Abstract
Background
The data on hepatocellular carcinoma (HCC) patients without liver cirrhosis is scarce.
Aims
To study the epidemiology, underlying etiology and fibrosis distribution in noncirrhotic HCC and compare the survival outcomes to cirrhotic HCC.
Methods
We conducted a retrospective study including all adult patients diagnosed with HCC at two US tertiary academic centers from 2000 to 2015. Univariable and multivariable Cox regression analyses were performed to evaluate the variables associated with patient survival.
Results
Two thousand two hundred and thirty-seven HCC patients were included in the final analysis, of which, 13% had no liver cirrhosis. The most common underlying liver disease in non-cirrhotic patients was cryptogenic cause (40%), followed by nonalcoholic fatty liver disease (NAFLD) (25.2%) and hepatitis C (19%). The percentage of F0–F1, F2, and F3 was 72%, 17%, and 11% (cryptogenic cause); 69%, 12%, and 19% (NAFLD); 50%, 17%, and 33% (alcohol); 33%, 39%, and 28% (hepatitis B); 20%, 40%, and 40% (hemochromatosis); and 12%, 40%, and 48% (hepatitis C), respectively. In non-cirrhotic compared to cirrhotic patients, the tumor was more likely to be larger and fell outside Milan criteria (all p < 0.001). Cirrhotic patients had significant shorter survival than non-cirrhotic patients (p < 0.001). On the multivariable analysis, having liver cirrhosis (HR 1.48; 1.21–1.82, p < 0.001), combined viral hepatitis and alcohol use (HR 1.51; 1.23–1.88, p < 0.001), morbid obesity (HR 1.31; 1.01–1.69, p = 0.040) and underweight (HR 2.06; 1.27–3.34, p = 0.004) were associated with worse patient survival.
Conclusions
The fibrosis distribution in non-cirrhotic HCC differed among each etiology of liver diseases. Despite more advanced HCC, patients without cirrhosis had significantly longer survival than those with cirrhosis.
Keywords: Liver cirrhosis, Liver neoplasms, Non-alcoholic fatty liver disease, Hepatitis B, Hepatitis C, Hemochromatosis
Introduction
Primary liver cancer is currently the fourth leading cause of cancer-related deaths worldwide [1]. Hepatocellular carcinoma (HCC) is the predominant type of primary liver cancer with an increasing incidence [2]. Liver cirrhosis, regardless of the etiology, is the main risk factor for HCC and regional variations in HCC incidence are largely attributed to geographical differences in the risk factors that lead to cirrhosis [3-6]. The rising incidence of nonalcoholic fatty liver disease (NAFLD) and the improved treatment of viral hepatitis have changed the epidemiology of liver cirrhosis and HCC.
Hepatocarcinogenesis is a multistep biological process and the molecular pathogenesis depends on the underlying etiological factors [7]. Despite the lack of apparent causative role of fibrosis in the promotion of HCC, there is evidence that liver fibrosis and the activation of hepatic stellate cells contribute to both direct and indirect development of HCC [8]. Strong correlation between advanced liver fibrosis and HCC development has been demonstrated in many studies in patients with liver cirrhosis.
HCC can develop in non-cirrhotic liver disease, which accounts for 1.7 to 28.7% of all HCC cases [9-14]. The mechanism associated with HCC development in patients with non-cirrhotic liver disease remains unclear and possibly is a combination of multiple complex mechanisms that vary by etiology of liver disease. Patients diagnosed with HCC in a non-cirrhotic liver background generally have large tumor size and approximately one-third have extrahepatic metastasis at presentation [9, 11]. More advanced disease is presumably due to lack of surveillance recommended by the current guidelines in non-cirrhotic liver disease. Currently, there are limited number of studies on HCC without liver cirrhosis and the regional differences in the etiology of HCC across these studies limit the generalizability of the results. NAFLD and metabolic syndrome are among the most commonly identified causes linked to HCC in non-cirrhotic patients in the US [12, 13], while viral hepatitis and alcohol are among the most commonly reported causes in Asian [15, 16], and European studies [9, 10]. However, among non-cirrhotic HCC patients, the degree of liver fibrosis and etiology of liver disease on the risk of HCC development have not been evaluated. Prior studies included small number of participants and reported fibrosis distribution on a single liver disease etiology [17, 18], while other large studies did not report on the fibrosis distribution [12, 13].
Therefore, we aim to study the epidemiology and the fibrosis distribution of noncirrhotic HCC at our institution, including two transplant referral centers in the US. Our secondary aim is to compare the survival outcomes of non-cirrhotic HCC to cirrhotic HCC and to determine if cirrhosis, the cause of liver disease and metabolic risk factors are associated with different survival outcome in patients with HCC.
Patients and Methods
Study Population
We identified all adult patients with the diagnosis of “Malignant neoplasm of liver, primary” and “Malignant neoplasm of liver, not specified as primary or secondary” presented at Cleveland Clinic Main Campus (Cleveland, Ohio) and the Cleveland Clinic Florida (Weston, Florida) between January 2000 and December 2015. Manual review of electronic medical records of each individual was performed. Patients were excluded if HCC was diagnosed prior to 2000, previously transplanted, had liver metastasis, cholangiocarcinoma, fibrolamellar HCC, hepatoblastoma, hepatocholangiocarcinoma, HCC arising in a background of hepatic adenoma, had HCC recurrent disease, uncertain or no HCC diagnosis, or had insufficient information to determine liver cirrhosis or non-cirrhosis status at the time of HCC diagnosis. The protocol was approved by the institutional ethical review board at the Cleveland Clinic.
Definitions
HCC was diagnosed by either pathological diagnosis or radiological diagnosis of Liver Imaging Reporting and Data System (LI-RADS) 5 criteria [19]. In individuals where the diagnosis did not meet the predefined definition, HCC diagnosis was determined based on a multidisciplinary team consensus.
The classification of cirrhosis and no cirrhosis in the present study was based on the criteria proposed by Mittal et al. [12] which had been validated previously [13]. Liver cirrhosis were considered present if the patient exhibit clinical signs of portal hypertension (ascites, varices, or hepatic encephalopathy), had histopathologic diagnosis of cirrhosis, radiological evidence of cirrhosis, or 2/3 abnormal following laboratory results; albumin < 3 g/L, platelet < 200,000/ml and INR > 1.1 within 6 months prior or at the time of HCC diagnosis. Patients without cirrhosis were categorized as patients with histologic diagnosis or with clinical diagnosis of no cirrhosis (categorized as level 1 evidence of no cirrhosis and level 2 evidence of no cirrhosis by Mittal et al., respectively [12]). In the non-cirrhosis group diagnosed histologically included patients who had no clinical or radiological features of cirrhosis, with pathologic evidence of no cirrhosis of the non-tumor liver within 1 year prior to or at the time of HCC diagnosis. On the other hand, in the non-cirrhosis group diagnosed clinically, patients were included if they had aspartate aminotransferase to platelet ratio index (APRI) < 1 on the laboratory test at the time of HCC diagnosis (patient who were diagnosed with alcoholic liver disease received an exemption for this criteria), and 2/3 of the following laboratory investigations were normal; albumin > 3.5 g/L, platelet > 200,000/ml and INR < 1.1, without clinical and radiological features of cirrhosis. Patients who had insufficient information to classify into the aforementioned categories were excluded from the final cohort.
Underlying liver disease were determined by extensive review of physician documentation, and the available laboratory investigations. The categories of liver diseases include hepatitis C, hepatitis B, hepatitis B and C, hepatitis B or C and alcohol use, alcoholic liver disease, NAFLD, hemochromatosis, autoimmune liver disease (primary biliary cholangitis, primary sclerosing cholangitis and autoimmune hepatitis), alpha-1 antitrypsin deficiency, unknown or cryptogenic cause, and other liver disease. NAFLD patients were defined by the histologic or radiologic confirmation of hepatic steatosis, or patients with metabolic risk factors with the exclusion of other causes of liver diseases, as documented by the attending physician. Cryptogenic cause of liver disease was classified in patients who had no other identified causes of cirrhosis.
Patient demographics, comorbidities, smoking and alcohol use status, tumor characteristics and laboratory results were collected. Major vascular invasion was defined as tumor extension into the major venous system in the liver as documented by radiology or pathology. In the non-cirrhosis group, patients who had detailed trichrome stain result available reported by the institution pathologist, Metavir fibrosis staging of the non-tumor liver was collected. The patients were categorized by body mass index (BMI) at the time of HCC diagnosis using WHO criteria as underweight (BMI < 18.5), normal to overweight (BMI of ≥ 18.5 and < 30), non-morbid obese (BMI of ≥ 30 and < 40), and morbid obese (BMI ≥ 40). The initial HCC treatment assigned to each patient were collected and liver transplantation at any time after the diagnosis of HCC was noted.
For the survival analysis, time to event was defined by the number of years from diagnosis of HCC to death. Data was censored at the time of last follow-up visit, the time when patient is last known to be alive, or 31 December 2019, whichever occurred first.
Statistical Analysis
Descriptive statistics were presented by mean and standard deviation or median and percentiles for continuous variables and frequencies for categorical variables. Statistical difference between the group with cirrhosis and without cirrhosis were tested using the Chi-square test, fisher exact test, student t test or Mann-Whiney U test as appropriate. To assess the variables associated with survival over time, multivariable Cox proportional hazard regression analysis was performed to adjust for the prespecified variables (Model 1: age, gender, race, Albumin-Bilirubin (ALBI) grade, INR, Alpha-fetoprotein (AFP), largest tumor size, major vascular invasion, and metastasis; Model 2: age, gender, race, ALBI grade, INR, AFP, largest tumor size, major vascular invasion, metastasis, tumor resection, liver transplantation, and treatment with palliative aim). Multivariable analysis was performed on all HCC cases and subgroups of patient with and without cirrhosis. Survival rate was estimated using Kaplan–Meier method, and the difference between the function of cirrhosis status were compared using log-rank test. All statistical analyses were performed using Stata version 15.1 (Stata corp, LCC, Texas) and p value of < 0.05 was considered statistically significant.
Results
Patient Demographics and Etiologies of Liver Disease
Of 4503 patients identified, exclusion criteria were met in 2266 patients. Our final HCC cohort comprised of 2237 patients (Fig. 1). There were 1947 HCC patients (87%) with liver cirrhosis and 290 patients (13%) without liver cirrhosis. The incidence rate of HCC in non-cirrhotic compared to all HCC decreased over time. The proportion of HCC in the non-cirrhotics to all HCC every 4 years from 2000–2015 were 22.1%, 16.3%, 13.4% and 10.6% (p for trend < 0.001). Histologic evidence of no cirrhosis was apparent in 243 patients (83.8%), while 47 patients (16.2%) had clinical diagnosis of no cirrhosis. Mean platelet count was higher in the clinical diagnosis group than histologic diagnosis group. Albumin, total bilirubin, INR, and APRI score were similar between the two groups. (Supplementary Table 1).
Fig. 1.
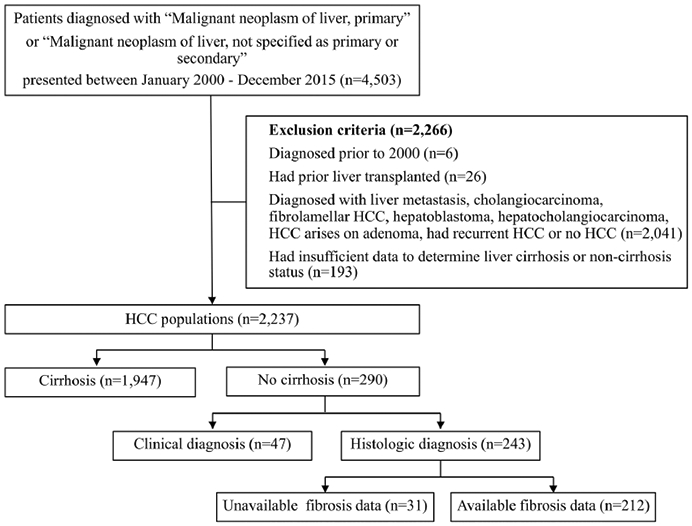
Flow diagram of patient selection
Demographics, etiologies of liver disease and laboratory data of HCC patients with and without cirrhosis are shown in Table 1. Compared to patients with cirrhosis, patients without liver cirrhosis were older (67.4 ± 12.5 vs. 62.7 ± 9.7, p < 0.001), had lower proportion of male gender (71.0% vs. 79.6%, p = 0.001), had more concurrent diagnosis of hypertension (64.5% vs. 56.8%, p = 0.014), hyperlipidemia (37.9% vs. 23.6%, p < 0.001) and 3 or more metabolic risk factors (31.5% vs. 25.4%, p = 0.027). In cirrhotic patients, significant alcohol use was more likely to be observed (32.4% vs. 20.8%, p < 0.001), while smoking status was similar. The median follow-up time was slightly longer in non-cirrhotic group (1.3 years (0.4, 4.1) vs. 1.0 years (0.2, 4.2), p = 0.092) with lower mortality (37.2% vs. 51.3%, p < 0.001).
Table 1.
Demographic and laboratory data
| All HCC (n = 2237) | Non-cirrhotic HCC (n = 290) | Cirrhotic HCC (n = 1947) | p value | |
|---|---|---|---|---|
| Age at HCC diagnosis, year, mean ± SD | 63.3 ± 10.3 | 67.4 ± 12.5 | 62.7 ± 9.7 | < 0.001 |
| Male gender, n(%) | 1755 (78.5) | 206 (71.0) | 1549 (79.6) | 0.001 |
| Diagnosis year, n(%) | 0.001 | |||
| 2000–2003 | 113 (5.1) | 25 (8.6) | 88 (4.5) | |
| 2004–2007 | 430 (19.2) | 70 (23.3) | 360 (18.5) | |
| 2008–2011 | 704 (31.5) | 95 (31.0) | 614 (31.5) | |
| 2012–2015 | 990 (44.3) | 105 (36.2) | 885 (45.5) | |
| Race, n(%) | 0.152 | |||
| Caucasian | 1762 (78.8) | 228 (78.6) | 1534 (78.8) | |
| African-American | 308 (13.8) | 38 (13.1) | 270 (13.9) | |
| Asian | 72 (3.2) | 16 (5.5) | 56 (2.9) | |
| Hispanic | 41 (1.8) | 4 (1.4) | 37 (1.9) | |
| Unknown | 54 (2.4) | 4 (1.4) | 50 (2.6) | |
| Body mass index, kg/m2, mean ± SD | 28.5 ± 5.9 | 27.9 ± 5.8 | 28.6 ± 6.0 | 0.064 |
| Etiology, n(%) | < 0.001 | |||
| Hepatitis C | 873 (39.0) | 55 (19.0) | 818 (42.0) | |
| Hepatitis C/alcohol | 201 (9.0) | 1 (0.3) | 200 (10.3) | |
| Hepatitis B | 129 (5.8) | 24 (8.3) | 105 (5.4) | |
| Hepatitis B/alcohol | 10 (0.5) | 0 | 10 (0.5) | |
| Hepatitis B and C | 40 (1.8) | 0 | 40 (2.1) | |
| Alcohol | 293 (13.1) | 16 (5.5) | 277 (14.2) | |
| NAFLD | 346 (15.5) | 73 (25.2) | 273 (14.0) | |
| Hemochromatosis | 40 (1.8) | 5 (1.7) | 35 (1.8) | |
| Autoimmune | 52 (2.3) | 0 | 52 (2.7) | |
| Alpha-1 antitrypsin deficiency | 9 (0.4) | 0 | 9 (0.5) | |
| Cryptogenic | 241 (10.8) | 116 (40.0) | 125 (6.4) | |
| Other | 3 (0.1) | 0 | 3 (0.2) | |
| Fibrosis stage, n(%)a | ||||
| F0 | 72 (34.0) | |||
| F1 | 38 (17.9) | |||
| F2 | 49 (23.1) | |||
| F3 | 53 (25.0) | |||
| Hypertension, n(%) | 1293 (57.8) | 187 (64.5) | 1106 (56.8) | 0.014 |
| Diabetes mellitus, n(%) | 814 (36.4) | 102 (35.2) | 712 (36.6) | 0.640 |
| Hyperlipidemia, n(%) | 569 (25.5) | 110 (37.9) | 459 (23.6) | < 0.001 |
| Having 3 or more metabolic risk factors, n(%) | 584 (26.2) | 91 (31.5) | 493 (25.4) | 0.027 |
| Ever smoker, n(%)a | 1614 (72.4) | 200 (69.0) | 1414 (72.9) | 0.164 |
| Significant alcohol use, n(%)a | 686 (30.9) | 60 (20.8) | 626 (32.4) | < 0.001 |
| Platelet count, k/mcL, mean ± SD | 140.1 ± 94.0 | 267.7 ± 95.4 | 121.1 ± 77.5 | < 0.001 |
| MPV, fL, mean ± SDa | 11.0 ± 2.9 | 10.3 ± 1.1 | 11.1 ± 3.1 | < 0.001 |
| AST, U/L, median (IQR) | 70 (45, 116) | 44 (26, 72) | 75 (48, 121.5) | < 0.001 |
| ALT, U/L, median (IQR) | 46 (29, 78) | 34 (20, 60) | 49 (30, 81) | < 0.001 |
| Albumin, g/dl, mean ± SD | 3.4 ± 0.7 | 4.0 ± 0.5 | 3.3 ± 0.7 | < 0.001 |
| Bilirubin, g/dl, median (IQR) | 1.1 (0.7, 2.1) | 0.5 (0.4, 0.7) | 1.3 (0.8, 2.4) | < 0.001 |
| INR, mean ± SD | 1.21 ± 0.44 | 1.01 ± 0.07 | 1.24 ± 0.47 | < 0.001 |
| Creatinine, mg/dl, median (IQR)a | 0.89 (0.71, 1.1) | 0.9 (0.8, 1.1) | 0.9 (0.7, 1.1) | 0.166 |
| Na, mmol/L, mean ± SDa | 136.8 ± 5.2 | 138.6 ± 3.3 | 136.5 ± 5.4 | < 0.001 |
| APRI score, median (IQR) | 1.7 (0.8, 3.1) | 0.4 (0.3, 0.7) | 1.9 (1.1, 3.4) | < 0.001 |
| ALBI Grade, n(%) | < 0.001 | |||
| Grade 1 | 590 (26.4) | 208 (71.7) | 382 (19.6) | |
| Grade 2 | 1166 (52.1) | 76 (27.2) | 1087 (55.8) | |
| Grade 3 | 481 (21.5) | 3 (1.0) | 478 (24.6) | |
| AFP, ng/dl, median (IQR)a | 28 (6.1, 430) | 17.4 (3.7, 589.3) | 29.1 (6.5, 413.2) | 0.012 |
| AFP > 10 ng/dl, n(%)a | 1412 (65.1) | 158 (56.4) | 1254 (66.4) | 0.001 |
| Follow-up time, year, median (IQR) | 1.0 (0.2, 4.2) | 1.3 (0.4, 4.1) | 1.0 (0.2, 4.2) | 0.092 |
| Death at last follow up, n(%) | 1106 (49.4) | 108 (37.2) | 998 (51.3) | < 0.001 |
HCC hepatocellular carcinoma, NAFLD non-alcoholic fatty liver disease, MPV mean platelet volume, AST aspartate aminotransferase, ALT alanine aminotransferase, APRI aspartate aminotransferase to platelet ratio index, ALBI albumin to bilirubin, AFP alpha-fetoprotein
Data not available for all subjects, data available for non-cirrhotic HCC groups: fibrosis stage 212; smoke 290; alcohol 288; MPV 275; Cr 289; Na 289, data available for cirrhotic HCC groups: smoke 1940; alcohol 1930; MPV 1751; Cr 1932; Na 1932
The most common underlying liver disease in non-cirrhotic patients was cryptogenic cause (40%) followed by NAFLD (25.2%) and HCV (19.0%). In cirrhotic patients, HCV (42.0%) was the most common cause, followed by alcoholic cirrhosis (14.2%) and NAFLD (14.0%). Cryptogenic cause accounts for 6.4% of cirrhotic patients.
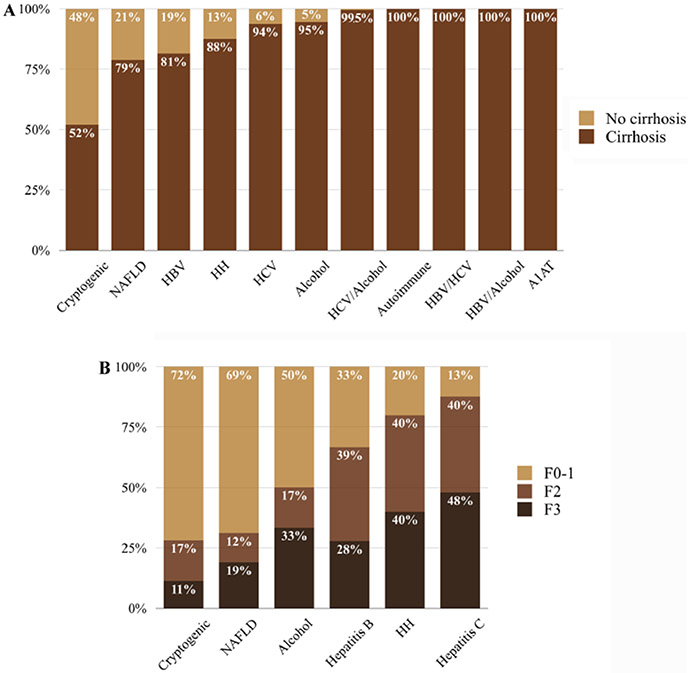
The percentage of HCC in non-cirrhotic and cirrhotic liver in each etiology of liver disease is shown in Fig. 2a. Almost all patients with combined hepatitis C and alcohol use as the underlying etiology of liver disease had HCC with background liver cirrhosis (99.5%). Hepatitis B and C coinfection, hepatitis B and alcohol use, autoimmune liver disease and alpha-1 antitrypsin deficiency were only observed in HCC patients with cirrhosis.
Fig. 2.
a Percentage of HCC in non-cirrhotic and cirrhotic liver to all HCC cases, b Metavir fibrosis stage distribution of non-tumor liver in patients with HCC by etiologies of liver disease; NAFLD non-alcoholic fatty liver disease, HBV hepatitis B, HH hereditary hemochromatosis, HCV hepatitis C, A1AT alpha 1 antitrypsin deficiency
In the non-cirrhotic group, laboratory results showed higher platelet count, albumin and sodium levels, lower mean platelet volume, transaminases, bilirubin, INR and APRI score compared to the cirrhotic group (all p < 0.05). Median AFP level was higher in the cirrhotic group (29.1 (6.5, 413.2) vs.17.4 (3.7, 589.3), p = 0.012).
Tumor Characteristics and Treatment
The tumor character and treatment received are shown in Table 2. In non-cirrhotic compared to cirrhotic patients, more patients had histologic confirmation of HCC (96.6% vs. 57.3%, p < 0.001), have significantly larger tumor size [7.8 (5, 11.5) vs. 3.7 (2.5, 6.1), p < 0.001] and fall outside Milan criteria at presentation (20.7% vs. 45.8%, p < 0.001). Major vascular invasion was more common in cirrhosis patients (16.4% vs. 7.2%, p < 0.001). More patients in the non-cirrhotic HCC group underwent liver resection (52.1% vs. 5.7%, p < 0.001), while more patients in the cirrhotic HCC group received other curative treatment, including tumor ablative therapy, liver transplantation, and locoregional palliative treatment (p < 0.05 for all).
Table 2.
Tumor characteristics and treatment
| All HCC (n=2237) |
Non-cirrhotic HCC (n = 290) |
Cirrhotic HCC(n = 1947) |
p value | |
|---|---|---|---|---|
| Histologic confirmation of HCC, n(%) | 1395 (62.4) | 280 (96.6) | 1115 (57.3) | < 0.001 |
| Largest tumor diameter, cm, median (IQR) | 4 (2.6, 7) | 7.8 (5, 11.5) | 3.7 (2.5, 6.1) | < 0.001 |
| Largest tumor size > 5 cm, n(%) | 868 (38.8) | 215 (74.1) | 653 (33.5) | < 0.001 |
| Major vascular invasion, n(%) | 340 (15.2) | 21 (7.2) | 319 (16.4) | < 0.001 |
| Metastasis, n(%) | 182 (8.1) | 28 (9.7) | 154 (7.9) | 0.310 |
| BCLC stage, n(%) | < 0.001 | |||
| Very early | 150 (6.7) | 11 (3.8) | 139 (7.1) | |
| Early | 674 (30.1) | 49 (16.9) | 625 (32.1) | |
| Intermediate | 749 (33.5) | 172 (59.3) | 577 (29.6) | |
| Advanced | 403 (18.0) | 54 (18.6) | 349 (17.9) | |
| Terminal | 261 (11.7) | 4 (1.4) | 257 (13.2) | |
| Tumor within Milan criteria, n(%) | 952 (42.6) | 60 (20.7) | 892 (45.8) | < 0.001 |
| Received transplant, n(%) | 520 (23.3) | 7 (2.4) | 513 (26.4) | < 0.001 |
| Initial treatment received, n(%) | ||||
| Resection | 261 (11.7) | 151 (52.1) | 110 (5.7) | < 0.001 |
| Curative locoregional therapya | 171 (7.6) | 12 (4.1) | 159 (8.2) | 0.013 |
| Liver transplantation | 212 (9.5) | 2 (0.7) | 210 (10.8) | < 0.001 |
| Bridging therapy and transplantation | 292 (13.1) | 3 (1.0) | 289 (14.8) | < 0.001 |
| Palliative locoregional therapyb | 578 (25.8) | 43 (14.8) | 535 (27.5) | < 0.001 |
| Systemic therapy | 161 (7.2) | 17 (5.9) | 144 (7.4) | 0.395 |
| Other | 7 (0.3) | 1 (0.3) | 6 (0.3) | 1.000 |
| Supportive care | 428 (19.1) | 57 (19.7) | 371 (19.1) | 0.808 |
| Unknown | 127 (5.7) | 4 (1.4) | 123 (6.3) | 0.001 |
Radiofrequency ablation, microwave ablation, direct ethanol injection
Trans arterial chemoembolization, Y90 radioembolization, stereotactic body radiation therapy
Fibrosis Stage Distribution Among HCC Patients Without Liver Cirrhosis
There were 212 non-cirrhotic patients that had fibrosis stage data available. The Metavir fibrosis stage distribution in each of the etiologies of liver disease are shown in Fig. 2b and Table 3. The percentage of non-significant liver fibrosis (F0-F1) in cryptogenic liver disease, NAFLD, alcoholic liver disease, hepatitis B (HBV), hereditary hemochromatosis and HCV were 72%, 69%, 50%, 33%, 20%, and 13%, respectively. The percentage of bridging fibrosis (F3) in HCV, hereditary hemochromatosis, alcoholic liver disease, HBV, NAFLD and cryptogenic liver disease were 48%, 40%, 33%, 28%, 19%, and 11%, respectively.
Table 3.
Metavir fibrosis stage of HCC patients without liver cirrhosis (only patients with available detailed trichrome stain data; n = 212)
| Liver disease, n(%) | F0 | F1 | F2 | F3 |
|---|---|---|---|---|
| Cryptogenic (n = 71) | 43 (60.6) | 8 (11.3) | 12 (16.9) | 8 (11.3) |
| NAFLD (n = 58) | 23 (39.7) | 17 (29.3) | 7 (12.0) | 11 (19.0) |
| Hepatitis C (n = 48) | 3 (6.3) | 3 (6.3) | 19 (39.6) | 23 (47.9) |
| Hepatitis B (n = 18) | 2 (11.1) | 4 (22.2) | 7 (38.9) | 5 (27.8) |
| Alcoholic liver disease (n = 12) | 1 (8.3) | 5 (41.7) | 2 (16.7) | 4 (33.3) |
| Hereditary hemochromatosis (n = 5) | 0 | 1 (20) | 2 (40) | 2 (40) |
NAFLD nonalcoholic fatty liver disease
Patient Survivals and Factors Associated with Patient Survivals
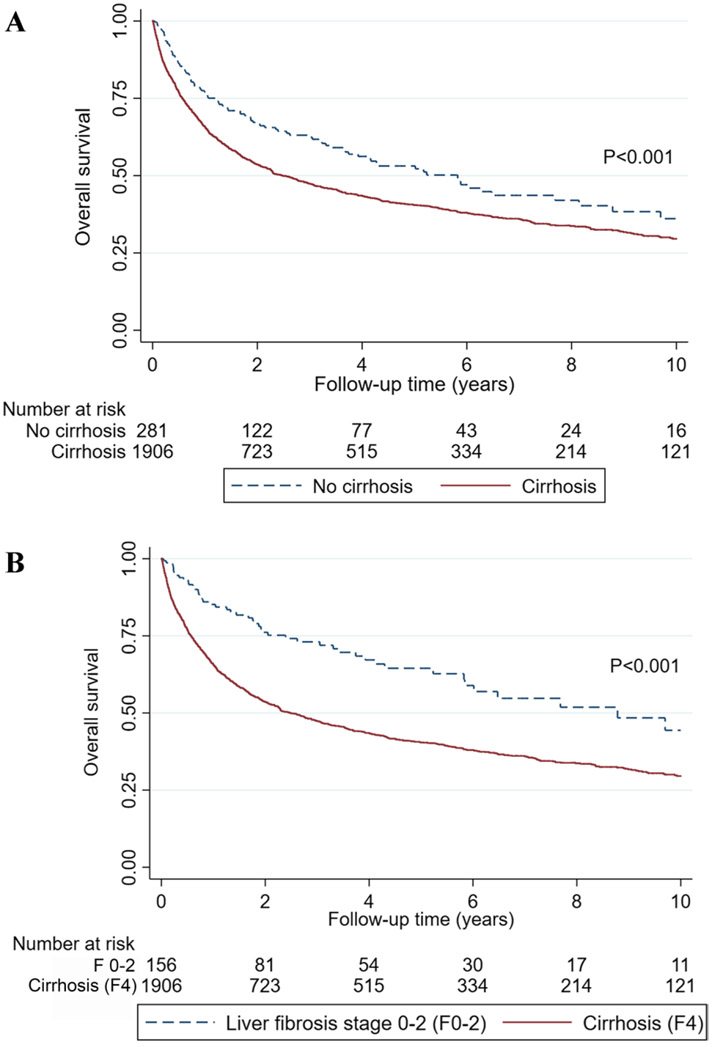
Liver cirrhosis was associated with significant shorter patient survivals (p < 0.001) (Fig. 3a). The median survival in patients with cirrhosis and those without were 2.5 years; 95% CI 2.2–3.0, and 5.8 years; 95%CI 3.7–8.1, respectively. Univariable and Multivariable analysis on patient survival in all HCC patients, and subgroup of patients with and without cirrhosis, are shown in Table 4, supplementary table 2 and 3, respectively. Having liver cirrhosis was associated with increased risk of death compared to patients with no cirrhosis after adjusting for baseline factors, tumor characteristics and treatment (adjusted HR 1.48; 95%CI 1.21–1.82, p < 0.001). Morbid obesity (adjusted HR 1.31; 95%CI 1.01–1.69, p = 0.040), underweight (adjusted HR 2.06; 95%CI 1.27–3.34, p = 0.004), and combined viral hepatitis and alcohol use (HR 1.51; 1.23–1.88, p < 0.001) were associated with worse patient’s survival after adjusting for baseline factors, tumor characteristics and treatment. Multivariate analysis in HCC patients with and without cirrhosis demonstrated that the cause of liver disease, except for combined viral hepatitis and alcohol use in patients with cirrhosis (HR 1.48; 1.21-1.81, p < 0.001), non-morbid obesity, diabetes, and having 3 or more metabolic risk factors were not associated with change in survival. Morbid obesity in non-cirrhotics (adjusted HR 2.55; 95%CI 1.08–6.00, p = 0.032) and cirrhotics (1.31; 95%CI 1.00–1.72, p = 0.049) and being underweight (adjusted HR 1.73; 1.05–2.86, p = 0.032) in cirrhotics were associated with significantly worse survival outcome.
Fig. 3.
Overall survival of HCC patients a stratified by no cirrhosis and cirrhosis, b stratified by liver fibrosis stage 0–2 (F0–2) and cirrhosis (F4)
Table 4.
Multivariable analysis of factors associated with patient survival
| Variables | Unadjusted HR (95% CI) |
p value | Model 1a Adjusted HR (95% CI) |
p value | Model 2b Adjusted HR (95% CI) |
p value |
|---|---|---|---|---|---|---|
| Liver cirrhosis | 1.45 (1.19–1.77) | < 0.001 | 1.74 (1.45–2.09) | < 0.001 | 1.48 (1.21–1.82) | < 0.001 |
| NAFLD | 0.98 (0.83–1.15) | 0.812 | 0.94 (0.80–1.12) | 0.499 | 1.08 (0.91–1.28) | 0.381 |
| Hepatitis C | 0.90 (0.80–1.02) | 0.094 | 1.06 (0.92–1.21) | 0.431 | 1.06 (0.93–1.21) | 0.373 |
| Alcoholic liver disease | 1.35 (1.14–1.59) | < 0.001 | 1.28 (1.08–1.52) | 0.004 | 1.07 (0.90–1.28) | 0.415 |
| Hepatitis B | 0.81 (0.62–1.06) | 0.122 | 1.00 (0.75–1.32) | 0.974 | 0.94 (0.72–1.24) | 0.677 |
| Viral hepatitis and alcohol | 1.62 (1.34–1.95) | < 0.001 | 1.71 (1.40–2.09) | < 0.001 | 1.51 (1.23–1.88) | < 0.001 |
| Underweight (BMI < 18.5 kg/m2)c | 1.97 (1.24–3.15) | 0.004 | 1.83 (1.13–2.97) | 0.015 | 2.06 (1.27–3.34) | 0.004 |
| Obesity (BMI ≥ 30 kg/m2)c | 1.01 (0.88–1.15) | 0.921 | 1.07 (0.94–1.23) | 0.306 | 1.06 (0.93–1.22) | 0.361 |
| Morbid obesity (BMI ≥ 40 kg/m2)c | 1.54 (1.20–1.98) | 0.001 | 1.40 (1.09–1.81) | 0.010 | 1.31 (1.01–1.69) | 0.040 |
| Diabetes mellitus | 0.95 (0.84–1.07) | 0.414 | 0.96 (0.85–1.09) | 0.557 | 1.04 (0.91–1.18) | 0.566 |
| Having 3 or more metabolic risk factors | 0.94 (0.82–1.07) | 0.365 | 0.95 (0.82–1.08) | 0.419 | 1.02 (0.89–1.17) | 0.780 |
NAFLD nonalcoholic fatty liver disease, BMI body mass index
Model 1: variables adjusted for age, gender, race, ALBI grade, INR, AFP, largest tumor size, major vascular invasion, and metastasis
Model 2: variables adjusted for age, gender, race, ALBI grade, INR, AFP, largest tumor size, major vascular invasion, metastasis, tumor resection, liver transplantation, and treatment with palliative aim
Compared to normal BMI (≥ 18.5 and < 30 kg/m2)
Given that there is a risk of misclassifying early cirrhosis changes as F3 on liver biopsy, sub-analysis comparing the survivals of patients with liver fibrosis stage 0–2 (F0-2) and cirrhosis (F4) was performed. Overall survival in patients with F0-2 and cirrhosis is shown in (Fig. 3b) (p < 0.001). Patients with cirrhosis had significantly shorter overall survival compared to patients with F0-2 as demonstrated by univariable analysis (HR 2.02; 95%CI 1.52–2.69), multivariable analysis after adjusting for baseline factors, and tumor characteristics (adjusted HR 2.78; 95%CI 2.03–3.80), and after adjusting for baseline factors, tumor characteristics and treatment (adjusted HR 1.88; 95%CI 1.33–2.66), all p < 0.001.
Discussion
There are four major findings in our study. First, 13% of HCC in our cohort developed in non-cirrhotic liver background with decreasing frequency overtime. Second, HCC patients without cirrhosis had significantly longer survival than those with cirrhosis despite larger tumor size and higher likelihood of being beyond Milan criteria on presentation. Third, fibrosis distribution of the non-tumor liver background in non-cirrhotic patients varied depending on the cause of liver disease. Fourth, both extreme ends of BMI were associated with worse overall survival in HCC patients.
The prevalence of 13% HCC cases occurring in non-cirrhotic in our study is similar to other US cohorts that used the same criteria to determine cirrhosis status [12, 13]. Also in line with previously published studies, the ratio of non-cirrhotic HCC to all HCC cases is declining [13]. One likely explanation is the increase in incidence of HCC in cirrhotic patients. HCV, NAFLD and alcoholic liver disease are the three main causes of liver disease in our cohort. NAFLD and alcohol related cirrhosis are on the rise and although HCV-related cirrhosis is declining, HCV remains one of the major causes of liver cirrhosis [20], and having the highest risk for developing HCC compared to other liver diseases [21]. Combined with decreasing rate of mortality in patients with liver cirrhosis [20] the number of HCC cases in liver cirrhosis is predicted to be on the rise.
Larger tumor size and less patients within Milan criteria in noncirrhotic HCC patients is likely related to lack of surveillance in this patient population. A prior study reported that only 14.7% of non-cirrhotic HCC patients received regular HCC surveillance within 2 years before HCC diagnosis [13]. In our study, major vascular invasion was more common in patients with liver cirrhosis. To date, the mechanism of portal vein tumor thrombosis remains largely unknown with direct tumor extension and a few molecular pathways have been proposed [22]. The higher proportion of cirrhotic patients with vascular invasion reported in our study might be related to different tumor microenvironment. It is also important to note that many of our patients were referred to our institution after their initial diagnosis which might partly account for the high percentage of vascular involvement in cirrhotic population. Future research is needed to confirm this finding.
Previous studies reported similar survival rate in HCC patients with and without liver cirrhosis [23] while others reported poorer prognosis in patients with cirrhosis [13, 24], which might be due to the difference in the inclusion criteria of these studies. The study that reported similar survival rate included only patients who were transplanted or had liver resection [23]. However, the studies which reported lower survival rate in HCC patients with cirrhosis compared to non-cirrhotic patients included all patients regardless of the treatment modality received [13] or only the cases received curative resection [24]. In our study, HCC patients without cirrhosis had longer survival despite larger tumor size at presentation. These patients were more likely to undergo hepatic resection as the non-cirrhotic patients tended to have better hepatic function. After adjusting for baseline factors, tumor character and treatment, HCC patients with liver cirrhosis are associated with approximately 1.5 fold higher risk of death compared to patients without cirrhosis, and 1.9 fold higher risk of death compared to patients with F0-F2. This could be due to worse hepatic function and reserve and increased risk of complications in cirrhotic patients.
It is known that HCC has complex hepatocarcinogenesis and various mechanisms contribute to HCC development, including genetic and epigenetic alteration, inflammation and immune response, oxidative stress [25] and premalignant environment, characterized by chronic hepatic cell death, inflammation and fibrosis [8]. The cellular and molecular mechanisms significantly differ across diverse etiologies of liver disease [25]. Our results showed that fibrosis distribution was different in each etiology of liver disease. This suggests that fibrosis plays a role in the pathogenesis of HCC. However, its role may vary among the different etiologies of liver disease. Our results showed that in patients with hepatitis B and C coinfection, combined viral hepatitis infection and alcohol use, autoimmune liver disease and alpha-1 antitrypsin deficiency, HCC develops mainly in patients with liver cirrhosis, which is a well-known risk factor of HCC. This might be explained by the accelerated process of developing liver fibrosis in patients with combined etiologies of liver disease. The low risk of HCC in autoimmune liver disease [26, 27] and alpha-1 antitrypsin deficiency patients [28] without cirrhosis was previously established. This is likely because the risk of HCC is due to cirrhosis and not due to the underlying liver disease, as the disease-specific pathogenesis might not be associated with HCC development. On the other hand, in patients with NAFLD, cryptogenic and alcoholic liver disease developed HCC without advanced fibrosis or cirrhosis. This suggests that a mechanism not related to fibrosis could be more prominent in these diseases. Inflammatory process associated with obesity and insulin resistance is one of the proposed tumorigenesis mechanism in NAFLD patients [29]. Oxidative stress induction, and interferences of host anti-tumor mechanisms by alcohol could facilitate HCC development [30]. There is extremely limited data in HCC patients with unknown cause of liver disease without cirrhosis. In hepatitis B endemic area, approximately half of the cryptogenic HCC without liver cirrhosis had occult hepatitis B infection [23]. Other possible causes in these patients are an exposure to chemical carcinogens, and germline mutations [31]. Therefore, in addition to focusing our treatment on preventing the progression of fibrosis to cirrhosis, identifying and further exploring the mechanisms by which these etiologies lead to the development of HCC are warranted in order to craft etiology-based HCC prevention strategies. Among non-cirrhotic patients, it is also important to note that despite the difference in fibrosis distribution in each etiology of liver disease, survival rates were similar among the different etiologies of liver disease after adjusting for tumor character and treatment.
Extreme BMI, on both ends of the spectrum, has been demonstrated to be independently associated with increased mortality in cirrhotic HCC patients in our cohort. There is substantial evidence that obesity, as defined by elevated BMI, is associated with poor prognosis in HCC patients [32]. In contrast, there is limited evidence showing that being underweight has a negative impact on HCC patient’s survival [33]. Underweight defined by BMI of < 18.5 is one of the parameters indicating malnutrition in patients with cirrhosis [34]. Cirrhotic patients with malnutrition are more prone to complications and infections [35]. Therefore, nutritional support aimed to improve nutritional status should be considered as part of the treatment regimen in this patient population. Diabetes mellitus is not associated with HCC survival in our study. Previous studies investigating HCC prognosis in diabetes patients showed discrepant results [36, 37] with varied results between the study populations. Diabetes was shown to worsen the prognosis in patients with earlier HCC [37, 38], and whom underwent hepatectomy [36, 39]. Metabolic syndrome is an emerging factor associated with HCC development. However, it is not independently associated with patient survival in our study. This finding is in concordance with what was previously described in the literature [40]. Morisco et al. demonstrated that patients with a greater number of metabolic risk factors had a better liver function despite having advanced tumor stage as compared to patients with lower number of metabolic risk factors [40]. Our study did not replicate previously reported advanced tumor stage in metabolic syndrome, however, we observed better liver function in this patient population. It has been previously suggested that in metabolic syndrome-related HCC, tumor may develop early before the patient develop severe liver fibrosis [41]. The better liver function may explain why the presence of metabolic syndrome does not affect survival in our study.
Our study had several limitations. First, our study population derived from a referral institution with transplant capability, which could limit generalizability of the data and generate possible referral biases. Second, the retrospective nature of our study resulted in a few missing data. Third, there is a potential risk of misclassifying F3 and early cirrhosis based on liver biopsy; however, laboratory data of patients with F3 in our cohort were cross-checked, and they are all in agreement with the robust laboratory criteria used for non-cirrhosis in our study which could minimize this risk. The strength of our study includes manual review of all patients’ electronic medical records to minimize misclassification biases, the inclusion of only patients with available trichrome stain as reported by our institution pathologist to curtail the heterogeneity of histologic reports of the non-tumor liver, and the inclusion of patients with pathological and clinical diagnosis of no-cirrhosis in our cohort could provide a more accurate representation of the true burden and survival of HCC in non-cirrhotic liver disease. Since many patients with less advanced disease underwent surgical resection and transplantation, the majority of the histologic data were obtained from the resected or explanted liver.
In conclusion, our study revealed different fibrosis distribution among the different etiologies of liver disease in non-cirrhotic HCC patients. This finding could further aide our understanding of how fibrosis is involved in HCC development in relation to the underlying cause of liver disease. Despite more advanced disease, HCC patients without cirrhosis had better prognosis than those with cirrhosis.
Supplementary Material
Funding
Supported in part by RO1 GM119174 (SD); RO1 DK113196 (SD); P50 AA024333 (AJM, SD); UO1 AA 021890 (AJM, SD); UO1 DK 061732 (AJM, SD).
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10620-021-07048-5.
Declarations
Conflict of interest The authors declare no conflict of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Hainaut P, Gores GJ et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirk GD, Lesi OA, Mendy M et al. The Gambia Liver Cancer Study: Infection with hepatitis B and C and the risk of hepatocellular carcinoma in West Africa. Hepatology 2004;39:211–219. [DOI] [PubMed] [Google Scholar]
- 4.Gao JD, Shao YF, Xu Y et al. Tight association of hepatocellular carcinoma with HBV infection in North China. Hepatobiliary Pancreat Dis Int 2005;4:46–49. [PubMed] [Google Scholar]
- 5.Ohishi W, Fujiwara S, Cologne JB et al. Risk factors for hepatocellular carcinoma in a Japanese population: a nested case-control study. Cancer Epidemiol Biomarkers Prev 2008;17:846–854. [DOI] [PubMed] [Google Scholar]
- 6.Dorfman JD, Schulick R, Choti MA et al. Differences in characteristics of patients with and without known risk factors for hepatocellular carcinoma in the United States. World J Gastroenterol 2007;13:781–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alqahtani A, Khan Z, Alloghbi A et al. Hepatocellular carcinoma: molecular mechanisms and targeted therapies. Medicina (Kaunas) 2019;55:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Affo S, Yu LX, Schwabe RF. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev Pathol 2017;12:153–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schutte K, Schulz C, Poranzke J et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol 2014;14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giannini EG, Marenco S, Bruzzone L et al. Hepatocellular carcinoma in patients without cirrhosis in Italy. Dig Liver Dis 2013;45:164–169. [DOI] [PubMed] [Google Scholar]
- 11.Kaczynski J, Hansson G, Wallerstedt S. Diabetes: one of few remarkable differences in clinicopathologic features between cirrhotic and noncirrhotic Swedes with hepatocellular carcinoma. Dig Dis Sci 2006;51:796–802. 10.1007/s10620-006-3209-9 [DOI] [PubMed] [Google Scholar]
- 12.Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14:124–131 e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gawrieh S, Dakhoul L, Miller E et al. Characteristics, aetiologies and trends of hepatocellular carcinoma in patients without cirrhosis: a United States multicentre study. Aliment Pharmacol Ther 2019;50:809–821. [DOI] [PubMed] [Google Scholar]
- 14.Leung C, Yeoh SW, Patrick D et al. Characteristics of hepatocellular carcinoma in cirrhotic and non-cirrhotic non-alcoholic fatty liver disease. World J Gastroenterol 2015;21:1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar M, Kumar R, Hissar SS et al. Risk factors analysis for hepatocellular carcinoma in patients with and without cirrhosis: a case-control study of 213 hepatocellular carcinoma patients from India. J Gastroenterol Hepatol 2007;22:1104–1111. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, Huang L, Li BK et al. Clinicopathologic features and longterm outcomes of Chinese patients with hepatocellular carcinoma in non-cirrhotic liver. Dig Surg 2008;25:376–382. [DOI] [PubMed] [Google Scholar]
- 17.Bengtsson B, Stal P, Wahlin S et al. Characteristics and outcome of hepatocellular carcinoma in patients with NAFLD without cirrhosis. Liver Int 2019;39:1098–1108. [DOI] [PubMed] [Google Scholar]
- 18.Albeldawi M, Soliman M, Lopez R et al. Hepatitis C virus-associated primary hepatocellular carcinoma in non-cirrhotic patients. Dig Dis Sci 2012;57:3265–3270. 10.1007/s10620-012-2260-y [DOI] [PubMed] [Google Scholar]
- 19.Marrero JA, Kulik LM, Sirlin CB et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 20.Orman ES, Roberts A, Ghabril M et al. Trends in characteristics, mortality, and other outcomes of patients with newly diagnosed cirrhosis. JAMA Netw Open 2019;2:e196412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ioannou GN, Green P, Lowy E et al. Differences in hepatocellular carcinoma risk, predictors and trends over time according to etiology of cirrhosis. PLoS One. 2018;13:e0204412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun JX, Shi J, Li N et al. Portal vein tumor thrombus is a bottleneck in the treatment of hepatocellular carcinoma. Cancer Biol Med 2016;13:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shim CW, Park JW, Kim SH et al. Noncirrhotic hepatocellular carcinoma: etiology and occult hepatitis B virus infection in a hepatitis B virus-endemic area. Therap Adv Gastroenterol 2017;10:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HW, Choi GH, Kim DY et al. Less fibrotic burden differently affects the long-term outcomes of hepatocellular carcinoma after curative resection. Oncology 2017;93:224–232. [DOI] [PubMed] [Google Scholar]
- 25.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 2006;6:674–687. [DOI] [PubMed] [Google Scholar]
- 26.Valean S, Acalovschi M, Dumitrascu DL et al. Hepatocellular carcinoma in patients with autoimmune hepatitis—a systematic review of the literature published between 1989–2016. Med Pharm Rep 2019;92:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lleo A, de Boer YS, Liberal R et al. The risk of liver cancer in autoimmune liver diseases. Ther Adv Med Oncol 2019;11:1758835919861914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Propst T, Propst A, Dietze O et al. Prevalence of hepatocellular carcinoma in alpha-1-antitrypsin deficiency. J Hepatol. 1994;21:1006–1011. [DOI] [PubMed] [Google Scholar]
- 29.Perumpail RB, Liu A, Wong RJ et al. Pathogenesis of hepatocarcinogenesis in non-cirrhotic nonalcoholic fatty liver disease: Potential mechanistic pathways. World J Hepatol 2015;7:2384–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sidharthan S, Kottilil S. Mechanisms of alcohol-induced hepatocellular carcinoma. Hepatol Int 2014;8:452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desai A, Sandhu S, Lai JP et al. Hepatocellular carcinoma in non-cirrhotic liver: a comprehensive review. World J Hepatol 2019;11:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta A, Das A, Majumder K et al. Obesity is independently associated with increased risk of hepatocellular cancer-related mortality: a systematic review and meta-analysis. Am J Clin Oncol 2018;41:874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Xing H, Liu D et al. Negative impact of low body mass index on liver cirrhosis patients with hepatocellular carcinoma. World J Surg Oncol 2015;13:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tandon P, Raman M, Mourtzakis M et al. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology 2017;65:1044–1057. [DOI] [PubMed] [Google Scholar]
- 35.Maharshi S, Sharma BC, Srivastava S. Malnutrition in cirrhosis increases morbidity and mortality. J Gastroenterol Hepatol 2015;30:1507–1513. [DOI] [PubMed] [Google Scholar]
- 36.Wang YG, Wang P, Wang B et al. Diabetes mellitus and poorer prognosis in hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One 2014;9:e95485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su YW, Liu PH, Hsu CY et al. Prognostic impact of diabetes mellitus on hepatocellular carcinoma: Special emphasis from the BCLC perspective. PLoS One 2017;12:e0174333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toyoda H, Kumada T, Nakano S et al. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma. Cancer 2001;91:957–963. [PubMed] [Google Scholar]
- 39.Ting CT, Chen RC, Chen CC et al. Diabetes worsens the surgical outcomes in cirrhotic patients with hepatocellular carcinoma. Tohoku J Exp Med 2012;227:73–81. [DOI] [PubMed] [Google Scholar]
- 40.Morisco F, Guarino M, Valvano MR et al. Metabolic disorders across hepatocellular carcinoma in Italy. Liver Int. 2018;38:2028–2039. [DOI] [PubMed] [Google Scholar]
- 41.Paradis V, Zalinski S, Chelbi E et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology 2009;49:851–859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.