Abstract
Background/Aim: The treatment of solitary brain metastasis is a challenging intervention since the incidence increases and prognosis is poor. This study investigated the role of perilesional edema in the overall mass effect of solitary brain metastasis.
Patients and Methods: We conducted a retrospective analysis on 88 patients with single supratentorial brain metastasis and concomitant perilesional edema undergoing en bloc resection. Each patient was evaluated for perilesional brain edema grading. We stratified patients into three groups based on the size of the metastatic lesion and the extent of perilesional edema.
Results: The grade of perilesional edema at 30 days after surgical removal did not correlate with the maximum diameter of the metastasis (Pearson’s correlation 0.098, p=0.494). In patients with a maximal metastatic diameter ≤2 cm, the grade of perilesional edema before surgical treatment was 1.63 (STD 0.43), while 30 days after removal it was significantly reduced; 0.47 (STD 0.26), p<0.001.
Conclusion: The overall mass effect of solitary supratentorial brain metastases is not correlated to the size of the lesion and the grade of the associated perilesional edema should be considered. Surgical en bloc resection can be considered the first choice of treatment in the presence of solitary metastasis ≤2 cm in maximal diameter but with high-grade edema, since this treatment reduces the overall mass effect.
Keywords: Brain metastasis, perilesional edema, en bloc resection, overall mass effect, size of lesion
Metastases are the most frequent neoplasms affecting the central nervous system (CNS) and their incidence varies depending on the primary tumor’s histotype (1). The prognosis is poor: less than 20% of patients with symptomatic brain metastases survive one year after the diagnosis (2). The prognosis depends on several factors such as sex, age, histotype of the primary tumor, the time elapsed from the primary tumor diagnosis, the number of metastatic lesions, the Karnofsky performance scale (KPS), the presence of extracranial metastases, and the response to systemic treatment (3). The most common primary tumors responsible for brain metastases are lung cancer, breast cancer, renal cell carcinoma, and melanoma (4-7). However, brain metastases have also been identified to originate from other primary tumors such as lymphomas, gastroesophageal, thyroid gland (8), gynaecological, and genitourinary.
Brain metastases are challenging to treat since their incidence increases and treatments strategies remain inadequate. One of the reasons why the treatment is ineffective is that the blood-brain barrier (BBB) and the blood-tumour barrier (BTB) restrict access to chemotherapy (9). The development of metastases depends on the crucial interactions between the invading metastatic tumor cells and the cerebral microenvironment during its development (10).
The type of metastatic infiltration at the metastasis/brain parenchyma (M/BP) interface is related to the origin of the primary tumor, which has prognostic value (11). En bloc resection of the metastasis and SRS appeared to reduce the rate of local brain relapse (12). It may be helpful to decrease the diffusion of micrometastases into the parenchyma and reduce the residue risk, however, it is necessary to confirm that en bloc resection is not always doable.
This study investigated the role of perilesional edema in the overall mass effect of solitary brain metastasis and shows that the perilesional edema of metastasis is reduced at 30 days after en bloc surgical resection of metastasis. Additionally, the perilesional edema reduction can be related to an improvement in the KPS and overall survival (OS).
Patients and Methods
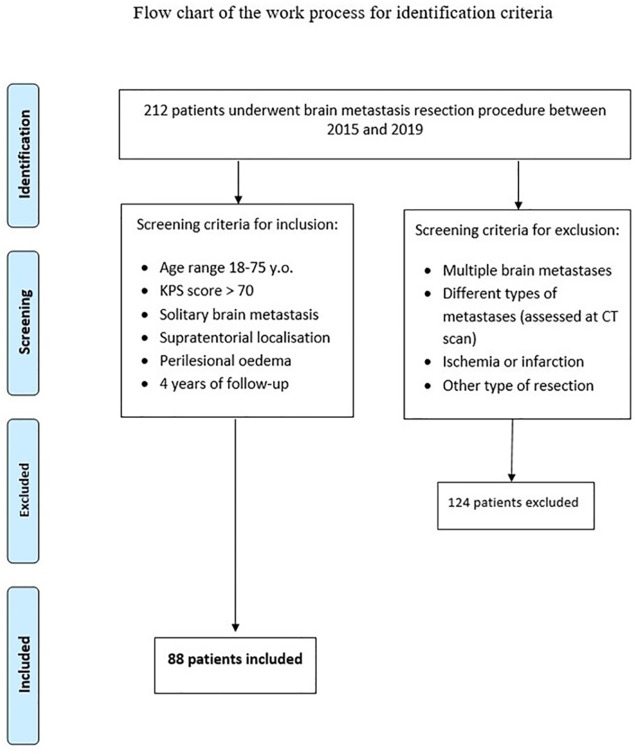
Patients. We conducted a retrospective analysis on 212 patients undergoing supratentorial cerebral metastases surgery from 01/01/2015 to 12/31/2019 at the Policlinico Umberto I University Hospital of Rome and at Sant’Andrea University Hospital Sapienza University of Rome. In this analysis, we included patients with solitary supratentorial brain metastasis and concomitant perilesional edema, undergoing an en bloc resection between 18 and 75 years, and excluded patients with multiple brain metastatic lesions, concomitant ischemic lesions, and patients with infratentorial metastases (Figure 1). In the light of examined patients, 88 of 212 complied with the inclusion criteria for this analysis (Figure 1).
Figure 1. Identification criteria for inclusion; flow chart of the work process.
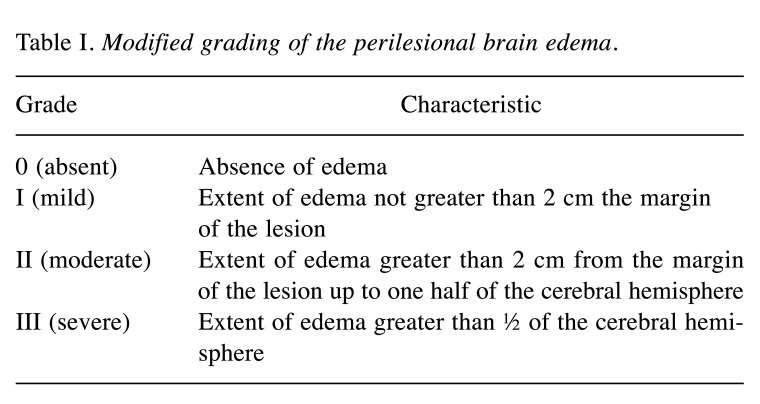
Demographics. Each patient was evaluated for sex, age, histological characteristics of the primary tumor, the localization of eloquent brain area, the maximal diameter of metastasis, KPS, presence of intra-metastatic bleeding, surgical complications, pre-operative edema, and post-operative edema at 30 days after surgery assessed with the modified grading of the perilesional brain edema (Table I).
Table I. Modified grading of the perilesional brain edema.
Imaging analysis. Magnetic resonance imaging (MRI) 1.5T or 3T sequences with contrast administration, T2 weighted sequences, DWI, PWI, FLAIR and spectroscopy were performed in all the patients included in this investigation in the immediate pre-operative and in the post-operative at 30 days after surgery. A functional MRI was performed in patients with lesions in the eloquent brain area. The extent of perilesional edema was evaluated on the FLAIR sequences of the pre-operative MRI and in the post-operative MRI at 30 days.
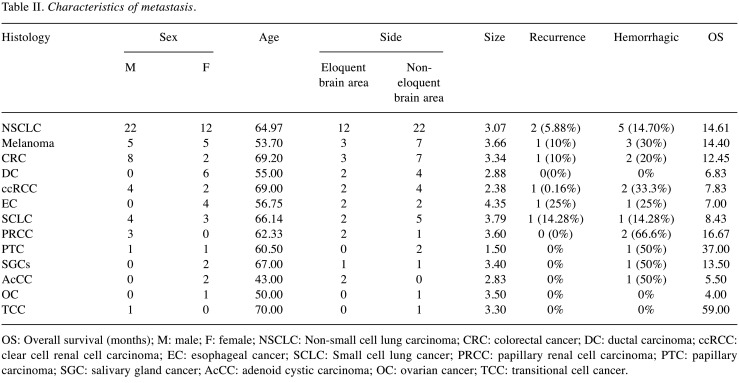
Inclusion and exclusion criteria. Patients who were included in this analysis presented a solitary supratentorial brain metastasis that was histologically characterized after resection. Lesions with unknown histological diagnosis were excluded. The characteristics of the metastasis were reported in Table II.
Table II. Characteristics of metastasis.
OS: Overall survival (months); M: male; F: female; NSCLC: Non-small cell lung carcinoma; CRC: colorectal cancer; DC: ductal carcinoma; ccRCC: clear cell renal cell carcinoma; EC: esophageal cancer; SCLC: Small cell lung cancer; PRCC: papillary renal cell carcinoma; PTC: papillary carcinoma; SGC: salivary gland cancer; AcCC: adenoid cystic carcinoma; OC: ovarian cancer; TCC: transitional cell cancer.
Grading. Perilesional edema was measured on T2-weighted MR images and graded according to the modified classification of Kazner et al. (13). This scale, introduced by Kazner et al. in 1982 and modified by Chernov in 2005 (14), is used in literature to define the perilesional edema grading of brain metastases. The authors standardized this score with a linear transformation obtaining a normal distribution with mean μ and unit variance σ2. Averages obtained by this standard score are reported specifying the standard deviation (Table III, Table IV, and Table V). We used the perilesional edema grading consisting of 4 degrees that span from degree 0, which corresponds to the absence of edema, up to grade 3 corresponding to edema covering an area that is greater than half of the cerebral hemisphere (Table I) (14).
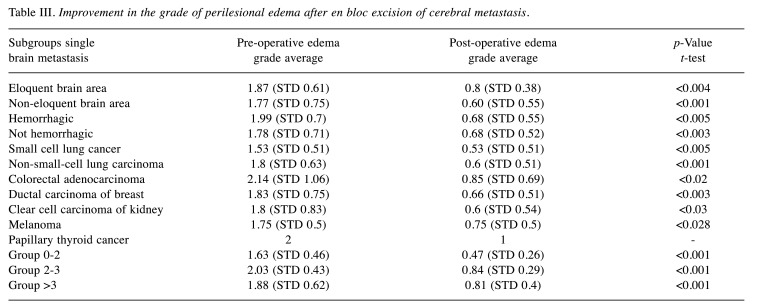
Table III. Improvement in the grade of perilesional edema after en bloc excision of cerebral metastasis.
Table IV. Pre-operative perilesional edema grade comparison among the different groups of patients divided according to the maximum diameter of the single metastasis.
Table V. Comparison of the grade of post-operative perilesional edema among the groups of patients divided according to the maximum diameter of the single metastasis.
Stratification. Based on the size of the metastatic lesion we identified three groups: in the first one the metastases had a maximal diameter of ≤2 cm (Group range 0-2), in the second group the diameter was >2 and ≤3 cm (Group 2-3), while in the third the diameter was >3 cm (Group >3). The maximal diameter of the single lesion and not the volume was employed as a parameter in classifying the groups since the diameter is an available value commonly used in clinical practice and suitable for its utilization in algorithms for the management of newly diagnosed brain metastases (15).
Surgical and clinicopathological correlation. Metastasis Brain metastases are extra-axial lesions that present a capsule and a cleavage plane from the brain parenchyma. En bloc resection following lesion wounds is always preferred, in the author’s opinion, since it can decrease the diffusion of micro metastases into the parenchyma and to reduce residue risk. In our experience, the “no internal touch” (12) asportation technique without damaging the surrounding brain structures can be performed. Transient neurological deficits can be observed post-operatively due to white matter fiber tracts micro traction and the temporary increasement of the perilesional edema. For this reason, the authors investigated the perilesional edema grade at 30 days after the resection of metastases and not immediately after surgery. Metastases not removed by both the en bloc technique and the “no internal touch” technique were excluded.
Statistical analysis. Univariate statistical analysis and linear correlation between the grading pre-operative edema and the size of metastasis was performed. A multivariate statistical analysis was also performed for the dependent variables: OS, KPS, the degree of post-operative edema, and the reduction of the degree of edema after surgical treatment. A simultaneous regression model was used in all the independent variables entered simultaneously in the regression equation. Multivariate simultaneous regression analysis was essential to understand the statistical inference of the single variables we examined and the correlations with clinical endpoints (OS, KPS, and recurrence-risk ratio). The parameters are shown by using Kaplan-Meier survival curves compared with log-rank tests, χ2, t-student test, and scatter plots dependent on the characteristics of the examined variable. The statistical analysis and graphs were obtained using the statistical software IBM SPSS Statistics for Windows, Version 25.0. (Armonk, NY, USA). Statistical analysis methodology follows the one used by Salvati et al. in 2020 (16).
Results
Metastatic mass effect. We conducted univariate and multivariate analyses on the examined variables: age, histological type, localization of metastases (eloquent brain area or non-eloquent brain area), hemorrhagic presentation, size of metastasis, the grade of recurrence, pre-operative and post-operative perilesional edema grade at 30 days after surgery, KPS, and OS. The analysis of the correlation between radiological parameters (size of lesion, pre-operative and post-operative edema) and clinical endpoints (KPS and OS) was particularly interesting. The data analysis clearly evidenced that the overall mass effect of solitary brain metastasis depends on dimension, maximal diameter of the metastasis, and the perilesional edema grade resistant to therapy. Particularly, the correlation analysis showed that lesions with maximal diameter ≤2 cm can also have a very important overall mass effect when they present a high grade of perilesional edema grade resistant to edema therapy. The median age of enrolled patients was 62.72 (STD=13.17), and 54.54% of patients were male and 45.46% female.
Metastatic types. The most frequent histological type of metastasis was NSCLC, which represented 39% (n=34) of the total cases (n=88), followed by melanoma and colorectal cancer (CRC) metastasis 11% (n=10), and SCLC 8% (n=7). Solitary brain metastases of primary pulmonary origin constituted 47% of the total cases of metastases. The histology of metastatic lesions, localization of metastases divided into eloquent brain areas and non-eloquent brain areas, grade of recurrence, and hemorrhagic presentation are shown in Table II.
Group stratification analysis. We identified three groups according to the size of the metastatic lesion: 21.59% of metastases had a diameter ≤2 cm (Group 0-2), 29.54% had a diameter >2 and ≤3 cm (Group 2-3), and 48.86% had a diameter >3 cm (Group >3). The metastases were localized in the eloquent brain area in 36% of the cases. In 22% of the samples, the metastases were hemorrhagic. Perilesional edema was evaluated by T2-weighted MR images and graded pre-operatively and post-operatively, 30 days after surgery (Table III). The average of overall pre-operative perilesional edema grade was 1.84 (STD=0.70), while the post-operative one at 30 days was 0.68 (STD=0.5), with a statistically significant difference (p<0.001). In the present analysis, the average lesion edema of single metastases after en bloc removal was reduced after thirty days. The sample set was divided into homogeneous groups (localization, maximum diameter of the lesion, hemorrhagic presentation, and histotype of the primary tumor) in order to verify whether a significant reduction in periwound edema was observed after thirty days. The average pre-operative perilesional edema grade of all lesions was 1.84 (STD=0.7) and distributed as follows: 1.87 (STD=0.61) in the eloquent brain area, 1.77 (STD=0.75) in non-eloquent brain area, 1.99 (STD=0.7) in hemorrhagic lesions, and 1.78 (STD=0.71) in non-hemorrhagic lesions (Table III). The average post-operative perilesional edema grade at follow-up 30 days after surgery was 0.68 (STD=0.75) and distributed as follows: 0.8 (STD=0.38) in the lesions in the eloquent brain area; 0.60 (STD=0.55) in the lesions in the non-eloquent brain area, 0.68 (STD=0.55) in the hemorrhagic lesions, and 0.68 (STD=0.52) in non-hemorrhagic lesions.
Pre and Post-operative edema features analysis. Thirty days after en bloc removal, the degree of edema significantly reduced in both haemorrhagic lesions (p<0.005) and non-haemorrhagic lesions (p<0.003) compared to that pre-operatively. We found a slight increase in pre-operative perilesional edema grade in hemorrhagic lesions compared to those in the control group (non-hemorrhagic lesions), with no statistical significance. After 30 days of en bloc removal of the metastasis, a significant reduction in periwound edema was observed both in metastases in the area located in the eloquent area (p<0.004) and in those located in non-eloquent areas (p<0.001) compared to those pre-operatively. The overall data of each subclass of the studied metastases are shown in Table III. The average pre-operative edema grade was higher for solitary metastases of CRC, NSCLC, ductal carcinoma of the breast, and melanoma. The histotype of the primary tumor was the main factor that affected the pre-operative perilesional edema development (Table III). The post-operative edema grade at 30 days after surgery varied according to the histotype and was higher for metastases of CRC, melanoma, and ductal carcinoma of the breast. This result was not statistically significant because of the examined subgroup heterogeneity and because it needs to be validated on a larger sample. Despite these differences, within all groups examined based on the histology of the primary tumor (SCLC, NSCLC, CRC, ductal carcinoma of the breast, clear cell carcinoma of the kidney, and melanoma) the degree of peri-wound edema showed a significant reduction after 30 days from en bloc removal.
Group comparison analysis. We compared the grade of pre-operative perilesional edema in the three groups of patients and no statistically significant differences were found (Table IV). The comparison of pre-operative edema grade average of Group 0-2 cm vs. Group 2-3 cm showed a strong trend towards significance, 1.67 (STD=0.46) vs. 2.03 (STD=0.43), respectively, p=0.05. The postoperative edema grade average 30 days after surgical removal was not related to the maximum diameter of the metastasis (Pearson’s correlation 0.098, p=0.494). In the comparison of the groups, a significant difference was found between Group 0-2 vs. Group 2-3 (0.47 vs. 0.84 p=0.02) and Group 0-2 vs. Group >3 (0.47 vs. 0.79 p=0.05), as reported in the data in Table V. We observed that post-operative edema at 30 days after surgery was larger after en bloc resection of metastasis with maximal diameter >3 cm and this variation could be explained by the surgical parenchymal injury effect.
In Figure 2, two examples of reduction of perilesional edema grade are reported. The first one belongs to Group 0-2 and the second one to Group 2-3. The correlation analysis (Figure 3) showed that there was no direct correlation between the maximum diameter of the metastasis and the grade of pre-operative cerebral edema (Pearson’s correlation 0.14, p=0.17). The grade of perilesional edema did not significantly vary with the size of the metastasis. Also, there was no difference between sexes. The average post-operative KPS was 72.1 (STD=15.73), in the case of the lesions in the eloquent brain area was 71.25 (STD=15.53), in non-eloquent brain area was 74.75 (STD=15.32), in hemorrhagic lesions was 68.45 (STD=15.63), and in non-hemorrhagic lesions 76.48 (STD=15.84). The KPS had a negative correlation with pre-operative perilesional edema grade (Pearson Correlation - 0.335, p=0.001), and the highest grades of perilesional edema were associated with significantly lowest grades of KPS (Figure 4). A high degree of pre-operative edema grade average was associated with a worse post-operative KPS. The subgroup of patients with pre-operative edema grade average equal to 3 had a KPS ≤70 in 42.10% of cases and when compared with the subgroup of patients with pre-operative edema grade average equal to 1, a statistically significant difference was found in terms of post-operative KPS (85.33 vs. 67.89; p=0.004), Figure 4. The post-operative KPS was strongly affected by the pre-operative perilesional edema grade and the patient group with perilesional edema grade >3 may also present KPS <70. The post-operative KPS represents an independent prognostic factor for OS and the group of patients with KPS between 80 and 100 had a higher OS (Figure 5). OS significantly correlated with tumor histology (Log-rank χ2=15.668, p=0.047) (Table II), and the patient’s age (Log-rank χ2=11.092, p=0.004). In addition, OS correlated with post-operative KPS (Breslow-Generalized Wilcoxon χ2=5.434 p=0.066) (Figure 5), and the degree of postoperative edema (Log-rank χ2=5.292, p=0.071) (Figure 6). However, these correlations did not reach statistical significance. No correlation between OS and metastasis maximum diameter was identified (Log-rank χ2=4.097, p=0.129) (Figure 7). The most frequent complications with en bloc resection were dural tear and neurological deficits (Table VI). The overall recurrence rate of locoregional metastases was 6.81%. It was 5.26% in Group 0-2, 7.69% in Group 2-3 and 6.97% in Group >3. No significant differences were found among these three groups.
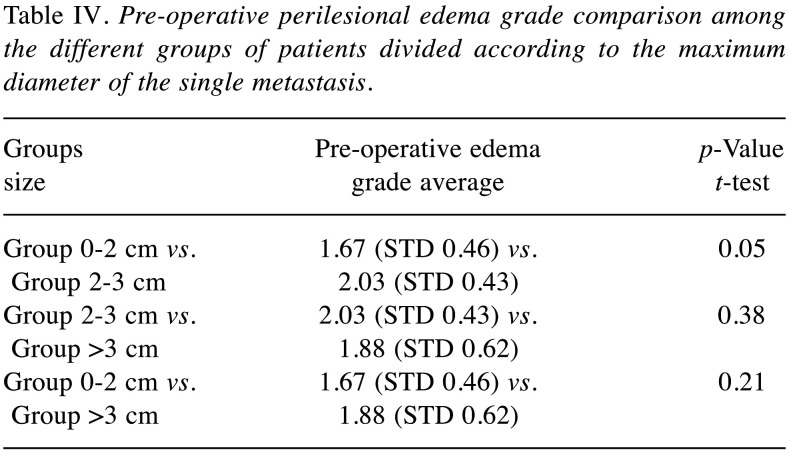
Figure 2. Reduction in perilesional edema after en bloc resection. A) Pre-operative FLAIR MRI of Group 0-2 cm metastases in the eloquent brain area with pre-operative grade II perilesional edema; B) Postoperative FLAIR MRI of Group 0-2 cm metastases in the eloquent area with post-operative grade 0 of perilesional edema; C) Pre-operative FLAIR MRI of Group 2-3 cm metastases in the non-eloquent brain area with pre-operative grade I perilesional brain; D) Postoperative FLAIR MRI of Group 2-3 cm metastases in the non-eloquent brain area with post-operative grade 0 perilesional edema. MRI: Magnetic resonance imaging; FLAIR: fluid attenuated inversion recovery.
Figure 3. Size distribution of solitary metastasis in relation to preoperative perilesional edema. No difference is found between female (in blue) and male (in red) patients regarding the size and the grade of edema.
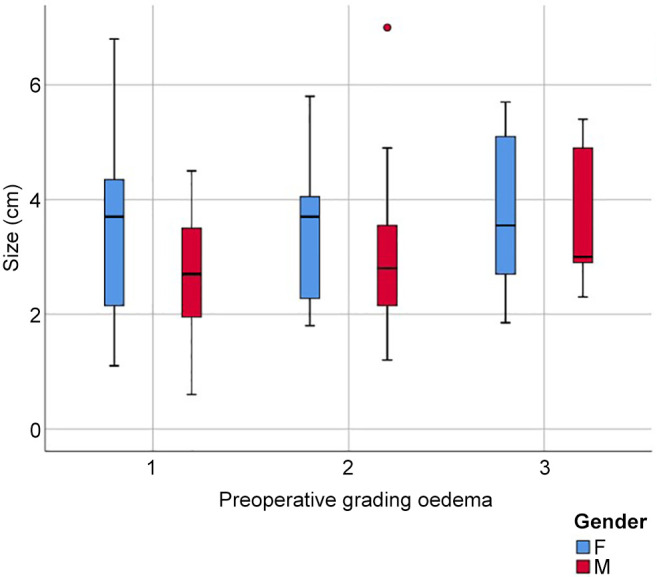
Figure 4. Distribution of post-operative Karnofsky performance scale in relation to pre-operative perilesional edema.
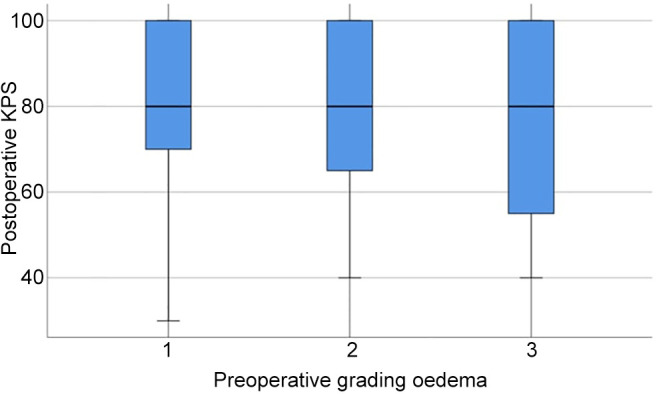
Figure 5. Kaplan-Meier curve of overall survival in relation to Karnofsky performance scale.
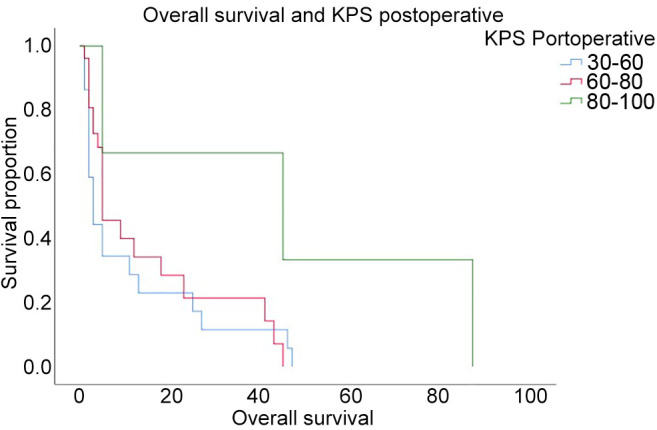
Figure 6. Kaplan-Meier curve of overall survival in relation to the grade of post-operative perilesional edema.
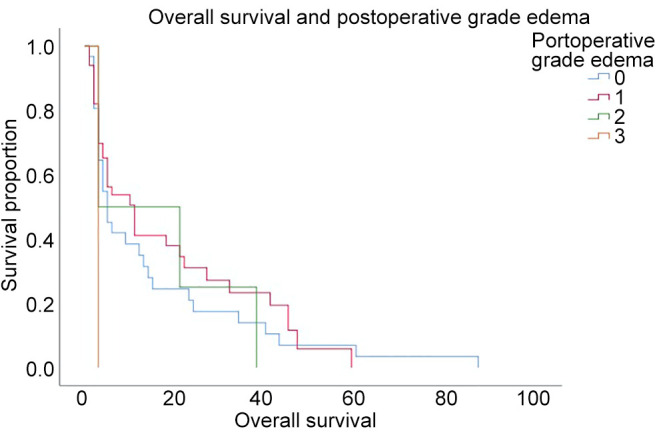
Figure 7. Kaplan-Meier survival curve of overall survival in relation to the size of the metastatic lesion.
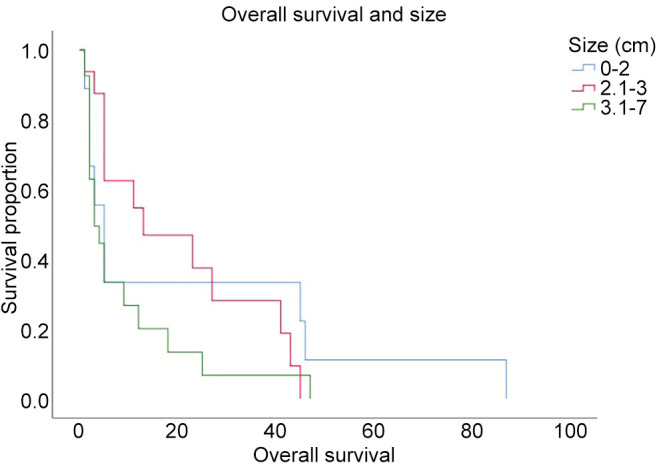
Table VI. Complications after en bloc excision of brain metastasis.
Discussion
This study shows that en bloc resection of solitary supratentorial metastasis reduces perilesional edema at 30 days after surgery. En bloc resection technique consists of the dissection of the tumor capsule in the tumor-brain interface described as the “no internal touch” and is recommended over piecemeal resection, because it has been shown to reduce the risk of local recurrence (17).
En bloc resection of solitary brain metastasis may offer several advantages over piecemeal resection; however, in some instances it is not performed in eloquent brain areas due to the risk of developing neurological deficits. However, recent studies have shown that postoperative complication rates are not increased by en bloc resection, including the removal of lesions in eloquent brain regions or large tumors. This stresses the idea that en bloc resection of brain metastasis is at least as safe as piecemeal resection (18).
Local brain invasion is a process based on cell motility, adhesion, and enzymatic remodelling of the extracellular matrix components. Brain invasion results from interactions between brain stromal and endothelial cells and the invading metastatic tumor cells. Proteolytic degradation of the extracellular matrix is a process thought to sustain tumor invasion by clearing a pathway for the invading tumor cells (19). Brain metastasis is an extra-axial lesion and tumor cells react with the surrounding brain parenchyma and blood vessels, resulting in a series of pathological and biochemical changes. Brain metastasis usually has a cleavage plane from the brain parenchyma, unlike gliomas, which diffusely infiltrate the brain parenchyma.
Some studies have investigated the infiltration of metastatic carcinoma cells into adjacent brain parenchyma and the reaction of the glial-defense system against the infiltrating carcinoma cells at the M/BP-interface with in vivo/in vitro models (20). Siam et al. demonstrated the presence of four types of metastatic infiltration at the M/BP-interface based on the pattern of infiltrating cancer cells with a significant glial reaction; the result supports the differences in the infiltration pattern related to the dependency to the primary tumor and to the metastatic infiltration pattern. The assessment of the immune cell infiltration at the tumor margin might have prognostic value and may be suitable for biological drug treatments. The interaction between the metastatic environment and glial-reaction can explain the different rates of metastasis recurrence based on the primary tumor histotype. It would be interesting to correlate in further studies the type of infiltration at the M/BP-interface and the perilesional edema grade, developed in this study.
En bloc resection can be considered a safe and effective technique in solitary brain metastases resection. However, taking into account the infiltration of metastatic cells into adjacent brain parenchyma, the extension of neurosurgical resection at the M/BP-interface can be considered in non-eloquent brain areas. Further studies are required to validate the supramarginal technique in non-eloquent areas and to understand whether a correlation exists with the reduction of local recurrence rate. In our study, the histotype of the primary tumor is the main factor associated with the pre-operative perilesional edema. However, at 30 days after en bloc resection, we found a statistically significant reduction in the degree of perilesional edema, with an overall reduction in the mass effect. All the examined subgroups had a significative reduction in the degree of perilesional edema compared to pre-operative, but higher for CRC, melanoma, ductal carcinoma of the breast, and ductal carcinoma of breast metastasis, as well as for hemorrhagic lesion. It is particularly interesting to note that the grade of pre-operative edema was not related to the size of the metastasis and that perilesional edema at 30 days was significantly reduced regardless of the size of the lesion.
Two-thirds of patients with brain metastases associated with peritumor edema developed cognitive problems due to white matter fibres damage (21). Neurological symptoms seem to be related to the general metastasis overall mass effect and not to the volume of the lesion. This can be explained by the lesion dimensions and perilesional edema, which is not reduced with anti-edema therapy. Metastases with a maximal diameter of less than 2 cm, but with a high grade of perilesional edema, resistant to corticosteroids and treated with stand-alone stereotaxic radiosurgery (SRS) may worsen the edema grade together with KPS and neurological symptoms.
The presence of peritumoral edema is associated with immune system activation and correlated with OS (22). The peritumor edema of brain metastases is vasogenic. This is due to the blood-brain barrier damage linked to a functional modification of the tumor vessels. This is mediated by a functional difference rather than a quantitative one of tumor-associated blood vessels (23). Activation of the immune system, occurrence of metastasis, and the development of perilesional edema are elements to consider, and these are correlated to the patient outcome.
Pre-operative perilesional edema in our study was negatively correlated with post-operative KPS (p=0.001), while OS was related to the histotype of the primary tumor, the patient’s age, and post-operative KPS. Other authors had identified a substantial prognostic value of perilesional edema in solitary brain metastases (24). In our study, perilesional edema was weakly correlated with OS, however, as mentioned, it negatively affected post-operative KPS as an important prognostic factor. The best results after surgical treatment were obtained in terms of OS in patients with KPS >70, younger age, control of the primary tumor, and diameter of brain metastases greater than 3 cm with mass effect and the possibility of complete resection (25). The surgery improved the outcome of patients with a single metastatic lesion, and a low KPS with limited number of extracranial metastases (26). In patients with unknown primary or multiple primary tumors, surgery helps establish the histological diagnosis (27). Surgery only, however, does not ensure the prevention of recurrence and 46% of patients treated with surgery alone reported disease recurrence (28). The presence of perilesional edema was also related to the tendency of the tumor to spread and this was linked to resistance to radiotherapy. Consequently, less edema was associated with less radioresistance, improving the response to SRS and decreasing the risk of relapse (29).
Whole-brain radiotherapy (WBRT) is taken into consideration for patients in which surgery or SRS is not recommended. This can be in the case of leptomeningeal dissemination, numerous metastases, or in the presence of other contraindications (30). Surgical treatment followed by WBRT in patients with single lesions has been shown effective in several trials. The combination of surgery and WBRT in the treatment of single brain metastases demonstrated a 70% reduction in local recurrences. There are several side effects of WBRT, including cognitive deficits, for which the use of SRS is preferred; recent studies agree that WBRT and SRS are equivalent in the treatment of single brain metastases, but SRS is associated with fewer side effects. En bloc resection followed by SRS reduces the risk of relapse. In our study, we reported a recurrence rate of 7% (7/10 days) that was associated with a higher degree of sustained periwound edema at 30 days. In addition, we observed that early local recurrence induces a shorter survival period; however, a larger cohort would provide a reliable estimate of the OS of recurrences cases.
SRS is the indicated procedure as a stand-alone treatment for solitary brain metastases smaller than 3 cm in diameter (31), and it has a favourable outcome for patients with a single lesion compared to surgery and WBRT (32). However, this does not lead to an immediate resolution for the edema, mass effect, and hydrocephalus. In contrast, SRS is excluded for lesions with dimensions greater than 3 cm and with a midline shift greater than 1 cm. For multiple metastases, the literature suggests using SRS for up to 4 lesions and WBRT for more than five metastases. SRS is indicated to treat solitary brain metastases smaller than 3 cm in diameter. Despite its effectiveness, SRS can lead to new-onset or worsening perilesional edema within weeks or months after treatment. High-dose steroids can be used for symptoms associated with edema (33). However, prolonged administration of corticosteroids is associated with substantial side effects (34).
Strengths of the study and methodology. This retrospective study investigated the radiological patterns and outcomes of patients enrolled after the clinical workup. We conducted a narrative literature review of the topic and we discussed with our Institutional Ethics panel about the formal approval of this research. We performed a random selection of the patients based on the criteria of inclusion. We analysed data to measure and control the parameters using appropriate statistical methodology to avoid potential confounders. Despite the exiguity of the samples that could result in a potential source of bias, an excellent post-hoc statistical power (1-β=0.914 for α=0.05 and effect size “f”=0.78) reached reliable conclusions concerning the selected endpoints. We obtained consent forms before the surgical procedure from all patients who gave written explicit consent with adequate information about their participation in the clinical investigation. All the data reported were completely anonymized. No randomization techniques were performed. All the procedures were consistent with the Declaration of Helsinki for human rights.
Limitations of the study. This investigation was limited because it was carried out retrospectively on a limited sample of patients from a single institution. It is necessary to design prospective and randomized clinical trials to validate the prognostic value of perilesional edema of single supratentorial brain metastases. In addition, the trials should be multicenter in order to recruit a large enough sample to allow the role of perilesional edema to be validated even for the different histotypes. It is interesting to compare the grade of perilesional edema at 30 days of supratentorial solitary brain metastases with a diameter of less than 2 cm, treated with stand-alone SRS or “en-bloc” excision.
Conclusion
This study showed that the histotype of the primary tumor is the main factor associated with pre-operative perilesional edema. En bloc resection is a safe and effective technique for solitary brain metastasis treatment including case of the removal of lesions in eloquent brain regions and for large tumors. We found that the perilesional edema is reduced at 30 days after en bloc resection of solitary brain metastasis. The overall mass effect of a solitary brain metastasis depends not only on the maximal diameter of the metastasis but also on the perilesional edema grade. However, we did not find evidence of a direct correlation between the maximal diameter of the metastasis and the grade of pre-operative brain edema. Pre-operative perilesional edema is associated with the histotype of the primary tumor and it has a negative correlation with the KPS of the patients. Furthermore, at 30 days after the en bloc resection, we found a reduction in the overall mass effect due to a substantial reduction in the degree of perilesional edema. En bloc resection technique can be considered as first choice of treatment in the presence of solitary metastasis lesions ≤2 cm in maximal diameter but with high-grade edema since this treatment reduces the overall mass effect. A multicenter randomized prospective study is required to evaluate the outcome of patients with a solitary supratentorial metastasis who present perilesional edema resistant to anti-edema therapy and treated with stand-alone SRS or with the en bloc surgical technique. Other studies are required to define the role of perilesional edema for a future defined therapeutic protocol of solitary brain metastases. There is the need to investigate the correlation between the types of metastatic infiltration at the metastasis/brain parenchyma-interface and perilesional edema in order to validate a supramarginal technique for non-eloquent areas.
Funding
This research was funded by Sapienza University Funds. Pierfrancesco Lapolla is supported by The Foundation Blanceflor Boncompagni Ludovisi, née Bildt scholarship.
Conflicts of Interest
The Authors declare no conflicts of interest in relation to this study.
Authors’ Contributions
Conceptualization, P.B., P.L. and G.D.; methodology, P.B. and A.F.; software, P.F. and G. D.; validation, G.F., A.S., A.F., G.D. and M.S.; formal analysis, A.D.; investigation, G.Z., P.F., P.B., A.M., M.K.; resources, G.Z. and A.D.; data curation, P.L., P.B. and A.D.; writing—original draft preparation, P.B., A.D. and P.L.; writing—review and editing, P.B., A.D. and P.L.; visualization, A.S., P.F. and A.M.; supervision, A.S. and P.F.; project administration, A.S. and G.F. All Authors have read and agreed to the published version of the manuscript.
Acknowledgements
We thank Dr. Veronica Bruzzaniti for editing the figures of this manuscript.
References
- 1.Ostrom QT, Wright CH, Barnholtz-Sloan JS. Brain metastases: epidemiology. Handb Clin Neurol. 2018;149:27–42. doi: 10.1016/B978-0-12-811161-1.00002-5. [DOI] [PubMed] [Google Scholar]
- 2.Yuzhalin AE, Yu D. Brain metastasis organotropism. Cold Spring Harb Perspect Med. 2020;10(5):a037242. doi: 10.1101/cshperspect.a037242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wanleenuwat P, Iwanowski P. Metastases to the central nervous system: Molecular basis and clinical considerations. J Neurol Sci. 2020;412:116755. doi: 10.1016/j.jns.2020.116755. [DOI] [PubMed] [Google Scholar]
- 4.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 5.Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain MC, Baik CS, Gadi VK, Bhatia S, Chow LQ. Systemic therapy of brain metastases: non-small cell lung cancer, breast cancer, and melanoma. Neuro Oncol. 2017;19(1):i1–i24. doi: 10.1093/neuonc/now197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gállego Pérez-Larraya J, Hildebrand J. Brain metastases. Handb Clin Neurol. 2014;121:1143–1157. doi: 10.1016/B978-0-7020-4088-7.00077-8. [DOI] [PubMed] [Google Scholar]
- 8.Relucenti M, Familiari P, Iacopino G, Bruzzaniti P, Miglietta S, Salvati M, Li X, Chen R, D’andrea G, Frati A, Di gioia C, Pernazza A, Della rocca C, Familiari G, Santoro A. RET/PTC3 translocation in a rare hemorrhagic brain metastasis of papillary thyroid cancer post Chernobyl radiation affects vessels ultrastructure. Interdisciplinary Neurosurgery. 2021;23:100889. doi: 10.1016/j.inat.2020.100889. [DOI] [Google Scholar]
- 9.Sprowls SA, Arsiwala TA, Bumgarner JR, Shah N, Lateef SS, Kielkowski BN, Lockman PR. Improving CNS delivery to brain metastases by blood-tumor barrier disruption. Trends Cancer. 2019;5(8):495–505. doi: 10.1016/j.trecan.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathoo N, Chahlavi A, Barnett GH, Toms SA. Pathobiology of brain metastases. J Clin Pathol. 2005;58(3):237–242. doi: 10.1136/jcp.2003.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siam L, Bleckmann A, Chaung HN, Mohr A, Klemm F, Barrantes-Freer A, Blazquez R, Wolff HA, Lüke F, Rohde V, Stadelmann C, Pukrop T. The metastatic infiltration at the metastasis/brain parenchyma-interface is very heterogeneous and has a significant impact on survival in a prospective study. Oncotarget. 2015;6(30):29254–29267. doi: 10.18632/oncotarget.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvati M, Cervoni L, Delfini R. Solitary brain metastases from non-oat cell lung cancer: clinical and prognostic features. Neurosurg Rev. 1996;19(4):221–225. doi: 10.1007/BF00314834. [DOI] [PubMed] [Google Scholar]
- 13.Steinhoff H, Lanksch W, Kazner E, Grumme T, Meese W, Lange S, Aulich A, Schindler E, Wende S. Computed tomography in the diagnosis and differential diagnosis of glioblastomas. A qualitative study of 295 cases. Neuroradiology. 1977;14(4):193–200. doi: 10.1007/BF00496983. [DOI] [PubMed] [Google Scholar]
- 14.Chernov MF, Kubo O, Hayashi M, Izawa M, Maruyama T, Usukura M, Ono Y, Hori T, Takakura K. Proton MRS of the peritumoral brain. J Neurol Sci. 2005;228(2):137–142. doi: 10.1016/j.jns.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 15.Sankey EW, Tsvankin V, Grabowski MM, Nayar G, Batich KA, Risman A, Champion CD, Salama AKS, Goodwin CR, Fecci PE. Operative and peri-operative considerations in the management of brain metastasis. Cancer Med. 2019;8(16):6809–6831. doi: 10.1002/cam4.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvati M, Bruzzaniti P, Relucenti M, Nizzola M, Familiari P, Giugliano M, Scafa AK, Galletta S, Li X, Chen R, Barbaranelli C, Frati A, Santoro A. Retrospective and randomized analysis of influence and correlation of clinical and molecular prognostic factors in a mono-operative series of 122 patients with glioblastoma treated with STR or GTR. Brain Sci. 2020;10(2):91. doi: 10.3390/brainsci10020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel AJ, Suki D, Hatiboglu MA, Abouassi H, Shi W, Wildrick DM, Lang FF, Sawaya R. Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurosurg. 2010;113(2):181–189. doi: 10.3171/2009.11.JNS09659. [DOI] [PubMed] [Google Scholar]
- 18.Patel AJ, Suki D, Hatiboglu MA, Rao VY, Fox BD, Sawaya R. Impact of surgical methodology on the complication rate and functional outcome of patients with a single brain metastasis. J Neurosurg. 2015;122(5):1132–1143. doi: 10.3171/2014.9.JNS13939. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, Ueno Y, Hayashi S, Fukushima T. The role of proteolysis in tumor invasiveness in glioblastoma and metastatic brain tumors. Anticancer Res. 2002;22(6C):4265–4268. [PubMed] [Google Scholar]
- 20.Chuang HN, Lohaus R, Hanisch UK, Binder C, Dehghani F, Pukrop T. Coculture system with an organotypic brain slice and 3D spheroid of carcinoma cells. J Vis Exp. 2013;(80):50881. doi: 10.3791/50881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8(6):344–356. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berghoff AS, Fuchs E, Ricken G, Mlecnik B, Bindea G, Spanberger T, Hackl M, Widhalm G, Dieckmann K, Prayer D, Bilocq A, Heinzl H, Zielinski C, Bartsch R, Birner P, Galon J, Preusser M. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology. 2015;5(1):e1057388. doi: 10.1080/2162402X.2015.1057388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran T, Jilaveanu L, Mahajan A, Goldberg S, Nguyen D, Chiang V, Kluger H. Perilesional edema and blood vessel characteristics in brain metastases and implications for treatment with immune therapy. Journal of Clinical Oncology. 2019;36(15_suppl):9572–9572. doi: 10.1200/jco.2018.36.15_suppl.9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spanberger T, Berghoff AS, Dinhof C, Ilhan-Mutlu A, Magerle M, Hutterer M, Pichler J, Wöhrer A, Hackl M, Widhalm G, Hainfellner JA, Dieckmann K, Marosi C, Birner P, Prayer D, Preusser M. Extent of peritumoral brain edema correlates with prognosis, tumoral growth pattern, HIF1a expression and angiogenic activity in patients with single brain metastases. Clin Exp Metastasis. 2013;30(4):357–368. doi: 10.1007/s10585-012-9542-9. [DOI] [PubMed] [Google Scholar]
- 25.Nahed BV, Alvarez-Breckenridge C, Brastianos PK, Shih H, Sloan A, Ammirati M, Kuo JS, Ryken TC, Kalkanis SN, Olson JJ. Congress of Neurological Surgeons systematic review and evidence-based guidelines on the role of surgery in the management of adults with metastatic brain tumors. Neurosurgery. 2019;84(3):E152–E155. doi: 10.1093/neuros/nyy542. [DOI] [PubMed] [Google Scholar]
- 26.Noordijk EM, Vecht CJ, Haaxma-Reiche H, Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N, Metsaars JA, Wattendorff AR. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys. 1994;29(4):711–717. doi: 10.1016/0360-3016(94)90558-4. [DOI] [PubMed] [Google Scholar]
- 27.Verger E, Gil M, Yaya R, Viñolas N, Villà S, Pujol T, Quintó L, Graus F. Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int J Radiat Oncol Biol Phys. 2005;61(1):185–191. doi: 10.1016/j.ijrobp.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 28.Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, Markesbery WR, Foon KA, Young B. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 29.Tini P, Nardone V, Pastina P, Battaglia G, Vinciguerra C, Carfagno T, Rubino G, Carbone SF, Sebaste L, Cerase A, Federico A, Pirtoli L. Perilesional edema in brain metastasis from non-small cell lung cancer (NSCLC) as predictor of response to radiosurgery (SRS) Neurol Sci. 2017;38(6):975–982. doi: 10.1007/s10072-017-2876-y. [DOI] [PubMed] [Google Scholar]
- 30.Suh JH, Kotecha R, Chao ST, Ahluwalia MS, Sahgal A, Chang EL. Current approaches to the management of brain metastases. Nat Rev Clin Oncol. 2020;17(5):279–299. doi: 10.1038/s41571-019-0320-3. [DOI] [PubMed] [Google Scholar]
- 31.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G. Stereotactic radiosurgery plus whole-brain radiation therapy vs. stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 32.Kalkanis SN, Kondziolka D, Gaspar LE, Burri SH, Asher AL, Cobbs CS, Ammirati M, Robinson PD, Andrews DW, Loeffler JS, McDermott M, Mehta MP, Mikkelsen T, Olson JJ, Paleologos NA, Patchell RA, Ryken TC, Linskey ME. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):33–43. doi: 10.1007/s11060-009-0061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanna A, Boggs DH, Kwok Y, Simard M, Regine WF, Mehta M. What predicts early volumetric edema increase following stereotactic radiosurgery for brain metastases. J Neurooncol. 2016;127(2):303–311. doi: 10.1007/s11060-015-2034-4. [DOI] [PubMed] [Google Scholar]
- 34.Vecht CJ, Hovestadt A, Verbiest HB, van Vliet JJ, van Putten WL. Dose-effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: a randomized study of doses of 4, 8, and 16 mg per day. Neurology. 1994;44(4):675–680. doi: 10.1212/wnl.44.4.675. [DOI] [PubMed] [Google Scholar]