Abstract
The virulence factors of the opportunistic human pathogen Staphylococcus epidermidis have been a main subject of research. In contrast, limited information is available on the mechanisms that allow the bacterium to accommodate to the conditions during carriage, a prerequisite for pathogenicity. Here, we tested the hypothesis that the adaptation of S. epidermidis at different anatomical sites is reflected by differential gene regulation. We used qPCR to profile S. epidermidis gene expression in vivo in nose and skin swabs of 11 healthy individuals. Despite some heterogeneity between individuals, significant site-specific differences were detected. For example, expression of the S. epidermidis regulator sarA was found similarly in the nose and on the skin of all individuals. Also, genes encoding colonization and immune evasion factors (sdrG, capC, and dltA), as well as the sphingomyelinase encoding gene sph, were expressed at both anatomical sites. In contrast, expression of the global regulator agr was almost inactive in the nose but readily present on the skin. A similar site-specific expression profile was also identified for the putative chitinase-encoding SE0760. In contrast, expression of the autolysine-encoding gene sceD and the wall teichoic acid (WTA) biosynthesis gene tagB were more pronounced in the nose as compared to the skin. In summary, our analysis identifies site-specific gene expression patterns of S. epidermidis during colonization. In addition, the observed expression signature was significantly different from growth in vitro. Interestingly, the strong transcription of sphingomyelinase together with the low expression of genes encoding the tricarboxylic acid cycle (TCA) suggests very good nutrient supply in both anatomical niches, even on the skin where one might have suspected a rather lower nutrient supply compared to the nose.
Keywords: in vivo gene expression, human colonization, nasal colonization, skin colonization, global regulators, bacterial adaptation, staphylococcal accessory regulatorA (sarA)
Introduction
The human nose is an important colonization site for two major staphylococcal species, Staphylococcus (S.) aureus and Staphylococcus epidermidis, the latter also being a ubiquitous colonizer of healthy skin (Wertheim et al., 2005; Du et al., 2021). While S. epidermidis is primarily considered as a commensal bacterium with beneficial properties, for example, in modulating the immune system (Naik et al., 2012) and protecting against colonization by pathogens (Cogen et al., 2010), it also possesses selective pathogenic potential to cause nosocomial infections associated with implanted medical devices (Harris et al., 2010; Tande and Patel, 2014).
The pathogenic lifestyle of this opportunistic pathogen has been the main subject of research, and its ability to form biofilm is recognized as a major virulence factor (Otto, 2009, 2014; Buttner et al., 2015). In contrast, there is limited information on how the bacterium adapts during colonization and how environmental factors influence its commensal lifestyle. In particular, the skin at various anatomical sites with differences in temperature, pH, moisture, and sebum content provides strongly contrasting microenvironments (Grice et al., 2009; Grice and Segre, 2011). The different properties of the skin sites influence the composition of the local microbial community, which varies depending on the skin region. For example, Staphylococci and Corynebacteria preferentially colonize moist areas of the skin (Byrd et al., 2018). Despite varying external influences, the composition of the skin’s microbial communities in one individual is largely stable over time (Oh et al., 2016).
The fact that S. epidermidis, as one of the most abundant bacterial species on the skin (Byrd et al., 2018), can act as either pathogen or commensal suggests dynamic and tunable virulence gene regulation. Gene regulation is facilitated by global regulators that are part of a complex network that integrates information such as cell density, nutritional, and other environmental signals, or stress such as exerted by antimicrobial peptides (AMPs) (Vuong et al., 2005; Li et al., 2007; Otto, 2009). Besides transcriptional regulation, the transition of S. epidermidis, from the commensal to infectious phenotype, also occurs through genomic changes. Both et al. (2021) demonstrated that the crucial gene loci can almost exclusively be assigned to the mobilome, which is defined as a group of exchangeable elements such as phage-encoded genes or mobile genetic elements. The adaptation to the infectious lifestyle is characterized by increased biofilm formation and enhanced growth on nutrient-poor media, as well as a reduced production of hemolysins (Both et al., 2021). The relevance of S. epidermidis gene regulation is illustrated by the fact that expression and secreting the protease EcpA aggravate the clinical symptoms in patients with atopic dermatitis (Cau et al., 2021). Thus, the bacterium acts context-dependent and not always beneficial. Recently, we could show that the integrity of the skin barrier determines whether S. epidermidis colonizes the skin as friend or foe. When the skin barrier is disrupted, S. epidermidis loses its protective function and its presence leads to increased colonization with S. aureus (Burian et al., 2017).
To gain insights into the commensal lifestyle of S. epidermidis and identify relevant determinants during colonization, we performed direct transcript analysis in the authentic human environment, considering its two major ecological niches: the nose and the skin. Using our direct in vivo qPCR approach, we here reveal for the first time the niche-specific expression pattern of S. epidermidis during its commensal lifestyle.
Materials and Methods
Ethics Statement
Skin and nasal swabs were obtained from healthy volunteers. This approach was approved by the local ethics committee of the Medical Faculty RWTH University of Aachen, Germany (EK 173/20). In accordance with the Declaration of Helsinki Principles guidelines, written consent was obtained from all participants involved in the study.
Study Population, Sampling, and Growth of Swab Material
Eleven healthy volunteers [nine females and two males, median age 31.4 years (range 22–44 years)] were included in this study. Volunteers with medical history of systemic diseases, such as autoimmune diseases, diabetes mellitus, and cancer as well as chronic skin diseases (eczema or psoriasis), immune deficiency, or recent antibiotic use, were excluded. Concurrent colonization with Staphylococcus aureus was also an exclusion criterion.
For in vivo transcript analysis, a cotton wool swab was moistened in 300 μl nuclease-free water and the left and right anterior nares of the human volunteers were swabbed. Skin swabs were obtained by rotating the swab in the area between the toes. The swab was vigorously vortexed and 10 μl of the suspension was used for bacteriological and quantitative analyses. The cotton wool was removed from the swab using sterile tweezers and both the suspension and the cotton wool were directly treated with 1 ml of TRIzol™ LS reagent (Thermo Fisher Scientific) containing 0.5 ml of zirconia/silica beads [0.1 mm in diameter (Carl Roth)].
For in vitro transcript analysis, the native swab material was grown overnight in TSB medium (Carl Roth), diluted to an initial optical density value at 600 nm (OD600) of 0.05 in fresh medium and grown with shaking (180 rpm) at 37°C to the exponential (OD600 = 0.5) and post-exponential (OD600 = 0.5 + 4 h) phase. Bacteria were harvested by centrifugation and dissolved in 1 ml TRIzol™ reagent containing 0.5 ml of zirconia/silica beads.
RNA Isolation, Reverse Transcription, and Quantitative Real-Time PCR
Bacteria were lysed using a high-speed homogenizer [FastPrep-24™ 5G (MP Biomedical)] twice at 6,500 rpm for 20 s and RNA isolation was performed as described previously (Burian et al., 2010b). To eliminate contaminating DNA, each in vivo RNA sample was digested with 8 U of RNase-free DNase I (Roche), 2 μl 10× incubation buffer (Roche), and 16 U of RNasin ribonuclease inhibitor (Promega) for 30 min at room temperature. DNase treatment was carried out twice for each sample. DNase I treatment was stopped using DNase inactivation reagent (Thermo Fisher Scientific). DNase treatment of in vitro samples was carried out using 0.05 μg of total RNA with adjusted amounts of reagents for a final volume of 50 μl.
Around 1 μl of in vitro and 3 μl of in vivo RNA were transcribed into cDNA using Super Script III Reverse Transcriptase (Thermo Fisher Scientific) and 200 ng of random hexamer primers (Thermo Fisher Scientific). Reverse transcription was performed as described in the instructions of the Superscript manufacturer. Complementary DNAs were diluted 1:3 with nuclease-free water (Thermo Fisher Scientific) and frozen at −20°C using Eppendorf LoBind Tubes (Eppendorf) for prolonged storage.
Relative quantification of S. epidermidis transcripts by qPCR was performed as described previously (Burian et al., 2021). Briefly, qPCR was carried out using the 7300 Real-Time PCR instrument (Applied Biosystems) in combination with the KAPA SYBR® FAST qPCR Master Mix (2×) ABI Prism (Merck). Master mixes were prepared as following: 8 μl KAPA SYBR® FAST qPCR Master Mix (2×) ABI Prism, 8 μl nuclease-free water, and 1 μl of each primer (see Supplementary Table 1). Finally, 2 μl of cDNA was added per reaction. Relative quantities of transcripts were calculated by a standard curve for each gene generated using 6-fold serial dilution of a S. epidermidis ATCC 12228 and RP62A wild-type cDNA mixture.
Data Visualization and Statistical Analysis
Figures depicting differential gene expression as the ratio between in vivo and minimum or maximum expression in vitro were visualized as heat map (generated by GraphPad Prism 9.0.2). For this purpose, the ratio was calculated based on either the expression value at OD = 0.5 or OD = 0.5 + 4 h, depending on the time point at which the gene reaches its maximum expression in vitro. Subsequently, the data were logarithmically transformed to base 2. Accordingly, the ratio of in vivo to minimal transcription in vitro was also calculated.
Statistical analysis was performed with the Prism 9.0.2 package (GraphPad Software) using the Kruskal-Wallis test. Value of p < 0.05 was considered to be statistically significant.
Results
In vivo Transcriptional Analysis During Human Staphylococcus epidermidis Colonization
For the analysis of S. epidermidis gene expression during human colonization, we obtained nose and skin swabs of 11 healthy individuals who were positive for S. epidermidis at both anatomical sites (Figure 1). The S. epidermidis load determined by culture in nose and skin material varied markedly between individuals (rangenose 4.2 × 104–2.20 × 106 CFU/ml and rangeskin 4.04 × 104–2.86 × 106 CFU/ml). No significant difference in abundance was observed between the two niches (mean nose 4.1 × 105 CFU/ml and mean skin 9.05 × 105 CFU/ml; Figure 2).
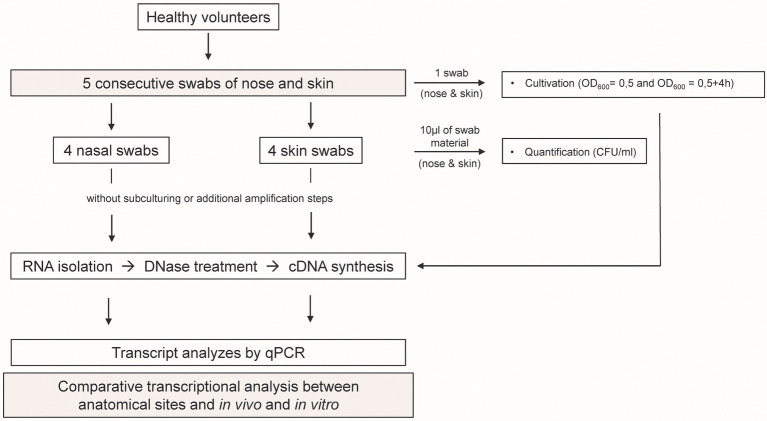
Figure 1.
Study design. Five swabs from the nose and skin were obtained from 11 healthy individuals. RNA was isolated directly from the material of four swabs, and bacterial transcription was profiled using qPCR. In parallel, one swab from each individual was used for cultivation to exponential (OD600 = 0.5) and post-exponential (OD600 = 0.5 + 4 h) growth phase. RNA was isolated and bacterial transcription was measured by qPCR. In vivo gene expression in nose and skin was then compared with the in vitro expression pattern.
Figure 2.
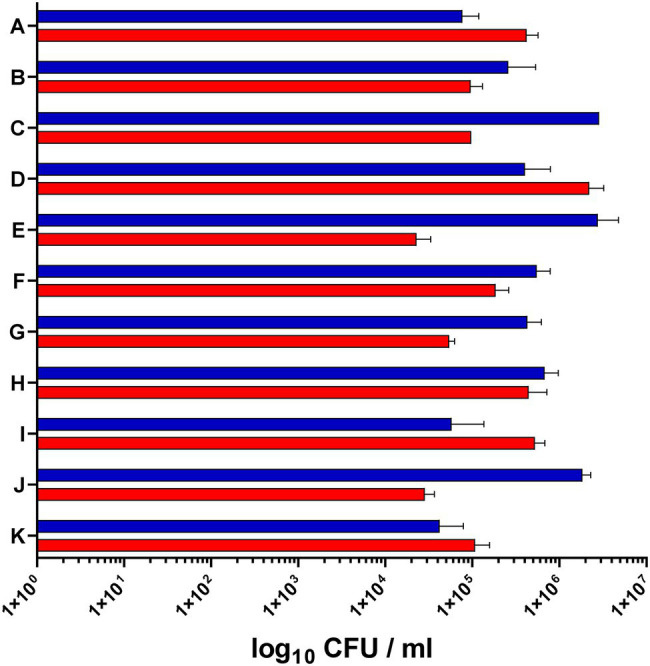
Staphylococcus epidermidis load in the nose and skin of 11 healthy volunteers. The bacterial load in the nose (red bars) and on the skin (blue bars) was determined by serial dilution and plating of swab material (CFU, colony forming units).
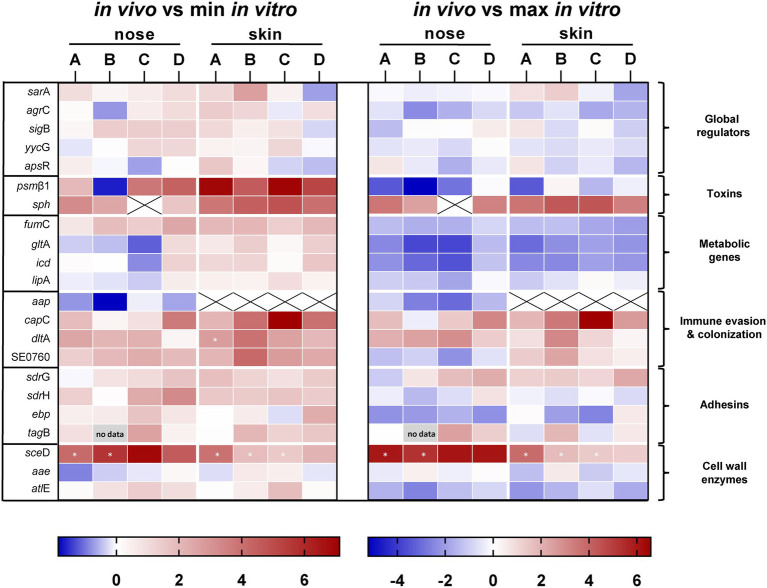
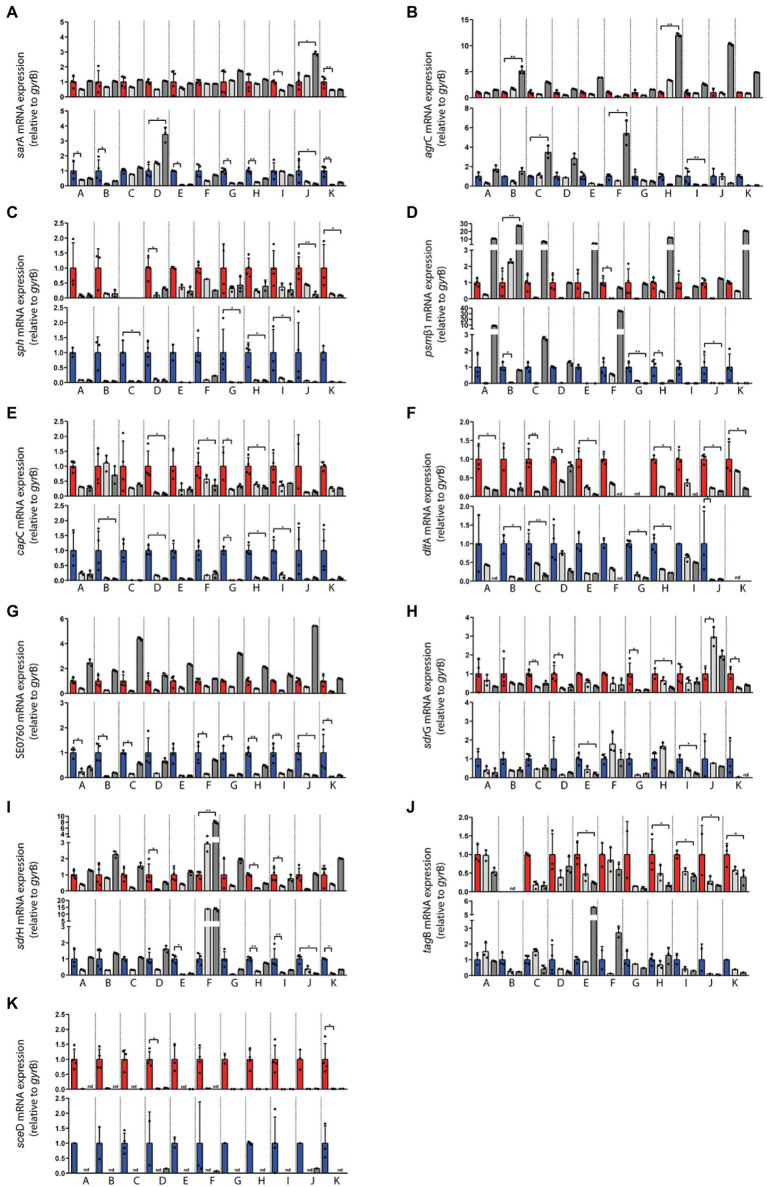
A total of five consecutive swabs were collected from each site, and four swabs were used directly for transcript analyses without sub-culturing or additional amplification steps (Figure 1). Initially, swabs were taken from four individuals and a total of 22 genes encoding bacterial factors involved in virulence regulation, toxin production, metabolism, adhesion, cell wall dynamics, immune evasion, and colonization were analyzed (see Supplementary Table 2). Results were visualized in a heat map as ratio between the expression in vivo and the minimum or maximum expression in vitro (Figure 3). No regulation or downregulation in vivo was detected for 11 genes. Therefore, we next focused on genes upregulated in vivo and genes with infection-associated biological significance. For these 11 remaining genes, swab samples from the four individuals mentioned above plus another seven individuals were analyzed. Results are shown as transcription in vivo (nose or skin) vs. transcription during growth in vitro (exponential and post-exponential growth phase) relative to the housekeeping gene gyrB (Figure 4). The main results can be functionally classified into the following groups.
Figure 3.
Direct transcript analysis of Staphylococcus epidermidis genes in the nose and skin of four healthy volunteers (A–D). Results are given as the ratio of transcription in vivo vs. minimal expression in vitro and vs. maximal expression in vitro. All data were log transformed (basis 2) and changes in gene expression were normalized in reference to the constitutively expressed gene gyrB. Genes colored red are those which were upregulated compared to in vitro and genes colored blue are those which were downregulated compared to in vitro. White cells indicate the same expression levels in vivo and in vitro. Results are the means of four separate samplings. White cells with an “x” have no values because gene expression was below the detection limit. Cells marked with an asterisk have no in vitro values because gene expression was below detection limit. For presentation reasons, the ratio was calculated from the mean value of the in vitro data of all subjects. The heat map was generated using GraphPad Prism 9.0.2.
Figure 4.
Transcriptional analysis of the indicated Staphylococcus epidermidis genes in swab material of the nose and skin of 11 healthy individuals (A–K). Expression levels in nose swabs (upper panels, red columns) and skin swabs (lower panels, blue columns) as well as during exponential growth in vitro (light gray) and post-exponential growth in vitro (dark gray) were normalized to the expression level of the house keeping gene gyrB. Values from four independent samplings (four swabs) were used to calculate the mean expression in vivo. In vitro values represent technical replicates from one cultivation. The in vitro values were normalized to the respective in vivo level (set to 1). Statistically significant differences are indicated. *p ≤ 0.05; **p ≤ 0.01. Relative transcription of (A) sarA, (B) agrC, (C) sph, (D) psmβ1, (E) capC, (F) dltA, (G) SE0760, (H) sdrG, (I) sdrH, (J) tagB, and (K) sceD. Gene name abbreviations, see Supplementary Table 2.
Global Regulators
Expression of five global regulators sarA, sigB, yycFG, apsXRS, and agrC of the agr quorum-sensing system was analyzed (Figure 3). In vivo transcriptional analyses of sarA revealed high expression in the nose, mostly within the range of maximal transcription in vitro (Figures 3, 4A). Moreover, sarA expression was even more pronounced in skin swabs, where it mostly exceeded the maximal in vitro levels (exception: individual C and D; Figure 4A), suggesting a general role of this regulatory protein during commensalism. In contrast to the high activity of sarA, the quorum-sensing regulator agr was predominantly not expressed during nasal colonization (Figures 3, 4B). On human skin, agr activity was very heterogeneous, as shown by the high transcription in six individuals (Figure 4B subjects E, G, H, I, J, K). A high individual heterogeneity was also observed for the alternative sigma factor B (sigB) and the two- and three-component regulatory systems yycFG and apsXRS, respectively (Figure 3; Supplementary Figure 1).
Toxins
Lysis of host cells and bacterial spread especially during biofilm formation is mediated by a number of S. epidermidis toxins (Bresco et al., 2017). Similar to the heterogeneous agr expression on human skin, psmß1 peptide was not uniformly expressed among individuals (Figures 3, 4D). While in the nose psmß1 was strongly transcribed in five individuals, expression on the skin was upregulated in eight individuals (Figure 4D).
Interestingly, the sphingomyelinase encoding gene sph (also annotated as hlb in S. aureus) was highly transcribed in all individuals during both nasal and skin colonization. Only in the nose of subject C sph was not expressed (Figures 3, 4C).
Metabolic Genes
Similar to S. aureus, S. epidermidis TCA cycle activity is de-repressed during stationary phase in vitro (Wang et al., 2018). In the authentic human environment, we found the TCA encoding genes fumC, gltA, and icd expressed at low levels during asymptomatic colonization. Similarly, the lipase encoding gene lipA was weakly transcribed during nasal colonization. lipA expression on skin exhibits high individual heterogeneity (Figure 3; Supplementary Figure 1).
Immune Evasion and Colonization
In addition to its ability to form biofilm, S. epidermidis can produce a second exopolymer, poly-γ-glutamic acid (PGA), which is synthesized by the gene products of the cap locus (Kocianova et al., 2005). In our study, we investigated the transcription of capC and aap, which is known to be involved in aggregation and biofilm formation (Hussain et al., 1997). While aap was only weakly transcribed and sometimes below the detection limit, capC was strongly induced in both niches (Figures 3, 4E). Similarly, the cell wall remodeling enzyme D-alanine-D-alanyl carrier protein ligase (dltA), which mediates D-alanylation of teichoic acids and thus resistance to AMPs (Simanski et al., 2013), was highly expressed in both anatomical niches (Figures 3, 4F). In contrast, the putative chitinase-encoding SE0760 (Zhang et al., 2003) lacked nasal expression but showed high expression on skin (Figures 3, 4G).
Adhesins
For the permanent colonization, factors involved in tissue adherence seem to be important. In the authentic environment, we found increased transcription of the adhesion sdrG at both anatomical sites and, with greater heterogeneity between individuals, for sdrH as well (Figures 3, 4H,I). In contrast, ebp encoding the elastin-binding protein was not induced under in vivo conditions indicating a selective regulation of adhesion molecules (Figure 3; Supplementary Figure 1).
Wall teichoic acid (WTA) of S. aureus is known to be an important colonization factor mediating attachment to epithelial and endothelial cells (Weidenmaier et al., 2005). In S. epidermidis, WTA is required for the primary attachment and accumulation phase of the S. epidermidis biofilm phenotype (Holland et al., 2011). Similar to S. aureus, a multitude of enzymes are involved in WTA biosynthesis. We have characterized the role of WTA during colonization by analyzing the expression of tagB. We detected high transcript levels in the nose, whereas on the skin tagB transcription was heterogeneous (Figure 4J).
Cell Wall Enzymes
Of the analyzed sceD, aae, and atlE genes belonging to the “cell wall enzymes” category, only autolysin sceD played a critical role in all individuals studied, as shown by its high transcription in vivo (Figures 3, 4K).
Niche-Specific Gene Expression of Staphylococcus epidermidis During Commensalism
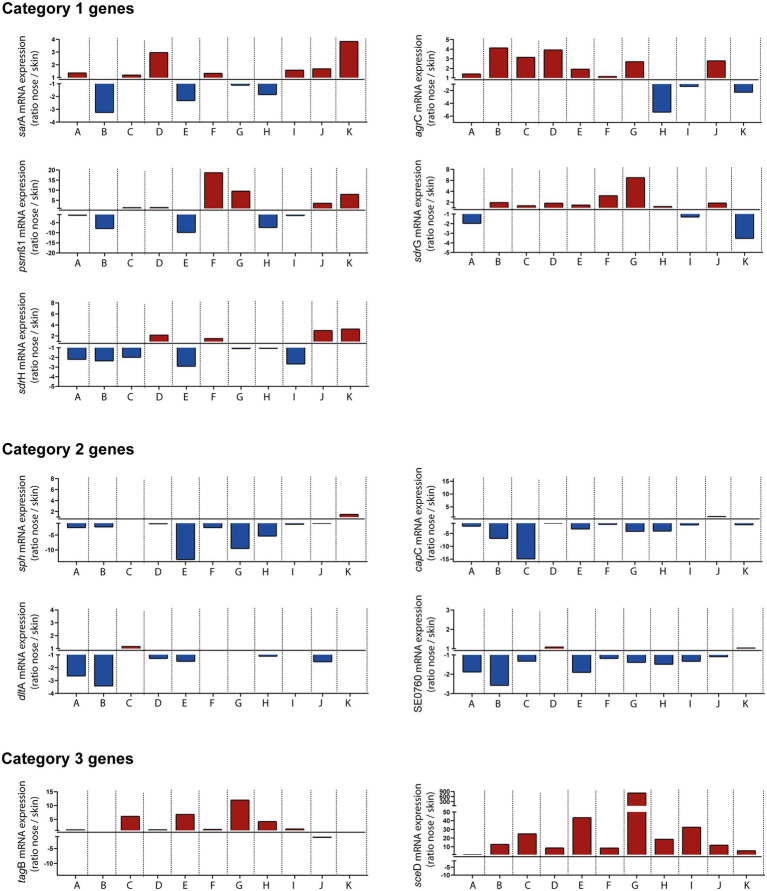
Collection of swab material both from nose and skin of all individuals also enabled a comparative analysis of transcript levels between both sites (without considering in vitro expression as a reference). This comparison revealed three distinct categories of genes: Category 1 included genes that were heterogeneously expressed between individuals and therefore sometimes showed higher transcription in the nose and sometimes on the skin, like sarA, agr, psmß1, sdrG, and sdrH (Figure 5). Category 2 included genes like sph, capC, dltA, and SE0760 whose transcription was higher on the skin. Finally, category 3 contained genes such as tagB and sceD that were increased during nasal but not skin colonization (Figure 5). In summary, our results revealed site-specific gene expression of S. epidermidis during colonization of different host niches.
Figure 5.
In vivo transcription of indicated Staphylococcus epidermidis genes in the nose vs. skin of 11 healthy individuals (A–K). Transcripts were quantified in reference to the constitutively expressed gene gyrB and the ratio of transcription between nose and skin was plotted. Red columns indicate high expression in the nose and blue columns indicate high expression on skin. Category 1 genes heterogeneously expressed between individuals. Category 2 genes increased transcription on skin. Category 3 genes increased transcription during nasal colonization. Gene name abbreviations, see Supplementary Table 2.
Discussion
Mounting evidence indicates that bacterial gene expression profiles significantly differ between in vitro growth conditions and in vivo consistent with differential availability of signals involved in gene regulation (Goerke et al., 2000; Joost et al., 2009). However, there is only a limited number of studies addressing gene expression of staphylococcal species in the authentic human environment and these studies mainly focused on the invasive lifestyle (Joost et al., 2009; Le Masters et al., 2021). In S. epidermidis research, the reported transcriptional analyses focused on in vivo gene expression analysis in models of foreign material infections (Vandecasteele et al., 2003). Given the mainly commensal lifestyle of S. epidermidis, however, this approach does not facilitate a truly comprehensive picture of its biology. In fact, analysis of gene expression profiles of colonizing S. epidermidis is critical to gain insights into niche adaptation events during colonization of, for example, the skin. Furthermore, such analysis provides important clues to better understand the evolution of S. epidermidis within the host during the transition from commensalism to invasive disease. Therefore, we established qPCR assays that allowed us to quantitatively monitor gene transcription of more than 20 S. epidermidis genes in vivo. The assay was employed to analyze specimens obtained from two environments dominated by S. epidermidis colonization and significantly differing in environmental conditions. The nose being a moist and aerobic habitat (Grice and Segre, 2011), compared to the toe web, a moist microaerophilic environment with an increased temperature (Roth and James, 1988). Intriguingly, we observed a general expression signature during colonization that was significantly different from growth conditions in vitro and gene expression profiles of S. epidermidis also showed significant differences depending on the colonized body site. Thus, adaptation to the human host appears to be finely tuned and specific to the respective niche. As the skin and nose are not colonized with a single clonal S. epidermidis strain, but rather with different clonal lineages (Zhou et al., 2020), a limitation of this study is that we did not type the strains, and therefore, we could not determine if the nose and skin isolates were genetically identical.
To obtain a comprehensive picture of the adaptation of S. epidermidis, we investigated: virulence regulators, toxins, metabolic genes, adhesins, cell wall enzymes, and immune evasion genes. To gain insight into the underlying regulatory network, we analyzed five regulatory elements. While gene expression levels of sigB, yycG, and apsR were subject to relevant individual heterogeneity, the staphylococcal accessory regulator A (sarA) was highly transcribed regardless of the colonized niche, suggesting a general role for this regulatory element during the asymptomatic lifestyle of S. epidermidis. So far, sarA is known to affect transcription of various genes as either an activator or repressor (Cue et al., 2012). For example, among the affected genes, the ica gene locus [encoding the polysaccharide intercellular adhesin (PIA)] is positively regulated by sarA (Tormo et al., 2005). While this study aimed to characterize S. epidermidis in its non-pathogenic state, it will be interesting to clarify in further studies whether sarA impacts the pathogenic lifestyle (for example, biofilm formation on medical devices) and proves to be a master regulator, or whether other regulators such as the alternative sigma factor B (sigB; which is also known to positively influence ica transcription; Handke et al., 2007) dominate.
In contrast to the global activity of sarA, the quorum-sensing system agr was active only on the skin. However, this habitat-dependent agr activity was heterogeneous among individuals, which might be explained by clonal diversity of colonizing S. epidermidis strains carrying different types of the agr quorum-sensing system. Olson et al. (2014) reported that cross-inhibitory interactions between the agr type I and II system as well as between the agr type I and III system exist. Because of these cross-inhibitory effects, agr may be highly transcribed on the skin in only 55% of individuals examined. In addition, using a porcine skin model, they demonstrated that a S. epidermidis wild-type strain (positive for agr) significantly enhanced skin colonization compared to an agr mutant (Olson et al., 2014). These data and the transcription observed here on the skin in some individuals suggest that agr contributes to skin colonization, although not as the only decisive factor in humans. Most recently, a modified agr typ of group III (IIIb) and a new agr group IV was found (Zhou et al., 2020). Moreover, Zhou et al. (2020) reported that the distribution of agr types is highly host dependent and that multiple agr types of S. epidermidis in a host niche suppress the expression of virulence factors to maintain homeostasis. Since there is also a cross talk between agr peptide pheromones produces by S. aureus and S. epidermidis (Otto et al., 2001), the agr activity in the presence of S. aureus might differ. The mechanisms leading to the partial activation on human skin and whether agr activity is switched on, for example, during co-colonization of the nose by S. aureus will be the subject of future investigations. Consistent with differential levels of agr transcription between individuals, the psmβ1 peptide was also heterogeneously transcribed. Among psm peptides, mainly β-type peptides are produced by S. epidermidis and are known to play a role in biofilm formation (Le et al., 2019). Since the β-type peptide does not have a cytolytic character (Otto, 2009), its heterogeneous function during asymptomatic colonization remains to be determined.
Interestingly, the sph gene encoding sphingomyelinase (also annotated as hlb in S. aureus) appears to be of central importance during its commensal lifestyle, although sph transcription was even more pronounced during skin colonization than in the nose. Recently, it has been reported that sphingomyelinase activity, on the one hand, makes nutrients available to the bacterium (by cleaving sphingomyelin into phosphocholine and ceramide) and, on the other hand, contributes to the formation of the skin barrier through ceramide (Zheng et al., 2022). In S. aureus, colonization was increased more than 50-fold in a mouse colonization model compared to the strain in which hlb was not produced, due to integration of the prophage ΦSa3mw. Here, the toxin promotes colonization by damaging keratinocytes (Katayama et al., 2013). Since the adaptation strategy of S. epidermidis is strongly influenced by the physiological milieu of the colonized niche, we also addressed transcription of genes involved in metabolism, such as the tricarboxylic acid cycle (TCA). Under nutrient-rich conditions, TCA activity is suppressed as the bacterial demand for biosynthetic intermediates is supplied exogenously. Only when environmental conditions change and nutrients become growth-limiting, such as during the post-exponential growth phase, catabolism of non-preferred carbon sources such as acetate begins (Somerville and Proctor, 2009). At this stage, the enzymatic activity of the TCA cycle is required (Somerville et al., 2003; Somerville and Proctor, 2009). The low transcription of the TCA cycle genes studied together with the high sph expression indicates a very good nutrient supply in both anatomical niches, even on the skin, where one might suspect a rather lower nutrient supply compared to the nose.
To ensure persistent colonization, S. epidermidis expresses molecules involved in adhesion and evasion of the immune system. For immune evasion, capC and dltA are present in both host niches at high transcription rates. Production of the exopolymer PGA (encoded by the cap locus) is known to mediate resistance to AMPs and phagocytosis (Kocianova et al., 2005). In addition to high salt concentrations, which also lead to the induction of cap (Kocianova et al., 2005), AMPs are present in both habitats (Cole et al., 2002; Schauber and Gallo, 2008). Besides PGA, resistance to AMPs is mediated by the expression of dltA, which leads to the d-alanylation of teichoic acids, thereby reducing the anionic charge of the bacterial surface (Simanski et al., 2013). Since the putative chitinase B (SE0760) is selectively expressed on skin and it is known to mediate skin invasion (Zhang et al., 2003), it would be interesting to investigate in further studies whether a SE0760 mutant is less able to colonize and persist on skin using, for example, our newly established human colonization model (Burian et al., 2021).
Tissue adherence by S. epidermidis seems to be mediated by a selective transcription of adhesive molecules. We found that WTA is highly expressed during nasal colonization, whereas sdrG is transcribed in both habitats. SdrG is mainly responsible for tissue adherence by binding to fibrinogen (Hartford et al., 2001). Given the low abundance of fibrinogen on human skin, the high expression in toe web spaces could indicate additional, so far uncharacterized functions of sdrG. The high expression of WTA was also demonstrated during nasal colonization of S. aureus (Burian et al., 2010a,b). Again, similar to persistent colonization of the human nose (Burian et al., 2010b) and early colonization of the skin (Burian et al., 2021) by S. aureus, we observed a strong expression of the autolysin sceD. Interestingly, although sceD was expressed in both niches, it was clearly overrepresented in the nose. This high sceD transcription in both species underscore the importance of the lytic transglycosylase and may therefore be a useful candidate for developing a targeted therapy by inhibition of sceD. Such therapy could play a role especially in atopic dermatitis when massive colonization with S. aureus is responsible for the severity of the disease (Totte et al., 2016) and S. epidermidis also has a deleterious effect by expressing, for example, the protease ecpA (Cau et al., 2021).
In summary, we have elucidated here for the first time the expression pattern of S. epidermidis in the authentic human environment. Overall, a general expression signature was observed during asymptomatic colonization that was significantly different from growth in vitro, and specific gene expression patterns were associated with colonization of different host niches. Thus, S. epidermidis is able to specifically adapt to different niches in its human host. It will be interesting to investigate in further studies, e.g., how the expression pattern of S. epidermidis changes in diseased skin compared to healthy skin and to investigate transcription more comprehensively, e.g., by using RNAseq analysis. Multidimensional phenotypic and genotypic studies are also needed to fully understand the transition from commensalism to invasion. In conclusion, our results significantly improve our understanding of the complex interplay between host and microbe especially during the commensal lifestyle of the bacterium.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Medical Faculty RWTH University of Aachen, Pauwelsstraße 30, 52074 Aachen. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HR, AY, and MB contributed to study concept and design. PT and MB designed and performed the experiments. PT, AB, CW, MH, HR, AY, and MB wrote the manuscript. PT, AB, HR, AY, and MB analyzed the data. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the START-Program of the Faculty of Medicine of the RWTH Aachen University and by CRC TRR 156 by the DFG (to AY).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb. 2022.896311/full#supplementary-material
References
- Both A., Huang J., Qi M., Lausmann C., Weisselberg S., Buttner H., et al. (2021). Distinct clonal lineages and within-host diversification shape invasive Staphylococcus epidermidis populations. PLoS Pathog. 17:e1009304. doi: 10.1371/journal.ppat.1009304, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresco M. S., Harris L. G., Thompson K., Stanic B., Morgenstern M., O’mahony L., et al. (2017). Pathogenic mechanisms and host interactions in Staphylococcus epidermidis device-related infection. Front. Microbiol. 8:1401. doi: 10.3389/fmicb.2017.01401, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burian M., Bitschar K., Dylus B., Peschel A., Schittek B. (2017). The protective effect of microbiota on S. aureus skin colonization depends on the integrity of the epithelial barrier. J. Invest. Dermatol. 137, 976–979. doi: 10.1016/j.jid.2016.11.024, PMID: [DOI] [PubMed] [Google Scholar]
- Burian M., Plange J., Schmitt L., Kaschke A., Marquardt Y., Huth L., et al. (2021). Adaptation of Staphylococcus aureus to the human skin environment identified using an ex vivo tissue model. Front. Microbiol. 12:728989. doi: 10.3389/fmicb.2021.728989, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burian M., Rautenberg M., Kohler T., Fritz M., Krismer B., Unger C., et al. (2010a). Temporal expression of adhesion factors and activity of global regulators during establishment of Staphylococcus aureus nasal colonization. J. Infect. Dis. 201, 1414–1421. doi: 10.1086/651619, PMID: [DOI] [PubMed] [Google Scholar]
- Burian M., Wolz C., Goerke C. (2010b). Regulatory adaptation of Staphylococcus aureus during nasal colonization of humans. PLoS One 5:e10040. doi: 10.1371/journal.pone.0010040, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner H., Mack D., Rohde H. (2015). Structural basis of Staphylococcus epidermidis biofilm formation: mechanisms and molecular interactions. Front. Cell. Infect. Microbiol. 5:14. doi: 10.3389/fcimb.2015.00014, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd A. L., Belkaid Y., Segre J. A. (2018). The human skin microbiome. Nat. Rev. Microbiol. 16, 143–155. doi: 10.1038/nrmicro.2017.157, PMID: [DOI] [PubMed] [Google Scholar]
- Cau L., Williams M. R., Butcher A. M., Nakatsuji T., Kavanaugh J. S., Cheng J. Y., et al. (2021). Staphylococcus epidermidis protease EcpA can be a deleterious component of the skin microbiome in atopic dermatitis. J. Allergy Clin. Immunol. 147, 955–966.e16. doi: 10.1016/j.jaci.2020.06.024, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen A. L., Yamasaki K., Muto J., Sanchez K. M., Alexander L. C., Tanios J., et al. (2010). Staphylococcus epidermidis antimicrobial delta-toxin (phenol-soluble modulin-gamma) cooperates with host antimicrobial peptides to kill group A Streptococcus. PLoS One 5:e8557. doi: 10.1371/journal.pone.0008557, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A. M., Liao H. I., Stuchlik O., Tilan J., Pohl J., Ganz T. (2002). Cationic polypeptides are required for antibacterial activity of human airway fluid. J. Immunol. 169, 6985–6991. doi: 10.4049/jimmunol.169.12.6985, PMID: [DOI] [PubMed] [Google Scholar]
- Cue D., Lei M. G., Lee C. Y. (2012). Genetic regulation of the intercellular adhesion locus in staphylococci. Front. Cell. Infect. Microbiol. 2:38. doi: 10.3389/fcimb.2012.00038, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Larsen J., Li M., Walter A., Slavetinsky C., Both A., et al. (2021). Staphylococcus epidermidis clones express Staphylococcus aureus-type wall teichoic acid to shift from a commensal to pathogen lifestyle. Nat. Microbiol. 6, 757–768. doi: 10.1038/s41564-021-00913-z, PMID: [DOI] [PubMed] [Google Scholar]
- Goerke C., Campana S., Bayer M. G., Doring G., Botzenhart K., Wolz C. (2000). Direct quantitative transcript analysis of the agr regulon of Staphylococcus aureus during human infection in comparison to the expression profile in vitro. Infect. Immun. 68, 1304–1311. doi: 10.1128/IAI.68.3.1304-1311.2000, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice E. A., Kong H. H., Conlan S., Deming C. B., Davis J., Young A. C., et al. (2009). Topographical and temporal diversity of the human skin microbiome. Science 324, 1190–1192. doi: 10.1126/science.1171700, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice E. A., Segre J. A. (2011). The skin microbiome. Nat. Rev. Microbiol. 9, 244–253. doi: 10.1038/nrmicro2537, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handke L. D., Slater S. R., Conlon K. M., O’donnell S. T., Olson M. E., Bryant K. A., et al. (2007). SigmaB and SarA independently regulate polysaccharide intercellular adhesin production in Staphylococcus epidermidis. Can. J. Microbiol. 53, 82–91. doi: 10.1139/w06-108, PMID: [DOI] [PubMed] [Google Scholar]
- Harris L. G., El-Bouri K., Johnston S., Rees E., Frommelt L., Siemssen N., et al. (2010). Rapid identification of staphylococci from prosthetic joint infections using MALDI-TOF mass-spectrometry. Int. J. Artif. Organs 33, 568–574. doi: 10.1177/039139881003300902, PMID: [DOI] [PubMed] [Google Scholar]
- Hartford O., O’brien L., Schofield K., Wells J., Foster T. J. (2001). The Fbe (SdrG) protein of Staphylococcus epidermidis HB promotes bacterial adherence to fibrinogen. Microbiology 147, 2545–2552. doi: 10.1099/00221287-147-9-2545, PMID: [DOI] [PubMed] [Google Scholar]
- Holland L. M., Conlon B., O’gara J. P. (2011). Mutation of tagO reveals an essential role for wall teichoic acids in Staphylococcus epidermidis biofilm development. Microbiology 157, 408–418. doi: 10.1099/mic.0.042234-0, PMID: [DOI] [PubMed] [Google Scholar]
- Hussain M., Herrmann M., Von Eiff C., Perdreau-Remington F., Peters G. (1997). A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect. Immun. 65, 519–524. doi: 10.1128/iai.65.2.519-524.1997, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joost I., Blass D., Burian M., Goerke C., Wolz C., Von Muller L., et al. (2009). Transcription analysis of the extracellular adherence protein from Staphylococcus aureus in authentic human infection and in vitro. J. Infect. Dis. 199, 1471–1478. doi: 10.1086/598484, PMID: [DOI] [PubMed] [Google Scholar]
- Katayama Y., Baba T., Sekine M., Fukuda M., Hiramatsu K. (2013). Beta-hemolysin promotes skin colonization by Staphylococcus aureus. J. Bacteriol. 195, 1194–1203. doi: 10.1128/JB.01786-12, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocianova S., Vuong C., Yao Y., Voyich J. M., Fischer E. R., Deleo F. R., et al. (2005). Key role of poly-gamma-DL-glutamic acid in immune evasion and virulence of Staphylococcus epidermidis. J. Clin. Invest. 115, 688–694. doi: 10.1172/JCI200523523, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Masters T., Johnson S., Jeraldo P. R., Greenwood-Quaintance K. E., Cunningham S. A., Abdel M. P., et al. (2021). Comparative transcriptomic analysis of Staphylococcus aureus associated with periprosthetic joint infection under in vivo and in vitro conditions. J. Mol. Diagn. 23, 986–999. doi: 10.1016/j.jmoldx.2021.05.011, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le K. Y., Villaruz A. E., Zheng Y., He L., Fisher E. L., Nguyen T. H., et al. (2019). Role of phenol-soluble modulins in Staphylococcus epidermidis biofilm formation and infection of indwelling medical devices. J. Mol. Biol. 431, 3015–3027. doi: 10.1016/j.jmb.2019.03.030, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Lai Y., Villaruz A. E., Cha D. J., Sturdevant D. E., Otto M. (2007). Gram-positive three-component antimicrobial peptide-sensing system. Proc. Natl. Acad. Sci. U. S. A. 104, 9469–9474. doi: 10.1073/pnas.0702159104, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S., Bouladoux N., Wilhelm C., Molloy M. J., Salcedo R., Kastenmuller W., et al. (2012). Compartmentalized control of skin immunity by resident commensals. Science 337, 1115–1119. doi: 10.1126/science.1225152, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J., Byrd A. L., Park M., Program N. C. S., Kong H. H., Segre J. A. (2016). Temporal stability of the human skin microbiome. Cell 165, 854–866. doi: 10.1016/j.cell.2016.04.008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. E., Todd D. A., Schaeffer C. R., Paharik A. E., Van Dyke M. J., Buttner H., et al. (2014). Staphylococcus epidermidis agr quorum-sensing system: signal identification, cross talk, and importance in colonization. J. Bacteriol. 196, 3482–3493. doi: 10.1128/JB.01882-14, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. (2009). Staphylococcus epidermidis--the ‘accidental’ pathogen. Nat. Rev. Microbiol. 7, 555–567. doi: 10.1038/nrmicro2182, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. (2014). Staphylococcus epidermidis pathogenesis. Methods Mol. Biol. 1106, 17–31. doi: 10.1007/978-1-62703-736-5_2, PMID: [DOI] [PubMed] [Google Scholar]
- Otto M., Echner H., Voelter W., Gotz F. (2001). Pheromone cross-inhibition between Staphylococcus aureus and Staphylococcus epidermidis. Infect. Immun. 69, 1957–1960. doi: 10.1128/IAI.69.3.1957-1960.2001, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. R., James W. D. (1988). Microbial ecology of the skin. Annu. Rev. Microbiol. 42, 441–464. doi: 10.1146/annurev.mi.42.100188.002301, PMID: [DOI] [PubMed] [Google Scholar]
- Schauber J., Gallo R. L. (2008). Antimicrobial peptides and the skin immune defense system. J. Allergy Clin. Immunol. 122, 261–266. doi: 10.1016/j.jaci.2008.03.027, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanski M., Glaser R., Koten B., Meyer-Hoffert U., Wanner S., Weidenmaier C., et al. (2013). Staphylococcus aureus subverts cutaneous defense by D-alanylation of teichoic acids. Exp. Dermatol. 22, 294–296. doi: 10.1111/exd.12114, PMID: [DOI] [PubMed] [Google Scholar]
- Somerville G. A., Proctor R. A. (2009). At the crossroads of bacterial metabolism and virulence factor synthesis in staphylococci. Microbiol. Mol. Biol. Rev. 73, 233–248. doi: 10.1128/MMBR.00005-09, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville G. A., Said-Salim B., Wickman J. M., Raffel S. J., Kreiswirth B. N., Musser J. M. (2003). Correlation of acetate catabolism and growth yield in Staphylococcus aureus: implications for host-pathogen interactions. Infect. Immun. 71, 4724–4732. doi: 10.1128/IAI.71.8.4724-4732.2003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tande A. J., Patel R. (2014). Prosthetic joint infection. Clin. Microbiol. Rev. 27, 302–345. doi: 10.1128/CMR.00111-13, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo M. A., Marti M., Valle J., Manna A. C., Cheung A. L., Lasa I., et al. (2005). SarA is an essential positive regulator of Staphylococcus epidermidis biofilm development. J. Bacteriol. 187, 2348–2356. doi: 10.1128/JB.187.7.2348-2356.2005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totte J. E., Van Der Feltz W. T., Hennekam M., Van Belkum A., Van Zuuren E. J., Pasmans S. G. (2016). Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta-analysis. Br. J. Dermatol. 175, 687–695. doi: 10.1111/bjd.14566, PMID: [DOI] [PubMed] [Google Scholar]
- Vandecasteele S. J., Peetermans W. E., Merckx R., Van Eldere J. (2003). Expression of biofilm-associated genes in Staphylococcus epidermidis during in vitro and in vivo foreign body infections. J. Infect. Dis. 188, 730–737. doi: 10.1086/377452, PMID: [DOI] [PubMed] [Google Scholar]
- Vuong C., Kidder J. B., Jacobson E. R., Otto M., Proctor R. A., Somerville G. A. (2005). Staphylococcus epidermidis polysaccharide intercellular adhesin production significantly increases during tricarboxylic acid cycle stress. J. Bacteriol. 187, 2967–2973. doi: 10.1128/JB.187.9.2967-2973.2005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Bojer M. S., George S. E., Wang Z., Jensen P. R., Wolz C., et al. (2018). Inactivation of TCA cycle enhances Staphylococcus aureus persister cell formation in stationary phase. Sci. Rep. 8:10849. doi: 10.1038/s41598-018-29123-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenmaier C., Peschel A., Xiong Y. Q., Kristian S. A., Dietz K., Yeaman M. R., et al. (2005). Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J. Infect. Dis. 191, 1771–1777. doi: 10.1086/429692, PMID: [DOI] [PubMed] [Google Scholar]
- Wertheim H. F., Melles D. C., Vos M. C., Van Leeuwen W., Van Belkum A., Verbrugh H. A., et al. (2005). The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5, 751–762. doi: 10.1016/S1473-3099(05)70295-4, PMID: [DOI] [PubMed] [Google Scholar]
- Zhang Y. Q., Ren S. X., Li H. L., Wang Y. X., Fu G., Yang J., et al. (2003). Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol. Microbiol. 49, 1577–1593. doi: 10.1046/j.1365-2958.2003.03671.x, PMID: [DOI] [PubMed] [Google Scholar]
- Zheng Y., Hunt R. L., Villaruz A. E., Fisher E. L., Liu R., Liu Q., et al. (2022). Commensal Staphylococcus epidermidis contributes to skin barrier homeostasis by generating protective ceramides. Cell Host Microbe 30, 301–313.e9. doi: 10.1016/j.chom.2022.01.004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Spoto M., Hardy R., Guan C., Fleming E., Larson P. J., et al. (2020). Host-specific evolutionary and transmission dynamics shape the functional diversification of Staphylococcus epidermidis in human skin. Cell 180, 454–470.e18. doi: 10.1016/j.cell.2020.01.006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.