Abstract
Background/Aim: High-dose chemotherapy is frequently administered to patients with hematologic malignancies, thereby causing severe adverse drug reactions (ADRs) at a relatively high frequency. To precisely monitor ADRs, we developed a medication instruction sheet (MIS) for patients who received rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) combination therapy for non-Hodgkin’s lymphoma (NHL). Herein, we evaluated the usefulness of the MIS for managing ADRs in patients who received R-CHOP therapy.
Patients and Methods: We included patients aged ≥20 years who received R-CHOP therapy as first-line treatment for NHL at the Department of Hematology, Kyushu University Hospital, between August 2014 and December 2018. Medical professionals evaluated the possible occurrence of ADRs according to the present MIS and ADRs were graded according to the Common Toxicity Criteria, version 4.0 (National Cancer Institute, Bethesda, MD, USA). Finally, the accuracy of the MIS in predicting the occurrence of ADRs of different grades and during definite periods was evaluated.
Results: Seventy-five patients with NHL were included in the present study. Overall, 359 ADR events were monitored, which were predicted ADR items listed in the MIS. Among these, 254 (71%) events occurred during the same period as those listed in the MIS. The onset timing of any grade of an infusion reaction and peripheral neuropathy precisely matched those listed in the MIS. However, the accuracy of the MIS was reduced in patients with thrombocytopenia (42%).
Conclusion: The present MIS could be useful for monitoring ADRs in patients with cancer undergoing R-CHOP therapy.
Keywords: Chemotherapy, medication instruction sheet, adverse drug reactions, non-Hodgkin’s lymphoma, R-CHOP therapy
Although several anticancer drugs confer beneficial effects against various types of cancers via distinct mechanisms of action, chemotherapeutic drugs, including cytotoxic agents and molecular target drugs, often induce undesirable effects. Several anticancer drugs are commonly used in clinical settings, potentially resulting in diverse adverse drug reactions (ADRs) with different onset times. Furthermore, the same anticancer drug can be employed in different chemotherapy regimens at different doses, thus complicating the occurrence of ADRs and their time-to-onset (i.e., ADR onset timing). Therefore, careful attention could help avoid or relieve serious ADRs during combination chemotherapy. Accordingly, clinical pharmacists are responsible for verifying prescription orders based on the chemotherapy regimen, providing safe and effective supportive care medications, extensive explanation of the onset, degree of symptoms, and duration of ADRs likely to occur, thus ensuring safe and effective chemotherapy and relieving patient anxiety (1-7).
To ensure the rapid and accurate monitoring of ADRs that could occur during combination chemotherapy, we have previously established an innovative medication instruction sheet (MIS) covering 300 chemotherapy regimens (1). These sheets have enabled medical professionals, including pharmacists, physicians, and nurses, to visually and promptly recognize the type of ADRs, as well as the extent of occurrence, when symptoms appear, and how long they persist after cancer chemotherapy. In addition, these sheets are useful for clinical pharmacists to educate patients regarding the risks and benefits of cancer chemotherapy.
Severe ADRs may develop at a relatively high frequency during cancer chemotherapy in patients with hematologic malignancies, as high-dose chemotherapy is commonly used in these patients. We provided pharmaceutical care by using MIS to monitor patients with non-Hodgkin’s lymphoma (NHL) who received combination chemotherapy, including rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP). Therefore, in the present study, we evaluated the usefulness of MIS in the clinical setting.
Patients and Methods
R-CHOP therapy. The R-CHOP regimen included rituximab (375 mg/m2, intravenous infusion for 3-6 h) on day 1, followed by doxorubicin (50 mg/m2, intravenous infusion for 1 min), vincristine (1.4 mg/m2 up to 2.0 mg/body weight, intravenous injection for 1 min), and cyclophosphamide (750 mg/m2, intravenous infusion for 2 h) on day 1 or 2, and prednisolone (100 mg/body weight) administered orally on days 1 to 5, every 21 days.
Preparation of medication instruction sheet (MIS). A MIS template was developed using Microsoft Excel® 2010 and a later version on the Microsoft Windows platform, which contained two parts: the treatment schedule section and the ADR monitoring section during cancer chemotherapy (Figure 1). The incidence rate of each ADR associated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone was obtained from the package insert and manufacturers’ brochures. ADRs with incidence rates >10% were listed in the MIS as those that need to be monitored by medical professionals. Data regarding the onset timing and duration of listed ADRs were obtained from scientific literature and retrospective investigations (8). The onset timings and durations of ADRs were marked in color to promptly and visually recognize ADRs that require extensive caution. Myelosuppression should be carefully monitored during R-CHOP therapy, as chemotherapy increases the risk of death due to infections associated with neutropenia and thrombocytopenia-induced hemorrhage (9,10). Leukopenia was monitored from 3 days before the expected day of maximal reduction in the leukocyte count to the day of recovery to the normal range. The monitoring periods for leukopenia associated with cyclophosphamide, doxorubicin, and vincristine were 7-21, 7-21, and 9-21, respectively. Premedication and supportive care medicines are listed in the MIS. A brief explanation, along with speech bubbles, was inserted in the MIS, which was determined after the approval of oncology specialists at our Department of Hematology. Illustrations inserted in the MIS were originally drawn by pharmacists at the Department of Pharmacy, Kyushu University Hospital.
Figure 1. Medication instruction sheet (MIS) for R-CHOP therapy. A typical MIS was prepared for R-CHOP therapy in patients with non-Hodgkin’s lymphoma. The upper part presents drug names, including supportive care medicines and chemotherapeutic agents, and treatment schedule, while the lower part presents predicted ADRs and their onset and duration. A brief explanation for each ADR is also described. The MIS was arranged to fit a 3-week cycle schedule in one sheet according to the treatment regimen. ADRs: Adverse drug reactions; ALT: alanine aminotransferase; AST: aspartate aminotransferase; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone.
Assessment of MIS. In total, 75 patients aged ≥20 years received R-CHOP therapy as the first-line treatment for NHL at the Department of Hematology, Kyushu University Hospital, between August 2014 and December 2018. Physicians, nurses, and pharmacists monitored ADRs using the MIS for R-CHOP and verified the prescription of supportive care drugs on the appearance of any sign of potential ADRs. Documented ADRs were graded according to the Common Toxicity Criteria, version 4.0 (National Cancer Institute, Bethesda, MD, USA).
In the present study, we evaluated the usefulness of MIS by comparing ADR items and their onset timings observed during chemotherapy with those presented in the MIS.
Data analysis. The predictive accuracy of the prepared MIS was evaluated by calculating the rate of matching of each ADR item and the MIS-predicted onset timing when compared with those observed during chemotherapy. Data are shown as the matching rate and 95% confidence interval (CI) for the population proportion, as reported by Rumsey (11). The Chi-square test was used to examine differences in the predictive accuracy of the MIS between mild to moderate events and severe ADRs. Data were analyzed using JMP Pro® 16 (SAS Institute, Cary, NC, USA), and a p-value <0.05 was considered statistically significant.
Results
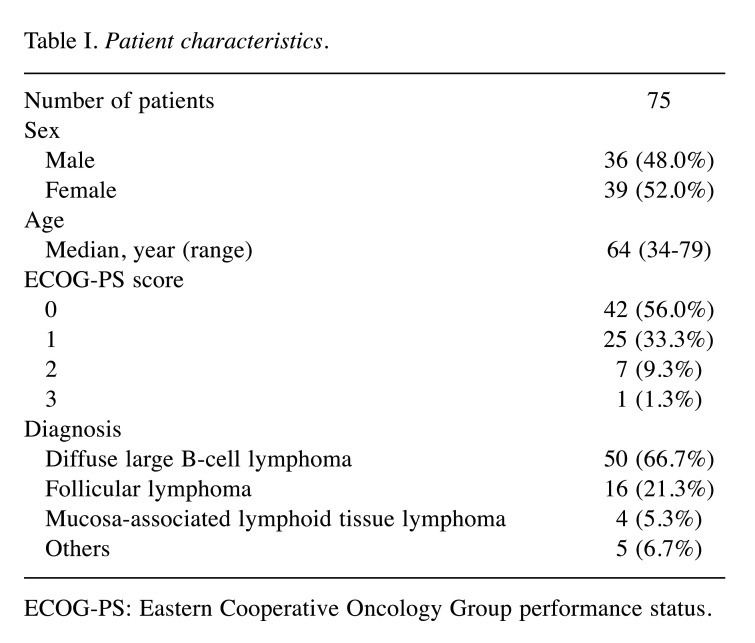
Baseline clinical characteristics of patients. A total of 75 patients with NHL were included in the present study. Table I presents the baseline clinical characteristics of included patients. The median age of the patients was 64 years (range=34-79 years). Most patients presented a good performance status (0-1). The most common disease was diffuse large B-cell lymphoma (66.7%), followed by follicular lymphoma (21.3%) and mucosa-associated lymphoid tissue lymphoma (5.3%).
Table I. Patient characteristics.
ECOG-PS: Eastern Cooperative Oncology Group performance status.
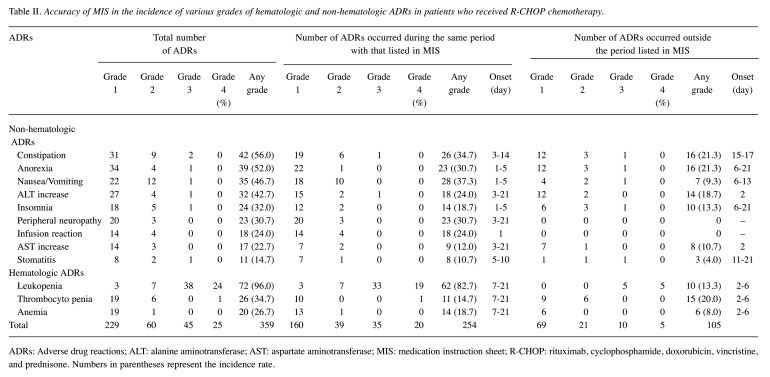
Evaluation of MIS for predicting ADR items and their time of onset. As shown in Table II, a total of 359 ADR events were monitored in 75 patients, including 42 patients with constipation (incidence rate, 56.0%), 39 with anorexia (52.0%), 35 with nausea/vomiting (46.7%), 32 presenting elevated alanine aminotransferase (42.7%), 24 with insomnia (32.0%), 23 exhibiting peripheral neuropathy (30.7%), 18 displaying infusion reactions (24.0%), 17 presenting elevated aspartate aminotransferase (22.7%), and 11 with stomatitis (14.7%) in terms of non-hematologic ADRs, along with 72 with leukopenia (96.0%), 26 with thrombocytopenia (34.7%), and 20 with anemia (26.7%).
Table II. Accuracy of MIS in the incidence of various grades of hematologic and non-hematologic ADRs in patients who received R-CHOP chemotherapy.
ADRs: Adverse drug reactions; ALT: alanine aminotransferase; AST: aspartate aminotransferase; MIS: medication instruction sheet; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone. Numbers in parentheses represent the incidence rate.
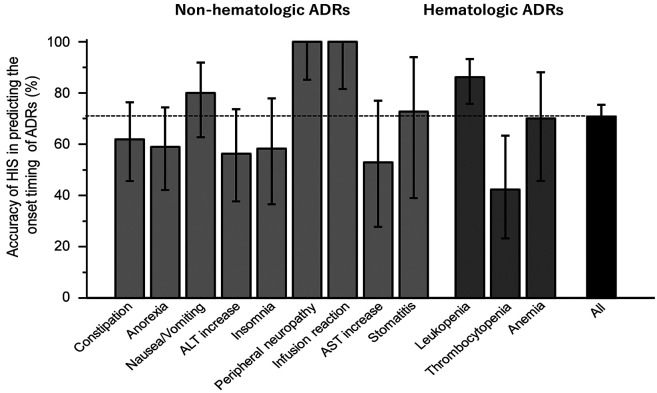
Notably, all documented ADRs were predictable ADRs listed in the MIS. Among all ADR events, 254 (70.8%) occurred during the same period as described in the MIS. Notably, the onset timings of the infusion reaction and peripheral neuropathy precisely matched those listed in the present MIS (Figure 2). The accuracy of the MIS for predicting the ADR onset timing was sufficiently high for leukopenia (86.1%, 95%CI=75.8-93.3%) and nausea/vomiting (80.0%, 62.7-91.9%), but moderate for stomatitis (72.7%, 39.0-94.0%) and anemia (70.0%, 45.7-88.1%). However, the prediction value was insufficient for the onset timing of thrombocytopenia (42.3%, 23.3-63.3%).
Figure 2. Accuracy of the present MIS for predicting the onset of any grade of ADRs occurring in patients with cancer receiving R-CHOP chemotherapy. Accuracy represents the rate of concordance between the onset timing of each ADR observed and that listed in the MIS. The ADRs observed in the present study were all predicted ADRs shown in MIS. The figures in parentheses represent the number of events. The upper and lower bars represent the upper and lower limits of the 95% confidence interval, respectively. ADRs: Adverse drug reactions; ALT: alanine aminotransferase; AST: aspartate aminotransferase; MIS: medication instruction sheet.
Unpredicted ADRs such as constipation, anorexia, nausea/vomiting, insomnia, and stomatitis appeared later than the respective onset timing listed in the MIS; however, other ADRs, including elevated serum aminotransferases and hematologic ADRs such as leukopenia, thrombocytopenia, and anemia, occurred earlier than the onset timings listed in the MIS.
No significant difference in the predictive accuracy of MIS was noted between mild to moderate (grades 1-2) events (68.9%: 199 of 289 events) and severe ADRs (78.6%: 55 of 70 events) (p=0.11, Chi-square test).
Discussion
In the present study, the predominant ADRs were leukopenia (96.0%), followed by constipation (56.0%), anorexia (52.0%), nausea/vomiting (46.7%), elevated serum alanine aminotransferase (42.7%), and thrombocytopenia (34.7%). In general, our data on the incidence of these ADRs were consistent with results reported by the randomized international phase III trial evaluating R-CHOP therapy, although less frequent (12).
Notably, the ADRs observed in the present study were all predicted by the prepared MIS. Moreover, the onset timings of these ADRs were nearly consistent with those described in the MIS. The accuracy rate of the present MIS in terms of the onset timing was estimated from the proportion of the number of ADR events that occurred during the predicted period described in the MIS to the total ADR events; overall, the rate was 70.8% (95%CI=65.7-75.4%).
In our previous report, the accuracy rate of the MIS for paclitaxel and carboplatin (TC) combination chemotherapy was 81.6% in patients with ovarian cancer (7). Thus, the present accuracy rate was slightly lower than that reported previously in patients receiving TC chemotherapy. The difference in the intensity of chemotherapy between R-CHOP (5 chemotherapy agents) and TC (2 agents) may explain the discrepancy in the predictive accuracy rate of the MIS.
Hematologic abnormalities are characteristic of NHL at the time of diagnosis, thereby indicating the need for discriminating chemotherapy-induced myelosuppression from pathological hematologic abnormalities when monitoring the bone marrow function of patients with NHL.
Herein, it should be noted that the accuracy rate of the MIS was 100% for peripheral neuropathy and infusion reactions. Furthermore, the onset timings of leukemia and nausea/vomiting were highly predictable, with an accuracy rate of 86.1% (75.8-93.3%) and 80.0% (62.7-91.9%), respectively. These findings suggest that the present MIS enables the precise on-target monitoring of such ADRs.
Among all observed ADRs, the lowest accuracy rate was associated with the occurrence of thrombocytopenia (42.3%, 95%CI=23.3-63.3%). In all cases, unpredicted thrombocytopenia (15 events) occurred earlier (on days 2-6) than the predicted timing (7-21 days). However, among these, leukopenia occurred during the predicted period (on days 7-21) in 13 patients (87%) and appeared at an unpredicted time (on days 2-6) in only 2 patients. Therefore, it is unlikely that myelosuppression occurred earlier in these 15 patients.
In patients with NHL, Conlan et al. (13) have reported that chemotherapy-induced leukopenia and thrombocytopenia are markedly affected by the involvement of bone marrow during pathogenesis, which may explain the difficulty in predicting the incidence of thrombocytopenia.
Furthermore, it has been shown that NHL is associated with liver damage at the time of diagnosis in several patients (14). Therefore, it remains unclear whether elevated aminotransferase levels can be associated with cancer chemotherapy of NHL. From an etiological perspective, serum aminotransferases should be monitored before and after chemotherapy.
In addition, the accuracy rate could be impacted by the symptom grade. The accuracy rate was 69.9% for grade 1, 65.0% for grade 2, 77.8% for grade 3, and 80.0% for grade 4 (data not shown). However, no significant difference in the accuracy rate was detected between mild to moderate (grades 1-2) events (68.9%) and severe ADRs (78.6%).
Serious ADRs can reduce the patients’ quality of life; thus, patients with cancer remain anxious regarding the type of ADRs that can occur and their onset timing. Therefore, a reliable MIS is required to monitor ADRs precisely, as different ADR grades appear at specific times during cancer chemotherapy. Accordingly, the present MIS may be potentially useful for monitoring and managing ADRs in patients with NHL who received R-CHOP therapy. Using the present MIS, medical professionals could provide sufficient information regarding the onset, timing, and degree of severity of ADRs, as well as countermeasures against ADRs. Furthermore, the present MIS was prepared as a clinical pathway and therefore, it is visually understandable for not only patients with cancer but also medical professionals, including pharmacists. Thus, the present MIS could enable medical professionals to achieve secure and timely monitoring of ADRs, regardless of the abundance of practical experience. Moreover, this sheet is assumed to be extremely important for the standardization and uniformity of pharmacists’ clinical practice.
The clinical usefulness of the MIS prepared to verify chemotherapy regimens, supportive care medicines, and ADRs during R-CHOP chemotherapy was evaluated. Herein, we reported, for the first time, that the predictive accuracy of the MIS was 70.8% (95%CI=65.7-75.4%) in patients with NHL receiving R-CHOP therapy. In addition, the ADR items observed in clinical settings were predicted using the MIS. Notably, the onset timings of peripheral neuropathy and infusion reactions precisely matched those listed in the MIS. Therefore, it can be concluded that the present MIS for R-CHOP therapy is potentially useful in clinical practice.
Limitations. The present study was undertaken in a small number of patients at a single institution, and data were retrospectively analyzed. A large-scale, multi-institutional study is needed to further confirm the accuracy of the developed MIS.
Conflicts of Interest
The Authors declare no conflicts of interest in relation to this study.
Authors’ Contributions
Mayako Uchida: Conceptualization, methodology, data collection, formal analysis, writing –original draft preparation. Rie Hisamitsu, Sayaka Mai, Shigeru Ishida, Hiroyuki Watanabe: Data collection. Rika Kawai, Takehiro Kawashiri, Koji Kato, Keiko Hosohata, Toshihiro Miyamoto, Nobuaki Egashira: Writing - Review and Editing. Tsutomu Nakamura, Koichi Akashi, Ichiro Ieiri: Writing - Review and Editing, Supervision.
Acknowledgements
The Authors are grateful to Professor Ryozo Oishi, Professor Emeritus at Kyushu University (Fukuoka, Japan), Professor Yoshinori Ito, Professor Emeritus at Gifu University (Gifu, Japan), and Dr. Hiroaki Ikesue at the Department of Pharmacy, Kobe City Medical Center General Hospital (Kobe, Japan). The Authors thank Ms Misui Kashiwagi, Ms. Nami Maegawa, and Ms. Aoi Takano for technical assistance.
References
- 1.Uchida M, Nakamura T, Watanabe H, Kato K, Miyamoto T, Akashi K, Masuda S. Usefulness of medication instruction sheets for sharing information on cancer chemotherapy within the health care team. Pharmazie. 2019;74(9):566–569. doi: 10.1691/ph.2019.9467. [DOI] [PubMed] [Google Scholar]
- 2.Patel H, Gurumurthy P. Implementation and evaluation of medicine and therapeutic information service by clinical pharmacists in oncology care setting. J Oncol Pharm Pract. 2019;25(1):60–67. doi: 10.1177/1078155217727141. [DOI] [PubMed] [Google Scholar]
- 3.Boşnak AS, Birand N, Diker Ö, Abdi A, Başgut B. The role of the pharmacist in the multidisciplinary approach to the prevention and resolution of drug-related problems in cancer chemotherapy. J Oncol Pharm Pract. 2019;25(6):1312–1320. doi: 10.1177/1078155218786048. [DOI] [PubMed] [Google Scholar]
- 4.Fornasier G, Taborelli M, Francescon S, Polesel J, Aliberti M, De Paoli P, Baldo P. Targeted therapies and adverse drug reactions in oncology: the role of clinical pharmacist in pharmacovigilance. Int J Clin Pharm. 2018;40(4):795–802. doi: 10.1007/s11096-018-0653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aziz MT, Rehman TU, Qureshi S, Andleeb S. Effects of multidisciplinary teams and an integrated follow-up electronic system on clinical pharmacist interventions in a cancer hospital. Int J Clin Pharm. 2017;39(6):1175–1184. doi: 10.1007/s11096-017-0530-7. [DOI] [PubMed] [Google Scholar]
- 6.Holle LM, Harris CS, Chan A, Fahrenbruch RJ, Labdi BA, Mohs JE, Norris LB, Perkins J, Vela CM. Pharmacists’ roles in oncology pharmacy services: Results of a global survey. J Oncol Pharm Pract. 2017;23(3):185–194. doi: 10.1177/1078155216629827. [DOI] [PubMed] [Google Scholar]
- 7.Ikesue H, Ishida M, Uchida M, Harada M, Haro T, Mishima K, Itoh Y, Kotsubo K, Yoshikawa M, Oishi R. Monitoring for potential adverse drug reactions in patients receiving chemotherapy. Am J Health Syst Pharm. 2004;61(22):2366,2368–2366,2369. doi: 10.1093/ajhp/61.22.2366. [DOI] [PubMed] [Google Scholar]
- 8.Makieda D, Hisaeda S, Kinoshita H, Uchida M, Ikesue H, Mishima K, Watanabe H, Sueyasu M, Miyamoto T, Egashira N, Oishi R. Investigation of adverse drug reactions in bortezomib therapy for relapsed multiple myeloma. Jpn J Pharmaceut Health Care Sci. 2010;36(4):270–276. doi: 10.5649/jjphcs.36.270. [DOI] [Google Scholar]
- 9.Carey PJ. Drug-induced myelosuppression: diagnosis and management. Drug Saf. 2003;26(10):691–706. doi: 10.2165/00002018-200326100-00003. [DOI] [PubMed] [Google Scholar]
- 10.Kaplow R. Innovations in antineoplastic therapy. Nurs Clin North Am. 2005;40(1):77–94. doi: 10.1016/j.cnur.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Rumsey DJ. How to determine the confidence interval for a population proportion. Available at: https://www.dummies.com/education/math/statistics/how-to-determine-the-confidenceinterval-for-a-population-proportion/ [Last accessed on November 1, 2021]
- 12.Flinn IW, van der Jagt R, Kahl BS, Wood P, Hawkins TE, Macdonald D, Hertzberg M, Kwan YL, Simpson D, Craig M, Kolibaba K, Issa S, Clementi R, Hallman DM, Munteanu M, Chen L, Burke JM. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944–2952. doi: 10.1182/blood-2013-11-531327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conlan MG, Armitage JO, Bast M, Weisenburger DD. Clinical significance of hematologic parameters in non-Hodgkin’s lymphoma at diagnosis. Cancer. 1991;67(5):1389–1395. doi: 10.1002/1097-0142(19910301)67:5<1389::aid-cncr2820670519>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/jco.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]