Summary
Background
Despite declines in deaths from rheumatic heart disease (RHD) in Africa over the past 30 years, it remains a major cause of cardiovascular morbidity and mortality on the continent. We present an investment case for interventions to prevent and manage RHD in the African Union (AU).
Methods
We created a cohort state-transition model to estimate key outcomes in the disease process, including cases of pharyngitis from group A streptococcus, episodes of acute rheumatic fever (ARF), cases of RHD, heart failure, and deaths. With this model, we estimated the impact of scaling up interventions using estimates of effect sizes from published studies. We estimated the cost to scale up coverage of interventions and summarised the benefits by monetising health gains estimated in the model using a full income approach. Costs and benefits were compared using the benefit–cost ratio and the net benefits with discounted costs and benefits.
Findings
Operationally achievable levels of scale-up of interventions along the disease spectrum, including primary prevention, secondary prevention, platforms for management of heart failure, and heart valve surgery could avert 74 000 (UI 50 000–104 000) deaths from RHD and ARF from 2021 to 2030 in the AU, reaching a 30·7% (21·6–39·0) reduction in the age-standardised death rate from RHD in 2030, compared with no increase in coverage of interventions. The estimated benefit–cost ratio for plausible scale-up of secondary prevention and secondary and tertiary care interventions was 4·7 (2·9–6·3) with a net benefit of $2·8 billion (1·6–3·9; 2019 US$) through 2030. The estimated benefit–cost ratio for primary prevention scale-up was low to 2030 (0·2, <0·1–0·4), increasing with delayed benefits accrued to 2090. The benefit–cost dynamics of primary prevention were sensitive to the costs of different delivery approaches, uncertain epidemiological parameters regarding group A streptococcal pharyngitis and ARF, assumptions about long-term demographic and economic trends, and discounting.
Interpretation
Increased coverage of interventions to control and manage RHD could accelerate progress towards eradication in AU member states. Gaps in local epidemiological data and particular components of the disease process create uncertainty around the level of benefits. In the short term, costs of secondary prevention and secondary and tertiary care for RHD are lower than for primary prevention, and benefits accrue earlier.
Funding
World Heart Federation, Leona M and Harry B Helmsley Charitable Trust, and American Heart Association.
Introduction
Although acute rheumatic fever (ARF) and rheumatic heart disease (RHD) are now rare in most high-income countries, they remain a major cause of cardiovascular disease in several regions, including Africa, where RHD causes more than 18 000 deaths per year.1 Africa is the region with the highest RHD prevalence in the world.2 ARF is caused by an inflammatory process in some individuals after infection with group A streptococcus, and some people with ARF go on to develop RHD.3 Rates of ARF and RHD remain high in Africa in part because of living conditions associated with poverty, including household overcrowding, and inadequate levels of coverage of high-quality health care.4-6
Substantial evidence exists for cost-effective strategies to prevent and manage ARF and RHD in low-income and middle-income settings.7-9 The incidence of ARF dropped significantly in Cuba, Costa Rica, and Tunisia over periods coinciding with concerted campaigns improving coverage of primary and secondary prevention.10-12 Primary prevention through treatment of children with group A streptococcal pharyngitis with antibiotics—often benzathine penicillin G (BPG) injection—has typically been delivered through primary health care or in schools to make care accessible to achieve necessary coverage.11,13 Monthly BPG injection for secondary prevention is recommended for several years after a case of ARF involving carditis, with varied duration depending partly on severity and age.14 Management of heart failure and other sequelae of severe disease, assessment for eligibility for surgical intervention to repair or replace damaged heart valves, and postoperative anticoagulation and follow-up benefit from integrated care strategies,15 yet access to these services in Africa has been low. The Global Rheumatic Heart Disease Registry (REMEDY) study found high mortality (about 17%) over an initial 2-year period.16 Few patients in Africa who meet criteria for surgical heart valve repair or replacement receive it.17
The World Heart Federation (WHF) has advocated for greater political and financing commitments at the global and national levels to address RHD. Intense effort by groups on the African continent over the past 15 years has built global momentum in research and advocacy around RHD, although progress has not been rapid.18,19 The Addis Ababa communiqué, which identified priority areas for action on RHD to address gaps in data, health systems, and policy, was endorsed by heads of state in the African Union (AU) in 2016.20 In 2017, member states of WHO adopted the RHD Resolution EB141.R1 at the World Health Assembly, calling for national, regional, and global actions to prevent and control ARF and RHD, with WHO and member states reporting on progress in 2021.21 The Pan African Society of Cardiology has convened a series of meetings with the WHO Regional Office for Africa (WHO/AFRO) to develop and endorse an RHD control programme that includes primary and secondary prevention.19,21,22 WHO/AFRO and partners have been exploring regional strategies for expanding integrated outpatient care for severe, chronic non-communicable diseases (NCDs) at district hospitals (PEN-Plus) that includes preliminary echocardiographic RHD diagnosis, medical treatment of RHD, and postoperative anticoagulation.23-25 Multidisciplinary care during pregnancy continuing into the post-partum period reduced maternal and fetal mortality in women with cardiovascular disease in a pilot study in South Africa.26
Many solutions to addressing the RHD burden are known—the main barriers to their implementation are lack of prioritisation and resources. To encourage funding and collective action by countries, foundations, and development agencies, the WHF commissioned this investment case for RHD in the AU. In this investment case, we use evidence about the costs and effects of interventions to estimate the health impact and total costs of scaling up programmes in countries in the AU to operationally feasible levels over the next 10 years.27 This work is intended to offer a way forward in addressing RHD as part of a broader NCD and injuries poverty agenda.28
Methods
Overview
We constructed a model to estimate the health effects, costs, and monetised health gains from increasing coverage of a set of RHD interventions in the scale-up period (2021–30) to estimate the benefit–cost ratio and net benefit. Our approach was informed by WHO guidance on investment cases for NCD prevention and control, as well as other recent global investment cases.27,29 We chose a 10-year period for its relevance to the policy cycle and alignment with the timeframe of the UN’s 2030 Sustainable Development Goals.
Health impact model
We constructed a cohort state-transition model with an underlying demographic projection model. Transitions between states occurred in cycles of 1 year, with prevention and treatment interventions altering the transition probabilities between states. For the demographic projections, we used levels of population and fertility estimates from the Global Burden of Disease Study (GBD) 20171 and trends in projected all-cause mortality and fertility from the UN Population Division World Population Prospects 2019 revision (appendix p 6).30,31 We did analyses by region of the AU (appendix p 5) and aggregated to present results for the AU as a whole and its component regions.
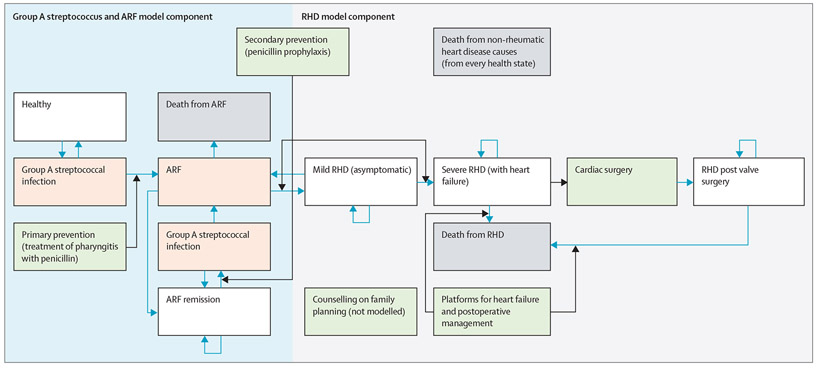
The structure of our model of disease progression, with labels for particular parameters, is shown in figure 1. The model was split into two parts, with the first covering the disease process from pharyngitis to RHD incidence, and the second for the disease process from the development of RHD through its long-term consequences. The first part of the model used inputs from meta-analyses on pharyngitis incidence among children and evidence on the risks of ARF and subsequent RHD collated by previous cost-effectiveness models to generate estimates of RHD incidence. The parameters governing pharyngitis and ARF were calibrated to the RHD incidence estimates from GBD 2017. These GBD 2017 estimates were informed by echocardiographic studies of RHD prevalence, which were more geographically specific and numerous than the studies on pharyngitis and ARF. The parameters describing movement between health states in the RHD part of the model were derived from a combination of GBD 2017 estimates and epidemiological studies from the literature in the AU. The parameters are described in further detail in the appendix (pp 8–21, 26–31). Atrial fibrillation and stroke are long-term sequelae often noted in relation to RHD. To retain a parsimonious model, we did not explicitly include these sequelae as health states in the model, although costs for managing patients with severe RHD include costs of additional therapies, such as anticoagulation, that these patients require. Pregnancy is a health state during which higher risk of complication or death from RHD can occur.32 Although we do not explicitly model the state of pregnancy, our model covers this population through management of severe disease generally. Increasing coverage of family planning services might have a larger population impact, mitigating RHD-related complications during pregnancy. Further study examining the dynamics of disease progression in pregnancy and the cost and effectiveness of screening programmes to identify women with mild RHD would be required to create reliable estimates of potential costs and impact (appendix pp 24–25). We included family planning and prenatal consultation in women of reproductive age with RHD as interventions because of the importance of the risks from RHD during pregnancy, but the costs and effects were not estimated in our model.
Figure 1: Health impact model structure.
Health states are represented by white rectangles (death in grey rectangles) with transitions shown by blue arrows. Green rectangles represent interventions with black arrows showing the pathways on which the interventions act. Medically managed heart failure that no longer meets criteria for heart failure remains in the RHD with heart failure category because it has advanced irreversibly to severe disease. Populations occupy the health states in white and grey rectangles after each step of the model. The health states in the group A streptococcus and ARF portion of the model shown in pink are simplified in this figure, and there are more complex transitions occurring in each model step. The more detailed structure of the model is described in the appendix (pp 4, 8) with labels corresponding to transition probabilities. Postoperative management here is included with heart failure management, because these are services provided by the same providers within the health system in our model of scale-up. ARF=acute rheumatic fever. RHD=rheumatic heart disease.
The transition pathways on which the interventions act, the interventions, their effect sizes, and the baseline and target coverages are shown in figure 1 and table 1. We increased coverage over time from the starting coverage in 2020 to the target coverage in 2030. Starting coverage was based on sparse data and assumptions (appendix pp 26–31). We selected target coverage up to 2030 based on operationally plausible goals, assuming sufficient levels of funding would be available (appendix pp 26–31). We estimated health benefits by comparing the results with intervention scale-up to the reference results under the baseline coverage levels (appendix p 31). We report incident cases of RHD averted, deaths averted from ARF and RHD, and percentage reductions in rates of incidence, prevalence, and deaths in the scale-up scenario relative to the reference scenario.
Table 1:
Intervention effect sizes and baseline and target coverage by intervention
| Intervention | Coverage definition | Affected outcome | Effect size | Baseline coverage |
Target coverage |
|
|---|---|---|---|---|---|---|
| 1 | Primary prevention (treatment of group A streptococcal pharyngitis, awareness raising, strengthening supply chains, provider training) | Percentage of group A streptococcal pharyngitis cases treated in ages 5–15 years | ARF and all subsequent health states | 68% (52–79) | 15·0% (3·8) | 40% |
| 2a | Secondary prevention (prophylactic penicillin after ARF with carditis—10 years or until age 20 years, whichever longer) | Percentage of people with ARF treated with prophylactic penicillin | ARF and all subsequent health states | 55% (8–78) | 5·0% (1·3) | 40% |
| 2b | Secondary prevention (prophylactic penicillin in asymptomatic RHD) | Percentage of people with asymptomatic RHD treated with prophylactic penicillin | Severe RHD and all subsequent health states | 55% (7–78) | 5·0% (1·3) | 40% |
| 3 | Platforms for heart failure management and anticoagulation, including management during pregnancy | Percentage of people with heart failure from RHD having heart failure medically managed | Deaths or prevalence of people with severe RHD or RHD post-valve surgery | 60% (30–80)* | 8·0% (2·0) | 55% |
| 4 | Cardiac surgery and postoperative care | Percentage of people with heart failure from RHD aged 10–40 years receiving cardiac surgery and postoperative care | Deaths or prevalence of people with severe RHD or RHD post-valve surgery | 85% (70–92)† | 5·0% (1·3) | 25% |
| 5 | Evaluation and counselling on family planning for women of reproductive age‡ | Percentage of women of reproductive age with RHD desiring contraceptive method who have access | Severe RHD and all subsequent health states | ·· | 45·0% (5·0) | 75% |
Data are % reduction (95% uncertainty interval), % (SD), or %. ARF=acute rheumatic fever. RHD=rheumatic heart disease.
Mortality risk reduction assumed to last 4 years, because heart failure management is not curative.
Initial 3% operative mortality assumed.
Intervention included here because of the risk that RHD poses during pregnancy, but effects not modelled. References for and descriptions of coverage estimates and effect sizes are given in the appendix (pp 22, 28). 15% reduction in RHD incidence assumed over the period from factors related to living conditions—reductions distributed in pharyngitis and ARF parameters (appendix p 11). Postoperative management coverage assumed 100% among people who have received surgeries (assumed that surgeries not done without care in place for long-term management).
Cost
We estimated costs for these interventions from the perspective of the health system by assembling published data on programmatic costs, estimates of health-care costs from the WHO Choosing Interventions that are Cost-Effective (WHO-CHOICE) project, and data on costs of medications and equipment that were necessary for each intervention.33-35 We followed the procedures recommended by the Global Health Cost Consortium to convert costs to 2019 US$.36 A full description of cost components and conversions is given in the appendix (pp 32–37).
For each of our projection scenarios, we calculated the net cost difference compared with the reference no scale-up scenario to obtain the cost of scale-up (appendix p 32). We calculated costs shared between interventions (such as equipment used in multiple interventions) once to prevent double counting and represent these as shared costs between the relevant interventions.
Monetised health gains and benefit–cost ratio
Benefit–cost analysis has been previously used in cases for investment around health interventions.27,29,37 To monetise health benefits, we used a full income approach, combining economic benefit through projected changes in gross domestic product (GDP) and in the value of the health itself.38 To quantify the value of health gained, we multiplied deaths averted by estimates of the value of a statistical life (VSL). We used an established approach to adjust a VSL estimate from the USA for the AU using per capita gross national income and assumptions about income elasticity.37 We estimated the projected increase in GDP by multiplying the projected population difference between the scale-up and baseline scenarios by the per capita GDP, adjusted for projected real growth. In addition, we estimated the number of hospitalisations from ARF that would be averted by the scale-up of interventions and multiplied these by estimated costs of hospitalisations for these conditions. Using the estimated costs of intervention scale-up and economic benefits from these three components, we calculated the net benefits and benefit–cost ratio. Given that policy makers might be interested in understanding the short-term versus long-term benefits of investment, we estimated the benefit–cost ratio for the 2021–30 period and the 2021–90 period by accruing the health benefits through 2090 from the costs of the initial 2021–30 investment. The calculations are described in detail in the appendix (pp 38–40). We report costs and benefits without discounting and benefit–cost ratios and net benefits with 3% discounting of benefits and costs.39
Uncertainty and sensitivity analyses
We examined uncertainty through probabilistic sensitivity analysis and deterministic sensitivity analysis of particularly influential or uncertain parameters. For the probabilistic sensitivity analysis, we included uncertainty about the transition probabilities, intervention effect sizes, starting coverage estimates, and cost components in the model, creating 1000 draws of the probabilities from uncertainty distributions associated with each parameter and running the model for 1000 randomly combined draws. We took the 2·5th and 97·5th percentiles of the draws to report uncertainty intervals (UIs). The UIs we present should not be interpreted as precise 95% CIs given limitations in inputs and assumptions used to derive them (appendix pp 41–42), and we treated the uncertainty reported as a range of plausible values. This uncertainty analysis did not incorporate uncertainty about forecasted demographic trends or economic indicators, nor did it capture uncertainty in the structure of our disease model.
For certain parameters, we captured uncertainty through deterministic sensitivity analysis. For the main results, we assumed that 80% of ARF cases are preceded by symptomatic group A streptococcal pharyngitis, that children aged 5–15 years have about 2·3 cases of pharyngitis per year at the peak age with 10% from group A streptococcus, that the delivery model for primary prevention is through health centres, and that all interventions in table 1 are scaled from baseline to target coverage through 2030. We reported results from alternative scenarios that varied these parameters, and compared results using different strategies for calibrating the transition probabilities to severe disease and death. Given the costly nature of treating the large number of childhood cases of pharyngitis, we included an alternative delivery model for primary prevention with community health workers (CHWs). A 10-year modelling period does not fully capture the impact of prevention, and we included alternative scenarios to capture benefits over a longer period. More complete results from various sensitivity analyses are in the appendix (pp 47–56).
Presentation of results
We present results for primary prevention separately from other integrated interventions (secondary prophylaxis, heart failure care, echocardiography, surgery, and postsurgical management), as well as combined with other interventions for several reasons. First, the delivery of primary prevention does not depend on the other RHD interventions from a health systems perspective. By contrast, there are shared human resources, equipment, and connections in the care cascade that strongly tie the other interventions together. For example, for a surgical programme to exist, it is necessary to have a programme established that can manage heart failure, determine eligibility for surgery using echocardiography, and manage anticoagulation after surgery. Second, primary prevention is delivered through primary health care at health centres or in the community, whereas the other interventions all require access to secondary or tertiary care. Although secondary prophylaxis should be administered through primary care for patient accessibility, established referral pathways to more advanced care are necessary for initial diagnosis and monitoring through registries.
All analyses were done with R, version 3.6.1.
Role of the funding source
Members of the WHF contributed to the study’s design and interpretation. The other funders played no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
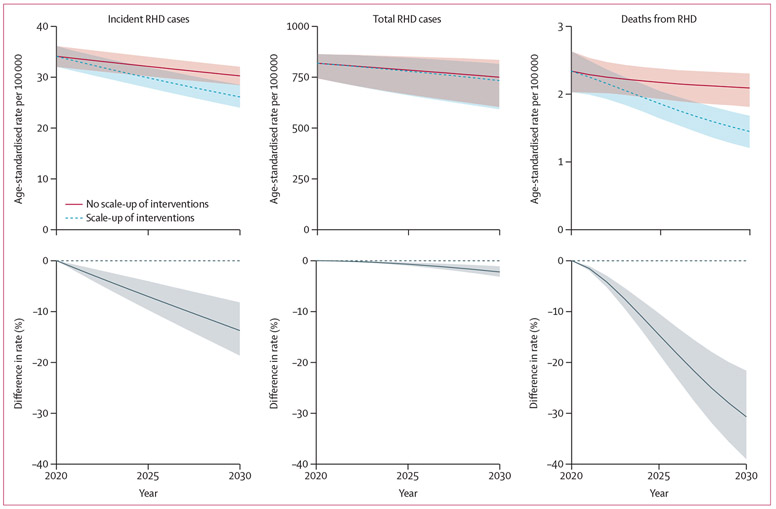
Scale-up in coverage of primary prevention was estimated to reduce the projected age-standardised incidence of RHD in 2030 by 7·6% (UI 4·7–10·1) compared with the reference no scale-up scenario, averting 187 200 (113 300–247 200) new cases of RHD from 2021 to 2030 (table 2). The death rate from ARF was estimated to be reduced by 8·5% (5·3–11·3) in 2030 compared with the value for 2030 estimated in the reference scenario, but estimated reductions in the age-standardised prevalence of RHD (1·3%, 0·8–1·8) and death rates from RHD (0·6%, 0·4–0·8) were smaller. Scale-up of secondary prophylaxis and integrated secondary and tertiary care were estimated to reduce incidence of RHD by 6·7% (1·2–11·3) in 2030. The projected age-standardised death rate from RHD was estimated to be reduced by 30·4% (21·4–38·7) in 2030 compared with the reference value, averting 59 500 (40 300–76 300) RHD deaths from 2021 to 2030.
Table 2:
Summary benefit and cost results from selected scenarios, 2021–30
| Primary prophylaxis only†* |
Integrated secondary and tertiary care only† |
All interventions‡ | |
|---|---|---|---|
| RHD incident cases averted (thousands) | 187·2 (113·3 to 247·2) | 184·5 (31·0 to 310·6) | 361·5 (207·2 to 497·3) |
| RHD deaths averted (thousands) | 0·8 (0·5 to 1·1) | 59·5 (40·3 to 76·3) | 60·0 (40·8 to 76·8) |
| ARF deaths averted (thousands) | 7·2 (1·5 to 19·6) | 7·1 (0·5 to 22·1) | 13·9 (2·4 to 38·3) |
| Cost (billions, US$) | 3·1 (1·9 to 4·3) | 1·0 (0·7 to 1·2) | 3·9 (2·7 to 5·1) |
| Cost per death averted (thousands, US$) | 526·1 (155·2 to 1389·4) | 14·8 (10·6 to 22·7) | 54·4 (33·8 to 83·5) |
| Full income benefit (billions, US$) | 0·5 (0·1 to 1·2) | 4·5 (3·0 to 5·9) | 4·9 (3·3 to 6·7) |
| Benefit–cost ratio to 2030 | 0·2 (<0·1 to 0·4) | 4·7 (2·9 to 6·3) | 1·3 (0·8 to 1·9) |
| Benefit–cost ratio to 2090§ | 0·7 (0·4 to 1·1) | 8·4 (4·8 to 12·1) | 3·2 (1·9 to 4·7) |
| Net benefit (billions, US$) | −2·1 (−3·1 to −1·2) | 2·8 (1·6 to 3·9) | 0·8 (−0·8 to 2·3) |
Data are mean (95% UI). Monetary values presented in 2019 US$. Costs and full income benefits presented without discounting. Benefit–cost ratio, net benefits, and cost per death averted based on discounted costs and benefits. Results reported for primary prevention delivered through health centre-based treatment, and results for additional sensitivity analyses, including for community-based delivery of primary prevention, are reported in the appendix (pp 47-56).
Health centre-based pharyngitis treatment.
Secondary prophylaxis, diagnosis, case management, and cardiac surgery for rheumatic fever and rheumatic heart disease.
Primary, secondary, and tertiary management.
Calculated using costs of scale-up 2021–30 and benefits accrued 2021–90; should be interpreted with caution because strongly dependent on assumed discount rates and inherently uncertain long-term projections of economic indicators.
Increases in coverage for all combined interventions to 2030 target levels were estimated to reduce the projected age-standardised death rate from RHD from 2·1 deaths per 100 000 (UI 1·8–2·3) in the reference scenario in 2030 to 1·4 deaths per 100 000 (1·2–1·7; figure 2) in the intervention scenario, a reduction of 30·7% (21·6–39·0). Cumulatively, we estimated 60 000 (40 800–76 800) RHD deaths averted and 13 900 (2400–38 300) ARF deaths averted from 2021 to 2030.
Figure 2: Impact of interventions scaled to target coverage on age-standardised rates of incidence, prevalence, and deaths from RHD, 2020–30.
Rates age-standardised to 2017 age structure of population in the African Union. Uncertainty intervals in rates reflect uncertainty in underlying epidemiological parameters as well as uncertainty about intervention effects; uncertainty in percent differences primarily reflects uncertainty in intervention effects. RHD=rheumatic heart disease.
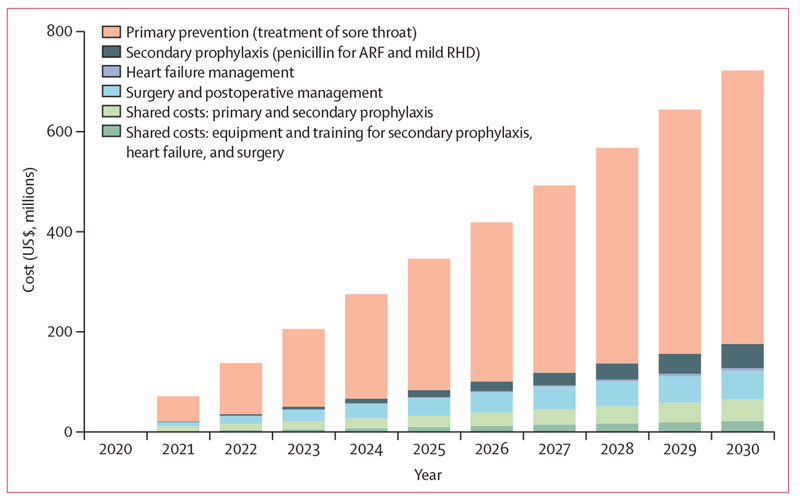
The total cost of scaling up all interventions from 2021 to 2030 was estimated at $3·9 billion (UI 2·7–5·1; 2019 US$). Whereas the cost per surgery was among the largest per-unit costs (appendix pp 34–35), the large number of cases of pharyngitis in the population caused the scale-up of primary prevention to be the largest component of the overall cost—$3·1 billion (1·9–4·3) on its own, more than 75% of the cost of all combined interventions. The components of the cost of scaling up interventions from 2021 to 2030 are shown in figure 3. Costs shared between multiple interventions because of overlaps in equipment and human resources made up a large portion of the costs outside of primary prevention (about 39%), as did surgery and postoperative care visits including anticoagulation (37%), with secondary prophylaxis visits and medication (21%) and heart failure management visits and medications (2%) making up the remainder.
Figure 3: Cost of interventions scaled to target coverage, 2020–30.
Costs in 2019 US$. Costs presented for scale-up of all interventions to target coverage. Shared costs for primary and secondary prophylaxis include mass media awareness and education campaign costs and costs of provider education, training, and mentorship to strengthen correct treatment of sore throat, referral of ARF for diagnosis, and administration of secondary prophylaxis at health centres. Shared costs for secondary prophylaxis, heart failure management, and surgery include first referral-level provider training and costs of equipment and supplies. ARF=acute rheumatic fever. RHD=rheumatic heart disease.
Costs were sensitive to several key inputs. The number of cases of pharyngitis per year was a large determinant of the overall cost of primary prevention because each case leads to the cost of a health-care visit, which includes provider time. Primary prevention delivered through a CHW model had potential to substantially reduce the cost of primary prevention (from $3·1 billion [UI 1·9–4·3] to $1·3 billion [0·8–1·9], assuming CHWs would see 12 clients per day with an initial visit for diagnosis and dispensation of oral antibiotics and a second for adherence support; appendix p 54).
The full income benefits from investing in all interventions—capturing increased economic activity and the intrinsic value of health—were estimated at $4·9 billion (UI 3·3 to 6·7). The majority (93%) of this benefit was from the VSL component. The benefit–cost ratio was estimated to be 1·3 (0·8 to 1·9) to 2030 with a 3% annual discount rate on costs and benefits, or 3·2 (1·9 to 4·7) accruing benefits of the increased 2021–30 coverage to 2090. Scaling up primary prevention alone was estimated to result in a low benefit–cost ratio (0·2, <0·1–0·4) and a negative net benefit (−$2·1 billion, −3·1 to −1·2) because of high cost and low short-term mortality impact. The benefit–cost ratio was estimated to be higher (0·7, 0·4 to 1·1) with benefits accrued to 2090. Scaling up other interventions without primary prevention was estimated to result in a higher benefit–cost ratio (4·7, 2·9 to 6·3) and net benefit $2·8 billion (1·6 to 3·9) in the short term because of the more direct and immediate impact on deaths. Incorporating long-term benefits through 2090, the estimated benefit–cost ratio grew to 8·4 (4·8 to 12·1) because of the effects of secondary prevention. The estimated long-term benefit–cost ratios were sensitive to discount rates and strongly depended on assumptions about economic growth through 2090 used to project VSL estimates (appendix pp 38–40, 55).
The short-term benefit–cost ratio through 2030 for primary prevention was estimated to be higher using a CHW model of delivering care, assuming CHWs could see 12 clients per day (0·4, UI 0·1–0·9), but remained low overall without the long-term benefits accrued. We modelled a high benefit, low cost scenario in which primary prevention was delivered by CHWs seeing 12 clients per day, children had one pharyngitis case per year with 10% from group A streptococcus, and coverage was increased to 100% in 2021, reverting to 0% coverage in 2031 to project benefits accrued to 2090. Under this scenario, the benefit–cost ratio to 2090 was estimated to be 4·2 (2·1–6·8). Projections to 2090 should be interpreted with caution (appendix pp 38–40, 55). Country-level and regional variation in epidemiology, demography, and economic productivity affects the estimated benefit–cost ratios (appendix pp 45–46, 56). Additional results for the various described scenarios are presented in the appendix (pp 47–56).
Discussion
We found substantial potential for reduction in cases of, and deaths from, RHD in the AU with scale-up in coverage of an evidence-informed bundle of related interventions from 2021 to 2030. Secondary prevention and treatment, targeting different stages of the disease process, are likely to avert substantial morbidity and mortality in the short term, whereas primary prevention is likely to accrue impact extending over a long time horizon. Key features of the RHD course and differing delivery strategies will influence cost and size of the effect of primary prevention.
Integrated prevention programmes have been linked to declines in ARF and RHD in parts of the AU and in other settings.11,12 However, evaluations have not been able to account for the effects of primordial prevention (improved living conditions) to isolate causal effects of specific interventions. Previous studies have suggested that prevention is cost-effective, although effects of primary prevention assumed in many cost-effectiveness studies are from specific populations in older trials among individuals presenting with pharyngitis.8,9,40 By contrast, our estimates of the benefit–cost ratio for primary prevention were relatively low. The ratio increased as we modelled benefits for a longer period, although the ratio remained comparatively low unless alternative cost and epidemiological assumptions were used (appendix pp 48–51, 54). The cost of primary prevention in our study was high, because it included the cost of increased health-care visits by many children aged 5–15 years with nongroup A streptococcal pharyngitis and the treatment of some of these children based on the imperfect specificity of a clinical decision rule.8,41 The health benefits of primary prevention were also limited by the proportion of ARF cases that were assumed to occur among people with preceding symptomatic pharyngitis (assumed 80%, although varied in sensitivity analyses) and cases occurring outside the intervention’s target age range.
Better evidence on the epidemiology of group A streptococcal pharyngitis in the AU and feasibility and cost-effectiveness studies on primary prevention delivery models, including potentially less costly CHW models, might be critical for understanding the costs and population-level impact of primary prevention. Primary prevention delivered at health centres might be more costly and might not be able to reach coverage levels as high as CHW-based delivery because of additional patient barriers to accessing care. However, treatment with BPG delivered at health centres might be more effective than oral antibiotics, and eliminates the concerns about antimicrobial resistance that come with adherence to courses of oral antibiotics. Primary prevention for RHD has not traditionally been delivered by CHWs; pilot programmes would improve logistical assumptions and estimates of both cost and effectiveness. Access to penicillin for both primary and secondary prevention efforts has been a critical part of success stories in RHD control, yet cost and availability of BPG have sometimes been barriers to its consistent use.12,42
Operationally, systems for secondary prevention, heart failure management, and valve surgery are interdependent. Investment in strengthening referral systems between levels of the health system, decentralising echocardiography for diagnosis of RHD, strengthening BPG supply chains, training of providers at health centres to administer penicillin prophylaxis and refer patients for higher-level care when necessary, strengthening and developing cardiac surgery centres, building awareness and education through multisectoral RHD initiatives, and strengthening surveillance and registry systems would benefit the coverage and quality of the continuum of care from secondary prophylaxis through cardiac surgery.20 Availability of long-term postoperative follow-up maximises the benefit of valve surgery, so it is advantageous for the scale-up of heart failure management and anticoagulation therapy to at least match the scale-up in cardiac surgery. Coordinated investment in facilities, equipment, medications, and human resources to provide integrated RHD services has been demonstrated through the PEN-Plus delivery model for severe, chronic NCDs such as type 1 and insulin-dependent type 2 diabetes, sickle cell disease, and advanced cardiovascular disease.23-25 The provider competencies needed for RHD management align with competencies required to manage other complex and chronic NCDs, including other causes of heart failure, creating an opportunity for integrated care and shared investments across disease priorities.15,43 Family planning services, preconception counselling and an option of safe abortion for women with RHD who might become pregnant, and adapted heart failure management strategies can reduce risk of maternal morbidity and mortality.26,44 Capacity for cardiac surgery in Africa has been growing, and although some continued investment in sending patients abroad for surgery might be necessary, there are several emerging cardiac surgery centres on the continent that would continue to grow with further investment and caseloads.45
There were several limitations to our modelling study. Data describing the complete epidemiological picture of ARF and RHD in the AU were lacking. We sought to anchor estimates to data from parts of the disease process with comparatively better evidence—eg, estimates of RHD incidence and prevalence from GBD 2017 informed by echocardiographic prevalence studies and characterisations of cohorts with RHD across sites with registries in the AU (appendix pp 57–63). However, questions remain about the natural history of subclinical RHD cases, and national-level RHD mortality data in Africa are largely restricted to South Africa.1,46 We reported ranges to reflect uncertainty in some of the input parameters, although our reported intervals should be interpreted with care given the limitations in the uncertainty estimates of inputs (appendix p 41). There were ways in which our model simplified the disease process, not explicitly including stroke, atrial fibrillation, and pregnancy, which can result from or interact with RHD. Our goal was to create a model that used available parameter estimates and included components most critical for costing and health benefit projection, but that was parsimonious. The long-term benefit–cost ratios presented to 2090 should be interpreted with caution, because our model does not capture uncertainties about long-term economic and demographic changes. The approach for estimating economic productivity benefits of lives saved is limited and does not account for individuals’ likelihood to contribute economically; however, the VSL is roughly 30 times the GDP per capita and contributes far more to the monetised health benefits. We sought to transparently describe the effects of model parameters and assumptions through sensitivity analyses, and we comment on limitations of specific components of the modelling throughout the appendix. This analysis shows a possible path for addressing RHD in the AU, although country-specific implementation strategies should be informed by more specific demographic and epidemiological information and assessment of local health system factors.
Our study did not account for the effects of the current COVID-19 pandemic. Elective surgeries have been postponed in many countries, travel slowed or halted, and resources diverted to manage COVID-19. Given the link between living standards and ARF and RHD, and the projected increase of as many as 400 million additional people pushed under the $1·90 poverty line globally, COVID-19 will probably continue to set back progress on RHD, making scale-up of these interventions even more critical.47 Our analysis suggests that primary prevention is not a high priority for countries with severe resource constraints in the wake of COVID-19, particularly given the high costs. However, there are a number of benefits of primary prevention outside of RHD, including preventing other sequelae of group A streptococcus and reducing inappropriate antimicrobial use. A comprehensive analysis of the benefits of primary prevention would likely find its benefits higher than its costs, but such an analysis was outside the scope of this study.48 Investing in the integrated implementation of prevention, management, and surgical interventions to address RHD can strengthen health systems, decentralise care, and engage multiple sectors to avert large amounts of morbidity and mortality, provide returns in economic welfare exceeding the costs, and accelerate progress towards eliminating RHD in the AU.
Supplementary Material
Research in context.
Evidence before this study
Rheumatic heart disease (RHD) remains a major cause of cardiovascular disease in the African Union (AU). We searched PubMed for English language articles published from database inception to Sept 14, 2020, using the terms "(rheumatic heart disease) AND (cost-effectiveness)". Although there have been debates about particular strategies, there are multiple studies documenting the cost-effectiveness of primary prevention through the treatment of group A streptococcal pharyngitis with antibiotics, and secondary prevention through penicillin prophylaxis in people who have a history of acute rheumatic fever (ARF). Several countries have shown marked declines in RHD and ARF after implementing control programmes, mostly in middle-income settings. Some delivery models have shown success with the decentralisation of heart failure management, which includes RHD management and postoperative anticoagulation for mechanical heart valves and atrial fibrillation. Research and advocacy have built momentum towards the data, health system, and policy gaps that need to be addressed to eliminate RHD in Africa.
Added value of this study
We have built on the evidence from earlier epidemiological, costing, and cost-effectiveness studies to construct an investment case for scale-up of prevention and management strategies to work towards RHD elimination in the AU.
We consider operationally plausible opportunities for investments in scaling up prevention, management, and surgical interventions and estimate the health impacts, cost, and returns on investment.
Implications of all the available evidence
We found potential for reducing RHD mortality by almost a third by increasing coverage of RHD interventions in regions of the AU to 2030. In the short term, investment in primary prevention would not avert a large number of deaths and would be costly because of the large number of childhood pharyngitis cases. Valve surgery, secondary prophylaxis, and medical management of established RHD cases through the Package of Essential Noncommunicable Disease Interventions-Plus (PEN-Plus) delivery strategy require overlapping investments in equipment, human resources, and referral pathways, making their coordinated scale-up operationally logical. Monetised health benefits from increasing coverage of secondary prevention, management of severe disease, and valve surgeries exceeded costs in the 2021–30 timeframe using a full income approach to quantify benefits. Costs of primary prevention exceeded the value of benefits in the next decade. We found that benefits accrued up to 2090 produced favourable benefit–cost ratios with lower-cost delivery models and more favourable assumptions about uncertain epidemiological parameters, and that long-term benefit–cost ratios were highly influenced by discounting and assumptions about economic growth.
Acknowledgments
Funding was provided by the WHF, the Leona M and Harry B Helmsley Charitable Trust, and the American Heart Association. GFK was supported in part by the National Institutes of Health (grant number 1K23HL140133).
Footnotes
Declaration of interests
DAW reports grants from the American Heart Association during the time of the study. GFK reports grants from the National Heart, Lung, and Blood Institute during the time of the study.
Data sharing
No primary data were collected for this study. Detailed input data used in modelling are described or displayed in the appendix, derived from published literature and secondary analysis of previously published estimates (such as those from the Global Burden of Disease Study, the World Bank, the International Monetary Fund, or the UN Population Division which are publicly available). Additional information about accessing underlying processed data from these sources used for inputs in the model is located in the appendix (p 2).
Contributor Information
Matthew M Coates, Division of Global Health Equity, Brigham and Women’s Hospital, Boston, MA, USA; Program in Global Noncommunicable Disease and Social Change, Department of Global Health and Social Medicine, Harvard Medical School, Boston, MA, USA.
Karen Sliwa, Cape Heart Institute and Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa; World Heart Federation, Geneva, Switzerland.
David A Watkins, Department of Medicine, University of Washington, Seattle, WA, USA; Department of Global Health, University of Washington, Seattle, WA, USA.
Liesl Zühlke, Division of Paediatric Cardiology, Department of Paediatrics, Red Cross Children’s Hospital, University of Cape Town, Cape Town, South Africa; Division of Cardiology, Department of Medicine, Groote Schuur Hospital, University of Cape Town, Cape Town, South Africa.
Pablo Perel, World Heart Federation, Geneva, Switzerland; Centre for Global Chronic Conditions, London School of Hygiene & Tropical Medicine, London, UK.
Florence Berteletti, World Heart Federation, Geneva, Switzerland.
Jean-Luc Eiselé, World Heart Federation, Geneva, Switzerland.
Sheila L Klassen, Program in Global Noncommunicable Disease and Social Change, Department of Global Health and Social Medicine, Harvard Medical School, Boston, MA, USA; Partners In Health, Boston, MA, USA.
Gene F Kwan, Program in Global Noncommunicable Disease and Social Change, Department of Global Health and Social Medicine, Harvard Medical School, Boston, MA, USA; Partners In Health, Boston, MA, USA; Section of Cardiovascular Medicine, Boston University School of Medicine, Boston, MA, USA.
Ana O Mocumbi, Instituto Nacional de Saúde, Maputo, Mozambique; Universidade Eduardo Mondlane, Maputo, Mozambique.
Dorairaj Prabhakaran, Department of Non-Communicable Disease Epidemiology, London School of Hygiene & Tropical Medicine, London, UK; Centre for Chronic Disease Control, New Delhi, India; Public Health Foundation of India, Gurgaon, India.
Mahlet Kifle Habtemariam, Africa Centres for Disease Control and Prevention, Addis Ababa, Ethiopia.
Gene Bukhman, Division of Global Health Equity, Brigham and Women’s Hospital, Boston, MA, USA; Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA, USA; Program in Global Noncommunicable Disease and Social Change, Department of Global Health and Social Medicine, Harvard Medical School, Boston, MA, USA; Partners In Health, Boston, MA, USA.
References
- 1.Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med 2017; 377: 713–22. [DOI] [PubMed] [Google Scholar]
- 3.Karthikeyan G, Guilherme L. Acute rheumatic fever. Lancet 2018; 392: 161–74. [DOI] [PubMed] [Google Scholar]
- 4.Carapetis JR, Beaton A, Cunningham MW, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers 2016; 2: 15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steer AC, Carapetis JR, Nolan TM, Shann F. Systematic review of rheumatic heart disease prevalence in children in developing countries: the role of environmental factors. J Paediatr Child Health 2002; 38: 229–34. [DOI] [PubMed] [Google Scholar]
- 6.Jaine R, Baker M, Venugopal K. Acute rheumatic fever associated with household crowding in a developed country. Pediatr Infect Dis J 2011; 30: 315–19. [DOI] [PubMed] [Google Scholar]
- 7.Michaud C, Rammohan R, Narula J. Cost-effectiveness analysis of intervention strategies for reduction of the burden of rheumatic heart disease. In: Narula J, Virmani R, Reddy K, Tandon R, eds. Rheumatic fever. Washington, USA: American Registry of Pathology, 1999: 485–97. [Google Scholar]
- 8.Irlam J, Mayosi BM, Engel M, Gaziano TA. Primary prevention of acute rheumatic fever and rheumatic heart disease with penicillin in South African children with pharyngitis: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes 2013; 6: 343–51. [DOI] [PubMed] [Google Scholar]
- 9.Watkins D, Lubinga SJ, Mayosi B, Babigumira JB. A cost-effectiveness tool to guide the prioritization of interventions for rheumatic fever and rheumatic heart disease control in African nations. PLoS Negl Trop Dis 2016; 10: e0004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arguedas A, Mohs E. Prevention of rheumatic fever in Costa Rica. J Pediatr 1992; 121: 569–72. [DOI] [PubMed] [Google Scholar]
- 11.Nordet P, Lopez R, Dueñas A, Sarmiento L, Dueñas A. Prevention and control of rheumatic fever and rheumatic heart disease: the Cuban experience (1986–1996–2002). Cardiovasc J Afr 2008; 19: 135–40. [PMC free article] [PubMed] [Google Scholar]
- 12.Yusuf S, Narula J, Gamra H. Can we eliminate rheumatic fever and premature deaths from RHD? Glob Heart 2017; 12: 3–4. [DOI] [PubMed] [Google Scholar]
- 13.Lennon D, Kerdemelidis M, Arroll B. Meta-analysis of trials of streptococcal throat treatment programs to prevent rheumatic fever. Pediatr Infect Dis J 2009; 28: e259–64. [DOI] [PubMed] [Google Scholar]
- 14.RHD Australia (ARF/RHD writing group). The 2020 Australian guildeline for prevention, diagnosis and management of acute rheumatic fever and rheumatic heart disease (3rd edn). Menzies School of Health Research, 2020. https://www.rhdaustralia.org.au/arf-rhd-guideline (accessed Sept 23, 2020). [Google Scholar]
- 15.Bukhman G, Kidder A, eds. The PIH guide to chronic care integration for endemic non-communicable diseases Rwanda edition cardiac, renal, diabetes, pulmonary, and palliative care. Boston, MA: Partners in health, 2011. [Google Scholar]
- 16.Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: two-year follow-up of the global rheumatic heart disease registry (the REMEDY Study). Circulation 2016; 134: 1456–66. [DOI] [PubMed] [Google Scholar]
- 17.Kingué S, Ba SA, Balde D, et al. The VALVAFRIC study: a registry of rheumatic heart disease in Western and Central Africa. Arch Cardiovasc Dis 2016; 109: 321–29. [DOI] [PubMed] [Google Scholar]
- 18.Mayosi B, Robertson K, Volmink J, et al. The Drakensberg declaration on the control of rheumatic fever and rheumatic heart disease in Africa. S Afr Med J 2006; 96: 246. [PubMed] [Google Scholar]
- 19.Mayosi BM, Gamra H, Dangou J-M, Kasonde J. Rheumatic heart disease in Africa: the Mosi-o-Tunya call to action. Lancet Glob Health 2014; 2: e438–39. [DOI] [PubMed] [Google Scholar]
- 20.Watkins D, Zuhlke L, Engel M, et al. Seven key actions to eradicate rheumatic heart disease in Africa: the Addis Ababa communiqué. Cardiovasc J Afr 2016; 27: 184–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO. A71/25 Rheumatic fever and rheumatic heart disease. 2018. https://apps.who.int/gb/ebwha/pdf_files/WHA71/A71_25-en.pdf (accessed May 15, 2020).
- 22.Ali SKM, Engel ME, Zühlke L, Jack SJ. Chapter 10. Primary prevention of acute rheumatic fever and rheumatic heart disease. In: Dougherty S, Carapetis J, Zühlke L, Wilson N, eds. acute rheumatic fever and rheumatic heart disease. San Diego, CA: Elsevier, 2020: 195–206. [Google Scholar]
- 23.Eberly LA, Rusingiza E, Park PH, et al. 10-Year heart failure outcomes from nurse-driven clinics in rural Sub-Saharan Africa. J Am Coll Cardiol 2019; 73: 977–80. [DOI] [PubMed] [Google Scholar]
- 24.Rusingiza EK, El-Khatib Z, Hedt-Gauthier B, et al. Outcomes for patients with rheumatic heart disease after cardiac surgery followed at rural district hospitals in Rwanda. Heart 2018; 104: 1707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Regional Office for Africa. PEN-Plus Meeting in Kigali—the management and treatment of non-communicable diseases at primary levels strengthened. https://www.afro.who.int/news/pen-plus-meeting-kigali-management-and-treatment-non-communicable-diseases-primary-levels (accessed Sept 16, 2019).
- 26.Sliwa K, Azibani F, Baard J, et al. Reducing late maternal death due to cardiovascular disease. A pragmatic pilot study. Int J Cardiol 2018; 272: 70–76. [DOI] [PubMed] [Google Scholar]
- 27.WHO, UN Development Programme. Non-communicable disease prevention and control: A guidance note for investment cases. Geneva: World Health Organization, 2019. https://www.who.int/ncds/un-task-force/publications/WHO-NMH-NMA-19·95/en/ (accessed Nov 2, 2020). [Google Scholar]
- 28.Bukhman G, Mocumbi AO, Atun R, et al. The Lancet NCDI Poverty Commission: bridging a gap in universal health coverage for the poorest billion. Lancet 2020; 396: 991–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stenberg K, Axelson H, Sheehan P, et al. Advancing social and economic development by investing in women’s and children’s health: a new global investment framework. Lancet 2014; 383: 1333–54. [DOI] [PubMed] [Google Scholar]
- 30.UN Department of Economic and Social Affairs, Population Division. World population prospects: the 2019 revision. United Nations, 2019. https://population.un.org/wpp/ (accessed April 10, 2020). [Google Scholar]
- 31.Murray CJL, Callender CSKH, Kulikoff XR, et al. Population and fertility by age and sex for 195 countries and territories, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1995–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mocumbi A, Beaton A, Soma-Pillay P, Dougherty S, Sliwa K. Chapter 9. Rheumatic heart disease in pregnancy. In: Dougherty S, Carapetis J, Zühlke L, Wilson N, eds. Acute rheumatic fever and rheumatic heart disease. San Diego, CA: Elsevier, 2021: 171–93. [Google Scholar]
- 33.Stenberg K, Lauer JA, Gkountouras G, Fitzpatrick C, Stanciole A. Econometric estimation of WHO-CHOICE country-specific costs for inpatient and outpatient health service delivery. Cost Eff Resour Alloc 2018; 16: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Management Sciences for Health, WHO. International medical products price guide. Medford, Masachusetts: Management Sciences for Health, 2015. https://www.msh.org/sites/default/files/msh-2015-international-medical-products-price-guide.pdf (accessed May 14, 2020). [Google Scholar]
- 35.Eberly LA, Rusangwa C, Ng’ang’a L, et al. Cost of integrated chronic care for severe non-communicable diseases at district hospitals in rural Rwanda. BMJ Glob Health 2019; 4: e001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner HC, Lauer JA, Tran BX, Teerawattananon Y, Jit M. Adjusting for inflation and currency changes within health economic studies. Value Health 2019; 22: 1026–32. [DOI] [PubMed] [Google Scholar]
- 37.Robinson LA, Hammit JK, Cecchini M, et al. Reference case guildelines for benefit-cost analysis in global health and development. 2019. https://cdn1.sph.harvard.edu/wp-content/uploads/sites/2447/2019/05/BCA-Guidelines-May-2019.pdf (accessed Sept 23, 2020).
- 38.Jamison DT, Summers LH, Alleyne G, et al. Global health 2035: a world converging within a generation. Lancet 2013; 382: 1898–955. [DOI] [PubMed] [Google Scholar]
- 39.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016; 316: 1093–103. [DOI] [PubMed] [Google Scholar]
- 40.Manji RA, Witt J, Tappia PS, Jung Y, Menkis AH, Ramjiawan B. Cost-effectiveness analysis of rheumatic heart disease prevention strategies. Expert Rev Pharmacoecon Outcomes Res 2013; 13: 715–24. [DOI] [PubMed] [Google Scholar]
- 41.Steinhoff MC, Walker CF, Rimoin AW, Hamza HS. A clinical decision rule for management of streptococcal pharyngitis in low-resource settings. Acta Paediatr 2005; 94: 1038–42. [DOI] [PubMed] [Google Scholar]
- 42.RHD Action. Global status of BPG report: the benzathine penicillin G report. Perth, WA: RHD Action, 2017. https://rhdaction.org/sites/default/files/RHD%20Action_Global%20Status%20of%20BPG%20Report_Online%20Version.pdf (accessed Sept 24, 2020). [Google Scholar]
- 43.WHO. WHO PEN and integrated outpatient care for severe, chronic NCDs at first referral hospitals in the African region (PEN-Plus). Report on regional consultation. Kigali, Rwanda, 29 July–1 August 2019. 2020. https://www.afro.who.int/publications/who-pen-and-integrated-outpatient-care-severe-chronic-ncds-first-referral-hospitals (accessed April 29, 2021). [Google Scholar]
- 44.Zühlke L, Acquah L. Pre-conception counselling for key cardiovascular conditions in Africa: optimising pregnancy outcomes. Cardiovasc J Afr 2016; 27: 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zühlke L, Mirabel M, Marijon E. Congenital heart disease and rheumatic heart disease in Africa: recent advances and current priorities. Heart 2013; 99: 1554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karthikeyan G. Measuring and reporting disease progression in subclinical rheumatic heart disease. Heart Asia 2016; 8: 74–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sumner A, Ortiz-Juarez E, Hoy C. Precarity and the pandemic: COVID-19 and poverty incidence, intensity, and severity in developing countries. Helsinki: UNU-WIDER, 2020. https://www.wider.unu.edu/publication/precarity-and-pandemic (accessed Dec 31, 2019). [Google Scholar]
- 48.Watkins DA, Beaton AZ, Carapetis JR, et al. Rheumatic heart disease worldwide: JACC scientific expert panel. J Am Coll Cardiol 2018; 72: 1397–416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.