Abstract
Background
Young survivors of cancer are at increased risk for cancers that are related to human papillomavirus (HPV), primarily caused by oncogenic HPV types 16 and 18. We aimed to examine the immunogenicity and safety of the three-dose series of HPV vaccine in young survivors of cancer.
Methods
We conducted an investigator-initiated, phase 2, single-arm, open-label, non-inferiority trial at five National Cancer Institute-designated comprehensive cancer centres in the USA. Eligible participants were survivors of cancer who were HPV vaccine-naive, were aged 9–26 years, in remission, and had completed cancer therapy between 1 and 5 years previously. Participants received three intramuscular doses of either quadrivalent HPV vaccine (HPV4; enrolments on or before March 1, 2016) or nonavalent HPV vaccine (HPV9; enrolments after March 1, 2016) over 6 months (on day 1, at month 2, and at month 6). We also obtained data from published clinical trials assessing safety and immunogenicity of HPV4 and HPV9 in 9–26-year-olds from the general population, as a comparator group. The primary endpoint was antibody response against HPV types 16 and 18 at month 7 in the per-protocol population. A response was deemed non-inferior if the lower bound of the multiplicity-adjusted 95% CI was greater than 0·5 for the ratio of anti-HPV-16 and anti-HPV-18 geometric mean titres (GMTs) in survivors of cancer versus the general population. Responses were examined separately in male and female participants by age group (ie, 9–15 years and 16–26 years). Safety was assessed in all participants who received at least one vaccine dose and for whom safety data were available. This study is registered with ClinicalTrials.gov, NCT01492582. This trial is now completed.
Findings
Between Feb 18, 2013, and June 22, 2018, we enrolled 453 survivors of cancer, of whom 436 received one or more vaccine doses: 203 (47%) participants had survived leukaemia, 185 (42%) were female, and 280 (64%) were non-Hispanic white. Mean age at first dose was 15·6 years (SD 4·6). 378 (83%) of 453 participants had evaluable immunogenicity data; main reasons for exclusion from per-protocol analysis were to loss to follow-up, patient reasons, and medical reasons. Data were also obtained from 26 486 general population controls. The ratio of mean GMT for anti-HPV types 16 and 18 in survivors of cancer versus the general population was more than 1 for all subgroups (ie, aged 9–15 years, aged 16–26 years, male, and female groups) in both vaccine cohorts (ranging from 1·64 [95% CI 1·12–2·18] for anti-HPV type 16 in female participants aged 9–15 years who received HPV9, to 4·77 [2·48–7·18] for anti-HPV type 18 in male participants aged 16–26 years who received HPV4). Non-inferiority criteria were met within each age and sex subgroup, except against HPV type 18 in female participants aged 16–26 years receiving HPV9 (4·30 [0·00–9·05]). Adverse events were reported by 237 (54%) of 435 participants; injection site pain was most common (174 [40%] participants). One serious adverse event (ie, erythema nodosum) was possibly related to vaccine (HPV9; 16–26 year female cohort).
Interpretation
Immunogenicity and safety of HPV vaccine three-dose series in survivors of cancer is similar to that in the general population, providing evidence for use in this clinically vulnerable population.
Funding
US National Cancer Institute, Merck, Sharp & Dohme, and American Lebanese Syrian Associated Charities.
Introduction
Persistent infection with oncogenic human papillomavirus (HPV; eg, types 16, 18, 31, 33, 45, 52, and 58) is strongly associated with development of HPV-related cervical, anogenital, and oropharyngeal neoplasms in the general population,1 with types 16 and 18 being responsible for most HPV-related cancers.2 Cancer treatment can result in extended immunosuppression,3 increasing the risk for HPV acquisition and persistence, which in conjunction with genotoxic therapy sets the stage for an elevated risk of invasive HPV-related subsequent neoplasms.4 Young survivors of cancer are at substantially higher risk for developing subsequent neoplasms that are related to HPV than are the general population.5 Fortunately, these neoplasms are now largely preventable through HPV vaccination, which offers protection against approximately 90% of HPV-related cervical, anal, vulvar, and vaginal cancers.6, 7 Prophylactic vaccination with the three-dose series of HPV vaccine is safe, highly immunogenic, and effective in preventing HPV infection and morbidity in healthy populations.6, 7, 8 However, the immunogenicity and safety of the three-dose series of HPV vaccine is unknown in young survivors of cancer.
Research in context.
Evidence before this study
Before starting this study, we searched PubMed for articles published between Jan 1, 2006 (ie, the year that the quadrivalent human papillomavirus [HPV] vaccine was first licensed in the USA), and July 19, 2012 (ie, initiation date of this study), with the terms “HPV vaccine” AND “clinical trial.” Included studies were those done in humans aged 9–26 years. No clinical trials were identified that aimed to understand the immunogenicity and safety of the HPV vaccine in young male and female survivors of cancer aged 9–26 years receiving conventional therapy or haematopoietic stem cell transplantation. Repeating this search through to May 3, 2021, also failed to yield any trials that were aimed at our targeted population; however, we identified one clinical trial that assessed immune response to HPV vaccination in 44 female survivors of cancer (aged 18–50 years) who received allogeneic haematopoietic stem cell transplantation.
Added value of this study
To our knowledge, we report results of the first phase 2 clinical trial to evaluate the safety and immunogenicity of the HPV vaccine in survivors of cancer aged 9–26 years who completed cancer treatment 1–5 years previously. In this non-inferiority trial, involving 436 young survivors of cancer, we noted that antibody responses against HPV types 16 and 18 were non-inferior to general population comparisons, except against HPV type 18 in older females (ie, aged 16–26 years) receiving nonavalent vaccine. The safety profile in survivors of cancer was similar to that in the general population.
Implications of all the available evidence
Our findings provide evidence for using the three-dose HPV vaccine series in young survivors of cancer, a population at increased risk for neoplasms related to HPV. Given that uptake of HPV vaccine among young survivors of cancer is notably lower than in the general population, these findings should remove hesitancy on the part of health-care providers in incorporating HPV vaccination into routine oncological follow-up care, with the ultimate goal of preventing subsequent neoplasms related to HPV in young survivors of cancer. These data support the further study of health-care provider-focused interventions to increase HPV vaccine uptake among young cancer survivors; one such intervention is currently being assessed in a cluster randomised trial of paediatric oncology practices (NCT04469569).
Therapy for childhood cancer can affect both innate and adaptive immunity, yet immune recovery following completion of treatment is understudied. Delays in immune reconstitution can go unrecognised, and the few existing small studies suggest that seroprotection against vaccine-preventable illnesses can be affected for months or years following cancer treatment.3 Our first aim of this study was to examine the determinants of HPV vaccine non-initiation using a cross-sectional survey, and we found that rates of HPV vaccine uptake among young survivors of cancer are significantly lower than in the general population.9 HPV vaccine non-initiation among young survivors of cancer is associated with absence of health-care provider recommendation for the vaccine, which might stem from a paucity of evidence regarding vaccine immunogenicity and safety in survivors.9
Our second aim is to address this knowledge gap by examining immunogenicity and safety of the three-dose series of HPV vaccine in young survivors of cancer with a non-inferiority trial design; non-inferiority trials establish immunogenicity when efficacy trials are not feasible.6, 10 We hypothesised that HPV vaccine would be safe among the survivors but that immunogenicity would be heterogeneous, with subpopulations who received the most intense cancer therapy showing lower immunogenicity than other cancer survivors.
Methods
Study design and participants
This investigator-initiated, single-arm, open-label, phase 2, non-inferiority trial was conducted at five National Cancer Institute-designated comprehensive cancer centres in the USA. We enrolled survivors of cancer with no previous history of HPV vaccination by self-report or report by parent or caregiver, who were aged 9–26 years, in remission, and had completed treatment for cancer 1–5 years previously. Participants were included if they spoke English or Spanish and had medical clearance from their treating clinician for study participation. Participants had to agree to return to a participating institution for three vaccine injections and had to provide informed consent or assent for participation. Participants were excluded if they had an allergy to any component of the vaccine, including yeast and aluminium; thrombocytopenia or coagulation disorder that would contraindicate intramuscular injection; transfusion of blood products or intravenous immunoglobulin within 3 months of study entry; were pregnant at time of enrolment; or were of childbearing potential and unwilling to avoid pregnancy during the vaccine phase of study.
We stratified enrolment by age (9–15 years; 16–26 years) and biological sex (female; male). The study was initially designed to evaluate quadrivalent HPV vaccine (ie, HPV4). However, nonavalent HPV vaccine (ie, HPV9) was licensed in the USA11 during HPV4 study enrolment, resulting in a protocol amendment adding evaluation of HPV9 on March 2, 2016. At study completion, we had enrolled two cohorts: HPV4 (enrolments on or before March 1, 2016) and HPV9 (enrolments after March 1, 2016).
Pooled data for general population comparisons were obtained from published licensing trials and package inserts that assessed the safety and immunogenicity of HPV412, 13, 14 and HPV97,15–17 vaccines in 9–26-year-olds (organised by vaccine type, sex, and age group and reflecting the antibody response to each HPV type included in each vaccine); these vaccine licensure trials were done between 2000 and 2015 in Asia Pacific, Europe, Latin America, Africa, North America, and the Middle East (appendix pp 1–9). The trials selected were those contributing to the safety and immunogenicity data included in the package insert for each vaccine.
The trial was conducted according to Good Clinical Practice principles. Institutional review boards at each site approved the protocol. All participants or parents provided written informed consent or assent according to site-specific requirements by the institutional review boards. A data safety monitoring committee monitored study safety findings. Consolidated Standards of Reporting Trials reporting guidelines for non-inferiority and equivalence trials were followed.18
Procedures
Three 0·5 mL doses of HPV4 (for participants enrolled on or before March 1, 2016) or HPV9 (for participants enrolled after March 1, 2016) were administered intramuscularly on day 1, at month 2 (≥8 weeks and ≤12 weeks from day 1), and at month 6 (≥24 weeks and ≤32 weeks from day 1 and ≥16 weeks from dose 2). HPV4 contains 20 μg of type 6, 40 μg of type 11, 40 μg of type 16, and 20 μg of type 18 HPV major capsid (L1) protein and 225 μg of amorphous aluminium hydroxyphosphate sulfate adjuvant per 0·5 mL.12 HPV9 contains 30 μg of type 6, 40 μg of type 11, 60 μg of type 16, and 40 μg of type 18 HPV-18 L1 protein; 20 μg of L1 protein of HPV types 31, 33, 45, 52, and 58; and 500 μg of amorphous aluminium hydroxyphosphate sulfate adjuvant per 0·5 mL.17 Participants were screened for acute illness and other medical contraindications before each dose. Female participants of childbearing potential agreed to take measures to avoid pregnancy during the initial 7 months of the study. The general population control group were administered the vaccine in the same way.
Antibody response against each HPV vaccine type (ie, anti-HPV types 6, 11, 16, and 18 for HPV4 and anti-HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58 for HPV9) was serologically assessed just before the first vaccine dose and at month 7 (ie, ≥4 and ≤8 weeks from dose 3) by use of a competitive Luminex immunoassay (Merck, Whitehouse Station, NJ, USA),19, 20 with results expressed as geometric mean titres (GMTs) in milliMerck units per mL (mMu/mL). The anti-HPV types 16 and 18 titres for the HPV4 cohort were converted to international units (IU/mL) according to Brown and colleagues’ method.21 There are no mMU/mL to IU/mL conversion factors available for the quadrivalent competitive Luminex immunoassays that were performed for anti-HPV types 6 and 11 in the HPV4 cohort or for any of the nonavalent competitive Luminex immunoassays that were performed in the HPV9 cohort. The antibody concentrations that were reported for the general population comparisons were also assayed by use of competitive Luminex immunoassay12, 17 and available mMU/mL to IU/mL conversion factors were applied.
Vaccine report cards were used to capture oral temperature and injection site status daily for 5 days after each dose and adverse symptoms until day 8 (for the HPV9 cohort) or day 15 (for the HPV4 cohort). Serious adverse events—defined as death, life-threatening conditions, unplanned admission to hospital for longer than 24 h, persistent or substantial disability, second cancer, or other medical event that was deemed by the investigator to jeopardise participant health—were reported in real time until month 24. Additionally, active queries regarding serious adverse events were conducted at study visits at month 7 and month 24. Site investigators were instructed to assign causality of adverse events (ie, definitely, probably, possibly, unlikely, or unrelated) to the vaccine.
Outcomes
The primary outcome was non-inferiority of antibody response in young survivors of cancer against HPV types 16 and 18 at study month 7 compared with the general population. Additional outcomes were clinical or host factors influencing immunogenicity and safety of HPV4 and HPV9 in young survivors of cancer. Exploratory outcomes included antibody response against the additional vaccine-associated HPV types for which antigens were included in the vaccine at study month 7 and seropositivity rates at month 7 for each vaccine-associated HPV type. Month 24 data (exploratory aim) will be reported separately.
Statistical analysis
This study was originally designed to evaluate immunogenicity and safety of HPV4, with targeted enrolment of 353 participants, estimated to yield 312 evaluable patients (ie, per-protocol sample). A sample of 312 patients was calculated to provide 80% power at an overall one-sided type I error of 0·025 (or 0·003125 for each of eight tests planned for anti-HPV types 16 and 18) to show non-inferiority with respect to anti-HPV type 16 or 18 response in survivors of cancer versus the general population; this sample size calculation was based on the mean GMT and SD that were reported in previous trials of male and female participants aged 9–15 years and 16–26 years from the general population,12 assuming a non-inferiority margin of 0·5 of the GMT of the comparisons.22 However, with impending withdrawal of HPV4 from the US marketplace, the study was amended to examine the same endpoints with the newly licensed HPV9. Due to budgetary limitations, planned enrolment in the HPV9 cohort was restricted to 200 additional patients, estimated to yield 176 evaluable patients. The HPV4 cohort was closed to enrolment on March 1, 2016; at that time, 265 (75%) of the planned 353 patients had enrolled. The HPV9 cohort opened for enrolment on March 2, 2016, and (due to operating constraints) closed on June 22, 2018, with 182 (91%) of the planned 200 patients enrolled.
Separate analyses were conducted for the HPV4 and HPV9 cohorts, with each cohort divided into four subgroups on the basis of age (ie, 9–15 years or 16–26 years) and biological sex (ie, female or male). Immunogenicity and seropositivity analyses were conducted in the per-protocol population (ie, survivors of cancer who were seronegative to the HPV type of interest on day 1, had received all three vaccine doses, and had blood drawn at month 7 within protocol-specified timeframes). Safety analyses were conducted in the intention-to-treat population (ie, survivors of cancer who received at least one vaccine dose and for whom there were safety data). All general population comparison studies also used the per-protocol population for immunogenicity and seropositivity analyses and the intention-to-treat population for safety analyses.12, 17
For the primary immunogenicity analysis, two-sided multiplicity-adjusted 95% CIs accounting for eight tests were computed by use of Fieller's method23 for the ratio of the mean anti-HPV type 16 and type 18 GMTs in survivors of cancer versus the general population7, 12, 15 by vaccine cohort (ie, HPV4 or HPV9) for each age–sex subgroup, for an overall type I error of 0·025 per HPV type. We used a non-inferiority margin of 0·5 for the ratio of the antibody GMTs in survivors of cancer versus general population comparisons, chosen to be consistent with the general population comparator trials at the time that our trial was initiated.10 We considered a response to be non-inferior if the lower bound of the two-sided multiplicity adjusted 95% CI, corresponding to 0·3125% lower bound (ie, divide 5% type I error by eight tests done for anti-HPV types 16 and 18 [5%/8=0·625%], then taking the lower bound [0·3125%] of the two-sided CI), exceeded 0·5.
Low immunogenicity was predefined as month 7 GMT for HPV type 16 or type 18 that was less than half of the mean GMT of the corresponding general population comparison group. We used logistic regression and a type 1 error of 0·05 to identify clinical or host factors influencing low immunogenicity. Patients who were baseline seropositive to HPV type 16 or 18 were excluded from the low immunogenicity analysis.
We compared vaccine-associated adverse events, including fever, headache, nausea, dizziness, fatigue, and injection site adverse events, by vaccine cohort in survivors of cancer versus general population comparisons.12, 13, 14, 16, 17 We used χ2 and Fisher's exact tests and calculated the 95% CI for the difference in the proportion of adverse events reported by the survivor and general population comparison groups.
We assessed antibody response against HPV types 6 and 11 in both vaccine cohorts, and HPV types 31, 33, 45, 52, and 58 in the HPV9 cohort. Two-sided non-multiplicity adjusted 95% CIs were calculated for these analyses.
Seropositivity was defined as antibody concentrations equal to or greater than a prespecified serostatus cutoff for a given HPV type (appendix p 54).10, 17 Tests of non-inferiority (ie, for HPV types 16 and 18) were conducted for each of four age–sex groups by constructing two-sided multiplicity adjusted 95% CIs accounting for eight tests (ie, for an overall type I error of 0·05 for both HPV types) for the difference in the prevalence of seropositivity between survivors of cancer and general population comparisons.7, 12, 15, 24 Seroconversion in survivors of cancer was considered non-inferior if the lower bound of the two-sided multiplicity-adjusted 95% CI for the difference was greater than –5 percentage points.
A data monitoring committee was used. Analysis for this study was done with SAS 9.4.
This trial is registered with ClinicalTrials.gov, NCT01492582.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
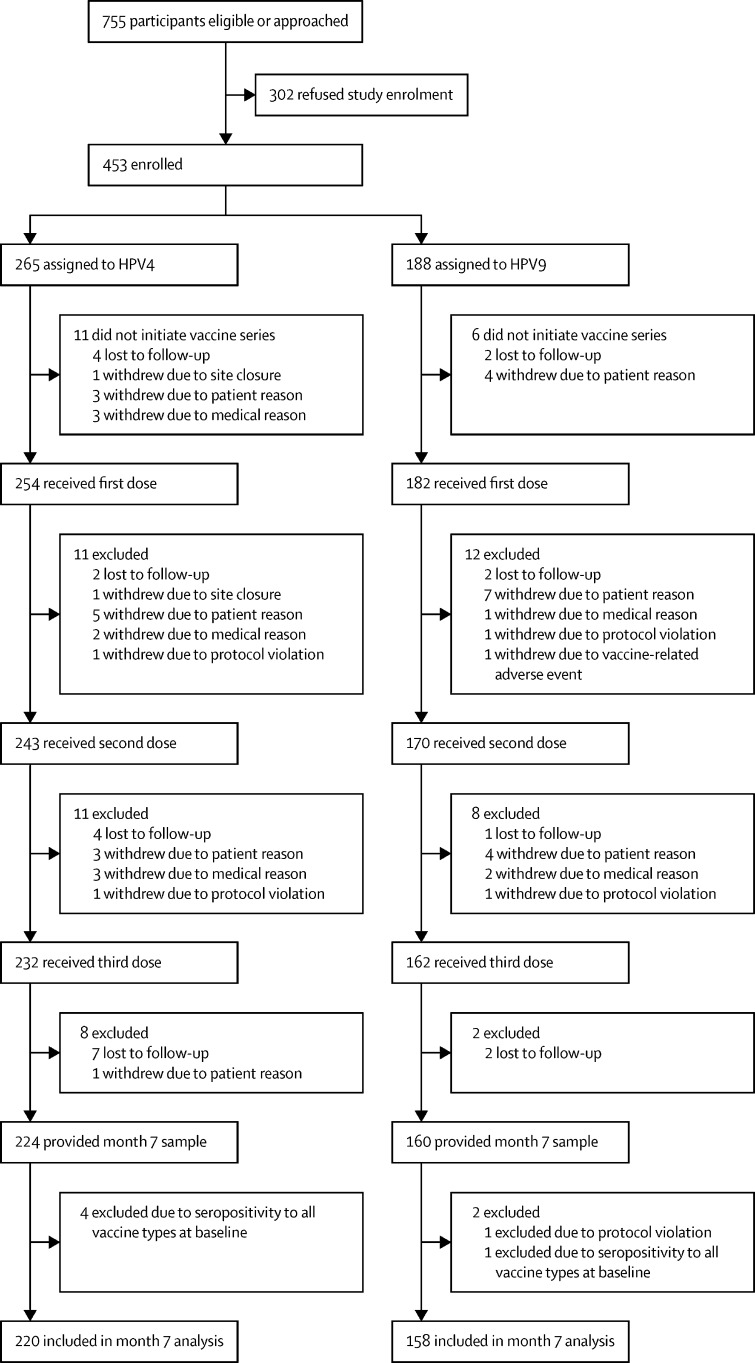
The trial began on July 19, 2012, with first patients enrolled on Feb 18, 2013, and last patients enrolled on June 22, 2018. Of 755 eligible patients who were approached, 453 (60%) consented to participate. 436 (96%) of participants who consented received at least one vaccine dose and 378 (87%) of those receiving at least one dose were evaluable in the per-protocol analysis at month 7 (ie, 220 in the HPV4 cohort and 158 in the HPV9 cohort; figure ). Reasons for exclusion of patients from the month 7 per-protocol analysis are presented in the appendix (p 10). Participants receiving at least one dose had a mean age of 15·6 years (SD 4·6) and were off therapy for 2·8 years (1·2) at dose 1; 185 (42%) of the 436 participants who received at least one dose were female, 280 (64%) were non-Hispanic white, 203 (47%) had a diagnosis of leukaemia, and 62 (14%) had undergone haematopoietic stem cell transplantation (HSCT; table 1 ). Compared with participants who enrolled, eligible patients who refused to enrol (n=302) were more likely to be of non-Hispanic white race or ethnicity and have a diagnosis of solid tumour (appendix p 11).
Figure.
Trial profile
HPV4=quadrivalent HPV vaccine. HPV9=nonavalent HPV vaccine.
Table 1.
Sociodemographic and clinical characteristics of study participants
|
Participants who received ≥1 vaccine dose |
Participants with evaluable immunogenicity data |
||||||
|---|---|---|---|---|---|---|---|
| Total | HPV4 | HPV9 | Total | HPV4 | HPV9 | ||
| Cohort of survivors of cancer | 436 | 254 | 182 | 378 | 220 | 158 | |
| Age at dose 1, years | |||||||
| Mean (SD) | 15·6 (4·6) | 16·0 (4·7) | 15·0 (4·4) | 15·4 (4·7) | 15·8 (4·8) | 14·8 (4·5) | |
| Median (IQR) | 15·0 (11·4–19·0) | 15·3 (11·8–19·8) | 14·2 (10·9–18·5) | 14·4 (11·2–18·9) | 14·9 (11·5–19·6) | 13·8 (10·7–18·3) | |
| Age at cancer diagnosis, years | |||||||
| Mean (SD) | 10·9 (5·2) | 11·2 (5·3) | 10·5 (5·0) | 10·8 (5·3) | 11·1 (5·4) | 10·3 (5·0) | |
| Median (IQR) | 10·9 (6·6–15·0) | 11·1 (6·7–15·3) | 10·7 (6·0–14·9) | 10·5 (6·2–14·9) | 10·7 (6·5–15·2) | 9·7 (5·9–14·8) | |
| Time from cancer diagnosis to dose 1, years | |||||||
| Mean (SD) | 4·6 (2·1) | 4·7 (2·2) | 4·5 (1·9) | 4·6 (2·1) | 4·7 (2·2) | 4·5 (1·8) | |
| Median (IQR) | 4·5 (3·0–5·9) | 4·5 (3·0–6·0) | 4·5 (2·8–5·8) | 4·5 (3·0–5·9) | 4·5 (3·0–6·1) | 4·5 (3·0–5·9) | |
| Time off cancer therapy at dose 1, years | |||||||
| Mean (SD) | 2·8 (1·2) | 2·9 (1·2) | 2·6 (1·3) | 2·8 (1·2) | 2·9 (1·1) | 2·6 (1·3) | |
| Median (IQR) | 2·6 (1·7–4·0) | 2·9 (1·8–4·0) | 2·4 (1·5–4·0) | 2·6 (1·7–4·0) | 2·7 (1·9–4·0) | 2·5 (1·5–3·8) | |
| Age group, n (%) | |||||||
| 9–15 years | 241 (55%) | 135 (53%) | 106 (58%) | 216 (57%) | 121 (55%) | 95 (60%) | |
| 16–26 years | 195 (45%) | 119 (47%) | 76 (42%) | 162 (43%) | 99 (45%) | 63 (40%) | |
| Sex, n (%) | |||||||
| Male | 251 (58%) | 152 (60%) | 99 (54%) | 221 (58%) | 134 (61%) | 87 (55%) | |
| Female | 185 (42%) | 102 (40%) | 83 (46%) | 157 (42%) | 86 (39%) | 71 (45%) | |
| Race or ethnicity, n (%) | |||||||
| Non-Hispanic white | 280 (64%) | 162 (64%) | 118 (65%) | 250 (66%) | 147 (67%) | 103 (65%) | |
| Hispanic | 50 (11%) | 30 (12%) | 20 (11%) | 44 (12%) | 25 (11%) | 19 (12%) | |
| Black | 91 (21%) | 50 (20%) | 41 (23%) | 70 (19%) | 37 (17%) | 33 (21%) | |
| Other | 15 (3%) | 12 (5%) | 3 (2%) | 14 (4%) | 11 (5%) | 3 (2%) | |
| Weight at dose 1, kg | |||||||
| Mean (SD) | 62·1 (24·6) | 63·4 (24·3) | 60·4 (24·9) | 61·2 (24·5) | 62·4 (24·1) | 59·6 (25·0) | |
| Median (IQR) | 57·7 (43·7–76·9) | 60·4 (45·8–77·2) | 54·5 (41·3–76·8) | 56·4 (42·0–76·4) | 58·7 (45·4–75·3) | 53·4 (40·4–77·0) | |
| Body-mass index at dose 1, kg/m2 | |||||||
| Mean (SD) | 23·8 (6·4) | 23·9 (6·2) | 23·5 (6·7) | 23·6 (6·4) | 23·7 (6·1) | 23·4 (6·7) | |
| Median (IQR) | 22·6 (19·0–27·0) | 22·9 (19·3–27·1) | 22·3 (18·8–26·8) | 22·5 (19·1–26·7) | 22·7 (19·3–26·7) | 22·1 (18·8–26·7) | |
| Diagnosis, n (%) | |||||||
| Leukaemia | 203 (47%) | 108 (43%) | 95 (52%) | 178 (47%) | 94 (43%) | 84 (53%) | |
| Lymphoma | 92 (21%) | 57 (22%) | 35 (19%) | 82 (22%) | 51 (23%) | 31 (20%) | |
| Solid tumour | 141 (32%) | 89 (35%) | 52 (29%) | 118 (31%) | 75 (34%) | 43 (27%) | |
| Chemotherapy, n (%) | |||||||
| No | 22 (5%) | 17 (7%) | 5 (3%) | 19 (5%) | 15 (7%) | 4 (3%) | |
| Yes | 414 (95%) | 237 (93%) | 177 (97%) | 359 (95%) | 205 (93%) | 154 (97%) | |
| Radiation, n (%) | |||||||
| No | 279 (64%) | 158 (62%) | 121 (66%) | 248 (66%) | 137 (62%) | 111 (70%) | |
| Yes | 157 (36%) | 96 (38%) | 61 (34%) | 130 (34%) | 83 (38%) | 47 (30%) | |
| Haematopoietic stem cell transplant, n (%) | |||||||
| No | 374 (86%) | 213 (84%) | 161 (88%) | 323 (85%) | 182 (83%) | 141 (89%) | |
| Yes | 62 (14%) | 41 (16%) | 21 (12%) | 55 (15%) | 38 (17%) | 17 (11%) | |
Final participant visits for immunogenicity were Feb 8, 2019 (ie, month 7), and for safety were July 20, 2020 (ie, month 24). For all age–sex subgroups in both vaccine cohorts, the GMTs were higher in survivors of cancer than in the general population (ratio of GMT >1; table 2 ). Non-inferiority criteria (ie, lower confidence bound of the multiplicity-adjusted 95% CI for survivor-to-general-population GMT ratio >0·5) was met in all age–sex subgroups except for anti-HPV type 18 among females aged 16–26 years who received HPV9.
Table 2.
GMTs for anti-HPV types 16 and 18 in survivors of cancer versus the general population, by subgroup
|
Survivors of cancer |
General population |
Survivors vs general population |
||||||
|---|---|---|---|---|---|---|---|---|
| n | GMT (95% CI), mMU/mL | GMT (95% CI), IU/mL* | n | GMT (95% CI), mMU/mL | GMT (95% CI), IU/mL* | Ratio of GMT (multiplicity-adjusted 95% CI) | ||
| HPV4 | ||||||||
| Female participants aged 9–15 years | ||||||||
| Anti-HPV type 16 | 51 | 15 209·7 (10 152·4–20 267·1) | 2083·7 (1390·9–2776·6) | 915 | 4918·5 (4556·6–5309·1) | 673·8 (624·3–727·4) | 3·09 (1·76–4·52) | |
| Anti-HPV type 18 | 54 | 2638·3 (1792·5–3484·1) | 496·0 (337·0–655·0) | 922 | 1042·6 (967·6–1123·3) | 196·0 (181·9–211·2) | 2·53 (1·48–3·66) | |
| Male participants aged 9–15 years | ||||||||
| Anti-HPV type 16 | 65 | 16 134·6 (11 944·7–20 324·5) | 2210·4 (1636·4–2784·5) | 882 | 6056·5 (5601·3–6548·7) | 829·7 (767·4–897·2) | 2·66 (1·75–3·66) | |
| Anti-HPV type 18 | 66 | 3472·2 (2407·9–4536·5) | 652·8 (452·7–852·9) | 887 | 1357·4 (1249·4–1474·7) | 255·2 (234·9–277·2) | 2·56 (1·52–3·68) | |
| Female participants aged 16–26 years | ||||||||
| Anti-HPV type 16 | 28 | 6107·3 (3149·1–9065·5) | 836·7 (431·4–1242·0) | 3249 | 2409·2 (2309·0–2513·8) | 330·1 (316·3–344·4) | 2·53 (1·00–4·09) | |
| Anti-HPV type 18 | 30 | 1009·6 (582·9–1436·3) | 189·8 (109·6–270·0) | 3566 | 475·2 (458·8–492·1) | 89·4 (86·3–92·5) | 2·12 (1·00–3·26) | |
| Male participants aged 16–26 years | ||||||||
| Anti-HPV type 16 | 65 | 8740·0 (6000·6–11 479·5) | 1197·4 (822·1–1572·7) | 1136 | 2403·3 (2243·4–2574·6) | 329·3 (307·4–352·7) | 3·64 (2·15–5·21) | |
| Anti-HPV type 18 | 68 | 1920·0 (1210·2–2629·9) | 361·0 (227·5–494·4) | 1175 | 402·6 (374·6–432·7) | 75·7 (70·4–81·3) | 4·77 (2·48–7·18) | |
| HPV9 | ||||||||
| Female participants aged 9–15 years | ||||||||
| Anti-HPV type 16 | 41 | 11 763·6 (8826·8–14 700·4) | NA | 2405 | 7159·9 (6919·7–7408·5) | NA | 1·64 (1·12–2·18) | |
| Anti-HPV type 18 | 41 | 3457·2 (2545·0–4369·4) | NA | 2420 | 2085·5 (2002·2–2172·3) | NA | 1·66 (1·10–2·23) | |
| Male participants aged 9–15 years | ||||||||
| Anti-HPV type 16 | 53 | 16 419·6 (11 743·7–21 095·5) | NA | 1076 | 8444·9 (8054·2–8854·5) | NA | 1·94 (1·23–2·68) | |
| Anti-HPV type 18 | 50 | 5559·8 (4081·9–7037·7) | NA | 1074 | 2620·4 (2474·3–2775·2) | NA | 2·12 (1·39–2·89) | |
| Female participants aged 16–26 years | ||||||||
| Anti-HPV type 16 | 23 | 11 522·9 (6301·3–16 744·5) | NA | 4361 | 3159·0 (3088·6–3231·1) | NA | 3·65 (1·61–5·70) | |
| Anti-HPV type 18 | 28 | 3483·3 (408·6–6557·9) | NA | 4884 | 809·9 (789·2–831·1) | NA | 4·30 (0·00–9·05) | |
| Male participants aged 16–26 years | ||||||||
| Anti-HPV type 16 | 32 | 10 770·4 (6127·8–15 412·9) | NA | 899 | 3346·0 (3158·9–3544·1) | NA | 3·22 (1·46–5·02) | |
| Anti-HPV type 18 | 32 | 3013·4 (1685·1–4341·6) | NA | 906 | 808·2 (754·9–865·4) | NA | 3·73 (1·65–5·89) | |
Anti-HPV type 16 and 18 GMTs with 95% CIs for cancer survivors and general population comparisons who received the HPV4 and HPV9 vaccines, with corresponding ratios and multiplicity-adjusted 95% CI. GMT=geometric mean titres. mMU=milli-Merck units. IU=international units. NA=not available.
There are no available conversion factors from mMU/mL to IU/mL for anti-HPV types 16 and 18 for the nonavalent competitive Luminex immunoassays performed in the HPV9 vaccine cohort.
Of the 356 patients evaluable for the low immunogenicity analysis (ie, those in the per-protocol population who were seronegative to HPV types 16 or 18 at baseline), 52 (15%) met the definition for low immunogenicity. In multivariable logistic regression analysis adjusted for age and sex, odds of low immunogenicity were higher among allogeneic HSCT recipients than in survivors who had not had allogeneic HSCT (11 [34·4%] of 32 allogeneic HSCT recipients had low immunogenicity vs 41 [12·7%] of 324 who had not had allogeneic HSCT; OR 3·6 [95% CI 1·6–8·1], p=0·0020). A further evaluation of all allogeneic HSCT recipients (n=32) suggested that receipt of HPV4 was associated with lower immunogenicity than with HPV9 (of the 11 allogeneic HSCT recipients with low immunogenicity, 10 [90·9%] had received HPV4 vs 1 [9·1%] had received HPV9; OR 9·1 [95% CI 0·98–84·3], p=0·052).
One or more adverse events were reported by 237 (54%) of 435 participants (129 [51%] of 253 in the HPV4 cohort [one of 254 patients who received dose 1 was lost to follow-up and did not contribute to safety data] and 108 [59%] of 182 in the HPV9 cohort); injection site pain was most common (table 3 ; appendix p 12). Compared with the general population, the proportion of participants reporting injection site adverse events (ie, pain, swelling, erythema) was significantly lower; the proportion reporting fever was not significantly different; the proportion reporting nausea was significantly higher; and the proportion reporting fatigue was significantly higher in patients who had survived cancer (table 3). All serious adverse events were deemed to be unrelated to the vaccine except for erythema nodosum, which occurred in one patient in the 16–26 year female HPV9 cohort and was deemed to be possibly related to the vaccine (appendix p 13).
Table 3.
Comparison of vaccine-related adverse events in survivors of cancer and the general population
|
HPV4 vaccine |
HPV9 vaccine |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Survivors of cancer, n (%) | General population, n (%) | Survivor–general population difference, % (95% CI) | p value | Survivors of cancer, n (%) | General population, n (%) | Survivor–general population difference, % (95% CI) | p value | ||
| Injection site, days 1–5, after any dose | |||||||||
| Vaccinated participants with safety follow-up data | 253 | 8181 | NA | NA | 182 | 15 776 | NA | NA | |
| Pain, any | 90 (36%) | 6168 (75%) | −40% (−46 to −34) | <0·0001* | 84 (46%) | 13 118 (83%) | −37% (−44 to −30) | <0·0001* | |
| Swelling, any | 15 (6%) | 1722 (21%) | −15% (−18 to −11) | <0·0001* | 15 (8%) | 5698 (36%) | −28% (−31 to −23) | <0·0001* | |
| Erythema, any | 15 (6%) | 1774 (22%) | −16% (−18 to −12) | <0·0001* | 17 (9%) | 4859 (31%) | −22% (−25 to −16) | <0·0001* | |
| Systemic, days 1–5, after any dose | |||||||||
| Vaccinated participants with safety follow-up data | 253 | 10 115 | NA | NA | 182 | 9354 | NA | NA | |
| Fever ≥37·8°C | 10 (4%) | 670 (7%) | −3% (−5 to 1) | 0·12* | 12 (7%) | 661 (7%) | 0% (−3 to 4) | 0·92* | |
| Systemic, 15 days after any HPV4 vaccine dose or 8 days after any HPV9 vaccine dose | |||||||||
| Vaccinated participants with safety follow-up data | 253 | 8181 | NA | NA | 182 | 15 776 | NA | NA | |
| Headache | 53 (21%) | NR | NA | NA | 33 (18%) | 2090 (13%) | 5% (0 to 11) | 0·1220* | |
| Nausea | 29 (12%) | 403 (5%) | 7% (3 to 11) | <0·0001* | 21 (12%) | 503 (3%) | 8% (5 to 14) | <0·0001* | |
| Dizziness | 5 (2%) | 241 (3%) | −1% (−2 to 2) | 0·48* | 2 (1%) | 355 (2%) | −1% (−2 to 2) | 0·45† | |
| Fatigue | 47 (19%) | NR | NA | NA | 24 (13%) | 294 (2%) | 11% (7 to 17) | <0·0001* | |
NA=not applicable. NR=not reported.
χ2 with Yates correction.
Fisher's exact test.
The GMTs in survivors of cancer were higher than GMTs in the general population (ratio of GMTs >1) for anti-HPV types 6 and 11 (ie, HPV4 and HPV9) and types 31, 33, 45, 52, and 58 (ie, HPV9; table 4 ). Seroconversion rates for anti-HPV types 16 and 18 and their differences (with multiplicity-adjusted 95% CI) versus the general population, and seroconversion rates for all other HPV vaccine types, are summarised in the appendix (pp 14–16). Seroconversion rates were 100% for all anti-HPV types among female and male participants aged 9–15 years across the HPV4 and HPV9 cohorts. Seroconversion rates were 100% among female and male participants aged 16–26 years across both vaccine cohorts for all anti-HPV types, with the exception of anti-HPV types 6 and 18 among 16–26-year-olds who received HPV4 (ie, seroconversion in 29 [96·7%; 95% CI 82·8–99·9] of 30 female participants and 67 [98·5%; 92·1–100·0] of 68 male participants for type 18; seroconversion in 29 [96·7%; 82·8–99·9] of 30 female participants and 65 [98·5%; 91·8–100·0] of 66 male participants for type 6).
Table 4.
GMTs for anti-HPV types 6 and 11 (HPV4 vaccine recipients) and 6, 11, 31, 33, 45, 52, and 58 (HPV9 recipients) in survivors of cancer versus the general population, by subgroup
|
Survivors of cancer |
General population |
Ratio of GMT, survivors vs general population (two-sided 95% CI) | ||||
|---|---|---|---|---|---|---|
| n | GMT (95% CI), mMu/mL | n | GMT (95% CI), mMu/mL | |||
| HPV4 | ||||||
| Female participants aged 9–15 years | ||||||
| Anti-HPV type 6 | 53 | 2050·9 (1285·1–2816·6) | 917 | 929·2 (874·6–987·3) | 2·21 (1·40–3·04) | |
| Anti-HPV type 11 | 53 | 3793·5 (1816·5–5770·5) | 917 | 1304·6 (1224·7–1389·7) | 2·91 (1·43–4·41) | |
| Male participants aged 9–15 years | ||||||
| Anti-HPV type 6 | 66 | 1715·5 (1337·1–2093·8) | 884 | 1037·5 (963·5–1117·3) | 1·65 (1·28–2·05) | |
| Anti-HPV type 11 | 65 | 2874·1 (2143·6–3604·7) | 885 | 1386·8 (1298·5–1481·0) | 2·07 (1·54–2·63) | |
| Female participants aged 16–26 years | ||||||
| Anti-HPV type 6 | 30 | 790·6 (409·7–1171·5) | 3329 | 545·0 (530·1–560·4) | 1·45 (0·78–2·12) | |
| Anti-HPV type 11 | 31 | 1338·5 (452·4–2224·5) | 3353 | 748·9 (726·0–772·6) | 1·79 (0·66–2·92) | |
| Male participants aged 16–26 years | ||||||
| Anti-HPV type 6 | 66 | 1106·4 (817·1–1395·7) | 1093 | 447·8 (418·9–478·6) | 2·47 (1·82–3·15) | |
| Anti-HPV type 11 | 66 | 2037·2 (1238·9–2835·5) | 1093 | 624·3 (588·4–662·3) | 3·26 (2·00–4·55) | |
| HPV9 | ||||||
| Female participants aged 9–15 years | ||||||
| Anti-HPV type 6 | 40 | 2811·1 (2150·7–3471·5) | 2349 | 1744·6 (1684·7–1806·7) | 1·61 (1·24–1·98) | |
| Anti-HPV type 11 | 41 | 2042·7 (1523·0–2562·5) | 2405 | 1289·7 (1244·3–1336·8) | 1·58 (1·19–1·98) | |
| Anti-HPV type 31 | 38 | 3297·2 (2362·7–4231·6) | 2397 | 1883·3 (1811·3–1958·1) | 1·75 (1·27–2·24) | |
| Anti-HPV type 33 | 41 | 1554·7 (1104·6–2004·8) | 2418 | 960·6 (927·5–994·9) | 1·62 (1·16–2·08) | |
| Anti-HPV type 45 | 42 | 1180·3 (830·2–1530·5) | 2430 | 728·7 (697·6–761·2) | 1·62 (1·15–2·10) | |
| Anti-HPV type 52 | 39 | 1266·8 (917·9–1615·7) | 2426 | 978·2 (942·8–1015·0) | 1·30 (0·95–1·64) | |
| Anti-HPV type 58 | 42 | 2036·9 (1478·3–2595·5) | 2397 | 1306·0 (1259·8–1354·0) | 1·56 (1·14–1·98) | |
| Male participants aged 9–15 years | ||||||
| Anti-HPV type 6 | 48 | 4756·2 (3027·7–6484·6) | 1055 | 2085·3 (1984·2–2191·6) | 2·28 (1·47–3·10) | |
| Anti-HPV type 11 | 51 | 3137·4 (2129·2–4145·6) | 1055 | 1469·2 (1397·7–1544·4) | 2·14 (1·46–2·82) | |
| Anti-HPV type 31 | 51 | 4502·3 (3120·2–5884·4) | 1069 | 2173·5 (2057·0–2296·6) | 2·07 (1·45–2·71) | |
| Anti-HPV type 33 | 51 | 2067·4 (1487·2–2647·5) | 1239 | 1178·6 (1120·9–1239·4) | 1·75 (1·27–2·25) | |
| Anti-HPV type 45 | 51 | 2045·1 (1265·1–2825·1) | 1079 | 841·7 (790·0–896·7) | 2·43 (1·52–3·36) | |
| Anti-HPV type 52 | 51 | 2140·1 (1381·5–2898·7) | 1077 | 1062·2 (1007·2–1120·2) | 2·01 (1·32–2·73) | |
| Anti-HPV type 58 | 53 | 2404·5 (1775·3–3033·7) | 1072 | 1545·8 (1470·6–1624·8) | 1·56 (1·15–1·97) | |
| Female participants aged 16–26 years | ||||||
| Anti-HPV type 6 | 25 | 3548·3 (764·0–6332·6) | 4321 | 893·7 (873·5–914·3) | 3·97 (1·04–6·90) | |
| Anti-HPV type 11 | 28 | 2297·5 (1203·6–3391·4) | 4327 | 669·3 (653·6–685·4) | 3·43 (1·88–4·99) | |
| Anti-HPV type 31 | 28 | 3688·8 (1119·0–6258·5) | 4806 | 664·8 (647·4–682·6) | 5·55 (1·89–9·22) | |
| Anti-HPV type 33 | 27 | 1267·7 (750·6–1784·8) | 5056 | 419·2 (409·6–429·1) | 3·02 (1·86–4·19) | |
| Anti-HPV type 45 | 28 | 1035·9 (81·3–1990·4) | 5160 | 254·1 (247·0–261·5) | 4·08 (0·52–7·64) | |
| Anti-HPV type 52 | 26 | 1019·7 (609·8–1429·6) | 4792 | 382·4 (373·0–392·0) | 2·67 (1·66–3·68) | |
| Anti-HPV type 58 | 28 | 1523·3 (731·2–2315·4) | 4818 | 489·2 (477·5–501·2) | 3·11 (1·58–4·65) | |
| Male participants aged 16–26 years | ||||||
| Anti-HPV type 6 | 33 | 2632·4 (1568·7–3696·1) | 847 | 782·0 (738·0–828·7) | 3·37 (2·06–4·71) | |
| Anti-HPV type 11 | 33 | 1881·1 (1074·6–2687·6) | 851 | 616·7 (582·4–653·0) | 3·05 (1·95–4·17) | |
| Anti-HPV type 31 | 34 | 2377·3 (1352·0–3402·6) | 908 | 708·5 (662·7–757·6) | 3·36 (1·96–4·79) | |
| Anti-HPV type 33 | 34 | 1137·8 (705·6–1570·1) | 901 | 384·8 (362·5–408·4) | 2·96 (1·87–4·07) | |
| Anti-HPV type 45 | 33 | 1113·5 (599·6–1627·4) | 909 | 235·6 (219·0–253·6) | 4·73 (2·63–6·90) | |
| Anti-HPV type 52 | 33 | 970·1 (606·9–1333·4) | 907 | 386·8 (363·4–411·6) | 2·51 (1·60–3·44) | |
| Anti-HPV type 58 | 33 | 1534·4 (832·4–2236·4) | 897 | 509·8 (479·9–541·6) | 3·01 (1·69–4·36) | |
GMTs for anti-HPV types 6 and 11 and anti-HPV types 6, 11, 31, 33, 45, 52, and 58 with two-sided 95% CIs for comparisons between survivors of cancer and the general population who received the HPV4 and HPV9 vaccines, with corresponding GMT ratios and two-sided 95% CIs. GMT=geometric mean titres. mMU=milli-Merck units.
Discussion
In this first report of immunogenicity and safety of the three-dose series of HPV4 and HPV9 in young survivors of cancer, we noted that 1 month after vaccine series completion, antibody responses against HPV types 16 and 18 in survivors of cancer were similar to those reported in several previously published clinical trials in the general population. The non-inferiority criterion was met for anti-HPV types 16 and 18 for all survivor subgroups, except for type 18 in female participants aged 16–26 years who received HPV9. Allogeneic HSCT recipients were more likely to have low immunogenicity than were cancer survivors who had not undergone allogeneic HSCT; these increased odds were driven primarily by allogeneic HSCT survivors who received HPV4.
Although it is reassuring that antibody responses in most survivors of cancer were similar to or higher than those seen in the general population comparisons, we noted that a third of allogeneic HSCT survivors met the criteria for low immunogenicity to HPV type 16 or 18 (ie, less than half of the mean GMT of the corresponding group in the general population). Among allogeneic HSCT survivors in our study, receipt of HPV4 was associated with low immunogenicity. A study examining response to HPV4 among 44 adult women (median age 33 years) who had undergone allogeneic HSCT reported that 86% (38 of 44) developed antibody responses to HPV types 16 and 18, compared with 100% of 20 healthy volunteers; women receiving immunosuppressive therapy at the time of vaccination were less likely to develop antibody responses than women who were not receiving this therapy (18 [78%] of 23 vs 20 [95%] of 21).25 Notably, compared with HPV4, HPV9 contains higher doses of HPV type 16 (40 μg vs 60 μg) and 18 (20 μg vs 40 μg) antigens and higher doses of adjuvant (225 μg vs 500 μg).26 Increasing vaccine dose or number of doses is a strategy that is commonly used to boost immune response in populations with low immunogenicity to vaccines.27 The increased dose of antigen and adjuvant contained in HPV9 might have been sufficient to overcome the low immunogenicity that is associated with receipt of HPV4 among the allogeneic HSCT recipients in our study. Furthermore, despite our finding that allogeneic transplantation was associated with low immunogenicity (almost exclusively among HPV4 recipients), all patients had seroconversion (ie, a marker that is commonly associated with adequate vaccine immunogenicity) to HPV type 16, and all but two patients (both of whom received HPV4) had seroconversion to HPV type 18. Thus, it seems reasonable to offer allogeneic HSCT survivors HPV9.
In the general population, age at vaccination is inversely proportional to vaccine-induced antibody response,15 and the three-dose series of HPV vaccine is recommended for people aged 15 years and older in the healthy general population.28 However, in younger (ie, aged 9–14 years) boys and girls in the general population, non-inferior antibody responses have been shown after a two-dose series (given at months 0 and 6–12), as compared with young adult (ie 16–26-year old) women, in whom efficacy has been shown after three doses.6, 29 On the basis of these data from general population studies, a two-dose series of HPV vaccine is recommended for healthy young people who are aged 9–14 years.28 Importantly, in our study we noted that, among young (ie, aged 9–15 years) survivors, immunogenicity of the HPV4 and HPV9 three-dose series was non-inferior to general population comparisons, and 100% seroconversion was reached for all HPV types, suggesting an opportunity for future research focusing on immunogenicity of the two-dose vaccine series in young survivors of cancer aged 9–14 years.
In older female participants (ie, aged 16–26 years) who received HPV9, the GMT ratio for HPV type 18 in survivors compared with the general population was 4·30; however, the non-inferiority response criterion was not met. This criterion was probably not met due to a small sample size and wide range of GMTs in this subgroup. Nevertheless, seroconversion to HPV type 18 was reached in 100% of older females who received HPV9. Although seroconversion analyses were exploratory due to insufficient power, the high seroconversion rates across all HPV types were reassuring. An additional exploratory sub-aim of this study that will evaluate the persistence of antibody response at 2 years post-vaccine initiation, and identify clinical and host factors influencing response persistence, will be reported separately.
The HPV vaccine series were safe and generally well tolerated in survivors of cancer, with lower prevalence of adverse events at the injection site, similar rates of fever, and higher rates of nausea and fatigue than reported in several previously published clinical trials in the general population. Survivors’ previous cancer therapy, which often involves repeated injections and medical procedures, might have contributed to their lower ratings of vaccine-related pain at the injection site than by the general population. Persistent symptoms after therapy, such as nausea and fatigue, have been reported in survivors of cancer30 and might have contributed to the higher proportion of survivors reporting nausea and fatigue after HPV9 than in general population peers. Similar to general population reports, a larger proportion of survivors receiving HPV9 reported adverse events at the injection site than survivors receiving HPV4, which might be attributable to higher concentrations of antigen and adjuvant in HPV9.26
This work should be considered in the context of its limitations. First, we were unable to conduct a randomised, placebo-controlled trial due to ethical concerns of withholding a vaccine with known efficacy in the general population; thus, the comparison group data are from the historical participants in vaccine licensing trials.7, 12, 13, 14, 15, 16, 17 Second, study participation was restricted to survivors who had completed cancer treatment within the previous 1–5 years, decreasing generalisability. Third, patients who enrolled were less likely to be non-Hispanic white and more likely to have a diagnosis of leukaemia than patients who refused to enrol. Fourth, due to licensing of HPV9 and withdrawal of HPV4 from the US marketplace during our trial, we were unable to reach the originally planned targeted enrolment for the HPV4 cohort, increasing type II error; however, CIs are provided to show the variability. Fifth, given the few patients with allogeneic HSCT in the cohort (ie, the main subgroup with low immunogenicity), findings from the low immunogenicity analysis should be interpreted with caution. Sixth, at the time of this report, we have not yet evaluated persistence of antibody response in this cohort. Finally, vaccine efficacy was not an endpoint in this study. The HPV vaccine is highly efficacious against acquisition of infection and development of premalignant and malignant lesions related to HPV in HPV-naive women and men in the general population.8, 26 Given that the protective mechanism afforded by HPV vaccine is via neutralising antibody production, and that antibody responses in survivors of cancer were similar to those seen in the general population, similar vaccine efficacy in survivors of cancer is probable.
Our study had many strengths, including enrolment of a large, diverse sample across the spectrum of diagnoses and treatments commonly seen in young survivors of cancer and excellent retention rates. Additionally, introduction of HPV9 provided an opportunity to evaluate immunogenicity and safety of HPV9 in survivors of cancer shortly after its implementation in the general population.
We noted that, among young survivors of cancer 1–5 years after completion of cancer treatment, the three-dose series of HPV vaccine was safe and well tolerated, and immunogenicity was similar to that in the general population, providing evidence for its use in survivors of cancer. Additionally, since the primary factor that was associated with low immunogenicity among allogeneic HSCT survivors was HPV4, clinicians can be reassured that low immunogenicity is unlikely among survivors who receive HPV9. As we have previously shown in part 1 of this study,9 uptake of HPV vaccine among young survivors of cancer is significantly lower than that in the general population. Thus, findings regarding safety and immunogenicity of the three-dose HPV vaccine from this trial should remove any hesitancy on the part of health-care providers in incorporating HPV vaccination into routine oncological follow-up care, with the ultimate goal of preventing subsequent neoplasms related to HPV in survivors of cancer.
Data sharing
Deidentified participant data that underlie the results reported in this paper (ie, text, tables, figures), will be made available on request to researchers who provide a methodologically sound proposal beginning 3 months and ending 5 years following publication.
Declaration of interests
JAC reports payment for a consulting or advisory role for X4 Pharmaceuticals. ARG reports payment for membership of a scientific advisory board and global advisory board for, honoraria from, and funds to their institution to conduct research studies unrelated to this research from Merck Sharp & Dohme. MMH reports payment for a consulting or advisory role for the Oncology Research Information Exchange Network Patient Advisory Committee, Princess Maxima Center Scientific Advisory Board, and SurvivorLink. WL reports non-financial support (provision of vaccine and laboratory analysis) to their institution related to this research from Merck Sharp & Dohme and payment for a consulting or advisory role for SurvivorLink. ML reports payment for consulting for Oncoceutics. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank Sean Phipps for his administrative and clinical research support throughout the study. All vaccine doses and competitive Luminex immunoassays that were used in this study were provided by Merck Sharp & Dohme without cost to the study. The opinions expressed in this Article are those of the authors and do not necessarily represent those of the organisations providing funding for this study. This work was supported by the US National Cancer Institute (R01CA166559 for principal investigators WL and JLK), the Investigator-Initiated Studies Program of Merck Sharp & Dohme (MISP #40083 for principal investigator WL), and the American Lebanese Syrian Associated Charities.
Contributors
WL, JLK, SB, MMH, LLR, and ARG came up with the concept and designed the study. WL, JLK, SB, MMH, FLW, JMY, JSF, HMH, PS, KA, KW-M, BC, RJ-R, ML, JAC, and SHA acquired, analysed, and interpreted the data. WL, JLK, SB, and FLW drafted the manuscript. All authors critically revised the manuscript for important intellectual content. WL, FLW, and JMY accessed and verified the data. FLW, WL, and SB statistically analysed the data. WL, JLK, and LLR obtained funding for the study. WL, JLK, SB, MMH, PS, and JMY provided administrative, technical, or material support. WL, JLK, SB, and MMH supervised the study. All authors had full access to all the data in the study and had final responsibility for decision to submit for publication.
Supplementary Material
References
- 1.Senkomago V, Henley SJ, Thomas CC, Mix JM, Markowitz LE, Saraiya M. Human papillomavirus-attributable cancers—United States, 2012–2016. MMWR Morb Mortal Wkly Rep. 2019;68:724–728. doi: 10.15585/mmwr.mm6833a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilcher GMT, Rivard L, Huang JT, et al. Immune function in childhood cancer survivors: a Children's Oncology Group review. Lancet Child Adolesc Health. 2021;5:284–294. doi: 10.1016/S2352-4642(20)30312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imburgia TM, Shew ML, Gravitt PE, Katzenellenbogen RA. Considerations for child cancer survivors and immunocompromised children to prevent secondary HPV-associated cancers. Transplantation. 2021;105:736–742. doi: 10.1097/TP.0000000000003444. [DOI] [PubMed] [Google Scholar]
- 5.Ojha RP, Tota JE, Offutt-Powell TN, et al. Human papillomavirus-associated subsequent malignancies among long-term survivors of pediatric and young adult cancers. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huh WK, Joura EA, Giuliano AR, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet. 2017;390:2143–2159. doi: 10.1016/S0140-6736(17)31821-4. [DOI] [PubMed] [Google Scholar]
- 7.Castellsagué X, Giuliano AR, Goldstone S, et al. Immunogenicity and safety of the 9-valent HPV vaccine in men. Vaccine. 2015;33:6892–6901. doi: 10.1016/j.vaccine.2015.06.088. [DOI] [PubMed] [Google Scholar]
- 8.Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klosky JL, Hudson MM, Chen Y, et al. Human papillomavirus vaccination rates in young cancer survivors. J Clin Oncol. 2017;35:3582–3590. doi: 10.1200/JCO.2017.74.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Block SL, Nolan T, Sattler C, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118:2135–2145. doi: 10.1542/peds.2006-0461. [DOI] [PubMed] [Google Scholar]
- 11.Petrosky E, Bocchini JA, Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64:300–304. [PMC free article] [PubMed] [Google Scholar]
- 12.Merck & Co . Merck & Co; Whitehouse Station, NJ: 2011. Gardasil [human papillomavirus quadrivalent (types 6, 11, 16, and 18) vaccine, recombinant] full prescribing information, updated April 2015. [Google Scholar]
- 13.Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J. 2007;26:201–209. doi: 10.1097/01.inf.0000253970.29190.5a. [DOI] [PubMed] [Google Scholar]
- 14.Moreira ED, Jr, Palefsky JM, Giuliano AR, et al. Safety and reactogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 viral-like-particle vaccine in older adolescents and young adults. Hum Vaccin. 2011;7:768–775. doi: 10.4161/hv.7.7.15579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen LK, Restrepo J, Moreira ED, Jr, et al. Impact of baseline covariates on the immunogenicity of the 9-valent HPV vaccine—a combined analysis of five phase III clinical trials. Papillomavirus Res. 2017;3:105–115. doi: 10.1016/j.pvr.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreira ED, Jr, Block SL, Ferris D, et al. Safety profile of the 9-valent HPV vaccine: a combined analysis of 7 phase III clinical trials. Pediatrics. 2016;138 doi: 10.1542/peds.2015-4387. [DOI] [PubMed] [Google Scholar]
- 17.Merck & Co . Merck & Co; Whitehouse Station, NJ: 2015. Gardasil 9 (human papillomavirus 9-valent vaccine, recombinant) prescribing information, updated December, 2015. [Google Scholar]
- 18.Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308:2594–2604. doi: 10.1001/jama.2012.87802. [DOI] [PubMed] [Google Scholar]
- 19.Dias D, Van Doren J, Schlottmann S, et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol. 2005;12:959–969. doi: 10.1128/CDLI.12.8.959-969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts C, Green T, Hess E, et al. Development of a human papillomavirus competitive Luminex immunoassay for 9 HPV types. Hum Vaccin Immunother. 2014;10:2168–2174. doi: 10.4161/hv.29205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown D, Müller M, Sehr P, et al. Concordance assessment between a multiplexed competitive Luminex immunoassay, a multiplexed IgG Luminex immunoassay, and a pseudovirion-based neutralization assay for detection of human papillomaviruse types 16 and 18. Vaccine. 2014;32:5880–5887. doi: 10.1016/j.vaccine.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Korn EL, Freidlin B. Conditional power calculations for clinical trials with historical controls. Stat Med. 2006;25:2922–2931. doi: 10.1002/sim.2516. [DOI] [PubMed] [Google Scholar]
- 23.Beyene J, Moineddin R. Methods for confidence interval estimation of a ratio parameter with application to location quotients. BMC Med Res Methodol. 2005;5:32. doi: 10.1186/1471-2288-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–226. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
- 25.Stratton P, Battiwalla M, Tian X, et al. Immune response following quadrivalent human papillomavirus vaccination in women after hematopoietic allogeneic stem cell transplant: a nonrandomized clinical trial. JAMA Oncol. 2020;6:696–705. doi: 10.1001/jamaoncol.2019.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 27.Pittet LF, Posfay-Barbe KM. Vaccination of immune compromised children-an overview for physicians. Eur J Pediatr. 2021;180:2035–2047. doi: 10.1007/s00431-021-03997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405–1408. doi: 10.15585/mmwr.mm6549a5. [DOI] [PubMed] [Google Scholar]
- 29.Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411–2421. doi: 10.1001/jama.2016.17615. [DOI] [PubMed] [Google Scholar]
- 30.Cameron CL, Cella D, Herndon JE, 2nd, et al. Persistent symptoms among survivors of Hodgkin's disease: an explanatory model based on classical conditioning. Health Psychol. 2001;20:71–75. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified participant data that underlie the results reported in this paper (ie, text, tables, figures), will be made available on request to researchers who provide a methodologically sound proposal beginning 3 months and ending 5 years following publication.