Abstract
Janus kinase (JAK) inhibitors baricitinib and tofacitinib are recommended by the US National Institutes of Health as immunomodulatory drugs for coronavirus disease 2019 (COVID‐19) treatment. In addition, baricitinib has recently received Emergency Use Authorization from the US Food and Drug Administration, although the instruction provided dosing information only for adults. Geriatrics with organ dysfunction are one of the most vulnerable cohorts when combating the pandemic. The aim of the present work was to evaluate current dosing strategies of baricitinib and tofacitinib for potential COVID‐19 treatment for White and Chinese geriatric patients with chronic renal impairment. An established physiologically‐based pharmacokinetic (PBPK) modeling framework for age‐dependent simulations was utilized. PBPK drug models adopted from literature were first verified. Several population models representing different age groups, ethnicities, and stages of renal impairment were used for prospective simulations. Notwithstanding the increase in systemic exposure of both drugs resulting from renal dysfunction was more pronounced for geriatrics than general White populations, our simulations confirmed their current dosage adjustments based on renal functions are broadly adequate. The exception being White older subjects with mild renal impairment where current recommendation of 4 mg baricitinib yielded a 2.31‐fold increase in systemic exposure, and reduction to 2 mg could mitigate the potential risk to an acceptable 1.15‐fold. Comparable relationships between systemic exposure and renal dysfunction were observed for both drugs in the Chinese population. In summary, PBPK modeling of both JAK inhibitors supports the rational and prudent dose adjustments of these COVID‐19 therapeutics among adult patients of different age groups and renal functions.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Janus kinase inhibitors baricitinib and tofacitinib are recommended by the National Institutes of Health (NIH) for coronavirus disease 2019 (COVID‐19) treatment. Current Emergency Use Authorization instruction issued by the US Food and Drug Administration (FDA) for baricitinib provides information only for adults, whereas geriatric patients with organ dysfunction are under‐represented when combating the pandemic.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ The research question is whether the current dosing regimens of baricitinib and tofacitinib have to be adjusted for potential COVID‐19 treatment among geriatric patients, especially those with renal impairment?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Current dosage adjustments based on renal functions are broadly adequate for geriatric patients. The exception being older White subjects with mild renal impairment where current recommendation of 4 mg baricitinib yielded a 2.31‐fold increase in systemic exposure, and reduction to 2 mg could mitigate the potential risk.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Physiologically‐based pharmacokinetic modeling provided prospective evidence for the rational dosing of baricitinib in a vulnerable cohort and supports dose adjustments of baricitinib and tofacitinib among adult patients with COVID‐19 of different age groups and renal functions.
The pathogenesis of coronavirus disease 2019 (COVID‐19) could be driven by a dysregulated immune/inflammatory response to severe acute respiratory syndrome‐coronavirus 2, which often appears at the later clinical course culminating in tissue damage. 1 , 2 Consequently, anti‐inflammatory/immunosuppressive therapies have been included in the US National Institutes of Health (NIH) COVID‐19 Treatment Guideline for hospitalized adults requiring oxygen. 3 The Janus kinase‐Signal Transducer and Activator of Transcription (JAK‐STAT) signaling pathway has been shown to be related to the proinflammatory cytokines and chemokines in the pathology of COVID‐19. 4 Treatment with corticosteroid dexamethasone has conferred a survival benefit of patients with COVID‐19, and for those on dexamethasone who also have rapidly increasing oxygen needs and systemic inflammation, JAK inhibitor baricitinib is recommended by the NIH as an immunomodulatory drug. 3 The US Food and Drug Administration (FDA) has also issued an Emergency Use Authorization (EUA) in November 2020 to permit the emergency use of baricitinib for the treatment of COVID‐19 in hospitalized adults and pediatric patients 2 years of age or older. 5 Another JAK inhibitor, tofacitinib, has been listed in the NIH guideline as an alternative if baricitinib is unavailable or not feasible to be prescribed. 3 Both of them are currently included in the Treatment Guidelines for COVID‐19 issued by Singapore National Centre for Infectious Diseases. 6 Approximately 70% and 30% of baricitinib and tofacitinib undergo renal clearance in humans, respectively. 7
The risk of severe COVID‐19 if an individual becomes infected is known to be higher in older individuals and the share of the population at increased risk was highest in countries with older populations. 8 Prescribing information of both baricitinib and tofacitinib suggested caution should be used for rheumatoid arthritis treatment among older adults, due to decreased renal function and increased incidence of adverse effects. 9 , 10 Moreover, current EUA for baricitinib is provided only for hospitalized adults and pediatric patients. 5 As advocated by a recently published white paper, a comprehensive plan is needed to ensure adequate evaluation of the safety and effectiveness of drugs in the geriatric population as aging can modify the pharmacokinetics (PKs), pharmacodynamics, and likelihood of adverse effects of a drug. 11 Hence, a question arose whether the current dosing regimens in adults for baricitinib and tofacitinib have to be adjusted for potential COVID‐19 treatment for the White and Chinese older patients, especially for those with chronic and notable renal impairment.
The physiologically‐based pharmacokinetic (PBPK) model is a mathematical framework by which the absorption, distribution, metabolism, and excretion (ADME of a drug can be simulated. It has been recommended by the health authorities to investigate clinical scenarios, which are difficult to study in clinical practice. The aim of the present work was to evaluate current dosing strategies and investigate potential dose adjustment of baricitinib and tofacitinib therapy of COVID‐19 in White and Chinese older adults, using our previously established PBPK modeling framework for age‐dependent simulations. 12 , 13
METHODS
Model development and verification
All PBPK modeling was performed using a population‐based absorption, distribution, metabolism, and excretion (simulator (Simcyp, version 19; Sheffield, UK)). Drug models were first developed based on two published PBPK models of baricitinib 14 and tofacitinib. 15 Key drug‐dependent parameters of baricitinib, tofacitinib, and probenecid necessary for PK simulation are reported in the original articles. Midazolam and simvastatin models were adopted from the Simcyp compound library and are presented in Table S1 as well.
Plasma concentration‐time profiles were simulated, and PK parameters were subsequently generated to verify the performance of the PBPK models. The participants, dose, and regimen selected for the simulations were matched to the corresponding clinical study designs. “Healthy Volunteers” and “Chinese Healthy Volunteers” provided in Simcyp were adopted as populations for the simulations for respective healthy subjects. A 10 × 10 trial was simulated each time. Model accuracy was assessed via fold error of the mean value of each simulated PK parameter to its corresponding observed clinical value.
Prospective simulations
Doses of baricitinib were selected based on its COVID‐19 EUA, which is 4 mg once daily (q.d.) for subjects with normal renal function. 5 Dosage of 5 mg twice daily (b.i.d.) for rheumatoid arthritis from FDA prescribing information of tofacitinib were utilized for simulations. 9 Several population models provided in Simcyp were utilized for prospective simulations of different clinical scenarios. “NEurCaucasian” (20–85 years) and “Chinese” (20–70 years) were used to represent the respective general populations. “Geriatric NEC” was used to simulate the White geriatric population (65–85 years), whereas the Chinese geriatric population model developed by Cui et al. 16 was utilized for Chinese geriatric patients. The methods that characterized key parameters of each population model are summarized in Table S2 . For patients with chronic renal failure, the population models were further modified to recapitulate the different stages of renal dysfunction based on previous studies. 12 , 13 , 17 Specifically, the default value of 60 million proximal tubular cells per gram kidney for a healthy population (creatinine clearance (CrCL) > 80 mL/min) was scaled down proportionally according to glomerular filtration rate (GFR) to 28.6, 17.6, and 8.8 million cells, for mild (CrCL 50–79 mL/min), moderate (CrCL 30–49 mL/min) and severe renal impairment (CrCL < 29 mL/min), respectively. Additionally, we applied a relative activity factor/relative expression factor of 0.73 and 0.41 for the baricitinib drug model in the moderate and severe renal failure populations to recapitulate the disproportionately greater deterioration in human organic anion transporter 3 (hOAT3)‐mediated secretion in comparison to GFR. 12 , 17 Such modification was not incorporated in the tofacitinib model due to the relatively minor contribution of renal transporters to the excretion of tofacitinib. 15 Simulated area under the curve (AUC; mean value with 95% confidence interval (CI)) of each scenario was compared with that in healthy subjects to derive the AUC ratio for evaluating the potential overexposure of baricitinib and tofacitinib. Specifically, an AUC ratio of 1.84, obtained from the DDI simulation between baricitinib and probenecid in healthy subjects, was predefined as the upper limit of acceptable in vivo exposure of baricitinib.
RESULTS
Model verification and validation
As outlined in Table 1 , all simulated PK parameters fell within 1.6‐fold error and were comparable to the clinically observed values. 14 , 16 , 18 , 19 , 20 The mechanistic PBPK models accurately recapitulated clinically observed PK profiles of both baricitinib and tofacitinib across different ethnicities and in the presence/absence of the hOAT3 inhibitor. Specifically, simulations of tofacitinib utilizing subjects with declined renal functions corroborated clinical observations of increase in tofacitinib systemic exposure. The Chinese geriatric population model was judiciously examined and verified via two probe substrates, simvastatin and midazolam.
Table 1.
Observed and predicted pharmacokinetic parameters (mean ± SD) for baricitinib, probenecid, tofacitinib, midazolam, and simvastatin
| Verification purpose | Substrate | Population | Dose | Parameter | Observed | Predicted | Fold error of mean | References |
|---|---|---|---|---|---|---|---|---|
| Baricitinib | Baricitinib | Healthy population | 4 mg single | AUC0–∞ (h*ng/mL) | 236.0 ± 51.9 | 223.8 ± 85.4 | 0.95 | 14 |
| C max (ng/mL) | 36.2 ± 8.0 | 39.6 ± 12.9 | 1.09 | |||||
| CL/F (L/hour) | 16.9 ± 3.7 | 21.3 ± 10.0 | 1.26 | |||||
| CLR (L/hour) | 11.0 ± 2.4 | 12.3 ± 5.6 | 1.12 | |||||
| 4 mg single with probenecid | AUC0–∞ (hour*ng/mL) | 480.0 ± 67.2 | 410.8 ± 121.7 | 0.86 | 14 | |||
| C max (ng/mL) | 37.3 ± 7.5 | 46.3 ± 14.1 | 1.24 | |||||
| CL/F (L/hour) | 8.3 ± 1.2 | 10.8 ± 4.0 | 1.30 | |||||
| CLR (L/hour) | 3.4 ± 0.7 | 5.1 ± 1.5 | 1.49 | |||||
| Chinese healthy population | 4 mg single | AUC0–∞ (hour*ng/mL) | 274.0 ± 45.2 | 245.3 ± 80.4 | 0.90 | 18 | ||
| C max (ng/mL) | 51.3 ± 20.4 | 42.8 ± 13.8 | 0.83 | |||||
| CL/F (L/hour) | 15.0 ± 2.5 | 18.2 ± 6.5 | 1.22 | |||||
| Probenecid | Healthy population | 1000 mg b.i.d. | C max (µg/mL) | 145.0 | 165.9 | 1.14 | 14 | |
| AUCss (µg/mL) | 115.0 | 122.4 | 1.06 | |||||
| Tofacitinib | Tofacitinib | Healthy population | 10 mg single | AUC0–∞ (hour*ng/mL) | 268.0 ± 71.5 | 261.5 ± 89.3 | 0.98 | 19 |
| C max (ng/mL) | 94.2 ± 25.3 | 71.1 ± 24.8 | 0.75 | |||||
| Subjects with mild RI | 10 mg single | AUC0–∞ (hour*ng/mL) | 370.0 ± 109.0 | 327.5 ± 105.3 | 0.89 | 19 | ||
| C max (ng/mL) | 87.3 ± 23.2 | 74.5 ± 24.8 | 0.85 | |||||
| Subjects with moderate RI | 10 mg single | AUC0–∞ (hour*ng/mL) | 396.0 ± 154.0 | 359.2 ± 142.9 | 0.91 | 19 | ||
| C max (ng/mL) | 104.0 ± 47.5 | 75.4 ± 26.1 | 0.72 | |||||
| Subjects with severe RI | 10 mg single | AUC0–∞ (hour*ng/mL) | 615.0 ± 214.0 | 512.8 ± 173.0 | 0.83 | 18 | ||
| C max (ng/mL) | 111.0 ± 28.6 | 86.8 ± 31.8 | 0.78 | |||||
| Chinese healthy population | 10 mg single | AUC0–∞ (hour*ng/mL) | 277.1 ± 37.0 | 354.3 ± 118.6 | 1.28 | 20 | ||
| C max (ng/mL) | 105.3 ± 42.0 | 90.3 ± 28.3 | 0.86 | |||||
| Chinese geriatric population | Midazolam | Chinese elderly population (66‐75 years) | 15 mg single | AUC0–∞ (hour*ng/mL) | 229.0 ± 67.3 | 285.5 ± 243.5 | 1.25 | 16 |
| C max (ng/mL) | 98.0 ± 41.5 | 89.2 ± 83.6 | 0.91 | |||||
| CL/F (L/hour) | 74.6 ± 25.7 | 103.7 ± 101.1 | 1.39 | |||||
| Chinese elderly population (>76 years) | 15 mg single | AUC0–∞ (hour*ng/mL) | 254.0 ± 87.3 | 319.5 ± 269.0 | 1.26 | 16 | ||
| C max (ng/mL) | 96.8 ± 57.5 | 97.3 ± 75.8 | 1.01 | |||||
| CL/F (L/hour) | 66.0 ± 23.2 | 95.5 ± 88.1 | 1.45 | |||||
| Simvastatin | Chinese elderly population (60–91 years) | 20 mg QD | AUC0–∞ (hour*ng/mL) | 17.2 | 22.1 | 1.29 | 16 | |
| CL/F (L/hour) | 1020.0 | 1630.8 | 1.60 |
AUC0 – ∞, area under the curve from zero to infinity; AUCss, area under the curve at steady‐state; C max, peak plasma concentration; CL/F, apparent clearance; CLR, renal clearance; RI, renal impairment.
Prospective simulations
Simulations of baricitinib among White and Chinese populations with different stages of chronic kidney disease are illustrated in Figure 1 a. Most of the AUC ratios were lower than the upper limit of 1.84, corroborating that current dosage adjustments based on GFR and in the presence of a strong hOAT3 inhibitor were adequate for both general and older populations. The AUC elevation resulting from renal dysfunction was more pronounced for geriatrics than general populations. Notably, for older White subjects with mild renal impairment, current recommendation of 4 mg dose of baricitinib could lead to a 2.31‐fold AUC increase (95% CI 2.10–2.55), and reduction to 2 mg could normalize the ratio to an acceptable 1.15‐fold (95% CI 1.05–1.27). Although a comparable trend of AUC fold‐change being correlated with renal dysfunction was observed for the Chinese population, the overall AUC increase was less pronounced than in the White population for both general and older populations. In the Chinese geriatric population with mild renal impairment receiving 4 mg dose, the AUC ratio of baricitinib was elevated to an acceptable 1.68‐fold (95% CI 1.52–1.85) and no dose adjustment simulation was performed.
Figure 1.
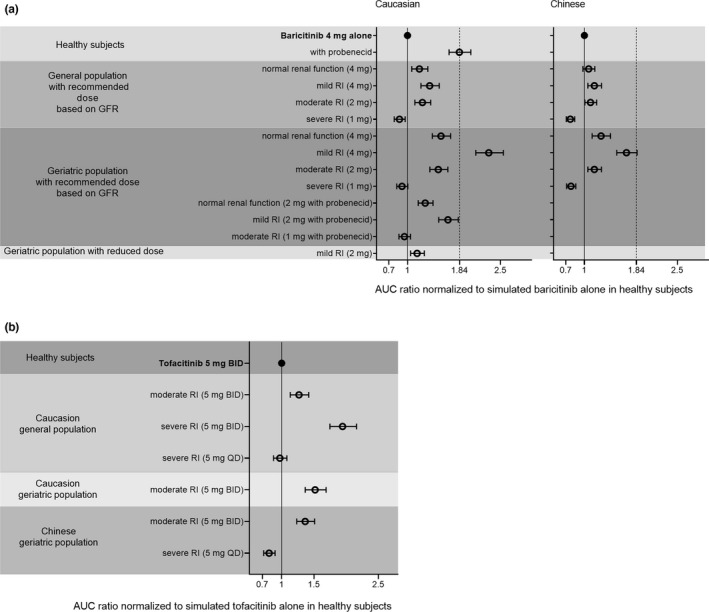
Simulated area under the curve (AUC) ratio of baricitinib (a) and tofacitinib (b) normalized to that in healthy subjects across different ethnicities, age groups, renal functions, and in the presence/absence of probenecid. Data are presented as mean value with 95% confidence interval (CI). Healthy subjects: Simcyp “Healthy Volunteers” or “Chinese Healthy Volunteers” population; General population: Simcyp “NEurCaucasian” (20–85 years) or “Chinese” (20–70 years) population; Geriatric population: Simcyp “Geriatric NEC” population or Chinese geriatric population based on Cui et al.’s work. 16 b.i.d., twice daily; GFR, glomerular filtration rate; q.d., once daily; RI, renal impairment.
Similar trends of increased systemic exposure with declined renal function were observed for tofacitinib, among both White and Chinese populations (Figure 1 b). As expected, older subjects yielded a higher AUC ratio of tofacitinib when compared with the general population. AUC ratios were 1.52 (95% CI 1.37–1.69) and 1.27 (95% CI 1.13–1.42) for older and general White adults with moderate renal impairment, respectively. Recommended dose reduction from 5 mg b.i.d. to 5 mg q.d. for older White subjects with severe renal impairment normalized AUC ratio to a comparable level observed with normal renal function (AUC ratio of 0.97, 95% CI 0.87–1.08).
DISCUSSION
Both baricitinib and tofacitinib are partially eliminated by the kidneys. 7 Baricitinib is predominantly eliminated unchanged in urine (70% of dose) where its renal tubular secretion is mainly mediated by basolaterally expressed OAT3 and several apically expressed transporters. 14 , 21 Probenecid, a strong hOAT3 inhibitor, increased the AUC of baricitinib by 2‐fold and decreased its renal clearance (CLR) to 69% of control in healthy subjects. 14 Our PBPK modeling reproduced the reduced CLR of baricitinib when it was co‐administered with probenecid. According to the dosage adjustment stipulated by EUA of baricitinib, 5 the dose should be reduced from 4 mg q.d. to 2 mg or 1 mg q.d. according to GFR and be further halved when co‐administered with strong hOAT3 inhibitors. 5 Our simulations among general White and Chinese adult populations rationalized these dosing recommendations. Current prescribing information of baricitinib for rheumatoid arthritis treatment recommends 2 mg q.d. as the baseline dose and does not recommend for its use in patients with severe renal impairment, 10 which are different from the EUA instruction. Our simulation results revealed that a dose reduction strategy could be applied to this patient cohort as well for adequate treatment of rheumatoid arthritis.
Renal excretion accounted for 30% of the elimination of tofacitinib. Both the FDA and European Medicines Agency (EMA) recommend dose adjustment from 5 mg b.i.d. to 5 mg q.d. of tofacitinib for subjects with severe renal impairment and the FDA recommends the same dosage reduction for moderate renal impairment. 9 , 22 Active renal secretion may contribute to renal elimination of tofacitinib, whereas the potential transporter has not been identified yet. 15 A PBPK simulation study by its manufacturer suggested limited contribution of active CLR secretion to overall clearance of tofacitinib. 15 The PBPK model was then adopted for our investigation and recapitulated the clinical‐observed increase in AUC of tofacitinib when renal function progressed from normal to severe. 19 The overall increase in AUC of tofacitinib due to renal impairment was relatively less pronounced than that observed for baricitinib. This is not unexpected due to the relatively minor contribution of renal elimination to the total clearance of tofacitinib.
The COVID‐19 pandemic underscored the challenge of under‐representation of older adults in clinical trials, especially those living in long‐term care facilities. A recent analysis of drug trials for COVID‐19 concluded that 23% excluded older adults based on a chronologic age restriction, and an additional 53% had indirect age‐related exclusions for comorbidities, functional impairments, lack of access to the internet or information technology, or other broad, poorly defined, or supported exclusions. 23 Addressing this challenge by the FDA, a workshop hosted in 2020, entitled “Roadmap to 2030 for New Drug Evaluation in Older Adults,” thoroughly discussed the deficits in the evaluation of drugs in clinical trials for older adults who are often accompanied by multiple chronic health conditions, polypharmacy, or frailty. 11 The under‐representation of this cohort sets similar barriers for making adequate decisions on optimal drug therapy of patients with COVID‐19.
Physiologically‐based pharmacokinetic modeling has previously demonstrated its predictive power by simulating the impact of aging in defining the PK of drugs in White and Chinese geriatric populations. 16 , 24 A combination of measured in vitro and clinically observed in vivo data could be applied to predict complex scenarios in young and older adults for dose adjustment recommendations. Using PBPK modeling, our laboratory reported that rivaroxaban, a substrate of hOAT3, could have a clinically significant increase in systemic exposure when it is co‐administered with amiodarone among older patients with moderate renal impairment, and proposed further dose reduction of rivaroxaban to ensure its efficacy and safety. 12 , 13 Our subsequent sensitivity analysis found that age and hOAT3 activity jointly contributed to the extent of this DDI and elevated systemic exposure of rivaroxaban. 12
Here, we applied validated PBPK models to simulate the PKs of baricitinib and tofacitinib in White and Chinese older subjects with different severities of renal impairment. Our results recapitulated the augmented AUC ratios of both JAK inhibitors in the presence of renal dysfunction where the older subjects are shown to be more susceptible. Extra caution is therefore needed during the treatment of COVID‐19 for geriatric patients with renal impairment using these drugs.
Although single doses up to 40 mg and multiple doses of up to 20 mg daily for 10 days of baricitinib have been administered in clinical trials without dose‐limiting toxicity, 10 concomitant probenecid would require dose modification of baricitinib due to its 2‐fold increase in systemic exposure. Our simulation results observed similar risk of overexposure when older adults with mild renal impairment received baricitinib (AUC ratio of 2.31). A 2 mg q.d. dose of baricitinib, which is not currently listed as a recommended dosage adjustment, yielded an acceptable AUC ratio of 1.15. For tofacitinib, caution has been suggested for its geriatric use due to a higher incidence of infections in the older population. 9 Our simulation illuminated another potential risk where there was a simulated 1.52‐fold increase in the AUC of tofacitinib when it was prescribed at 5 mg b.i.d. to older White subjects with moderate renal impairment. The increase in AUC was expectedly higher than those observed for general adults, and substantiated the dosage reduction of tofacitinib recommended by the FDA.
Exposure of baricitinib and tofacitinib in Chinese subjects were less affected by renal dysfunction as compared with their White counterparts, especially among older adults. AUC only increased by 1.68‐fold (95% CI 1.52–1.85) in older subjects with mild renal impairment receiving 4 mg q.d. baricitinib, and 1.36‐fold (95% CI 1.23–1.51) in those with moderate renal impairment receiving 5 mg b.i.d. tofacitinib. Notably, the age‐related distribution of physiological parameters was different between the Chinese and White geriatric populations (Table S2 ). Although geriatric populations were set as 65–85 years in our simulations, mean age distribution for the White population was 75.9 years (95% CI 66.7–84.1 years), whereas a shift was observed for Chinese population with less variability (mean 69.3 years, 95% CI 65.3–76.3 years). Such mean age distribution disparity possibly yielded differences in key renal function parameters, such as kidney weight and serum creatinine. Another possibility is that there is currently no validated PBPK model for the Chinese geriatric population with chronic renal disease and parameters were derived from White population models. 17 Taken together, further optimization and verification of the renal impairment population model for Chinese geriatric patients are warranted.
The geriatric population with chronic organ dysfunction is one of the most vulnerable cohorts when combating the COVID‐19 pandemic, for whom treatment indications might not be well‐represented in proportion to the prevalence of infection. Our proposed dose reduction of baricitinib in White older patients with mild renal impairment provided prospective evidence for the rational dosing of this cohort. In conclusion, PBPK modeling of both JAK inhibitors supports the rational and prudent dose adjustments of these COVID‐19 therapeutics among adult patients of different age groups and renal functions.
FUNDING
Z.W. received postdoctoral fellowship from the National University of Singapore. E.C.Y.C. received research grant funding from the Joseph Lim Boon Tiong Urology Cancer Research Initiative (Grant: A‐0002678‐01‐00).
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
Z.W. and E.C.Y.C. designed the research and wrote the manuscript. Z.W. performed the research and analyzed the data.
Supporting information
Table S1
Table S2
- 1. Zhang, W. et al. The use of anti‐inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID‐19): the perspectives of clinical immunologists from China. Clin. Immunol. 214, 108393 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stebbing, J. et al. COVID‐19: combining antiviral and anti‐inflammatory treatments. Lancet Infect. Dis. 20, 400–402 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Institutes of Health . Coronavirus disease 2019 (COVID‐19) treatment guidelines <https://www.covid19treatmentguidelines.nih.gov/> (2021). [PubMed]
- 4. Luo, W. et al. Targeting JAK‐STAT signaling to control cytokine release syndrome in COVID‐19. Trends Pharmacol. Sci. 41, 531–543 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. U.S. Food and Drug Administration . Baricitinib EUA letter of authorization <https://www.fda.gov/media/143822/download> (2021).
- 6. National Centre for Infectious Diseases . Treatment guidelines for COVID‐19 <https://www.ncid.sg/Health‐Professionals/Diseases‐and‐Conditions/Documents/Treatment%20Guidelines%20for%20COVID‐19%20v7%20Final%20%20%2828‐7‐2021%29.pdf> (2021).
- 7. Nakayamada, S. , Kubo, S. , Iwata, S. & Tanaka, Y. Chemical JAK inhibitors for the treatment of rheumatoid arthritis. Expert Opin. Pharmacother. 17, 2215–2225 (2016). [DOI] [PubMed] [Google Scholar]
- 8. Clark, A. et al. Global, regional, and national estimates of the population at increased risk of severe COVID‐19 due to underlying health conditions in 2020: a modelling study. Lancet Global Health 8, e1003–e1017 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. U.S. Food and Drug Administration . XELJANZ prescribing information <https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf>.
- 10. U.S. Food and Drug Administration . OLUMIANT prescribing information <https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/207924s002lbl.pdf>.
- 11. Liu, Q. et al. Roadmap to 2030 for drug evaluation in older adults. Clin. Pharmacol. Ther. 112, 210–223 (2022). [DOI] [PubMed] [Google Scholar]
- 12. Wang, Z. , Cheong, E.J.Y. , Kojodjojo, P. & Chan, E.C.Y. Model‐based risk prediction of rivaroxaban with amiodarone for moderate renal impaired elderly population. Cardiovasc. Drugs Ther. 10.1007/s10557-021-07266-z. [e‐pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13. Cheong, E.J.Y. , Goh, J.J.N. , Hong, Y. , Kojodjojo, P. & Chan, E.C.Y. Rivaroxaban with and without amiodarone in renal impairment. J. Am. Coll. Cardiol. 71, 1395–1397 (2018). [DOI] [PubMed] [Google Scholar]
- 14. Posada, M.M. et al. Prediction of transporter‐mediated drug‐drug interactions for baricitinib. Clin. Transl. Sci. 10, 509–519 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tse, S. , Dowty, M.E. , Menon, S. , Gupta, P. & Krishnaswami, S. Application of physiologically based pharmacokinetic modeling to predict drug exposure and support dosing recommendations for potential drug‐drug interactions or in special populations: an example using tofacitinib. J. Clin. Pharmacol. 60, 1617–1628 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cui, C. et al. Development of a physiologically based pharmacokinetic (PBPK) population model for Chinese elderly subjects. Br. J. Clin. Pharmacol. 87, 2711–2722 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsueh, C.‐H. et al. PBPK modeling of the effect of reduced kidney function on the pharmacokinetics of drugs excreted renally by organic anion transporters. Clin. Pharmacol. Ther. 103, 485–492 (2018). [DOI] [PubMed] [Google Scholar]
- 18. Zhao, X. et al. Pharmacokinetics, safety, and tolerability of single‐ and multiple‐dose once‐daily baricitinib in healthy Chinese subjects: a randomized placebo‐controlled study. Clin. Pharmacol. Drug Dev. 9, 952–960 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krishnaswami, S. , Chow, V. , Boy, M. , Wang, C. & Chan, G. Pharmacokinetics of tofacitinib, a Janus kinase inhibitor, in patients with impaired renal function and end‐stage renal disease. J. Clin. Pharmacol. 54, 46–52 (2014). [DOI] [PubMed] [Google Scholar]
- 20. Krishnaswami, S. et al. Single‐ and multiple‐dose pharmacokinetics of tofacitinib in healthy Chinese volunteers. Clin. Pharmacol. Drug Dev. 4, 395–399 (2015). [DOI] [PubMed] [Google Scholar]
- 21. Shi, J.G. et al. The pharmacokinetics, pharmacodynamics, and safety of baricitinib, an oral JAK 1/2 inhibitor, in healthy volunteers. J. Clin. Pharmacol. 54, 1354–1361 (2014). [DOI] [PubMed] [Google Scholar]
- 22. European Medicines Agency . Xeljanz product information <https://www.ema.europa.eu/en/medicines/human/EPAR/xeljanz>.
- 23. Helfand, B.K.I. et al. The exclusion of older persons from vaccine and treatment trials for coronavirus disease 2019–missing the target. JAMA Intern. Med. 180, 1546 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stader, F. et al. Repository describing an aging population to inform physiologically based pharmacokinetic models considering anatomical, physiological, and biological age‐dependent changes. Clin. Pharmacokinet. 58, 483–501 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


