Abstract
Background/Aim: We have previously shown that the water extract of Agrimonia eupatoria L. (AE) is a valuable source of polyphenols with excellent antioxidant properties and has clinical potential for the prevention and/or adjuvant therapy of cardiovascular complications associated with diabetes. Inspired by our previously published data, in the present study we examined whether AE improves skin wound healing in a series of in vitro and in vivo experiments.
Materials and Methods: In detail, we investigated the ability of the AE extract to induce fibroblast to myofibroblast conversion, extracellular matrix (ECM) deposition, and keratinocyte proliferation/ differentiation, in vitro. In parallel, in an animal model, we measured wound tensile strength (TS) and assessed the progression of open wounds using basic histology and immunofluorescence.
Results: The AE extract induced the myofibroblast-like phenotype and enhanced ECM deposition, both in vitro and in vivo. Furthermore, the wound TS of skin incisions and the contraction rates of open excisions were significantly increased in the AE-treated group.
Conclusion: The present data show that AE water extract significantly improves the healing of open and sutured skin wounds. Therefore, our data warrant further testing in animal models that are physiologically and evolutionarily closer to humans.
Keywords: Skin tissue, extracellular matrix, repair, regeneration, phytotherapy
In recent decades, interest in the potential health benefits of phytomedicine has increased. Many of the health effects of extracts are associated with the presence of polyphenols (1,2). In this context, we previously identified the presence of several phenolic constituents in the water infusion of Agrimonia eupatoria L. (AE) using HPLC-MS analysis (3). Specifically, the previously performed analysis revealed the presence of apigenin, kaempferol, quercetin derivatives, catechin and oligomeric proantocyanidins, with the most frequently identified compounds being quercetin glycosides and proantocyanidin trimers. Flavonoids belong to the group of polyphenols and are thought to be responsible, at least in part, for the biological properties of various medicinal plants, e.g., anti-inflammatory, antiviral, antibacterial, neuroprotective, antiulcerogenic, antispasmodic, antithrombotic, anticancer, antidiabetic, and/or antioxidant (3-6). For example, AE aqueous extracts exhibit “insulin-like” activities (7) and show hepatoprotective (8) and neuroprotective effects (9) due to their antioxidant properties.
We have previously shown that agrimony possesses good anti-glucosidase, anti-glycation, and anti-hyperglycemic activities (6). Moreover, our experiments on isolated aortas showed improved vasodilatation in diabetic rats. Notably, our in vivo study (3) revealed wound healing-promoting effects, which led us to extend the evaluation to fibroblasts and keratinocytes, as well as open and sutured wounds in rats. Thus, here we test our hypothesis in a series of in vitro and in vivo experiments to demonstrate the beneficial effects of AE on skin wound repair.
Clearly, the clinical presentation of non-healing wounds requires a better understanding of the basic biological mechanisms underlying the repair processes of higher organisms (10). Our previous experimental work on wound therapy (11-15) has shown that the identical treatment protocol for sutured and open wounds leads to different outcomes, which could be an aspect of crucial importance for clinical practice and also helped to explain why we performed a comparative analysis using rat skin as a suitable model (16). Therefore, in light of our previously published evidence, we report here new data related to the modulatory effect of AE in two basic models (incision vs. excision) of skin wound healing in vivo. To complete the series of experiments, we also studied fibroblasts and keratinocytes in vitro after treatment with AE extract.
Materials and Methods
Plant material and preparation of the aqueous extract. The dry extract of Agrimonia eupatoria L. (AE) was a gift from Phytopharma a.s. (Malacky, Slovak Republic). A water extract was prepared by pouring 100 ml of boiling distilled water over 10 g of dried plant material. The extract was then filtered (0.2 μm) and analyzed by gradient HPLC and TLC. The data from the analysis were published previously (3). For the in vitro study, the 10% w/v water extract was mixed with the culture medium at a ratio of 1:10 to obtain a final concentration of 1 % w/v.
Fibroblasts. The standard laboratory cell line of NIH-3T3 fibroblasts and the primary culture of human dermal fibroblasts (HDFs) were used for the experiment. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Biochrom, Berlin, Germany) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics (streptomycin and penicillin; Biochrom). Cells were seeded at a density of 3,000 cells/cm2 on glass coverslips and cultured for 24 h. Medium containing the tested concentration of AE extract (five concentrations were tested, i.e., 1, 1/4, 1/16, 1/64, 1/256; concentration 1 refers to 1% w/v) was then added to the cells and the cells were cultured for 4 (3T3) and 7 (HDF) days.
The HDFs were isolated from residual skin samples. They were obtained from the Prague Burn Center of the Third Faculty of Medicine of Charles University according to the criteria of the Declaration of Helsinki with the informed consent of the patients and approved by the Ethics Committee of the Královské Vinohrady University Hospital.
In vitro cultivation of HaCaT cells. The HaCaT cell line (human keratinocytes) was obtained from Cell Lines Service (Eppelheim, Germany) (17). Cells were cultured in DMEM (Biochrom) supplemented with 10% FBS (Biochrom) and 1% antibiotics (streptomycin and penicillin; Biochrom). Cells were seeded on glass coverslips at a density of 5,000 cells/cm2 and cultured for 24 h. The medium containing the tested concentrations of the AE extract (five concentrations were tested, i.e., 1, 1/4, 1/16, 1/64, 1/256) was subsequently added to the cells, which were then cultured for 4 days (the medium was changed once during the experiment).
Immunofluorescence of 3T3 cells, HDFs, HaCaTs, and wounds (frozen sections). In brief, cells/frozen sections were fixed with 2% buffered paraformaldehyde (pH 7.2) for 5 min and washed with PBS. Cell membranes were then permeabilized with Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) and sites for antigen-independent binding of antibodies were blocked with porcine serum albumin (DAKO, Glostrup, Denmark). Commercial antibodies were diluted according to manufacturers` recommendations. The primary and secondary antibodies used in this study are summarized in Table I. Specificity of the immunochemical reaction was ensured by replacing the specific first-step antibody with an irrelevant antibody, by omitting the first-step antibody during processing and by processing a positive control sample. Cell nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). All samples were embedded in Vectashield (Vector Laboratories, Burlingame, CA, USA) and examined with an Eclipse 90i fluorescence microscope (Nikon, Tokyo, Japan) equipped with filter blocks for fluorescein isothiocyanate (FITC), tetramethylrhodamine isothiocyanate (TRITC), and DAPI and a Cool-1300Q CCD camera (Vosskühler, Osnabrück, Germany). Data were analyzed using the image analysis system LUCIA 5.1 (Laboratory Imaging, Prague, Czech Republic).
Table I. Reagents used for immunofluorescence.
Animal model. The experimental conditions met the requirements of the European rules for ethical standards of animal treatment and welfare. The experiment was approved by the Ethics Committee of the Faculty of Pharmacy of Comenius University and by the State Veterinary and Food Administration of the Slovak Republic on 28 July 2015 (Ro-2617/15-221b).
Male Sprague-Dawley rats (n=21) weighing 400±40 g were obtained from the Laboratory of Research Biomodels of P. J. Šafárik University and used for the experiment. Animals were housed individually under standard conditions (55±5% humidity, 22±2˚C, 12/12 h light-dark cycle) in Plexiglas cages and had free access to standard laboratory diet and tap water ad libitum.
Rats were randomly divided into 3 groups. For general anesthesia, a combination of 33 mg/kg ketamine (Calypsol, Richter Gedeon, Budapest, Hungary), xylazine 11 mg/kg (Rometar a.u.v., Spofa, Prague, Czech Republic), and tramadol (Tramadol-K, Krka d.d., Novo Mesto, Slovenia) 5 mg/kg was administered intramuscularly to the rats. A 4-cm full-thickness skin incision and a round (1-cm in diameter) full thickness skin excision were made on the back of each rat under aseptic conditions. The incision was immediately closed with an intradermal running suture (Chiraflon 5/0, Chirmax, Prague, Czech Republic). Open wounds were left without dressing. The animals were euthanized with an overdose of anesthetics on the 7th and 14th day after surgery.
Wound treatment. In the control group, the water extract was not applied, leaving the wounds untreated. In the negative control group (to exclude the effect of wound cleansing and moist healing), the wounds were daily (during the first 3 days after surgery) treated topically (using an eye dropper) with sterile water. Similarly, in the experimental group, the extract (10% w/v) was applied topically, 3 times daily during the first 3 days after operation. Preliminary experiment involved comparison of two concentrations (1% and 10% w/v) of AE aqueous extract. However, the 1% AE concentration demonstrated no remarkable wound healing activity (data not shown).
Wound tensile strength measurement. The device for measuring wound-breaking strength was constructed in our laboratory (18). Briefly, it is based on an appropriately shaped horizontal arm that pulls one side of a specimen, while the opposite side is attached to a sensor tip of a force gage (OMEGA Engineering, Inc., Stamford, CT, USA). The moving arm is driven by a high-precision MDI-17 stepper motor (Intelligent Motion Systems, Inc., Marlborough, CT, USA) via a linear slider.
The technique for measuring wound tensile strength (TS) was described in detail previously (19). In brief, two 1-cm-wide strips of skin were removed from each incision and placed lengthwise between the clamps of the TS testing device. Pulling was performed perpendicular to the original direction of the incision. The maximum breaking strength was measured for each sample. TS was calculated using the following formula: TS=MBS/A, where TS is tensile strength (g/mm2), MBS is maximum breaking strength (g) and A represents wound area (mm2).
Histology and semi-quantitative scoring of histological sections. Wounds were routinely processed for light microscopy. In brief, fixation in 4% buffered formaldehyde, dehydration with increasing concentrations of alcohol, embedding in paraffin, sectioning (5-μm thick), and staining with hematoxylin-eosin (HE). The second set of wound specimens was cryoprotected using Tissue-Tek (Sakura Finetek Europe B.V.) and frozen in liquid nitrogen. Cryosections (10-μm thick) were first mounted on the surface of poly-l-lysine-treated slides (Sigma-Aldrich) and proceed for immunofluoresent staining (see Immunofluorescence of 3T3 cells, HDFs, HaCaTs, and wounds (frozen sections)).
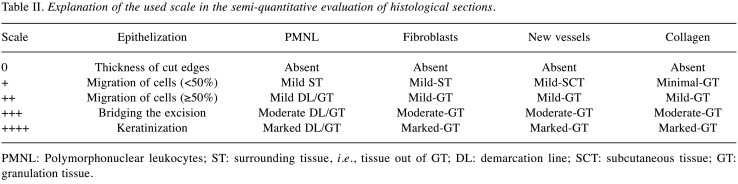
Re-epithelialization of the epidermis, the presence of inflammatory cells (polymorphonuclear leukocytes [PMNLs], fibroblasts, luminized vessels, and new collagen were evaluated in a blinded fashion (Table II). Similarly, fibronectin, collagen-3 and α-SMA were examined by a semi-quantitative method ranking the fluorescence signal intensities to the scale: absent (–), mild (+), moderate (++), and marked (+++).
Table II. Explanation of the used scale in the semi-quantitative evaluation of histological sections.
PMNL: Polymorphonuclear leukocytes; ST: surrounding tissue, i.e., tissue out of GT; DL: demarcation line; SCT: subcutaneous tissue; GT: granulation tissue.
Statistical analysis. Mean values with standard deviations (mean±SD) were calculated for all quantitative parameters. Semiquantitative data are expressed as median [represented as: – (0), + (1), ++ (2), +++ (3), and ++++ (4)]. To compare the difference in cell parameters (in vitro), wound TS and contraction rate at day 14 one-way ANOVA, followed by Tukey-Kramer multiple comparison test was used. The Kruskal-Wallis test was used to compare the non-parametric semi-quantitative data. The significance was assumed at p<0.05.
Results
3T3 fibroblasts and HDFs. The presence of AE in the culture medium resulted in the formation of a fibronectin-rich extracellular matrix (ECM) scaffold in both studied fibroblasts, 3T3 (Figure 1a-e, Figure 2e) and HDFs (Figure 2a, b, e). Interestingly, the most prominent newly synthesized ECM network was observed on the coverslips with cells exposed to an extract concentration of 1/256 (Figure 1b, Figure 2b, e). At that concentration cells showed the highest proliferation activity. As the concentration of the tested extract increased, cell proliferation and ECM deposition gradually decreased (Figure 2e).
Figure 1. Effect of different Agrimonia eupatoria L. (AE) concentrations on the studied cell lines. AE-induced formation of extracellular matrix in 3T3 fibroblasts (fibronectin, a-e) and induction of keratin 19 (K19, f-j) and Ki67 (k-o) expression in HaCaT keratinocytes after 4 days of culture (magnification 200×, scale bar 100 μm, representative images from three independent experiments). Graphs show the quantitative analysis of Ki67 (p) and K19 (q) expression.
Figure 2. Effect of Agrimonia eupatoria L. (AE) on primary cultures of human dermal fibroblasts (HDF). AE-induced formation of extracellular matrix (fibronectin, a-b) and induction of myofibroblast-like phenotype (α-smooth muscle actin; α-SMA, c-d) after 7 days of culture (magnification 200×, scale bar 100 μm). Graphs show the quantitative analysis of fibronectin (e) and α-SMA (f) expression.
In parallel, we also examined whether the AE extract induces fibroblast to myofibroblast transition in the studied human dermal fibroblasts. We observed the presence of α-smooth muscle actin (α-SMA)-positive fibers in HDFs cultured in the presence of AE at three tested concentrations (1/256, 1/64 and 1/16) (Figure 2d, f) whereas cells in the control medium were α-SMA free (Figure 2c).
HaCaTs. Compared to the control culture, treatment with AE resulted in a concentration-dependent modulation of Ki67 expression (Figure 1k-p). In particular, the two lower tested concentrations of AE (1/256 and 1/64, Figure 1l, m) increased Ki67 expression and accelerated cell proliferation. On the other hand, AE-treated cells at the highest tested concentration (AE-1, Figure 1o) showed to inhibited cell growth with low expression of Ki67, while proliferation of AE-1/16-treated keratinocytes (Figure 1n) remained comparable to the control culture (Figure 1k).
The majority of HaCaT cells expressed keratin-14 under all tested conditions (not shown), while keratin-10 expression was minimal (not shown). Interestingly, weak expression of keratin-19 was characteristic for the control culture (Figure 1f); whereas AE-treated cells showed increased expression of keratin-19 (Figure 1g-j).
Animal study. During the postoperative period, all animals remained healthy and with no clinical signs of infection. Semiquantitative analysis of histological sections is summarized in Table III. Representative (micro) photographs and data from measurements of wound TS and wound contraction are shown in Figure 3a-h. A detailed description of the results obtained at each time point is provided below.
Table III. Results of the semi-quantitative evaluation of histological sections.
C: Control; WC: water/negative control; AE: group treated with 10% w/v Agrimonia eupatoria L.; PMNL: polymorphonuclear leukocytes; α-SMA: α-smooth muscle actin; *p<0.05, **p<0.01.
Figure 3. Immunofluorescence: Agrimonia eupatoria L. (AE)-induced proliferation of keratinocytes of epidermis (a-b) during re-epithelization and conversion of fibroblasts into myofibroblasts (c-d) in the granulation tissue 7 days after surgery as well as collagen-3 deposition (e-f) in the granulation tissue 14 days after surgery. Wound tensile strength (g) and wound contraction (h) measurements: AE-increased wound tensile strength in male rats, 14 days following surgery. AE-induced wound contraction, 14 days after surgery. C: Control group; AC: aqueous/negative control group; AE: Agrimonia eupatoria L. extract-treated group; *p<0.05; D: dermis; E: epidermis; GT: granulation tissue; N: necrosis.
Wound TS of sutured skin incisions. Wounds treated with the AE extract had significantly higher TS compared to the two control groups (Figure 3g).
Contraction rate of open wounds. Wounds treated with AE extract had significantly increased contraction rates compared to both control groups (Figure 3h).
Histology of open wounds. Seven days after the procedure, the skin edges separated by the open wound in vivo were not yet completely bridged by a new epithelial layer (Figure 3a). A positive effect on wound re-epithelialization was observed in rats treated with AE (Figure 3b); however, with no significant difference. The wounds were only slightly infiltrated with PMNL (not shown). The newly formed granulation tissue (GT) was rich in fibronectin, fibroblasts, and high-caliber vessels (Figure 3c, d). The most prominent difference between the groups was seen in the presence of α-SMA-expressing fibroblasts. While wounds treated with AE were rich in this cell type (Figure 3d), both control groups contained no or very low number of this cell population (untreated, Figure 3c; treated with water, not shown).
Continued observation on day 14 revealed the presence of a keratin layer in the wounds, indicating a normal course of keratinocyte differentiation and a completed process of epidermal regeneration (not shown). The number of luminized vessels in the GT decreased (not shown). The content of fibronectin in GT decreased (not shown), while the content of collagen increased (Figure 3e, f). In GT, myofibroblasts were not present in any of the groups (not shown).
Discussion
To the best of our knowledge, the current study is the first to show that wound treatment with AE extract significantly increased the TS of skin incisions. It is well known that the amount, structure and composition of newly formed matrix in the incision correlates with wound stiffness (22). In this context we observed prominent ECM deposition by fibroblasts in vitro after treatment with AE. ECM proteins are produced and organized mainly by fibroblasts, one of the principal components of granulation tissue (23). Interestingly, one of the biologically active and dominant compounds of the AE extract, kaempferol, has been shown to remarkably increase wound TS in non-diabetic and diabetic (streptozotocin-induced) rats (24).
We next analyzed cell proliferation by assessing re-epithelialization and granulation tissue formation in vivo and by Ki67 staining in vitro. We observed an increased number of fibroblasts at the injury site and an accelerated process of epidermal regeneration after wound treatment with AE. The expression of Ki67 showed a concentration-dependent nature: except for the highest tested concentration of AE that rather inhibited cell growth, all other tested concentrations increased cell proliferation with the most prominent to be the lowest tested concentration of AE (1/256). Interestingly, the expression of keratin-19, one of the putative epidermal stem cell markers (25), was increased in treated cells.
As we have previously demonstrated, the potential healing-promoting mechanism of AE also involves antioxidant activity (3). Specifically, we have found increased expression of antioxidant enzymes, catalase, and superoxide dismutase, in an environment forced to oxidative stress (with H2O2). Flavonoids, such as luteolin, apigenin, kaempferol, and quercetin derivatives, as well as caffeoylquinic acids, caffeic acid, and their conjugates have been identified in traditional Polish medicine as effective agents against inflammatory skin diseases and wound-healing agents (26). Our extract also contained several phenolic constituents, mainly apigenin, kaempferol, and quercetin derivatives, as well as catechin and oligomeric proantocyanidins (3). The mixture of flavonoids acts as an effective free radical-scavenger that also promotes wound healing (27). Of note, quercetin also targets estrogen receptors (28), which may contribute to the positive wound-healing effect (29) of the tested extract.
In addition, antibacterial tests have shown that AE, incorporated into a two layer cotton material coated with poly(vinyl alcohol)-chitosan nanofibers, effectively inhibited the growth of Staphylococcus aureus and Pseudomonas aeruginosa (30). Moreover, a pulverized mixture of four herbs including AE, Nelumbo Nucifera Gaertn, Boswellia Carteri and Pollen Typhae Angustifoliae significantly increased the expression of TGF-β1 and Smad2/3 mRNA during the early phase of wound healing, but decreased the expression of TGF-β1 and Smad2/3 after two weeks (31). Since we observed a direct effect of AE on the fibroblast-to-myofibroblast transition in vivo, further experiments should be performed at the in vitro level aimed at finding the exact underlying mechanism of action, including interaction with TGF-β1 signaling.
Conclusion
The reported data provide a strong argument for further efforts to treat incisional and excisional wounds with natural products. Direct comparison of the two basic wound models showed that healing rates were significantly increased after treatment with AE. Therefore, this extract could be useful in improving the healing of acute skin wounds. Extrapolation from this experimental to clinical situation is not possible due to interspecies variability, but the general molecular regulation of wound healing is likely to be similar. Therefore, appropriate investigations are suggested by the present study in the rodent model.
Conflicts of Interest
The Authors have no conflicts of interest to declare.
Authors’ Contributions
Conceptualization, Tomáš Vasilenko and Peter Gál; Formal analysis, Karel Smetana Jr. and Peter Gál; Funding acquisition, Karel Smetana Jr. and Peter Gál; Investigation and methodology, Tomáš Vasilenko, Ivan Kováč, Martin Slezák, Ján Ďurkáč, Vlasta Peržeľová, Matúš Čoma, Miriam Kaňuchová, Lukáš Urban, Pavol Szabo, Barbora Dvořánková, Andrej Vrzgula, Robert Zajíček, Karel Smetana Jr. and Peter Gál; Writing – original draft, Peter Gál. All Authors red and approved the final version of the manuscript.
Acknowledgements
The project “Center for Tumor Ecology – Research of the Cancer Microenvironment Supporting Cancer Growth and Spread” (reg. no. CZ.02.1.01/0.0/0.0/16_019/0000785) is supported by the Operational Program Research, Development and Education and by Charles University (PROGRES Q28 and Q37). The Grant Agency of the Ministry of the Education, Science, Research and Sport of the Slovak Republic (under the contract No. VEGA-1/0561/18, 1/0319/20 and 1/0455/22), and the Agency for Science and Research (under the contract No. APVV-20-0017) are also appreciated for support. Part of the study was realized at the Medical University Science Park in Košice (MediPark, Košice - Phase II) ITMS2014+ 313011D103 supported by the Operational Program “Research and Innovations”, funded by the ERDF. We are grateful to Dr. Takáčová for English revision.
References
- 1.Benvenuto M, Focaccetti C, Ciuffa S, Fazi S, Bei A, Miele MT, Albonici L, Cifaldi L, Masuelli L, Bei R. Polyphenols affect the humoral response in cancer, infectious and allergic diseases and autoimmunity by modulating the activity of TH1 and TH2 cells. Curr Opin Pharmacol. 2021;60:315–330. doi: 10.1016/j.coph.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Melguizo-Rodríguez L, de Luna-Bertos E, Ramos-Torrecillas J, Illescas-Montesa R, Costela-Ruiz VJ, García-Martínez O. Potential effects of phenolic compounds that can be found in olive oil on wound healing. Foods. 2021;10(7):1642. doi: 10.3390/foods10071642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuczmannová A, Gál P, Varinská L, Treml J, Kováč I, Novotný M, Vasilenko T, Dall’Acqua S, Nagy M, Mučaji P. Agrimonia eupatoria L. and Cynara cardunculus L. water infusions: phenolic profile and comparison of antioxidant activities. Molecules. 2015;20(11):20538–20550. doi: 10.3390/molecules201119715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correia H, González-Paramás A, Amaral MT, Santos-Buelga C, Batista MT. Polyphenolic profile characterization of Agrimonia eupatoria L. by HPLC with different detection devices. Biomed Chromatogr. 2006;20(1):88–94. doi: 10.1002/bmc.533. [DOI] [PubMed] [Google Scholar]
- 5.Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med. 1997;22(5):749–760. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- 6.Kuczmannová A, Balažová A, Račanská E, Kameníková M, Fialová S, Majerník J, Nagy M, Gál P, Mučaji P. Agrimonia eupatoria L. and Cynara cardunculus L. water infusions: comparison of anti-diabetic activities. Molecules. 2016;21(5):564. doi: 10.3390/molecules21050564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray AM, Flatt PR. Actions of the traditional anti-diabetic plant, Agrimony eupatoria (agrimony): effects on hyperglycaemia, cellular glucose metabolism and insulin secretion. Br J Nutr. 1998;80(1):109–114. doi: 10.1017/s0007114598001834. [DOI] [PubMed] [Google Scholar]
- 8.Yoon SJ, Koh EJ, Kim CS, Zee OP, Kwak JH, Jeong WJ, Kim JH, Lee SM. Agrimonia eupatoria protects against chronic ethanol-induced liver injury in rats. Food Chem Toxicol. 2012;50(7):2335–2341. doi: 10.1016/j.fct.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Lee KY, Hwang L, Jeong EJ, Kim SH, Kim YC, Sung SH. Effect of neuroprotective flavonoids of Agrimonia eupatoria on glutamate-induced oxidative injury to HT22 hippocampal cells. Biosci Biotechnol Biochem. 2010;74(8):1704–1706. doi: 10.1271/bbb.100200. [DOI] [PubMed] [Google Scholar]
- 10.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gál P, Mokrý M, Vidinský B, Kilík R, Depta F, Harakalová M, Longauer F, Mozes S, Sabo J. Effect of equal daily doses achieved by different power densities of low-level laser therapy at 635 nm on open skin wound healing in normal and corticosteroid-treated rats. Lasers Med Sci. 2009;24(4):539–547. doi: 10.1007/s10103-008-0604-9. [DOI] [PubMed] [Google Scholar]
- 12.Lacjaková K, Bobrov N, Poláková M, Slezák M, Vidová M, Vasilenko T, Novotný M, Longauer F, Lenhardt L, Bober J, Levkut M, Sabol F, Gál P. Effects of equal daily doses delivered by different power densities of low-level laser therapy at 670 nm on open skin wound healing in normal and corticosteroid-treated rats: a brief report. Lasers Med Sci. 2010;25(5):761–766. doi: 10.1007/s10103-010-0791-z. [DOI] [PubMed] [Google Scholar]
- 13.Vasilenko T, Slezák M, Kovác I, Bottková Z, Jakubco J, Kostelníková M, Tomori Z, Gál P. The effect of equal daily dose achieved by different power densities of low-level laser therapy at 635 and 670 nm on wound tensile strength in rats: a short report. Photomed Laser Surg. 2010;28(2):281–283. doi: 10.1089/pho.2009.2489. [DOI] [PubMed] [Google Scholar]
- 14.Novotný M, Vasilenko T, Varinská L, Smetana K Jr, Szabo P, Sarišský M, Dvořánková B, Mojžiš J, Bobrov N, Toporcerová S, Sabol F, Matthews BJ, Gál P. ER-α agonist induces conversion of fibroblasts into myofibroblasts, while ER-β agonist increases ECM production and wound tensile strength of healing skin wounds in ovariectomised rats. Exp Dermatol. 2011;20(9):703–708. doi: 10.1111/j.1600-0625.2011.01284.x. [DOI] [PubMed] [Google Scholar]
- 15.Gál P, Vasilenko T, Kováč I, Čoma M, Jakubčo J, Jakubčová M, Peržeľová V, Urban L, Kolář M, Sabol F, Luczy J, Novotný M, Majerník J, Gabius HJ, Smetana KJ. Human galectin 3: Molecular switch of gene expression in dermal fibroblasts in vitro and of skin collagen organization in open wounds and tensile strength in incisions in vivo. Mol Med Rep. 2021;23(2):99. doi: 10.3892/mmr.2020.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorsett-Martin WA. Rat models of skin wound healing: a review. Wound Repair Regen. 2004;12(6):591–599. doi: 10.1111/j.1067-1927.2004.12601.x. [DOI] [PubMed] [Google Scholar]
- 17.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabol F, Vasilenko T, Novotný M, Tomori Z, Bobrov N, Zivčák J, Hudák R, Gál P. Intradermal running suture versus 3M™ Vetbond™ tissue adhesive for wound closure in rodents: a biomechanical and histological study. Eur Surg Res. 2010;45(3-4):321–326. doi: 10.1159/000320837. [DOI] [PubMed] [Google Scholar]
- 19.Gál P, Toporcer T, Vidinský B, Hudák R, Zivcák J, Sabo J. Simple interrupted percutaneous suture versus intradermal running suture for wound tensile strength measurement in rats: a technical note. Eur Surg Res. 2009;43(1):61–65. doi: 10.1159/000219214. [DOI] [PubMed] [Google Scholar]
- 20.Gál P, Kilik R, Mokry M, Vidinský B, Vasilenko T, Mozes S, Bobrov N, Tomori Z, Bober J, Lenhardt L. Simple method of open skin wound healing model in corticosteroid-treated and diabetic rats: Standardization of semi-quantitative and quantitative histological assessments. Veterinarni Medicina. 2008;53(12):652–659. doi: 10.17221/1973-Vetmed. [DOI] [Google Scholar]
- 21.Gál P, Vasilenko T, Kostelníková M, Jakubco J, Kovác I, Sabol F, André S, Kaltner H, Gabius HJ, Smetana K Jr. Open wound healing in vivo: Monitoring binding and presence of adhesion/growth-regulatory galectins in rat skin during the course of complete re-epithelialization. Acta Histochem Cytochem. 2011;44(5):191–199. doi: 10.1267/ahc.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinz B. Myofibroblasts. Exp Eye Res. 2016;142:56–70. doi: 10.1016/j.exer.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- 24.Özay Y, Güzel S, Yumrutaş Ö, Pehlivanoğlu B, Erdoğdu İH, Yildirim Z, Türk BA, Darcan S. Wound healing effect of kaempferol in diabetic and nondiabetic rats. J Surg Res. 2019;233:284–296. doi: 10.1016/j.jss.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Dvoránková B, Smetana K Jr, Chovanec M, Lacina L, Stork J, Plzáková Z, Galovicová M, Gabius HJ. Transient expression of keratin 19 is induced in originally negative interfollicular epidermal cells by adhesion of suspended cells. Int J Mol Med. 2005;16(4):525–531. [PubMed] [Google Scholar]
- 26.Mainka M, Czerwińska ME, Osińska E, Ziaja M, Bazylko A. Screening of antioxidative properties and inhibition of inflammation-linked enzymes by aqueous and ethanolic extracts of plants traditionally used in wound healing in Poland. Antioxidants (Basel) 2021;10(5):698. doi: 10.3390/antiox10050698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Domaszewska-Szostek A, Puzianowska-Kuźnicka M, Kuryłowicz A. Flavonoids in skin senescence prevention and treatment. Int J Mol Sci. 2021;22(13):6814. doi: 10.3390/ijms22136814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yigitaslan S, Erol K, Ozatik F, Ozatik O, Sahin S, Cengelli C. Estrogen-like activity of quercetin in female rats. Erciyes Tıp Dergisi/Erciyes Medical Journal. 2019;38(2):53–58. doi: 10.5152/etd.2016.0005. [DOI] [Google Scholar]
- 29.Čoma M, Lachová V, Mitrengová P, Gál P. Molecular changes underlying Genistein treatment of wound healing: a review. Curr Issues Mol Biol. 2021;43(1):127–141. doi: 10.3390/cimb43010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouro C, Dunne CP, Gouveia IC. Designing new antibacterial wound dressings: development of a dual layer cotton material coated with poly(vinyl alcohol)_chitosan nanofibers incorporating Agrimonia eupatoria L. extract. Molecules. 2020;26(1):83. doi: 10.3390/molecules26010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou Q, He WJ, Hao HJ, Han QW, Chen L, Dong L, Liu JJ, Li X, Zhang YJ, Ma YZ, Han WD, Fu XB. The four-herb Chinese medicine ANBP enhances wound healing and inhibits scar formation via bidirectional regulation of transformation growth factor pathway. PLoS One. 2014;9(12):e112274. doi: 10.1371/journal.pone.0112274. [DOI] [PMC free article] [PubMed] [Google Scholar]