Abstract
Background/Aim: The aim of this study was to assess the acute effects of physical vascular therapy (PVT) on the autonomous nervous system by heart rate variability (HRV) and heart rate asymmetry (HRA) analysis. The low-frequency, pulsed electromagnetic field (<35 μTesla) with a patented BEMER pattern can improve vasomotion and microcirculation. A non-invasive confirmation of the instant effects of PVT may provide an opportunity to give an immediate feedback to the patient and therapist.
Patients and Methods: Altogether 48 patients on inward rehabilitation with coronary heart disease (CHD) were involved, their treatment included PVT with B.Box Professional and B.Body Pro applicator (BEMER International AG, Triesen, Lichtenstein). After 15 min of postural adaptation, 6-min electrocardiograms (ECG) were taken immediately before, in the first and in the last 6 min of the 20-min PVT, and one hour after the treatment. Of the 48 patients, the last twenty patients received sham PVT with the same protocol. Off-line analysis was blinded. We used linear mixed statistical model to compare HRV and HRA parameters.
Results: The time domain parameters did not show any statistically significant differences between the changes in the real PVT and sham groups but, in the first stage of the treatment, Porta and Guzik indices significantly rose everywhere except in the sham group.
Conclusion: PVT significantly increases the Guzik and Porta indices in chronic ischemic heart disease patients reflecting a delicate autonomic response. HRA as a measure of autonomic regulation seems to be more sensitive than time domain parameters.
Keywords: Pulsed electromagnetic field, physical vascular therapy (BEMER), rehabilitation, heart rate variability, heart rate asymmetry
Many ancient civilizations such as the Greeks, Egyptians, Chinese, Mayas, and the Romans were aware of magnetism. They used magnetic stones for relieving pain, preserving beauty and healing. According to ancient writings, Hippocrates referred to magnetism as "iron-attractive stone". The intention to better understand the properties of magnets began in the 17th century. One of the earliest scientific accounts can be found in a book entitled De Magnete, written in 1600 by William Gilbert, personal physician of the Queen of England (1). Franz Anton Mesmer, who was a German physician, was the first scientist to declare that the magnetic properties of magnets are suitable for healing diseases. Initially, physicians used static magnetic fields (2). The recognition that magnetic fields can be generated by electricity was a great milestone in magnetotherapy. This phenomenon became widely recognized when Hans Christian Oersted conducted a series of similar experiments in 1820 (2). In the middle of the 20th century, there was a growing interest in effective electromagnetic therapy stemming from Japan, and then spreading to Europe. The therapies which utilize electromagnetic fields, including pulsed electromagnetic fields (PEMF), became more widespread than treatments using static magnetic fields. During the 1970s, Bassett confirmed that PEMF therapy has a positive effect on the healing of bone fractures (3). Furthermore, PEMF therapy seems to have clinical benefits in low back pain according to a recent meta-analysis (4).
The mechanism of action of the electromagnetic fields on organisms is still unclear. The relative permeability of biological tissues is very close to one; hence, the organism does not influence static magnetic fields, which means that this field penetrates into the body easily and without attenuation. We can apply quasi-static field theory at low frequencies (wavelength >> object size), because electric and magnetic fields are uncoupled in this case (5). The main actions of a time-varying magnetic field on a living body are related to the induced electric field and eddy currents within the tissues. As it is difficult to measure induced current densities within living tissues, scientists use different in silico simulations and phantom models are used instead (5,6). In a human model study with 50 μTesla 50 Hz external magnetic field, the change in the internal magnetic field was negligible, whereas the induced current density was as high as 615 μA/m2 in the brain, 403 μA/m2 in the heart, and 48 μA/m2 in the kidney (7). Even this relatively small current can influence the conformation and function of plasma membrane proteins, those acting as “antennae”. This mechanism is responsible for altered membrane transport of calcium-ion via conformational changes, electron tunnelling and/or other quantum effects resulting in alteration of their resonant behaviour (8,9). In addition, reactive oxygen species with their triplet orientation to magnetic field lines can act as sensitive elements, similarly to the inducible nitric oxide synthase enzyme (8). Besides calcium signalling and nitric oxide synthase, mitogen activated protein kinase and other signalling pathways may be included in the interaction of magnetic fields and osteogenic differentiation, mineralization and angiogenesis at the transcriptional level (9). Regarding the DNA-level, Blank and co-workers identified a sequence in the human DNA as a low-frequency magnetic field sensitive element (10).
The physical vascular therapy (PVT, i.e. BEMER) has been developed since the end of the last century. This device operates with specific parameters using a low-frequency, weak magnetic field (<35 μTesla) with the so-called BEMER pattern (US Patent 8808159) (11). PVT improves the microcirculation by restoring the physiological vasomotion of small arterioles and venules, which increases the number of open capillaries, hence improving net blood flow (12). Intravital microscopy demonstrated these changes in microcirculation. Furthermore, the changes were also measured by laser reflection spectroscopy (13). The cyclic variation of small vessel diameter is a complex process both at the tissue and organ level. This is driven by the synchronized contraction and relaxation of the smooth muscle cells resulting in a low-frequency oscillation (14). Different pathophysiological phenomena may be involved in this vasomotion: the sympathetic neural activity oscillations (arising from the nerves in the outer adventitial connective tissue layer surrounding arterioles) (15); synchronous calcium oscillations of smooth muscle cells (16) and the endothelium-derived factors (endothelium-derived relaxing factor (NO) and endothelium-derived hyperpolarizing factor) (17). This arteriolar vasomotion is neither strictly periodic nor random at frequencies of 5-25 cycle per min (cpm) in <30 μm arterioles and 0.3-3 cpm in 30-80 μm arterioles; which controls and maintains the adequate microcirculation (18).
PVT has many clinical benefits in musculoskeletal diseases: traditional physiotherapy combined with PVT reduces the low back pain in the short-term and may have long-term beneficial effects in osteoarthritis of the knee (19). It was published in 2011, that PVT reduces fatigue in patients with multiple sclerosis in the long-term (20). PVT also decreases resistance of cancer cells to radiotherapy (21). However, PVT was not found effective in reducing pain, and improving function or stiffness in patients with fibromyalgia (22). According to a recent publication from Tamulevicius et al. (23), adjunctive PEMF treatment during a 6-day preseason training significantly improved the ventilatory threshold in 8 athletes compared to the 6 controls without PEMF.
The heart rate variability (HRV) is a result of the summation of momentary autocrine, paracrine, and neuroendocrine actions on the sinus node. Due to the different time-constants of feedback-loops, there are different spectral bands in this beat-to-beat fluctuation (24-27). In many diseases, HRV analysis has prognostic and predictive value that highlights its importance in clinical practice and patient follow-up. Wolf et al. (28) were the first to describe the connection between reduced HRV and increased post-infarction mortality in 1978. By analysing one-minute electrocardiogram (ECG) recordings in acute myocardial infarction, they established that patients with sinus variability had lower mortality compared to those without this variability. In 1987, Kleiger et al. (29) published their pioneering work which demonstrated that simple HRV measures may help identify patients with a higher risk of sudden cardiac death after previous acute myocardial infarction. The Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology formulated guidelines on HRV standards of measurement, physiological interpretation, and clinical use in 1996 (27). Since then, several papers have verified the impact of HRV analysis in the prognosis and follow-up of several diseases, among others, heart failure (30), stroke (31), and multiple sclerosis (32). Acute changes of HRV were proved in several conditions, for example after carbon dioxide treatment (33) and during the investigation of the effects of transcutaneous electrical nerve stimulation in motion sickness (34). HRV analysis can also aid diagnosis with tilt test in patients with cardio inhibitory vasovagal syncope (35). Koppel et al. (36) found increased average heart rate and reduced total power of HRV during exposure to 50 Hz sinusoidal magnetic field at 4 μTesla.
Investigating the acute effects of PVT on the human autonomic nervous system by HRV and HRA analysis can give deeper insight into its mechanism of action. On the other hand, non-invasive evidence of the instant effects of PVT may provide an opportunity to give an immediate feedback both to the patient and to the therapist.
Patients and Methods
The present study was performed at the ISO 9001 accredited Cardiology Rehabilitation Inpatient Unit in the Harkány Vilmos SPA Hospital, Harkány, Hungary from September 2018 until June 2019. Due to organizational issues, the study was carried out in three phases. Regional Ethics Committee of the University of Pécs in accordance with the 2008 Helsinki declaration approved the study protocol under file number 6422-PTE2018. The forty-eight patients (30 men), consecutively enrolled within the particular phases, underwent a 3-week-long inward rehabilitation. Informed written consent was obtained from every patient. Exclusion criteria were previous PVT within one year before the study, permanent arrhythmia, discontinuation of rehabilitation and withdrawal of consent. To reveal latent infections, white blood cell count and C-reactive protein (CRP) levels were analysed on admission. CRP levels were determined in the clinical laboratory of Harkány Vilmos SPA Hospital, Harkány with automated analyser according to the manufacturer’s protocol (Cobas 8000; Roche Diagnostics GmbH, Mannheim, Germany).
During the 3-week-long inward rehabilitation, patients received a combination of physiotherapies designed individually after assessing cardiovascular status on admission. This inward rehabilitation included nine PVT (20 min pro session, BEMER B.Box Professional, B.Body Pro mattress, Triesen, Lichtenstein, Ref: 420200) too. Increasing flux density was used, ranging from 3.5 μTesla (level 1) to 35 μTesla (level 10), progressively. Output signal frequency was 30 Hz (US Patent 8808159) (11). The mattress has 2×3 coils, the flux density map can be found in the paper of Multanen and co-workers (22). Data acquisition was performed in the preceding 6 min of PVT, immediately from the start and during the last 6 min of PVT, straightway and one hour after finishing the treatment, thus providing five records at each patient. During the study, patients were in supine position at least 15 min before and throughout the recording preventing orthostatic actions. Of the 48 patients, the last twenty patients were enrolled to start with an additional sham treatment with the same protocol. Randomization and double blinding was not possible due to logistical reasons. The patients did not receive any medication besides their regular antihypertensive drugs including beta-blocker.
ECG signal acquisition was accomplished using a hand-held, battery-powered device based on a PIC18F46J50 microcontroller (Microchip Technology Inc., Chandler, AZ, USA) and INA333 instrumentation amplifier chip (Texas Instruments, Dallas, TX, USA). The instrument contains analogue circuitry for a single-channel (three-electrode) ECG amplifier among others. The recordings were transferred to a PC on an SD card for further analysis. After extracting the 1-ms temporal resolution ECG signal from the stored data, automatic detection of RR-intervals and manual checking was achieved by ECGRdet v2.1 software. A 300-sec-long artefact-free part of the recording was then selected manually adhering to standards in HRV analysis (25,27). Particular attention was paid to select a portion containing exclusively normal sinus beats in order to obtain correct HRV parameters. Varian v2.2 software was used to calculate the standard time-domain parameters: mean RR interval (meanRRI), standard deviation of normal-to-normal RR intervals (SDNN) and root mean square of successive RR-differences (RMSSD), Poincaré-plot parameters of SDSA (standard deviation of the projection of the plot perpendicular to the line of identity) and SDLA (standard deviation of the projection of the plot along the line of identity), as well as heart rate asymmetry (HRA) parameters: Porta and Guzik indices. Both the hardware and software (ECGRdet and Varian) were developed by L. Hejjel (37,38). We used Karmakar’s definition at HRA indices (39). Non-invasive systolic and diastolic blood pressure was registered in the middle of each of the five stages of the study. Average respiration frequency was computed by fast Fourier transformation (FFT) from the tachograms in Varian v2.2.
We analysed the HRV and HRA parameters applying a mixed model for repeated measurements (MMRM), using time, treatment and time-treatment interaction as fix effects and subjects as random effect. Right-skewed variables were log-transformed before the analysis and were transformed back to the original scale after the analysis for the graph illustrations. The log-transformed variables are SDNN, RMSSD, SDLA, and SDSA. We investigated the goodness of the fitted model by visualizing the joint distribution of the standardized residuals and the fitted values, and we additionally investigated the normal distribution of the random effects. These diagnostic tools showed good model fitting. Based on the fitted model, we computed the difference between the treatments in the change from baseline for the four post-baseline measurements. To account for multiple testing, we applied the single step adjustment method, which implements simultaneous tests for general linear hypotheses. We used R version 3.5.3 (40) to all analysis (R Core Team 2019), using the package nlme to fit the linear mixed effects models (41).
Results
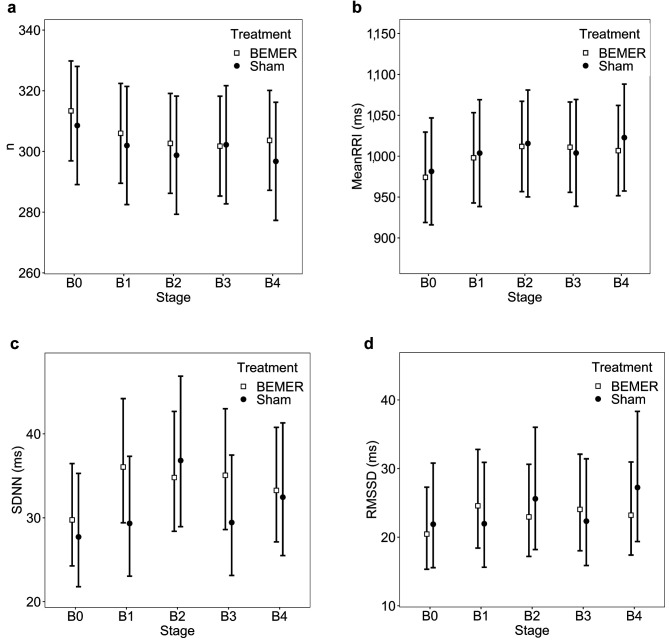
Clinical characteristics of the involved patients can be seen in Table I. Considering the relatively small number of included patients, we did not proceed to any statistical comparisons. Figure 1a shows no significant differences in the number of detected RR-intervals in the 5-minute-long epochs. This graph changes reciprocally to the Mean RR-interval graph (Figure 1b), as expected. Neither of the time domain parameters changed significantly between the treatment (BEMER) and sham groups relative to the baseline values; however, SDNN and RMSSD increased at the beginning of the real treatment (Figure 1b-c). SDSA and SDLA demonstrated similar alterations according to their time domain nature (Figure 2a-b). Interestingly, SDNN and RMSSD significantly rose at the last min of sham treatment and then both fell, while SDNN remained elevated in the treatment group during the observation period.
Table I. Clinical characteristics of the patients. Values are presented as mean±standard deviation, and the % value is given in parentheses when applicable.
PVT: Physical vascular therapy (BEMER) group; BMI: body mass index; EF: (left ventricular) ejection fraction; CRP: C-reactive protein level; WBC: white blood cell count; Hgb: haemoglobin level.
Figure 1. Conformation of the number (n) of detected peaks (a) and the time domain parameters: Mean pulse rate interval (MeanRR) (b), standard deviation of NN-intervals (SDNN, c), and the root mean square of successive RR-interval differences (RMSSD, d). There are no significant differences in the change from baseline level between the treatment (BEMER) and sham groups, however, SDNN and RMSSD show tendency of elevated variability on the beginning of exposure in the real treatment group and one stage later a milder increase in the sham group. There are no statistically significant changes from baseline either in the treatment (BEMER) or in the sham group; however, SDNN and RMSSD show a tendentiously elevated variability at the onset of exposure in the treatment group and, in the sham group, they show a slight increase one stage later. The error bars represent the 95% confidence intervals.
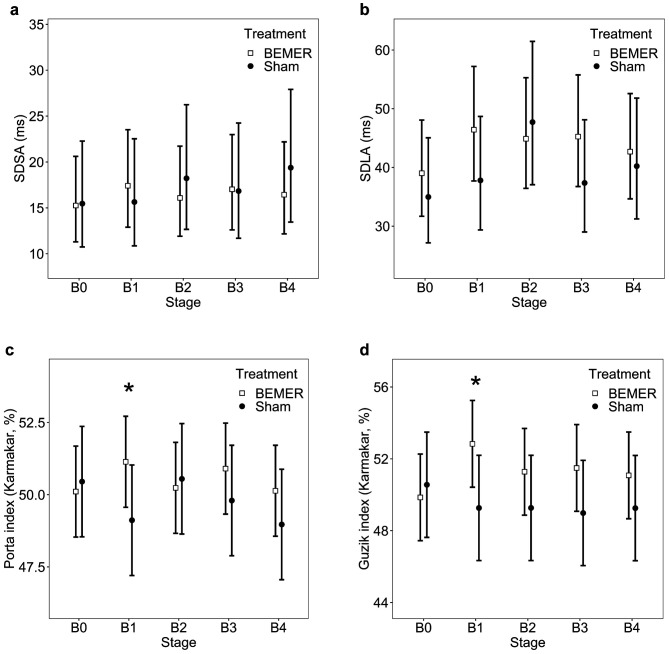
Figure 2. Poincaré plot related parameters: standard deviation along the short axis (SDSA, part a), standard deviation along the long axis (SDLA, b), Porta index (c) and Guzik index according to Karmakar’s definition (d). Porta and Guzik indices show significant increase in the PVT group compared to the sham group in the change from baseline at the beginning of the first stage. The asterisk represents significant difference, and the error bars denote the 95% confidence intervals.
The systolic and diastolic blood pressures measured in the middle of the particular stages were not significantly different. The changes in the mean respiration frequency calculated by spectral analysis of the tachograms did not reach statistically significant differences between the particular stages.
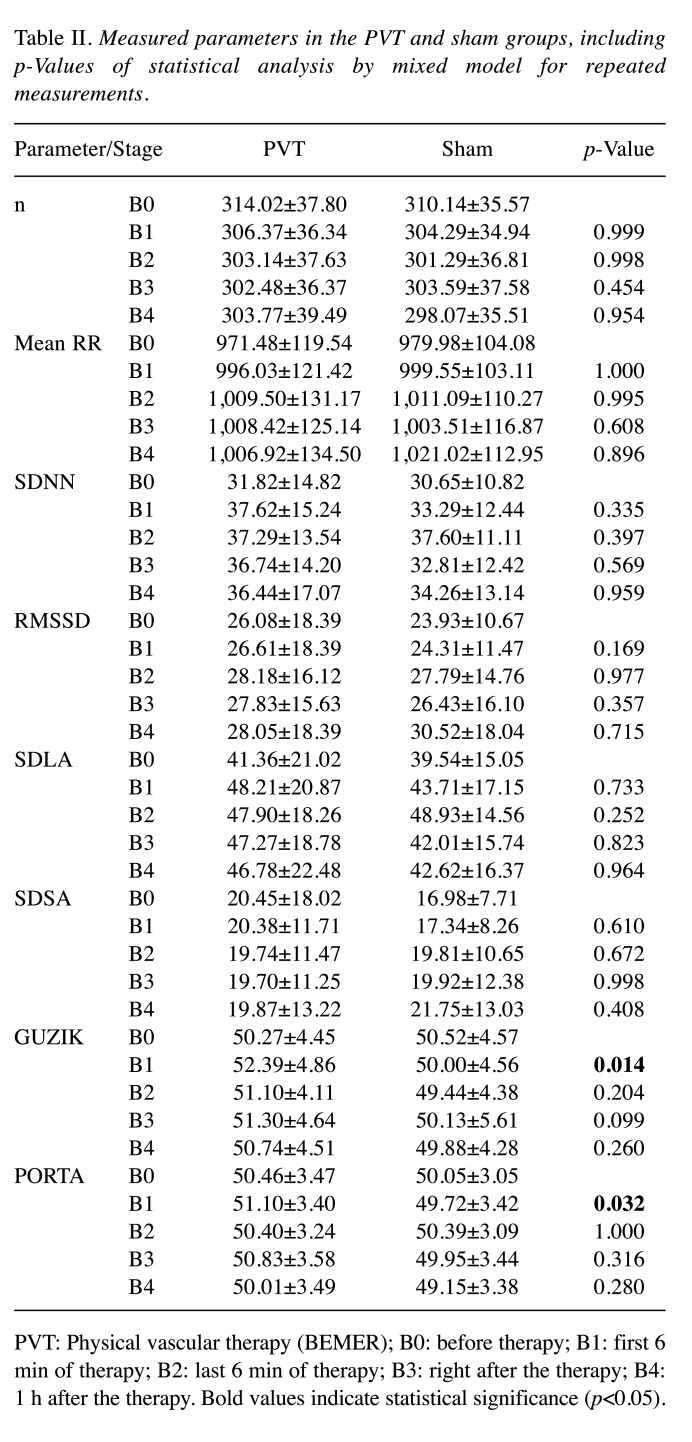
Porta and Guzik indices show a statistically significant increase in the first stage of BEMER treatment, and the Guzik index remained higher compared to the sham cohort throughout the observation (Figure 2c-d). The detailed results for HRV and HRA parameters are shown in Table II.
Table II. Measured parameters in the PVT and sham groups, including p-Values of statistical analysis by mixed model for repeated measurements.
PVT: Physical vascular therapy (BEMER); B0: before therapy; B1: first 6 min of therapy; B2: last 6 min of therapy; B3: right after the therapy; B4: 1 h after the therapy. Bold values indicate statistical significance (p<0.05).
Discussion
In the present study, we conducted HRV and HRA analysis to investigate the acute effects of PVT on the autonomous nervous system. The used MMRM statistical method compares the changes from the within-group baseline values in the treatment and sham groups; this compensates possible orthostatic or other effects (however, after 15 min it is not probable). Our protocol was designed to minimize the effects of diurnal changes, body position, vocalization and movements on HRV and HRA parameters (25,27).
To the best of our knowledge, this is the first study to investigate the immediate effects of PVT on HRV and HRA parameters. Statistically significant changes of Guzik and Porta indices and the tendentious alterations of time domain parameters were observed in our study indicating complex balanced sympathetic and parasympathetic effects. The high inter-individual differences in HRV parameters can be responsible for the non-significant changes of SDNN and RMSSD, however, the higher number of participants may counterbalance these changes. The inherently lesser scattering of HRA parameters could have facilitated the statistically significant differences. The baseline characteristics showed no substantial differences among the groups, except diabetes, but their relatively small percentage (~10% in sham and ~20% in PVT group) makes it ignorable.
The mean RR-interval was relatively stable around 1,000 ms (~60 beat/minute) during the study, corresponding to the relaxed, supine position after 15 min of orthostatic adaptation. This reflects a sympatho-vagal equilibrium since there is no need to change heart rate during steady state conditions. A little shorter mean RR-interval during the first stage might be related to the stress at the beginning of the treatment. The beat-to-beat variability parameters, RMSSD and SDSA reflects parasympathetic actions on the heart rate (25,27). These parameters showed only milder changes in the first min of the real treatment and then dropped in the last min of PVT. At the same time, these parameters rose in the sham group. Global (SDNN) and low-frequency (SDLA) variability reflecting both sympathetic and vagal effects increased upon the start of treatment and gradually returned to near the basal level one hour after the treatment. On the other hand, in the sham group, these parameters elevated exclusively during the last 6 min of sham treatment or in the 15+6+17=38th minute of supine position. The time course of the short- and long-range HRV parameters during real treatment demonstrates a different profile and reflects a short increase in vagal and – to maintain steady heart rate – in sympathetic effects. Then vagal activity falls back, which is followed by similar fall in sympathetic activity as well, resulting in steady heart rate and still higher overall variability but not beat-to-beat variability.
Guzik and Porta indices showed a transient but significant increase at the beginning of PVT. On the contrary, a decrease was observed in the same stage of the sham group. Guzik index is proportional to the HRV fraction during inspiration periods (38). At the onset of real PVT, mild bradypnea was proven by FFT, which may cause the changes of Guzik index; however, we need more investigations to clarify the particular physiopathological link, that is identifying the exact sympatho-vagal interactions behind the changes.
The effects of full-body PVT can be related to direct brain stem actions or effects on the sinus node, actions on vegetative ganglia, or indirectly to the improvement of peripheral microcirculation achieved by the increased vasomotion. However, we did not detect systemic blood pressure changes during the study. A local BEMER applicator can identify the “antenna” region within the body.
Study limitations. For logistical and infrastructural reasons, it was not possible to conduct a double-blinded randomized study. However, this pilot study confirms the need of a larger study with more participants, in order to compensate the inherently high dispersion of HRV parameters.
Conclusion
PVT (BEMER) significantly increases the Porta and Guzik indices, and tendentiously elevates time domain HRV parameters in chronic ischemic heart disease patients. To the best of our knowledge, this is the first study which proves that PVT has immediate vegetative actions in humans. The PVT probably decreases sympathetic activity without permanently influencing vagal actions. We encourage to conduct further randomized studies with recording additional parameters with local PVT applicator as well to elucidate the exact pathomechanism and localize the receptor area in the body.
Conflicts of Interest
The Authors declare no conflicts of interest.
Authors’ Contributions
Conception and design – LH, ZA; resources – LH, ZA; data collection – ZK, BA, BN; data analysis – ZK, PM, BA, BN, LH; drafting manuscript – ZK, PM, BN, BA, LH; editing manuscript – LH, PM, IK, ZA; final approval – ZK, PM, BN, BA, IK, LH, ZA.
Acknowledgements
The Authors would like to thank all the nurses of the Cardiology ward for their invaluable help.
References
- 1.Gilbert W. New York, Dover Publications. 1893. De Magnete. Translated by P. F. Mottelay. [Google Scholar]
- 2.de Andrade Martins R. Dordrecht, Springer. 2007. Ærsted, Ritter and Magnetochemistry. In: Hans Christian Ørsted and the romantic legacy in science. Brain RM, Cohen RS and Knudsen O (eds). Boston studies in the philosophy of science, vol 241. [DOI] [Google Scholar]
- 3.Bassett CA, Pawluk RJ, Pilla AA. Augmentation of bone repair by inductively coupled electromagnetic fields. Science. 1974;184(4136):575–577. doi: 10.1126/science.184.4136.575. [DOI] [PubMed] [Google Scholar]
- 4.Andrade R, Duarte H, Pereira R, Lopes I, Pereira H, Rocha R, Espregueira-Mendes J. Pulsed electromagnetic field therapy effectiveness in low back pain: A systematic review of randomized controlled trials. Porto Biomed J. 2016;1(5):156–163. doi: 10.1016/j.pbj.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durney CH, Christensen DA. Boca Raton, FL, CRC Press. 1999. Basic introduction to bioelectromagnetics. [Google Scholar]
- 6.Barchanski A. Dissertation, Darmstadt. 2007. Simulations of low-frequency electromagnetic fields in the human body. [Google Scholar]
- 7.Abd-allah MA. 2006 IEEE PES Power Systems Conference and Exposition. 2017. Interaction of ELF magnetic fields with human body organs model underneath EHV transmission lines; p. pp. 1967. [DOI] [Google Scholar]
- 8.Funk RH. Coupling of pulsed electromagnetic fields (PEMF) therapy to molecular grounds of the cell. Am J Transl Res. 2018;10(5):1260–1272. [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan J, Xin F, Jiang W. Underlying signaling pathways and therapeutic applications of pulsed electromagnetic fields in bone repair. Cell Physiol Biochem. 2018;46(4):1581–1594. doi: 10.1159/000489206. [DOI] [PubMed] [Google Scholar]
- 10.Blank M, Goodman R. Electromagnetic fields may act directly on DNA. J Cell Biochem. 1999;75(3):369–374. doi: 10.1002/(sici)1097-4644(19991201)75:3<369::aid-jcb2>3.3.co;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Gelim P, Klopp R. Apparatus for stimulating local and higher homeostatic autoregulatory mechanisms in the organism, US Patent 8808159, 2014 [Google Scholar]
- 12.Klopp RC, Niemer W, Schulz J, Ruhnau KJ. Influence of a specific, biorhythmically defined physical stimulus on deficient vasomotion in small-caliber arterioles in the subcutis in patients with diabetic polyneuropathy. J Complement Integr Med. 2013;10(Suppl):S21–7, S23-9. doi: 10.1515/jcim-2013-0033. [DOI] [PubMed] [Google Scholar]
- 13.Klopp RC, Niemer W, Schmidt W. Effects of various physical treatment methods on arteriolar vasomotion and microhemodynamic functional characteristics in case of deficient regulation of organ blood flow. Results of a placebo-controlled, double-blind study. J Complement Integr Med. 2013;10(Suppl):S39–46, S41-9. doi: 10.1515/jcim-2013-0035. [DOI] [PubMed] [Google Scholar]
- 14.Cole CW, Gordon RG, Braun PA. Cellular and ionic mechanisms of arterial vasomotion. In: Smooth muscle spontaneous activity, advances in experimental medicine and biology. Hashitani H and Lang J (eds) Springer Nature Singapore Pte Ltd, 2019:pp. 297. doi: 10.1007/978-981-13-5895-1_12. [DOI] [PubMed] [Google Scholar]
- 15.Mauban JR, Lamont C, Balke CW, Wier WG. Adrenergic stimulation of rat resistance arteries affects Ca(2+) sparks, Ca(2+) waves, and Ca(2+) oscillations. Am J Physiol Heart Circ Physiol. 2001;280(5):H2399–H2405. doi: 10.1152/ajpheart.2001.280.5.H2399. [DOI] [PubMed] [Google Scholar]
- 16.Meininger GA, Zawieja DC, Falcone JC, Hill MA, Davey JP. Calcium measurement in isolated arterioles during myogenic and agonist stimulation. Am J Physiol. 1991;261(3 Pt 2):H950–H959. doi: 10.1152/ajpheart.1991.261.3.H950. [DOI] [PubMed] [Google Scholar]
- 17.Aalkjær C, Boedtkjer D, Matchkov V. Vasomotion - what is currently thought. Acta Physiol (Oxf) 2011;202(3):253–269. doi: 10.1111/j.1748-1716.2011.02320.x. [DOI] [PubMed] [Google Scholar]
- 18.Davis MJ, Hill MA, Kuo L. Elsevier Inc. 2008. Local regulation of microvascular perfusion. In: Microcurculation. 2nd edition. Tuma RF, Durán WN and Ley K (eds.) p. pp. 161. [Google Scholar]
- 19.Gyulai F, Rába K, Baranyai I, Berkes E, Bender T. BEMER therapy combined with physiotherapy in patients with musculoskeletal diseases: a randomised, controlled double blind follow-up pilot study. Evid Based Complement Alternat Med. 2015;2015:245742. doi: 10.1155/2015/245742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haase R, Piatkowski J, Ziemssen T. Long-term effects of Bio-Electromagnetic-Energy Regulation therapy on fatigue in patients with multiple sclerosis. Altern Ther Health Med. 2011;17(6):22–28. [PubMed] [Google Scholar]
- 21.Storch K, Dickreuter E, Artati A, Adamski J, Cordes N. BEMER electromagnetic field therapy reduces cancer cell radioresistance by enhanced ROS formation and induced DNA damage. PLoS One. 2016;11(12):e0167931. doi: 10.1371/journal.pone.0167931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Multanen J, Häkkinen A, Heikkinen P, Kautiainen H, Mustalampi S, Ylinen J. Pulsed electromagnetic field therapy in the treatment of pain and other symptoms in fibromyalgia: A randomized controlled study. Bioelectromagnetics. 2018;39(5):405–413. doi: 10.1002/bem.22127. [DOI] [PubMed] [Google Scholar]
- 23.Tamulevicius N, Wadhi T, Oviedo GR, Anand AS, Tien JJ, Houston F, Vlahov E. Effects of acute low-frequency pulsed electromagnetic field therapy on aerobic performance during a preseason training camp: a pilot study. Int J Environ Res Public Health. 2021;18(14):7691. doi: 10.3390/ijerph18147691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 25.Hejjel L, Gál I. Heart rate variability analysis. Acta Physiol Hung. 2001;88(3-4):219–230. doi: 10.1556/APhysiol.88.2001.3-4.4. [DOI] [PubMed] [Google Scholar]
- 26.Malik M, Camm AJ. Components of heart rate variability – what they really mean and what we really measure. Am J Cardiol. 1993;72(11):821–822. doi: 10.1016/0002-9149(93)91070-x. [DOI] [PubMed] [Google Scholar]
- 27.Heart rate variability. standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 28.Wolf MM, Varigos GA, Hunt D, Sloman JG. Sinus arrhythmia in acute myocardial infarction. Med J Aust. 1978;2(2):52–53. doi: 10.5694/j.1326-5377.1978.tb131339.x. [DOI] [PubMed] [Google Scholar]
- 29.Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59(4):256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 30.La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, Ferrari R, Franchini M, Gnemmi M, Opasich C, Riccardi PG, Traversi E, Cobelli F. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107(4):565–570. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- 31.Lakusic N, Mahovic D, Babic T. Gradual recovery of impaired cardiac autonomic balance within first six months after ischemic cerebral stroke. Acta Neurol Belg. 2005;105(1):39–42. [PubMed] [Google Scholar]
- 32.Mahovic D, Lakusic N. Progressive impairment of autonomic control of heart rate in patients with multiple sclerosis. Arch Med Res. 2007;38(3):322–325. doi: 10.1016/j.arcmed.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Kreska Z, Németh B, Kiss I, Péter I, Ajtay Z, Hejjel L. Transcutaneous carbon dioxide treatment affects heart rate variability – a pilot study. In Vivo. 2018;32(5):1259–1264. doi: 10.21873/invivo.11374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu H, Li MH, Juan SH, Chiou WY. Effects of transcutaneous electrical nerve stimulation on motion sickness induced by rotary chair: a crossover study. J Altern Complement Med. 2012;18(5):494–500. doi: 10.1089/acm.2011.0366. [DOI] [PubMed] [Google Scholar]
- 35.Miranda CM, Silva RMFLD. Analysis of heart rate variability before and during tilt test in patients with cardioinhibitory vasovagal syncope. Arq Bras Cardiol. 2016;107(6):568–575. doi: 10.5935/abc.20160177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koppel T, Vilcane I, Ahonen M. 2018 EMF-Med 1st World Conference on Biomedical Applications of Electromagnetic Fields (EMF-Med) 2022. 50 Hz magnetic field affects heart rate variability – an experimental study; p. pp. 1. [DOI] [Google Scholar]
- 37.Hejjel L, Roth E. What is the adequate sampling interval of the ECG signal for heart rate variability analysis in the time domain. Physiol Meas. 2004;25(6):1405–1411. doi: 10.1088/0967-3334/25/6/006. [DOI] [PubMed] [Google Scholar]
- 38.Klintworth A, Ajtay Z, Paljunite A, Szabados S, Hejjel L. Heart rate asymmetry follows the inspiration/expiration ratio in healthy volunteers. Physiol Meas. 2012;33(10):1717–1731. doi: 10.1088/0967-3334/33/10/1717. [DOI] [PubMed] [Google Scholar]
- 39.Karmakar CK, Khandoker AH, Gubbi J, Palaniswami M. Defining asymmetry in heart rate variability signals using a Poincaré plot. Physiol Meas. 2009;30(11):1227–1240. doi: 10.1088/0967-3334/30/11/007. [DOI] [PubMed] [Google Scholar]
- 40.R Core Team R A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria, 2019. Available at: https://www.R-project.org/ [Last accessed on March 20, 2022]
- 41.Pinheiro J, Bates D, DebRoy S, Sarkar D, EISPACK , Heisterkamp S, Van Willigen B. R core team nlme: linear and nonlinear mixed effects models. R package version 3.1-145, 2020. . Available at: https://CRAN.R-project.org/package=nlme. [Last accessed on March 20, 2022]