accessed August 17, 2021. https://www.alzint.org/u/WorldAlzheimerReport2015.pdf
- 2.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326-1334. doi: 10.1056/NEJMsa1204629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzheimer’s Association . 2021 Alzheimer’s Disease Facts and Figures: Special Report: Race, Ethnicity and Alzheimer’s in America.; 2021. Accessed September 16, 2021. https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf
- 4.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794. doi: 10.1016/S1474-4422(14)70136-X [DOI] [PubMed] [Google Scholar]
- 5.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828. doi: 10.1016/S1474-4422(11)70072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . WHO Guidelines: Risk Reduction for Cognitive Decline and Dementia. Accessed August 17, 2021. https://www.who.int/mental_health/neurology/dementia/Dementia_Guidelines_Evidence_Profiles.pdf?ua=1
- 7.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agaku IT, Odani S, Okuyemi KS, Armour B. Disparities in current cigarette smoking among US adults, 2002-2016. Tob Control. 2020;29(3):269-276. doi: 10.1136/tobaccocontrol-2019-054948 [DOI] [PubMed] [Google Scholar]
- 9.US Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System . Published August 2020. Accessed January 1, 2021. https://www.cdc.gov/brfss/index.html
- 10.Liang Z, Li A, Xu Y, Qian X, Gao X. Hearing loss and dementia: a meta-analysis of prospective cohort studies. Front Aging Neurosci. 2021;13:695117. doi: 10.3389/fnagi.2021.695117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. LWW; 2011. [Google Scholar]
- 12.Kaiser HF. The application of electronic computers to factor analysis. Educ Psychol Meas. 1960;20(1). doi: 10.1177/001316446002000116 [DOI] [Google Scholar]
- 13.Ford AH, Hankey GJ, Yeap BB, Golledge J, Flicker L, Almeida OP. Hearing loss and the risk of dementia in later life. Maturitas. 2018;112:1-11. doi: 10.1016/j.maturitas.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 14.Yu JT, Xu W, Tan CC, et al. Evidence-based prevention of Alzheimer’s disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry. 2020;91(11):1201-1209. doi: 10.1136/jnnp-2019-321913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Zissimopoulos JM. Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimers Dement (N Y). 2018;4:510-520. doi: 10.1016/j.trci.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millett C, Gray J, Bottle A, Majeed A. Ethnic disparities in blood pressure management in patients with hypertension after the introduction of pay for performance. Ann Fam Med. 2008;6(6):490-496. doi: 10.1370/afm.907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byrd AS, Toth AT, Stanford FC. Racial disparities in obesity treatment. Curr Obes Rep. 2018;7(2):130-138. doi: 10.1007/s13679-018-0301-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care. 1999;22(3):403-408. doi: 10.2337/diacare.22.3.403 [DOI] [PubMed] [Google Scholar]
- 19.Harris MI. Racial and ethnic differences in health insurance coverage for adults with diabetes. Diabetes Care. 1999;22(10):1679-1682. doi: 10.2337/diacare.22.10.1679 [DOI] [PubMed] [Google Scholar]
- 20.Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25(3):187-195. doi: 10.1097/WAD.0b013e318211c6c9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukadam N, Cooper C, Livingston G. Improving access to dementia services for people from minority ethnic groups. Curr Opin Psychiatry. 2013;26(4):409-414. doi: 10.1097/YCO.0b013e32835ee668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espino DV, Lewis R. Dementia in older minority populations. issues of prevalence, diagnosis, and treatment. Am J Geriatr Psychiatry. 1998;6(2)(suppl 1):S19-S25. doi: 10.1097/00019442-199821001-00003 [DOI] [PubMed] [Google Scholar]
- 23.Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is the risk of developing Alzheimer’s disease greater for women than for men? Am J Epidemiol. 2001;153(2):132-136. doi: 10.1093/aje/153.2.132 [DOI] [PubMed] [Google Scholar]
- 24.Mayer F, Di Pucchio A, Lacorte E, et al. An estimate of attributable cases of Alzheimer disease and vascular dementia due to modifiable risk factors: the impact of primary prevention in Europe and in Italy. Dement Geriatr Cogn Dis Extra. 2018;8(1):60-71. doi: 10.1159/000487079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukadam N, Sommerlad A, Huntley J, Livingston G. Population attributable fractions for risk factors for dementia in low-income and middle-income countries: an analysis using cross-sectional survey data. Lancet. 2019;7(5):E596-E603. doi: 10.1016/S2214-109X(19)30074-9 [DOI] [PubMed] [Google Scholar]
- 26.Ashby-Mitchell K, Burns R, Shaw J, Anstey KJ. Proportion of dementia in Australia explained by common modifiable risk factors. Alzheimers Res Ther. 2017;9(1):11. doi: 10.1186/s13195-017-0238-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira D, Jun Otuyama L, Mabunda D, et al. Reducing the number of people with dementia through primary prevention in Mozambique, Brazil, and Portugal: an analysis of population-based data. J Alzheimers Dis. 2019;70(s1):S283-S291. doi: 10.3233/JAD-180636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olfert MD, Barr ML, Charlier CM, et al. Self-reported vs. measured height, weight, and BMI in young adults. Int J Environ Res Public Health. 2018;15(10):E2216. doi: 10.3390/ijerph15102216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodge JM, Shah R, McCullough ML, Gapstur SM, Patel AV. Validation of self-reported height and weight in a large, nationwide cohort of US adults. PLoS One. 2020;15(4):e0231229. doi: 10.1371/journal.pone.0231229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiels W, Baeken C, Engelborghs S. Depressive symptoms in the elderly—an early symptom of dementia? a systematic review. Front Pharmacol. 2020;11:34. doi: 10.3389/fphar.2020.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hulsegge G, Looman M, Smit HA, Daviglus ML, van der Schouw YT, Verschuren WM. Lifestyle changes in young adulthood and middle age and risk of cardiovascular disease and all-cause mortality: the doetinchem cohort study. J Am Heart Assoc. 2016;5(1):e002432. doi: 10.1161/JAHA.115.002432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berstad P, Botteri E, Larsen IK, et al. Lifestyle changes at middle age and mortality: a population-based prospective cohort study. J Epidemiol Community Health. 2017;71(1):59-66. doi: 10.1136/jech-2015-206760 [DOI] [PubMed] [Google Scholar]
- 33.Rothman K, Greenland S, Lash T. Modern Epidemiology. 3rd ed. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 34.Darrow LA, Steenland NK. Confounding and bias in the attributable fraction. Epidemiology. 2011;22(1):53-58. doi: 10.1097/EDE.0b013e3181fce49b [DOI] [PubMed] [Google Scholar]
- 35.Oates GR, Jackson BE, Partridge EE, Singh KP, Fouad MN, Bae S. Sociodemographic patterns of chronic disease: how the mid-south region compares to the rest of the country. Am J Prev Med. 2017;52(1S1)(suppl 1):S31-S39. doi: 10.1016/j.amepre.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Search strategy
eTable 1. Variables, questions and values in the 2018 BRFSS
eTable 2. Sex- and race/ethnicity-specific uniqueness for each modifiable risk factor using the 2018 BRFSS, U.S.
eTable 3. Unadjusted and combined Population Attributable Risk (PAR) for 8 Modifiable dementia risk factors by sex, 2018, U.S.
eTable 4. Unadjusted and combined Population Attributable Risk (PAR) for 8 Modifiable dementia risk factors by race/ethnicity, 2018, U.S.
eFigure. Unadjusted and combined Population Attributable Risk (PAR) for 8 Modifiable dementia risk factors by sex and race/ethnicity, 2018, U.S.