Abstract
Backgrounds
Hepcidin has been identified as a systemic iron-regulatory hormone. Recent studies have suggested that iron metabolism disorders may be involved in the pathogenesis of acute respiratory distress syndrome and multiple organ dysfunction in coronavirus disease 2019 (COVID-19).
Objectives
To re-evaluate the hepcidin-related iron metabolism parameters and explore the relationship between hepcidin-mediated iron dysmetabolism and COVID-19 severity.
Methods
COVID-19 is classified as mild and moderate as non-severe, severe and critical as severe. A meta-analysis was conducted. Four bibliographic databases were comprehensively searched up to December 31st 2021.
Results
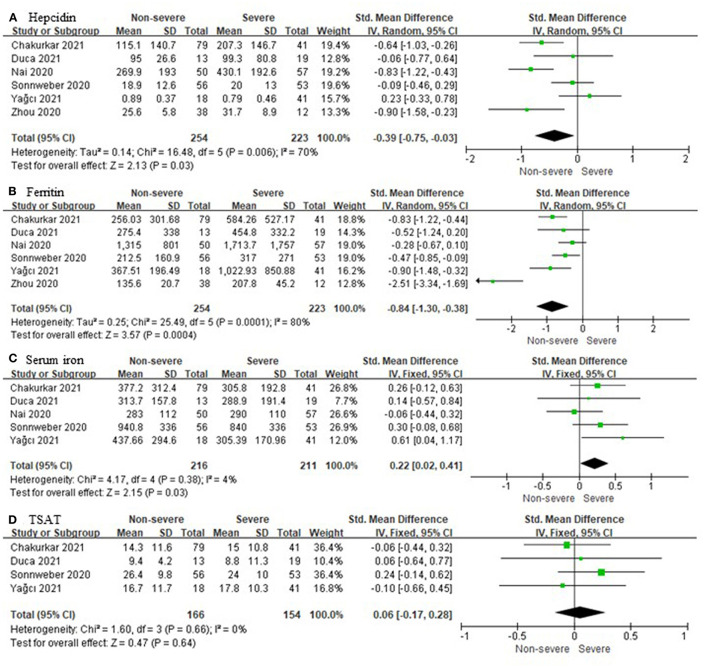
Six unique studies with data from 477 COVID-19 patients were included. Compared to non-severe cases, severe cases had higher hepcidin (standardized mean difference (SMD), −0.39; 95% Confidence Interval (CI) [−0.76, −0.03]; P = 0.03) and ferritin (SMD, −0.84; 95% CI [−1.30, −0.38]; P = 0.0004). In five out of six studies, a total of 427 patients were tested for serum iron, and there were significant differences in their levels between severe and non-severe cases (SMD, 0.22; 95% CI [0.02, 0.41]; P = 0.03). A total of 320 patients from four out of six studies were tested for transferrin saturation, and the statistical difference was not significant (SMD, 0.06; 95% CI [−0.17, 0.28]; P = 0.64).
Conclusion
Severe COVID-19 cases had higher serum levels of hepcidin and ferritin, and lower serum iron, without significant differences in transferrin saturation. Further studies are needed to verify whether targeting the hepcidin-mediated iron metabolism axis may influence the outcome and treatment of COVID-19.
Keywords: COVID-19, ferritin, hepcidin, iron metabolism, severity
Introduction
Since the outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1), the number of confirmed cases has increased rapidly around the world (2, 3). Acute respiratory distress syndrome (ARDS) and multiple organ dysfunction are the main clinical manifestations and leading causes of mortality in severe COVID-19 cases (4). Iron homeostasis is crucial for host immune defense and inflammatory response. Disorders of iron metabolism are mainly manifested as iron deficiency and/or overload, both of which can cause cellular and organ dysfunction (5). Low serum iron (SI) concentration restricts hemoglobin synthesis and causes anemia and systemic hypoxemia. Emerging data have found that hypoferremia is an independent risk factor for hypoxic respiratory failure and death in COVID-19 patients (6, 7). The rapid and excessive accumulation of intracellular iron, especially in macrophages, causes cell and tissue damage, presumably through the production of reactive oxygen species (ROS) catalyzed by iron. In addition, iron overload may also trigger a unique iron-dependent form of non-apoptotic cell death termed ferroptosis (8). Serum ferritin concentration has been proven to reflect the individual's iron storage status. Studies have found that increased serum ferritin levels are associated with adverse outcomes (9–11). Although studies on transferrin saturation (TSAT) have been far fewer than that of ferritin and hypoferritemia, low TSAT has been consistently reported in severe COVID-19, and significantly correlated to lung aeration loss, inflammatory markers and worse outcomes (7, 12–14).
The hepatic peptide hepcidin has been identified as the systemic iron-regulatory hormone and plays an important role in maintaining iron homeostasis (15). Hepcidin regulates intestinal iron absorption, SI concentrations, and tissue iron redistribution by inducing the degradation of its receptor, the sole iron exporter ferroportin (14, 16), and blocking ferroportin export activity (17). Serum hepcidin measurement may be a promising tool for assessing the status of iron metabolism (18), but has not been widely implemented in clinical practice (14, 19, 20). Notably, unlike serum ferritin testing that has been documented in guidelines, hepcidin has been rarely measured in COVID-19 patients (13, 21). As expected in severe inflammatory diseases, recent studies have reported that most COVID-19 patients showed varying degrees of upregulation of hepcidin levels (16, 21–25). More importantly, hepcidin levels have significantly negatively correlated with the ratio of arterial oxygen partial pressure (PaO2) and fraction of inspired oxygen (FiO2), which can be used to predict COVID-19 severity and mortality (22). To adopt the results for clinical use, these interesting findings need to be validated in wider cohorts, and the relationship between hepcidin-mediated iron dysmetabolism and COVID-19 severity also needs to be further analyzed. In view of the particularity of the prevention and control of the COVID-19 epidemic and the randomness of its incidence, large-scale, multi-center, prospective cohort studies are very difficult to achieve. Here we classify mild and moderate as non-severe, severe, and critical as severe, and conduct a meta-analysis to re-evaluate the hepcidin-related iron metabolism parameters (hepcidin, ferritin, iron, and TSAT) in COVID-19 patients and explore the relationship between iron dysmetabolism and severity.
Methods
Research Strategy
This meta-analysis was performed following a recently published guideline and reported according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (26). We searched comprehensively MEDLINE (National Library of Medicine, US), EMBASE (Elsevier, Netherlands), Cochrane (Cochrane Collaboration, UK), and the WHO COVID-19 database to identify relevant articles up to December 31, 2021. Keywords include “COVID-19,” “SARS-CoV-2,” and iron metabolism biomarkers, including “hepcidin,” “ferritin,” “serum iron,” “transferrin,” “soluble transferrin receptor,” “unsaturated iron binding capacity,” and “transferrin saturation,” The detailed search strategy is given in Supplementary File 1.
Study Selection and Eligibility Criteria
We follow the international guidelines for the diagnosis and treatment of COVID-19. The detailed severity classification criteria used in the evaluation of original studies and meta-analyses include: (1) Mild: asymptomatic or mild clinical symptoms, and no pneumonia on imaging, or outward treatment; (2) Moderate: fever, respiratory tract symptoms, pneumonia on imaging, and inward treatment; (3) Severe: dyspnea, or respiratory rate >30 breaths/min at rest, or oxygen saturation <93%, or PaO2/FiO2 < 300 mmHg; (4) Critical: respiratory failure and need for mechanical ventilation, or shock, or combined with other organ failure should be treated in the Intensive Care Unit. Severe and critical categories were defined as severe, mild, and moderate as non-severe in data analysis.
Inclusion criteria:
(1) All observational studies (e.g., cross-sectional, longitudinal, case-control, and cohort) with prospective or retrospective designs.
(2) Studies published in English, including preprints.
(3) Studies investigating hepcidin-mediated iron metabolism in COVID-19 cases.
Exclusion criteria:
(1) Duplicate studies, or repeated published studies.
(2) Studies in which accurate data cannot be obtained or some data are missing.
(3) Studies not strictly grouped by severity.
Data Extraction
The extraction methodology was discussed and formulated by the team. Two of us (ZL and HW) independently screened the full text according to the selection criteria, and recorded information about the author's name, publication time, study location, study design, sample size, laboratory results of iron metabolism parameters, and COVID-19 severity in the data file. All laboratory values were converted to conventional units based on the US National Institute of Standards and Technology conversion factors. For studies that only reported the median and interquartile range, we converted these values into mean and standard deviation according to the methods reported by Hozo et al. (27) and Wan et al. (28).
Risk of Bias Assessment
Two authors (LZ and YG) independently used the Newcastle-Ottawa scale to assess the quality of the included studies (29). A third author (DP) ruled in case consensus was not reached. The scale was developed for non-random and observational studies including case-control, cross-sectional, longitudinal, and cohort studies (if applicable). The quality was assessed using a 9-star system. Studies rated ≥6 stars were defined as “acceptable quality” and proceeded to the final meta-analysis step.
Statistical Analysis
This meta-analysis used RveMan5.4 (Cochrane Collaboration), with inverse variance as the statistical method to calculate standardized mean differences (SMDs) and corresponding 95% confidence intervals (CIs) for continuous variables. A P < 0.05 was determined to be statistically significant. The I2 statistic was used to assess the heterogeneity among the analyzed studies. An I2 > 50% or P < 0.10 indicated heterogeneity, for which the random effects model was used. Otherwise, the fixed effects model was used. In addition, visual inspection of funnel plots was used to evaluate publication bias.
Results
Study Identification and Selection
Sixty-four unique citations were identified, of which 48 non-observational studies were excluded and 16 were selected for full-text eligibility assessment. Of these, six observational studies including 477 COVID-19 patients were included in the final data analysis and synthesis. The flow chart and details of study selection results can be found in Figure 1 and Supplementary File 1. The detailed characteristics of the included studies were shown in Table 1.
Figure 1.
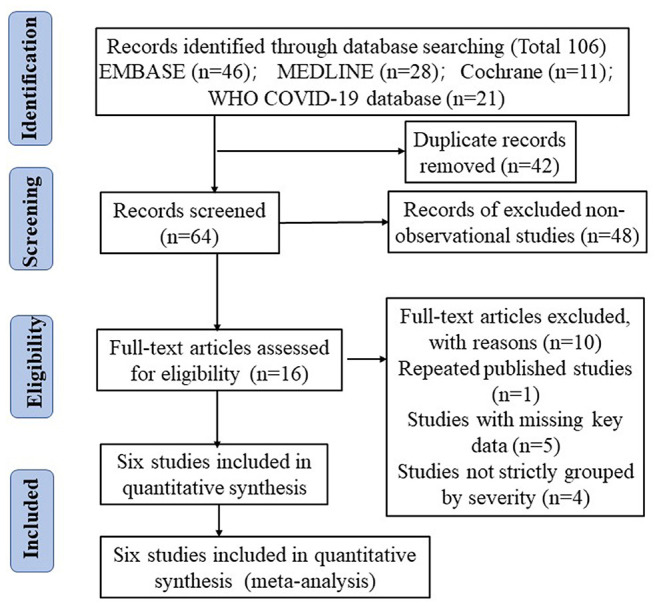
The literature selection process based on the PRISMA flow diagram.
Table 1.
Detailed characteristics of the eligible studies.
| References | Region | Center | Design | Sample size | Cohort settings | Iron parameter | NOS score |
|---|---|---|---|---|---|---|---|
| Chakurkar et al. (24) | India | Single | Prospective, cohort, longitudinal | 120 | Mild (n = 22); Moderate (n = 57); Severe (n = 41) |
Hepcidin, Ferritin, SI, TSAT | 7* |
| Duca et al. (25) | Italy (Piacenza) | Single | Prospective, cohort, longitudinal | 32 | PaO2/FiO2 ratio: >300 mmHg (n = 13) >200, <300 (n = 14) <100 (n = 5) |
Hepcidin, Ferritin, SI, TSAT | 6* |
| Nai et al. (22) | Italy (Milano) | Single | Retrospective, cohort, cross -sectional | 107 | PaO2/FiO2 ratio: >300 (n = 50) <300 (n = 57) |
Hepcidin, Ferritin, SI | 7* |
| Sonnweber et al. (16) | Austria | Multiple | Prospective, cohort, cross -sectional | 109 | Mild (n = 22); Moderate (n = 34); Severe (n = 53) |
Hepcidin, Ferritin, SI, TSAT | 8* |
| Yagci et al. (23) | Turkey | Single | Retrospective, cohort, cross -sectional | 59 | Mild (n = 18); Severe (n = 19); Critical (n = 22) |
Hepcidin, Ferritin, SI, TSAT | 7* |
| Zhou et al. (21) | China | Single | Retrospective, cohort, cross -sectional | 50 | Mild (n = 38); Severe (n = 12) |
Hepcidin, Ferritin | 6* |
FiO2, fraction of inspired oxygen; NOS, Newcastle-Ottawa quality assessment scale; PaO2, arterial oxygen partial pressure; SI, serum iron; TSAT, transferrin saturation. The symbol * means stars.
Serum Levels of Iron Metabolism Parameters
Six unique studies with data from 477 COVID-19 patients were included. Hepcidin and ferritin were assessed in six out of six (100%) studies. Heterogeneity was assessed at P = 0.006, I2 = 70% and P = 0.0001, I2 = 80%, respectively (Figures 2A,B). Both of them had significant heterogeneity, and the random effects model was used. Compared to non-severe cases, severe cases have higher hepcidin (SMD, −0.39; 95% CI [−0.76, −0.03]; P = 0.03) and ferritin (SMD, −0.84; 95% CI [−1.30, −0.38]; P = 0.0004). In five out of six (83.3%) studies, a total of 427 patients were tested for SI, and there were significant differences in their levels between severe and non-severe cases (SMD, 0.22; 95% CI [0.02, 0.41]; P = 0.03). A total of 320 patients from four out of six (66.7%) studies were tested for TSAT, and the statistical difference was insignificant (SMD, 0.06; 95% CI [−0.17, 0.28]; P = 0.64). Heterogeneity was assessed at P = 0.38, I2 = 4% and P = 0.66, I2 = 0%, respectively (Figures 2C,D). Neither of them was significantly heterogenous, and the fixed effects model was used.
Figure 2.
Comparison of iron metabolism parameters between non-severe and severe COVID-19 cases. (A) Hepcidin; (B) Ferritin; (C) Serum iron; (D) Transferrin saturation (TSAT).
Quality of Studies and Publication Bias
All six included studies had NOS scores ≥6 stars, and their quality was acceptable. The funnel plots of SMDs in hepcidin and ferritin (both included six studies) were asymmetric, suggesting the possible presence of publication bias. For SI and TAST, five and four studies were included, respectively, and we found no evidence of publication bias (Supplementary File 1).
Discussion
To our knowledge, this study is the first meta-analysis of the relationship between hepcidin-mediated iron dysmetabolism and COVID-19 severity. However, there is significant heterogeneity among six studies included in the data synthesis of hepcidin and ferritin. The possible reasons are analyzed as follows: (a) The time point and time course of laboratory testing were different. Some studies collected blood samples at admission (21–23), another 2 months after onset (16), and others throughout the hospital stay (24, 25). In addition, the detection methods and the reagents used were not the same. Enzyme-linked immunosorbent assay (ELISA) kits (16, 21–24) or competitive enzyme immunoassay (EIA) kits (25) were used to detect hepcidin. (b) Iron metabolism is strongly influenced by gender, with females generally having lower SI and hepcidin levels than males (22). Also, males have a higher ratio of severe COVID-19 cases. There were potential effects on heterogeneity due to differences in the composition of males and females between studies. (c) Our study classified mild and moderate as non-severe, severe and critical as severe. Differences in the proportions and sample sizes of cases of distinct severity between studies contributed to heterogeneity. Unfortunately, hepcidin and its related iron metabolism parameters are rarely tested simultaneously in COVID-19, and there are even fewer original studies analyzing the relationship between hepcidin and disease severity. Also, subgroup analysis could not be performed due to the lack of access to raw data. Furthermore, only the Sonnweber 2020 study tested hepcidin and ferritin 2 months after onset. We removed this study and performed a new meta-analysis and found no significant change in the final overall effect (Supplementary Figure 1). Therefore, from the results of the meta-analysis, differences in blood collection at different stages of COVID-19 between studies may not affect the fact that severe cases have higher levels of hepcidin and ferritin. This also suggests that hepcidin-mediated iron dysmetabolism throughout the course of COVID-19 may be some very promising therapeutic targets. Comprehensively, these studies met rigid criteria for inclusion and were homogeneous in terms of study design and objectives, as well as cohort settings. Therefore, the final overall effect generated by the random effects model brings novel insights into the biological effects of COVID-19.
Hepcidin, the key iron metabolism regulatory hormone, sequesters iron and prevents iron efflux in enterocytes and macrophages, resulting in increased intracellular ferritin and hypoferremia (30, 31). Physiologically, hepcidin synthesis by hepatocytes is reactively upregulated or downregulated by high or low SI, respectively. Other hepcidin agonists are inflammatory cytokines represented by interleukin-6 (IL-6). Conversely, hepcidin is antagonized by hypoxemia, with hypoxia induced factors (HIF) release (15, 32). Our study found that severe COVID-19 cases had higher serum levels of hepcidin and ferritin, and lower SI, without significant differences in TSAT.
These findings were consistent with most of other studies. Unexpectedly, hepcidin levels in severe COVID-19 cases did not appear to be downregulated by hypoferremia in a feedback manner and antagonized by systemic hypoxemia. Hyperferritinemia inevitably promotes the production of ROS and lipoperoxidation, which ultimately leads to extensive cell and tissue damage possibly through the ferroptosis pathway, and cascade-amplified inflammation (32, 33). In turn, hyperinflammation will transcriptionally upregulate hepcidin, further exacerbating iron dysmetabolism. We speculate that the potential intervention targets for this vicious circle may lie in the hepcidin-mediated iron metabolism axis.
Cytokine storm is a hallmark of the hyperinflammatory state of COVID-19 and is closely linked to disturbances in iron metabolism (30, 34). A growing body of studies have suggested that several components of elevated inflammatory status may be effective therapeutic targets, notably the administration of IL-6 receptor antagonists such as tocilizumab, which significantly reduces the mortality of severe COVID-19 cases (35, 36). However, the effective suppression of the excessive inflammatory response has also raised concerns about prolonged SARS-CoV-2 clearance. Furthermore, data on whether IL-6 receptor antagonists can correct hepcidin-mediated iron dysmetabolism in COVID-19 cases have been lacking to date.
Ehsani highlighted the striking similarity between the amino acid sequence of the SARS-CoV-2 spike glycoprotein and the hepcidin protein (37). This observation provides ideas for vaccine design and bioengineered antibody development for SARS-CoV-2. Hepcidin-mimetic action of SARS-CoV-2 may markedly increase circulating and tissue ferritin, while inducing SI deficiency (32, 33). However, whether SARS-CoV-2 utilizes ferroportin on the cell membrane as another binding receptor for the spike protein to invade host cells requires further analysis. Moreover, whether SARS-CoV-2 directly degrades ferroportin through mimicking hepcidin or indirectly upregulates hepcidin through inflammation to mediate iron dysmetabolism also needs to be carefully investigated. In animal models, hepcidin-neutralizing monoclonal antibodies have been shown to reverse inflammatory anemia (38). Recently, an antibody targeting ferroportin has been described and hypothesized to reduce ferroportin degradation by interfering with hepcidin binding, thereby increasing SI (39). The application of hepcidin antibodies to block viral entry and correct iron metabolism disturbances in COVID-19 cases may be a very promising therapeutic approach in the future.
Hyperferritinemia in COVID-19 lies downstream of the hepcidin-mediated iron metabolism axis. Iron chelation has been shown to reduce viral replication and exert anti-inflammatory effects in viral infections (32, 40, 41). Multiple evidences have shown that deferoxamine reduces the levels of IL-6, the main inflammatory mediator that triggers cytokine storm, mimics HIF, and downregulates hepcidin (40, 42). However, the application of iron chelators in COVID-19 has brought great controversy (42–44). The iron dysmetabolism of COVID-19 is essentially abnormal iron distribution; the coexistence of low SI and local intracellular iron overload, and total iron in the body may not increase. Therefore, the safety and efficacy of systemic iron chelator administration has been challenged (43), and hepcidin antagonists may be preferred as supportive treatments for COVID-19 compared with iron chelators. Four clinical trials of iron chelation are currently underway (NCT04333550, NCT04361032, NCT04389801, IRCT20200506047323N4) and the results are pending.
There are some limitations to this study that need to be noted. First, most of the studies were single-center, retrospective, with small sample sizes, and may be subject to confounding and bias. Second, the six studies used for hepcidin and ferritin data synthesis were heterogeneous, subgroup analyses were not performed, and the results from random-effects models may be inaccurate. Third, the literature search may not be completely comprehensive, resulting in the omission of a few relevant studies. Fourth, this included preprint is a preliminary manuscript version. There are certain risks to the validity and applicability of the data it provides.
Conclusions
Severe COVID-19 cases had higher serum levels of hepcidin and ferritin, and lower SI, without significant differences in TSAT. Compared with other clinically applied therapeutic options targeting the iron metabolism axis, such as IL-6 receptor antagonists and iron chelators, hepcidin antibody may be more promising, but further studies are needed to verify whether targeting the hepcidin-mediated iron metabolism axis may influence the outcome and treatment of COVID-19.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
DP was responsible for methodology, investigation, formal analysis, data curation, writing the original draft, and visualization. YG and LZ for investigation, formal analysis, and data curation. YL for conceptualization, investigation, review and editing, and supervision. ZL and HW worked on conceptualization, formal analysis, investigation, and data curation. All authors have read and approved the final manuscript version to be submitted.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Professor Hai Rao, School of Medicine, Southern University of Science and Technology, China, and Cindy Acon Chen, USA, for reviewing this manuscript.
Glossary
Abbreviations
- ARDS
acute respiratory distress syndrome
- CI
confidence interval
- COVID-19
coronavirus disease 2019
- FiO2
fraction of inspired oxygen
- HIF
hypoxia induced factor
- NOS
Newcastle-Ottawa quality assessment scale
- PaO2
arterial oxygen partial pressure
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SMD
standardized mean difference
- SI
serum iron
- TSAT
transferrin saturation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.881412/full#supplementary-material
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. (2020) 382:1199–207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. (2020) 180:934–43. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. (2020) 46:846–48. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol. (2015) 15:500–10. 10.1038/nri3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah A, Frost JN, Aaron L, Donovan K, Drakesmith H. Systemic hypoferremia and severity of hypoxemic respiratory failure in COVID-19. Crit Care. (2020) 24:320. 10.1186/s13054-020-03051-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Serum iron level as a potential predictor of coronavirus disease 2019 severity and mortality: a retrospective study. Open Forum Infect Dis. (2020) 7:ofaa250. 10.1093/ofid/ofaa250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. (2017) 171:273–85. 10.1016/j.cell.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lv Y, Chen L, Liang X, Liu X, Gao M, Wang Q, et al. Association between iron status and the risk of adverse outcomes in COVID-19. Clin Nutr. (2021) 40:3462–9. 10.1016/j.clnu.2020.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. (2020) 14:1753466620937175. 10.1177/1753466620937175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kappert K, Jahić A, Tauber R. Assessment of serum ferritin as a biomarker in COVID-19: bystander or participant? Insights by comparison with other infectious and non-infectious diseases. Biomarkers. (2020) 25:616–25. 10.1080/1354750X.2020.1797880 [DOI] [PubMed] [Google Scholar]
- 12.Bolondi G, Russo E, Gamberini E, Circelli A, Meca MCC, Brogi E, et al. Iron metabolism and lymphocyte char- acterisation during Covid-19 infection in ICU patients: an observational cohort study. World J Emerg Surg. (2020) 15:41. 10.1186/s13017-020-00323-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hippchen T, Altamura S, Muckenthaler MU, Merle U. Hypoferremia is associated with increased hospitalization and oxygen demand in COVID-19 patients. Hemasphere. (2020) 4:e492. 10.1097/HS9.0000000000000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girelli D, Marchi G, Busti F, Vianello A. Iron metabolism in infections: focus on COVID-19. Semin Hematol. (2021) 58:182–7. 10.1053/j.seminhematol.2021.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. (2012) 1823:1434–43. 10.1016/j.bbamcr.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonnweber T, Boehm A, Sahanic S, Pizzini A, Aichner M, Sonnweber B, et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients' performance: a prospective observational cohort study. Respir Res. (2020) 21:276. 10.1186/s12931-020-01546-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aschemeyer S, Qiao B, Stefanova D, Valore EV, Sek AC, Ruwe TA, et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood. (2018) 131:899–910. 10.1182/blood-2017-05-786590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoffel NU, Lazrak M, Bellitir S, Mir NE, Hamdouchi AE, Barkat A, et al. The opposing effects of acute inflammation and iron deficiency anemia on serum hepcidin and iron absorption in young women. Haematologica. (2019) 104:1143–9. 10.3324/haematol.2018.208645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girelli D, Nemeth E, Swinkels DW. Hepcidin in the diagnosis of iron disorders. Blood. (2016) 127:2809–13. 10.1182/blood-2015-12-639112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lasocki S, Lefebvre T, Mayeur C, Mebazaa A, Gayat E; FROG-ICU study group. Iron deficiency diagnosed using hepcidin on critical care discharge is an independent risk factor for death and poor quality of life at one year: an observational prospective study on 1161 patients. Crit Care. (2018) 22:314. 10.1186/s13054-018-2253-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou C, Chen Y, Ji Y, He X, Xue D. Increased serum levels of hepcidin and ferritin are associated with severity of COVID-19. Med Sci Monit. (2020) 26:e926178. 10.12659/MSM.926178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nai A, Lore NI, Pagani A, De Lorenzo R, Di Modica S, Saliu F, et al. Hepcidin levels predict Covid-19 severity and mortality in a cohort of hospitalized Italian patients. Am J Hematol. (2021) 96:E32–5. 10.1002/ajh.26027 [DOI] [PubMed] [Google Scholar]
- 23.Yagci S, Serin E, Acicbe Ö, Zeren MI, Odabaşi MS. The relationship between serum erythropoietin, hepcidin, and haptoglobin levels with disease severity and other biochemical values in patients with COVID-19. Int J Lab Hematol. (2021) 43(Suppl. 1):142–51. 10.1111/ijlh.13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakurkar V, Rajapurkar M, Lele S, Mukhopadhyay B, Lobo V, Injarapu R, et al. Increased serum catalytic iron may mediate tissue injury and death in patients with COVID-19. Sci Rep. (2021) 11:19618. 10.1038/s41598-021-99142-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duca L, Nava I, Vallisa D, Vadacca G, Magnacavallo A, Vercelli A . COVID-19, inflammatory response, iron homeostasis and toxicity: a prospective cohort study in the Emergency Department of Piacenza (Italy). Research Square (2021) (preprint). Available online: https://www.researchsquare.com/article/rs-1085949/v1 [DOI] [PMC free article] [PubMed]
- 26.Muka T, Glisic M, Milic J, Verhoog S, Bohlius J, Bramer W, et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur J Epidemiol. (2020) 35:49–60. 10.1007/s10654-019-00576-5 [DOI] [PubMed] [Google Scholar]
- 27.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 30.Edeas M, Saleh J, Peyssonnaux C. Iron: innocent bystander or vicious culprit in COVID-19 pathogenesis? Int J Infect Dis. (2020) 97:303–5. 10.1016/j.ijid.2020.05.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daher R, Manceau H, Karim Z. Iron metabolism and the role of the iron-regulating hormone hepcidin in health and disease. Presse Med. (2017) 46(12 Pt 2):e272–8. 10.1016/j.lpm.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 32.Cavezzi A, Troiani E, Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. (2020) 10:1271. 10.4081/cp.2020.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirschhorn T, Stockwell BR. The development of the concept of ferroptosis. Free Radic Biol Med. (2019) 133:130–43. 10.1016/j.freeradbiomed.2018.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. (2020) 395:1033–4. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. (2020) 368:473–4. 10.1126/science.abb8925 [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. (2020) 55:105954. 10.1016/j.ijantimicag.2020.105954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehsani S. COVID-19 and iron dysregulation: distant sequence similarity between hepcidin and the novel coronavirus spike glycoprotein. Biol Direct. (2020) 15:19. 10.1186/s13062-020-00275-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasu BJ, Cooke KS, Arvedson TL, Plewa C, Ellison AR, Sheng J, et al. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. (2010) 115:3616–24. 10.1182/blood-2009-09-245977 [DOI] [PubMed] [Google Scholar]
- 39.Sheetz M, Barrington P, Callies S, Berg PH, McColm J, Marbury T, et al. Targeting the hepcidin-ferroportin pathway in anaemia of chronic kidney disease. Br J Clin Pharmacol. (2019) 85:935–48. 10.1111/bcp.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu W, Zhang S, Nekhai S, Liu S. Depriving iron supply to the virus represents a promising adjuvant therapeutic against viral survival. Curr Clin Microbiol Rep. (2020) 20:1–7. 10.1007/s40588-020-00140-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abobaker A. Can iron chelation as an adjunct treatment of COVID-19 improve the clinical outcome? Eur J Clin Pharmacol. (2020) 76:1619–20. 10.1007/s00228-020-02942-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perricone C, Bartoloni E, Bursi R, Cafaro G, Guidelli GM, Shoenfeld Y, et al. COVID-19 as part of the hyperferritinemic syndromes: the role of iron depletion therapy. Immunol Res. (2020) 68:213–24. 10.1007/s12026-020-09145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrick MD, Ghio AJ. Iron chelation may harm patients with COVID-19. Eur J Clin Pharmacol. (2021) 77:265–6. 10.1007/s00228-020-02987-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abobaker A. Reply: iron chelation may harm patients with COVID-19. Eur J Clin Pharmacol. (2021) 77:267–8. 10.1007/s00228-020-02988-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.