This randomized clinical trial showed that use of ultrahigh-dose methylcobalamin in Japanese patients in the early stages of amyotrophic lateral sclerosis with moderate progression can slow functional decline and had a good safety profile.
Key Points
Question
Does twice-weekly intramuscular injection of 50-mg ultrahigh-dose methylcobalamin slow clinical progression in early-stage amyotrophic lateral sclerosis?
Findings
In this randomized phase 3 clinical trial that included 130 participants who were enrolled within 1 year from symptom onset and presented with a 1- or 2-point decrease on the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale total score during 12 weeks of observation, the changes in the score were −2.66 with methylcobalamin vs −4.63 with placebo during the 16-week treatment, which significantly differed.
Meaning
Ultrahigh-dose methylcobalamin may slow functional decline in early-stage amyotrophic lateral sclerosis with moderate progression rate.
Abstract
Importance
The effectiveness of currently approved drugs for amyotrophic lateral sclerosis (ALS) is restricted; there is a need to develop further treatments. Initial studies have shown ultrahigh-dose methylcobalamin to be a promising agent.
Objective
To validate the efficacy and safety of ultrahigh-dose methylcobalamin for patients with ALS enrolled within 1 year of onset.
Design, Setting, and Participants
This was a multicenter, placebo-controlled, double-blind, randomized phase 3 clinical trial with a 12-week observation and 16-week randomized period, conducted from October 17, 2017, to September 30, 2019. Patients were recruited from 25 neurology centers in Japan; those with ALS diagnosed within 1 year of onset by the updated Awaji criteria were initially enrolled. Of those, patients fulfilling the following criteria after 12-week observation were eligible for randomization: 1- or 2-point decrease in the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) total score, a percent forced vital capacity greater than 60%, no history of noninvasive respiratory support and tracheostomy, and being ambulatory. The target participant number was 64 in both the methylcobalamin and placebo groups. Patients were randomly assigned through an electronic web-response system to methylcobalamin or placebo.
Interventions
Intramuscular injection of methylcobalamin (50-mg dose) or placebo twice weekly for 16 weeks.
Main Outcomes and Measures
The primary end point was change in ALSFRS-R total score from baseline to week 16 in the full analysis set.
Results
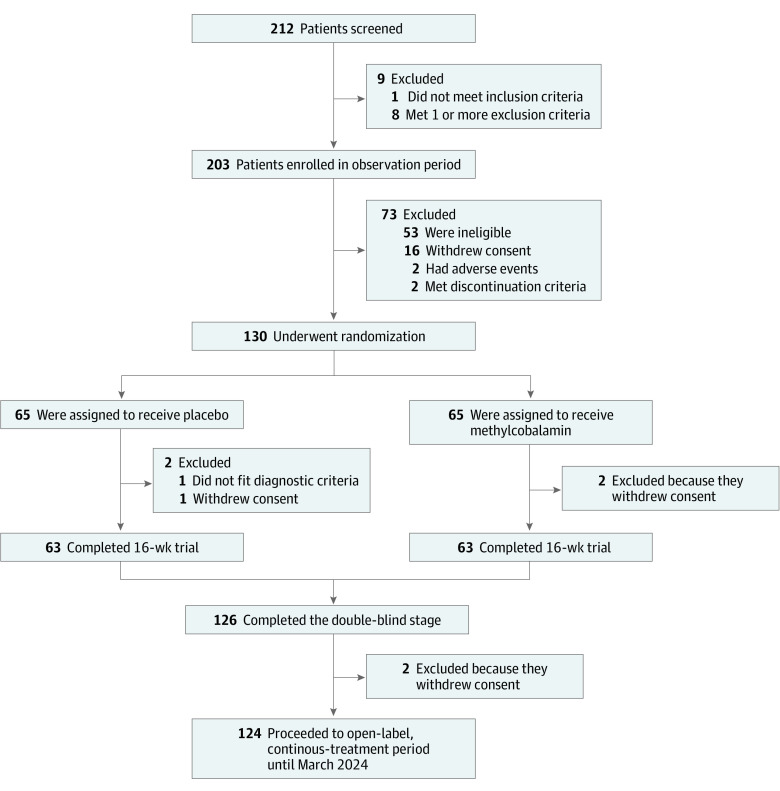
A total of 130 patients (mean [SD] age, 61.0 [11.7] years; 74 men [56.9%]) were randomly assigned to methylcobalamin or placebo (65 each). A total of 129 patients were eligible for the full analysis set, and 126 completed the double-blind stage. Of these, 124 patients proceeded to the open-label extended period. The least square means difference in ALSFRS-R total score at week 16 of the randomized period was 1.97 points greater with methylcobalamin than placebo (−2.66 vs −4.63; 95% CI, 0.44-3.50; P = .01). The incidence of adverse events was similar between the 2 groups.
Conclusions and Relevance
Results of this randomized clinical trial showed that ultrahigh-dose methylcobalamin was efficacious in slowing functional decline in patients with early-stage ALS and with moderate progression rate and was safe to use during the 16-week treatment period.
Trial Registration
ClinicalTrials.gov Identifier: NCT03548311
Introduction
Amyotrophic lateral sclerosis (ALS) is an intractable disease affecting the upper and lower motor neurons and resulting in progressive systemic muscle weakness and atrophy. The duration from symptom onset to the use of invasive respiratory support or death is 20 to 48 months.1 Although riluzole2,3 and edaravone4 have been approved by the US Food and Drug Administration to modify the disease progression of ALS, the effectiveness of these drugs is restricted, warranting the development of further treatments.
In vivo studies have shown that ultrahigh-dose methylcobalamin injections inhibited the progression of motor symptoms and neuropathological changes in a wobbler mouse model of ALS.5 A clinical pilot study demonstrated that intramuscular administration of ultrahigh-dose methylcobalamin increased the amplitude of compound muscle action potentials in patients with ALS.6 A phase 2/3 clinical trial including 373 Japanese patients with ALS within 3 years of clinical onset diagnosed by the El Escorial/Revised Airlie House Criteria (the Airlie House criteria) found that ultrahigh-dose methylcobalamin (25 mg or 50 mg) was safe and tolerable, although it did not show significant efficacy in the overall cohort.7 Nonetheless, post hoc analyses of patients who were enrolled within 1 year from symptom onset and had a 1- or 2-point decrease in ALSFRS-R total score during 12 weeks observation before treatment revealed dose-dependent efficacy of ultrahigh-dose methylcobalamin. These patients were most likely classified as the moderate progressors in a Japanese ALS cohort.8 Intramuscular injection of methylcobalamin (50 mg twice weekly) prolonged the intervals to primary events (full ventilation support or death) by more than 600 days compared with placebo. Additionally, the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) total score significantly differed between the 2 groups by 2.6 points at 16 weeks and by 5.3 points at 182 weeks, in favor of methylcobalamin. These results suggest that this treatment is beneficial for patients with ALS in the early stage and with moderate progression rate. Because ALS is a disease with heterogeneity, patient stratification especially by disease stages and progression rates is important when assessing the efficacy of a compound in clinical trials.9 Therefore, we conducted a phase 3 clinical trial to confirm the efficacy of ultrahigh-dose methylcobalamin (50 mg intramuscularly twice a week) to slow the progression of clinical symptoms for patients with ALS in the early stage and with moderate progression (the Japan Early-Stage Trial of Ultrahigh-Dose Methylcobalamin for ALS [JETALS]).
Methods
Study Design and Participants
This randomized, double-blind, placebo-controlled, investigator-initiated trial was conducted at 25 neurology centers in Japan from October 17, 2017, to September 30, 2019. The trial protocol was approved by the institutional review board of each site before trial initiation. The trial design and protocol have been published previously.10 The trial protocol and statistical analysis plan are available in Supplement 1 and Supplement 2, respectively. This trial comprised 3 stages: the observation period (12 weeks), the treatment period (16 weeks), and the open-label extended period (until March 2024); the latter 2 included randomized participants. This trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Ambulatory patients 20 years or older who were diagnosed as having sporadic or familial ALS with definite, probable, or probable laboratory-supported categories using the updated Awaji criteria11 (eAppendices 1, 2, 3, and 4 in Supplement 3) and within 1 year of symptom onset were enrolled for the observation period (primary enrollment). Patients who remained ambulatory and presented with a 1- or 2-point decrease in the ALSFRS-R total score during the 12-week observation period were entered into the 16-week treatment period (secondary enrollment). Patients were excluded before randomization if they met any of the following conditions: no change or a decrease of 3 or more points in ALSFRS-R total score during the observation period, a forced vital capacity (FVC) of 60% or less, or a history of noninvasive respiratory support or tracheostomy. Concomitant stable use of riluzole was allowed during the double-blind period. Use of edaravone was prohibited from 4 weeks before enrollment in the observation period and throughout the double-blind period (eAppendices 1, 2, and 4 in Supplement 3). All patients provided written informed consent.
Amendments to the trial protocol were made when needed and were approved by each institutional review board. The major revisions were the addition of investigational sites, changes of investigators, and the addition of prohibited concomitant drugs and therapies. An overview of the trial design is provided in eFigure 1 in Supplement 3.
Randomization, Masking, and Procedures
At the end of the observation period, the patients were randomly assigned in a 1:1 ratio to receive the investigational drug (either methylcobalamin 50 mg or placebo) with an electronic web-response randomization system on the basis of a complete randomization scheme prepared by the independent randomization expert (Supplement 1). Efficacy was evaluated by neurologists blinded to the treatment (R.O., K. Kanai, T. Tsunemi, Y.H., N. Atsuta, T. Shimizu, K.S., K.I., O.K., K. Nishinaka, Y.K., M.O., K. Komai, H.K., N.K., M.U., Y.N., M.N., S. Shimohama, T. Shimohata, T.I., S.I., N. Araki, M.M.) and safety was assessed by unblinded neurologists; both groups of neurologists were prohibited from sharing information that may have led to patient identification. The investigational drug contained lyophilized masses and powders with or without methylcobalamin 50 mg. The drug was stored in a light-shielded vial, and the vial was packaged and guaranteed to be indistinguishable from its appearance. Each vial of investigational drug was dissolved in 2.2 mL of saline, and 2.0 mL was administered into the muscles of 2 of the following areas: thigh, buttock, and deltoid (4.0 mL total). Trained physicians or nurses not involved in the efficacy evaluations injected the investigational drug; patients or caregivers could not observe it throughout the administration process. The patients were informed that administration of the investigational drug might cause reddening of the urine, but that there should be no health problems. Patients were not informed whether methylcobalamin or placebo would cause this coloration. To avoid bias, the evaluators at each institution were requested not to question the patient or caregivers about the color of the urine. Eligible patients for secondary enrollment were administered the investigational drug intramuscularly twice weekly during the 16-week treatment period. Efficacy and safety outcomes were evaluated at weeks 0 and 12 during the observation period and at weeks 4, 8, and 16 during the treatment period. Patients who wanted to receive methylcobalamin after week 16 of treatment were entered into the open-label extended period and were allowed to continue treatment until March 2024.
Outcomes
The primary end point was the change in the ALSFRS-R total score from the allocation day (baseline) to week 16 of the treatment period. We set the treatment period to 16 weeks because a significant effect was detected at as early as 16 weeks in the post hoc analysis of the previous trial (eAppendix 4 in Supplement 3). This also minimized patient exposure to the placebo. The secondary end points were time from the allocation day to the onset of an event (24-hour use of noninvasive respiratory support, use of invasive respiratory support, or death), or a change in FVC, plasma homocysteine concentration, manual muscle test total score, left and right grip strength, Norris scale total score, and ALS assessment questionnaire (ALSAQ-40) total score. The safety end points were adverse events, laboratory test results, electrocardiogram results, and vital sign measurements.
Sample Size
Based on post hoc analysis of the previous trial,7 the required number of patients for a type I error probability to 2.5% or less in 1-sided tests and a statistical power of 80% or more was a minimum of 60 patients per group. Considering that there would be dropout during the trial, the target number of patients was 64 patients per group (eAppendix 3 in Supplement 3).
Statistical Analysis
The primary efficacy analysis set was the full analysis set. The full analysis set included eligible patients who received the investigational drug at least once. The safety analysis set included eligible patients who received the investigational drug at least once, excluding those who had no assessable safety data. Analysis of the primary end point was performed using a linear mixed-effect model for repeated measures with an unstructured covariance structure of the error variance to estimate the change in the ALSFRS-R total score from baseline. Response variables were changes in ALSFRS-R total score at 4, 8, and 16 weeks. Missing values were not imputed. In the mixed model repeated measure model, we estimated the least square means difference between methylcobalamin and placebo in the change from baseline to week 16. Fixed effects included the treatment group, time points, minimization factors, and interactions between treatment groups and time points. The significance level was set at a 1-tailed P < .025. Sensitivity analysis was also performed for the per protocol set, excluding patients with deviation from the protocol. We also compared the slope between groups using the ALSFRS-R total score at the preinitiation, 4-, 8-, and 16-week time points as response variables, fitting the primary regression equation to the time points and response variables, and analyzed the data with a mixed model with intercept and slope as varying effects. An independent data and safety monitoring board periodically reviewed the efficacy and safety data. The data were obtained using electronic data capture (Supplement 2). Statistical analyses were performed with SAS, version 9.4 (SAS Institute).
Results
Study Participants
Between October 17, 2017, and September 30, 2019, we entered 203 patients in the observation period, 130 (mean [SD] age, 61.0 [11.7] years; 74 men [56.9%]; 56 women [43.1%]) of whom were enrolled for the treatment period and were randomly assigned to the methylcobalamin group (n = 65) or the placebo group (n = 65) (Figure 1). A total of 129 patients were included in the full analysis set and safety analysis set; 1 patient in the placebo group was excluded from the full analysis set and safety analysis set owing to a change in their initial ALS diagnosis (using the updated Awaji and Airlie House criteria) to cervical spinal canal stenosis based on clinical course and examination after randomization. In addition, 1 patient in the placebo group and 2 patients in the methylcobalamin group discontinued treatment owing to withdrawal of consent, 126 patients (63 patients in each group; 97%) completed the double-blind stage, and 124 patients proceeded to the open-label extended period. The baseline demographic and disease characteristics were similar between the groups, without significant differences (Table 1).12
Figure 1. Screening, Randomization, and Follow-up.
One patient in the placebo group was excluded from the full analysis set and safety analysis set as the patient was initially diagnosed with probable amyotrophic lateral sclerosis but was later rediagnosed with cervical spinal canal stenosis. A total of 126 patients (97%) ultimately completed the 16-week trial.
Table 1. Baseline Demographic and Clinical Characteristics (Full Analysis Set).
| Characteristic | Placebo (n = 64) | Methylcobalamin (n = 65) | Total (n = 129) |
|---|---|---|---|
| Male sex, No. (%) | 40 (63) | 34 (52) | 74 (57) |
| Age, mean (SD), y | 60.8 (12.1) | 61.2 (11.4) | 61.0 (11.7) |
| Period from ALS onset at the enrollment of the observation period, mean (SD), mo | 8.5 (2.3) | 8.2 (2.4) | 8.3 (2.3) |
| ALSFRS-R total score at baseline, mean (SD) | 42.3 (2.7) | 42.4 (2.6) | 42.4 (2.6) |
| FVC at baseline, mean (SD), % | 90.6 (16.9) | 93.4 (16.9) | 92.0 (16.9) |
| Body mass index, mean (SD) | 22.6 (3.9) | 21.8 (2.8) | 22.2 (3.4) |
| Vitamin B12 level at the enrollment of the observation period, mean (SD), pg/mL | 571.8 (719.9) | 585.9 (373.0) | 578.9 (570.2) |
| Disease type, No. (%) | |||
| Upper extremity | 32 (50) | 33 (51) | 65 (50) |
| Lower extremity | 13 (20) | 13 (20) | 26 (20) |
| Bulbar | 19 (30) | 19 (29) | 38 (30) |
| ALS type, No. (%) | |||
| Familial ALS | 0 (0) | 1 (2) | 1 (1) |
| Sporadic ALS | 64 (100) | 64 (98) | 128 (99) |
| Concomitant use of riluzole, No. (%) | 58 (91) | 58 (89) | 119 (92) |
| History of edaravone use, No. (%) | 6 (9) | 4 (6) | 10 (8) |
| ALS diagnosis of updated Awaji criteria, No. (%)a | |||
| Definite | 16 (25) | 23 (35) | 39 (30) |
| Probable | 32 (50) | 30 (46) | 62 (48) |
| Probable laboratory-supported | 16 (25) | 12 (19) | 28 (22) |
| ALS severity at baseline, No. (%)b | |||
| Grade 1 | 21 (33) | 21 (32) | 42 (33) |
| Grade 2 | 43 (67) | 44 (68) | 87 (67) |
| Change in ALSFRS-R total score in the observation period, No. (%)c | |||
| −2 | 28 (44) | 31 (48) | 59 (46) |
| −1 | 36 (56) | 34 (52) | 70 (54) |
Abbreviations: ALS, amyotrophic lateral sclerosis; ALSFRS-R, Revised Amyotrophic Lateral Sclerosis Functional Rating Scale; FVC, forced vital capacity.
The updated Awaji criteria, adopted as the ALS diagnostic criteria in this trial, comprised the categories of definite, probable, probable laboratory-supported, and possible. Patients with ALS who met the criteria of definite, probable, or probable laboratory-supported categories were eligible for enrollment.12
ALS severity: The severity of ALS symptoms was graded according to the Japan ALS severity classification of grades 1 to 5, with grade 5 being the most severe.
Change in the ALSFRS-R total score during the 12-week observation period before randomization. ALSFRS-R ranges from 0 to 48, with a lower score indicating more severe symptoms.
Efficacy Outcomes
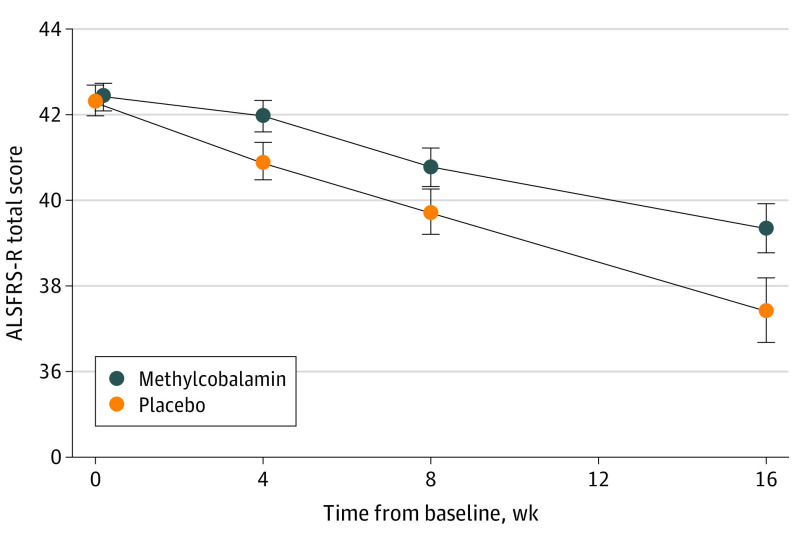
The mean (SD) least square mean difference in the ALSFRS-R total score at week 16 was −2.66 (0.61) in the methylcobalamin group and −4.63 (0.60) in the placebo group, and the difference was 1.97 in favor of methylcobalamin (95% CI, 0.44-3.50; P = .01) (Table 2). In 116 patients (90%) using riluzole concomitantly, the least squares mean difference in ALSFRS-R score was 2.11 in favor of methylcobalamin (95% CI, 0.46-3.76; P = .01) (eTable 4 in Supplement 3). The difference in the ALSFRS-R total score between the methylcobalamin and placebo groups amounted to 43% in all of the patients and 45% in the patients with concomitant use of riluzole. There were no deaths, 24-hour use of noninvasive respiratory support, or use of invasive respiratory support during the 16-week treatment period (Table 2). In the sensitivity analysis, the slope of the ALSFRS-R total score through the treatment period was significantly smaller in the methylcobalamin group (−0.12; 95% CI, −0.22 to −0.02, P = .02) (Figure 2). Additional analyses related to changes in the ALSFRS-R score are shown in eTables 1, 2, 3, and 4 in Supplement 3. The least square means difference in the plasma homocysteine concentration at week 16 were significantly lower in the methylcobalamin group (−1.71; 95% CI, −1.14 to −2.29; P < .001) (eFigures 2 and 3 in Supplement 3). The least square means difference in FVC, Norris scale total score, and manual muscle test total score did not show significant differences between the methylcobalamin and placebo groups.
Table 2. Primary and Secondary Efficacy Outcomes (Full Analysis Set).
| End pointa | Mean (SE) | Difference (95% CI) | P value | |
|---|---|---|---|---|
| Placebo (n = 64) | Methylcobalamin (n = 65) | |||
| Change in ALSFRS-R total score from baseline to week 4, 8, and 16 | ||||
| Change value from baseline to week | ||||
| 4 | −1.19 (0.35) | −0.20 (0.36) | 0.99 (0.34 to 1.65) | .003 |
| 8 | −2.33 (0.43) | −1.34 (0.44) | 0.99 (0.04 to 1.95) | .04 |
| 16 (Primary end point) | −4.63 (0.60) | −2.66 (0.61) | 1.97 (0.44 to 3.50) | .01 |
| Secondary end points (change from baseline to week 16) | ||||
| Events (24-h use of noninvasive respiratory support, use of invasive respiratory support, or death)b | ||||
| Change in plasma homocysteine concentration | 0.00 (0.28) | −1.71 (0.29) | −1.71 (−2.23 to −1.20) | <.001 |
| Change in %FVC | −9.4 (1.8) | −7.4 (1.8) | 2.0 (−1.9 to 5.8) | .31 |
| Change in manual muscle test total score | −3.7 (0.7) | −2.9 (0.7) | 0.8 (−0.6 to 2.3) | .27 |
| Change in Norris scale total score | −9.9 (1.5) | −7.0 (1.6) | 2.9 (−0.5 to 6.3) | .10 |
| Change in grip strength (right) | −2.5 (0.7) | −2.7 (0.7) | −0.2 (−1.6 to 1.3) | .83 |
| Change in grip strength (left) | −2.5 (0.6) | −2.1 (0.7) | 0.4 (−0.9 to 1.7) | .54 |
| Change in ALSAQ-40 total score | 18.2 (3.5) | 15.4 (3.7) | −2.8 (−10.0 to 4.5) | .46 |
Abbreviations: ALS, amyotrophic lateral sclerosis; ALSAQ-40, amyotrophic lateral sclerosis assessment questionnaire; FVC, forced vital capacity.
Primary end point and all secondary end points are the change from baseline to week 16.
No predefined events (24-hour use of noninvasive respiratory support, use of invasive respiratory support, or death) occurred throughout the 16-week treatment period.
Figure 2. Primary Efficacy Outcomes.
The mean and slope of the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) total score in the full analysis set are shown. Data are shown as mean (SE). Error bars indicate 95% CIs.
Safety Outcomes
Adverse events were reported in 62% of patients (40 of 65) in the methylcobalamin group and in 66% of patients (42 of 64) in the placebo group (Table 3). Three patients experienced serious adverse events that were not causally related to the investigational drugs: cerebral infarction in the methylcobalamin group and hemorrhoid surgery and stenosis of the tracheostoma after laryngotracheal separation in the placebo group. Regarding the tracheal stenosis, the patient underwent a laryngotracheal separation for dysphagia owing to ALS progression at week 5 of the treatment period and developed the stenosis at week 13 of the treatment period. No other patients underwent a tracheostomy during the treatment period. There were no adverse events leading to discontinuation. Events reported by at least 5% of patients (3 of 64 or 65) in either group are shown in Table 3. Details of adverse events are shown in eTables 5, 6, and 7 in Supplement 3. No notable differences in changes of laboratory measurements, electrocardiogram parameters, and vital signs were observed between the 2 groups (eTable 8 in Supplement 3).
Table 3. Adverse Events (Safety Analysis Set)a.
| Variable | Patients, No. (%) | |
|---|---|---|
| Placebo (n = 64) | Methylcobalamin (n = 65) | |
| Total adverse events | 42 (66) | 40 (62) |
| Adverse drug reactions | 1 (2) | 5 (8) |
| Severe adverse events | 1 (2) | 1 (2) |
| Severe adverse drug reactions | 0 | 0 |
| Adverse events leading to discontinuation | 0 | 0 |
| Adverse drug reactions leading to discontinuation | 0 | 0 |
| Serious adverse event | 2 (3) | 1 (2) |
| Serious adverse drug reactions | 0 | 0 |
| Adverse events reported by ≥5% of patients in either groupb | ||
| Constipation | 4 (6) | 3 (5) |
| Nasopharyngitis | 7 (11) | 4 (6) |
| Contusion | 7 (11) | 5 (8) |
| Fall | 2 (3) | 4 (6) |
| Back pain | 4 (6) | 3 (5) |
| Insomnia | 4 (6) | (2) |
Adverse events that emerged during the observation and treatment periods are shown.
Events are shown according to the preferred term in the Japanese translation of the Medical Dictionary for Regulatory Activities (MedDRA), version 22.1 (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use).
Discussion
This randomized clinical trial demonstrated that use of ultrahigh-dose methylcobalamin resulted in a 43% reduction in clinical deterioration as evaluated with the ALSFRS-R total score throughout the 16-week treatment period in the patients with early-stage ALS. The reduction ratio was virtually equivalent to that observed in the post hoc analysis in the previous phase 2/3 trial (45%).7 Our results indicate disease-modifying, reproducible, and clinically meaningful13 effects of ultrahigh-dose methylcobalamin for patients in the early stages of ALS and with moderate progression rate. In the ALSFRS-R subscores, a decrease in the fine motor, gross motor, and total limb (the sum of both) functions were significantly smaller with methylcobalamin; changes in bulbar and respiratory functions did not differ, probably because they would be more affected in later stages. Our results also confirmed that ultrahigh-dose methylcobalamin was safe during the 16-week treatment.
Ultrahigh-dose methylcobalamin also reduced the decline of ALSFRS-R score by 45% in the patients using riluzole during the treatment period, implying that the combination of riluzole and methylcobalamin has a greater therapeutic effect than riluzole alone. Correspondingly, an human in vitro ALS model using astrocytes expressing the G93A SOD1 gene variation showed that combination therapy with methylcobalamin and riluzole enhanced the survival of motor neurons compared with monotherapy of either drug alone.14
Methylcobalamin acts as a coenzyme of methionine synthase, which is required for the formation of methionine from homocysteine in the methylation cycle. The methylation cycle in central nervous tissue seems to have an indispensable role in the elimination of homocysteine.12 Multiple lines of evidence suggest homocysteine is neurotoxic,15 which provides a promising therapeutic target in neurological disorders, such as stroke16 and dementia.17 In particular, homocysteine induces excitotoxicity, oxidative stress, mitochondrial dysfunction, activation of inflammation, and motor neuron death.18,19 In fact, plasma homocysteine levels are reported to be elevated in patients with ALS.20 The current trial showed that plasma homocysteine levels indeed significantly decreased with methylcobalamin. On the other hand, changes in plasma homocysteine levels were not correlated with those in ALSFRS-R scores in the treatment period. It should be noted, however, that homocysteine levels are affected by several factors,21 such as diet, smoking, methylenetetrahydrofolate reductase genetic polymorphisms, and baseline B-vitamin status, which were not adjusted in our trial. Meanwhile, methylcobalamin may also exert a therapeutic effect independent of lowering homocysteine. Cobalamin exhibits antioxidant and anti-inflammatory effects in homocysteine-independent systems.22,23 Moreover, methylcobalamin protects against glutamate neurotoxicity.24 We also note that the gut microbiome has been indicated to play a disease-modifying role in SOD1 gene model mice25 and to be conceivably changed in patients with ALS.26 It would thus be interesting to speculate that methylcobalamin, which may modulate the gut microbiome, could exert its effect via microbiome in patients with ALS. Collectively, methylcobalamin potentially antagonizes many adverse cellular processes likely involved in ALS. The anti-ALS effect might be related to the attenuation of multiple processes rather than any single process.
Our trials showed that methylcobalamin should be used in the ultrahigh-dose form (50 mg) rather than 25 mg.6 It is suggested that methylcobalamin at a higher concentration paradoxically upregulates gene transcription and thereby protein synthesis in vitro.27 In vivo models of rat peripheral neuropathy also demonstrate methylcobalamin, when used in high, but not low, concentrations, promotes nerve regeneration.28,29 These results seem to reinforce the necessity of ultrahigh-dose methylcobalamin for ALS treatment. We decided on using the 50-mg dose because the post-hoc analysis of the previous trial with placebo and methylcobalamin 25 mg and 50 mg groups showed that methylcobalamin prolonged survival and inhibited ALSFRS-R decline in a dose-dependent manner. The effect of methylcobalamin on ALS may correlate with increasing dose in the nervous system. As the nervous system can retain an extremely small portion of the total dose, it would need much higher dose than other tissues.
Our results show the benefit of post hoc analyses of clinical trials. Based on the post hoc analysis, we adopted the 16-week treatment period. Although it was shorter than the usual 24-week period, it could reduce the site visits, likely reduce the dropouts during the intervention, allow for early enrollment in the open-label period, and provide for cost savings. Furthermore, because the post hoc analysis indicated that early diagnosis and enrollment would be critical, we used the updated Awaji criteria to efficiently enroll patients with early-stage ALS. In parallel, we also evaluated the categories in the Airlie House criteria; 12 of the 203 patients enrolled in the observation period satisfied the updated Awaji criteria but not the Airlie House criteria, which suggest that the updated Awaji criteria played a critical role in successful patient enrollment.
Limitations
This trial was optimized to replicate the results of the post hoc analysis in the previous trial,7 and the trial design had several limitations. First, because the trial was designed to enroll patients in the early stages of ALS and with moderate progression,8 efficacy of ultrahigh-dose methylcobalamin remains unvalidated in patients with other profiles; the previous trial enrolling 373 patients with ALS within 3 years from symptom onset failed to show efficacy.7 Second, the inclusion criteria for patients in the early stage of ALS posed a risk of inclusion of ALS mimics30; we actually detected, via careful monitoring, 1 patient with cervical spinal canal stenosis. Third, the treatment duration of 16 weeks was different from the duration of 24 weeks adopted in most other clinical trials for ALS. Fourth, the sample size was relatively small for a phase 3 trial, although it was twice as large as that in the post hoc analysis. Fifth, because the patients were in the early stages of ALS and without rapid progression, no 24-hour use of noninvasive respiratory support, use of invasive respiratory support, or death was observed during the 16-week treatment period, and other secondary end points did not attain significance. Meanwhile, the least square means difference in FVC, the Norris scale total score, and the manual muscle test total score showed a tendency toward smaller decline with methylcobalamin. Lastly, the following biomarkers were not evaluated: neurofilament light chain,31 phosphorylated neurofilament heavy chain,32 urinary p75,33 motor unit number index,34 homocysteine in the cerebrospinal fluid,35 and genetic factors.36
Conclusions
This phase 3 randomized clinical trial enrolling patients with early-stage ALS and moderate progression rate showed that ultrahigh-dose methylcobalamin significantly slowed clinical progression of the disease as assessed with the ALSFRS-R total score in the 16-week treatment period. The safety of ultrahigh-dose methylcobalamin for patients with ALS was also reproduced.
Trial Protocol
Statistical Analysis Plan
eFigure 1. Trial Design
eFigure 2. Secondary Efficacy Outcomes (Full Analysis Set)
eFigure 3. Association Between Changes in ALSFRS-R and Homocysteine
eTable 1. Change in ALSFRS-R Total Score in the FAS (Sensitivity Analysis)
eTable 2. The Slope of ALSFRS-R Total Score in the FAS (Sensitivity Analysis)
eTable 3. Summary Statistics of ALSFRS-R Total Score and Subscore in the FAS
eTable 4. ALSFRS-R Total Score in Subset in the FAS
eTable 5. Summary of Severe Adverse Events by System Organ Class and Preferred Term
eTable 6. Summary of Treatment-Emergent Adverse Events by System Organ Class and Preferred Term
eTable 7. Summary of Electrocardiogram Parameter Before and After Administration
eTable 8. Summary of the Patients Who Met Possible and Suspected Grade by the El Escorial
eAppendix 1. Inclusion and Exclusion Criteria
eAppendix 2. Diagnostic Criteria
eReferences
eAppendix 3. Sample Size
eAppendix 4. Rationale for the Treatment Period of 16 Weeks
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Chiò A, Logroscino G, Hardiman O, et al. ; Eurals Consortium . Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler. 2009;10(5-6):310-323. doi: 10.3109/17482960802566824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4(3):191-206. doi: 10.1080/14660820310002601 [DOI] [PubMed] [Google Scholar]
- 3.Fang T, Al Khleifat A, Meurgey JH, et al. Stage at which riluzole treatment prolongs survival in patients with amyotrophic lateral sclerosis: a retrospective analysis of data from a dose-ranging study. Lancet Neurol. 2018;17(5):416-422. doi: 10.1016/S1474-4422(18)30054-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abe K, Aoki M, Tsuji S, et al. ; Writing Group; Edaravone (MCI-186) ALS 19 Study Group . Safety and efficacy of edaravone in well-defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505-512. doi: 10.1016/S1474-4422(17)30115-1 [DOI] [PubMed] [Google Scholar]
- 5.Ikeda K, Iwasaki Y, Kaji R. Neuroprotective effect of ultrahigh-dose methylcobalamin in wobbler mouse model of amyotrophic lateral sclerosis. J Neurol Sci. 2015;354(1-2):70-74. doi: 10.1016/j.jns.2015.04.052 [DOI] [PubMed] [Google Scholar]
- 6.Kaji R, Kodama M, Imamura A, et al. Effect of ultrahigh-dose methylcobalamin on compound muscle action potentials in amyotrophic lateral sclerosis: a double-blind controlled study. Muscle Nerve. 1998;21(12):1775-1778. doi: [DOI] [PubMed] [Google Scholar]
- 7.Kaji R, Imai T, Iwasaki Y, et al. Ultrahigh-dose methylcobalamin in amyotrophic lateral sclerosis: a long-term phase II/III randomised controlled study. J Neurol Neurosurg Psychiatry. 2019;90(4):451-457. doi: 10.1136/jnnp-2018-319294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe H, Atsuta N, Hirakawa A, et al. A rapid functional decline type of amyotrophic lateral sclerosis is linked to low expression of TTN. J Neurol Neurosurg Psychiatry. 2016;87(8):851-858. doi: 10.1136/jnnp-2015-311541 [DOI] [PubMed] [Google Scholar]
- 9.Kiernan MC, Vucic S, Talbot K, et al. Improving clinical trial outcomes in amyotrophic lateral sclerosis. Nat Rev Neurol. 2021;17(2):104-118. doi: 10.1038/s41582-020-00434-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oki R, Izumi Y, Nodera H, et al. ; JETALS . The Japanese Early-Stage Trial of High-Dose Methylcobalamin for Amyotrophic Lateral Sclerosis (JETALS): protocol for a randomized controlled trial. JMIR Res Protoc. 2018;7(12):e12046. doi: 10.2196/12046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geevasinga N, Loy CT, Menon P, et al. Awaji criteria improves the diagnostic sensitivity in amyotrophic lateral sclerosis: a systematic review using individual patient data. Clin Neurophysiol. 2016;127(7):2684-2691. doi: 10.1016/j.clinph.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein JD. The metabolism of homocysteine: pathways and regulation. Eur J Pediatr. 1998;157(2)(suppl 2):S40-S44. doi: 10.1007/PL00014300 [DOI] [PubMed] [Google Scholar]
- 13.Castrillo-Viguera C, Grasso DL, Simpson E, Shefner J, Cudkowicz ME. Clinical significance in the change of decline in ALSFRS-R. Amyotroph Lateral Scler. 2010;11(1-2):178-180. doi: 10.3109/17482960903093710 [DOI] [PubMed] [Google Scholar]
- 14.Ito S, Izumi Y, Niidome T, Ono Y. Methylcobalamin prevents mutant superoxide dismutase-1-induced motor neuron death in vitro. Neuroreport. 2017;28(2):101-107. doi: 10.1097/WNR.0000000000000716 [DOI] [PubMed] [Google Scholar]
- 15.Obeid R, Herrmann W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 2006;580(13):2994-3005. doi: 10.1016/j.febslet.2006.04.088 [DOI] [PubMed] [Google Scholar]
- 16.Liakishev AA. Homocysteine lowering with folic acid and B vitamins in vascular disease. Article in Russian. Kardiologiia. 2006;46(5):70. doi: 10.1016/S0749-4041(08)70686-9 [DOI] [PubMed] [Google Scholar]
- 17.de Jager CA, Oulhaj A, Jacoby R, Refsum H, Smith AD. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int J Geriatr Psychiatry. 2012;27(6):592-600. doi: 10.1002/gps.2758 [DOI] [PubMed] [Google Scholar]
- 18.Hemendinger RA, Armstrong EJ III, Brooks BR. Methyl vitamin B12, but not methylfolate, rescues a motor neuron-like cell line from homocysteine-mediated cell death. Toxicol Appl Pharmacol. 2011;251(3):217-225. doi: 10.1016/j.taap.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 19.Zoccolella S, Bendotti C, Beghi E, Logroscino G. Homocysteine levels and amyotrophic lateral sclerosis: a possible link. Amyotroph Lateral Scler. 2010;11(1-2):140-147. doi: 10.3109/17482960902919360 [DOI] [PubMed] [Google Scholar]
- 20.Zoccolella S, Simone IL, Lamberti P, et al. Elevated plasma homocysteine levels in patients with amyotrophic lateral sclerosis. Neurology. 2008;70(3):222-225. doi: 10.1212/01.wnl.0000297193.53986.6f [DOI] [PubMed] [Google Scholar]
- 21.Hainsworth AH, Yeo NE, Weekman EM, Wilcock DM. Homocysteine, hyperhomocysteinemia and vascular contributions to cognitive impairment and dementia (VCID). Biochim Biophys Acta. 2016;1862(5):1008-1017. doi: 10.1016/j.bbadis.2015.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birch CS, Brasch NE, McCaddon A, Williams JHH. A novel role for vitamin B(12): cobalamins are intracellular antioxidants in vitro. Free Radic Biol Med. 2009;47(2):184-188. doi: 10.1016/j.freeradbiomed.2009.04.023 [DOI] [PubMed] [Google Scholar]
- 23.Suarez-Moreira E, Yun J, Birch CS, Williams JHH, McCaddon A, Brasch NE. Vitamin B(12) and redox homeostasis: cob(II)alamin reacts with superoxide at rates approaching superoxide dismutase (SOD). J Am Chem Soc. 2009;131(42):15078-15079. doi: 10.1021/ja904670x [DOI] [PubMed] [Google Scholar]
- 24.Akaike A, Tamura Y, Sato Y, Yokota T. Protective effects of a vitamin B12 analog, methylcobalamin, against glutamate cytotoxicity in cultured cortical neurons. Eur J Pharmacol. 1993;241(1):1-6. doi: 10.1016/0014-2999(93)90925-8 [DOI] [PubMed] [Google Scholar]
- 25.Blacher E, Bashiardes S, Shapiro H, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572(7770):474-480. doi: 10.1038/s41586-019-1443-5 [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Huang T, Debelius JW, Fang F. Gut microbiome and amyotrophic lateral sclerosis: a systematic review of current evidence. J Intern Med. 2021;290(4):758-788. doi: 10.1111/joim.13336 [DOI] [PubMed] [Google Scholar]
- 27.Pfohl-Leszkowicz A, Keith G, Dirheimer G. Effect of cobalamin derivatives on in vitro enzymatic DNA methylation: methylcobalamin can act as a methyl donor. Biochemistry. 1991;30(32):8045-8051. doi: 10.1021/bi00246a024 [DOI] [PubMed] [Google Scholar]
- 28.Watanabe T, Kaji R, Oka N, Bara W, Kimura J. Ultrahigh-dose methylcobalamin promotes nerve regeneration in experimental acrylamide neuropathy. J Neurol Sci. 1994;122(2):140-143. doi: 10.1016/0022-510X(94)90290-9 [DOI] [PubMed] [Google Scholar]
- 29.Okada K, Tanaka H, Temporin K, et al. Methylcobalamin increases Erk1/2 and Akt activities through the methylation cycle and promotes nerve regeneration in a rat sciatic nerve injury model. Exp Neurol. 2010;222(2):191-203. doi: 10.1016/j.expneurol.2009.12.017 [DOI] [PubMed] [Google Scholar]
- 30.Hardiman O, van den Berg LH, Kiernan MC. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7(11):639-649. doi: 10.1038/nrneurol.2011.153 [DOI] [PubMed] [Google Scholar]
- 31.Lu CH, Macdonald-Wallis C, Gray E, et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology. 2015;84(22):2247-2257. doi: 10.1212/WNL.0000000000001642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boylan KB, Glass JD, Crook JE, et al. Phosphorylated neurofilament heavy subunit (pNF-H) in peripheral blood and CSF as a potential prognostic biomarker in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2013;84(4):467-472. doi: 10.1136/jnnp-2012-303768 [DOI] [PubMed] [Google Scholar]
- 33.Shepheard SR, Wuu J, Cardoso M, et al. Urinary p75ECD: a prognostic, disease progression, and pharmacodynamic biomarker in ALS. Neurology. 2017;88(12):1137-1143. doi: 10.1212/WNL.0000000000003741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuwirth C, Barkhaus PE, Burkhardt C, et al. Tracking motor neuron loss in a set of six muscles in amyotrophic lateral sclerosis using the Motor Unit Number Index (MUNIX): a 15-month longitudinal multicentre trial. J Neurol Neurosurg Psychiatry. 2015;86(11):1172-1179. doi: 10.1136/jnnp-2015-310509 [DOI] [PubMed] [Google Scholar]
- 35.Valentino F, Bivona G, Butera D, et al. Elevated cerebrospinal fluid and plasma homocysteine levels in ALS. Eur J Neurol. 2010;17(1):84-89. doi: 10.1111/j.1468-1331.2009.02752.x [DOI] [PubMed] [Google Scholar]
- 36.Mejzini R, Flynn LL, Pitout IL, Fletcher S, Wilton SD, Akkari PA. ALS genetics, mechanisms, and therapeutics: where are we now? Front Neurosci. 2019;13:1310. doi: 10.3389/fnins.2019.01310 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eFigure 1. Trial Design
eFigure 2. Secondary Efficacy Outcomes (Full Analysis Set)
eFigure 3. Association Between Changes in ALSFRS-R and Homocysteine
eTable 1. Change in ALSFRS-R Total Score in the FAS (Sensitivity Analysis)
eTable 2. The Slope of ALSFRS-R Total Score in the FAS (Sensitivity Analysis)
eTable 3. Summary Statistics of ALSFRS-R Total Score and Subscore in the FAS
eTable 4. ALSFRS-R Total Score in Subset in the FAS
eTable 5. Summary of Severe Adverse Events by System Organ Class and Preferred Term
eTable 6. Summary of Treatment-Emergent Adverse Events by System Organ Class and Preferred Term
eTable 7. Summary of Electrocardiogram Parameter Before and After Administration
eTable 8. Summary of the Patients Who Met Possible and Suspected Grade by the El Escorial
eAppendix 1. Inclusion and Exclusion Criteria
eAppendix 2. Diagnostic Criteria
eReferences
eAppendix 3. Sample Size
eAppendix 4. Rationale for the Treatment Period of 16 Weeks
Nonauthor Collaborators
Data Sharing Statement