Abstract
Picrorhiza kurroa (P.K) usually familiar as kutki is a well-known plant in the Ayurvedic system of medicine due to its reported activities including antidiabetic, antibacterial, antioxidant, antitumor, anti-inflammatory, and hepatoprotective. The current research was intended to evaluate the antioxidant, inhibition activity of the ethanolic, methanolic, and aqueous extracts of P.K roots against α-amylase and α-glucosidase in vitro, after the phytochemical analysis. For this purpose, P.K roots were extracted with ethanol (EthPk), methanol (MthPk), and distilled water (AqPk) and phytochemical study of the extracts were performed to recognize the total phenolic content (TPC) and total flavonoids content (TFC). Antioxidant capability of the extracts was assessed by FRAP, ABTS, and DPPH assay. α-amylase inhibitory and α-glucosidase inhibitory activities were also determined. Software SPSS-23 was used to statistically analyze with One Way ANOVA and results were stated as mean standard deviation. Result of the study showed that MthPk contained the maximum concentration of TPC and TFC than EthPk and AqEh. Antioxidants in terms of DPPH (lowest IC50 = .894 ± .57), FRAP (612.54 ± 11.73) and ABTS (406.42 ± 4.02) assay was also maximum in MthPk. MthPk was also showed maximum inhibition activity against α-amylase and α-glucosidase with lowest IC50 (.39 ± .41; .61 ± .24), respectively. The extracts α-amylase and α-glucosidase inhibitory activities order was as MthPk > EthPk> AqPk. Results clearly specified that the methanolic extract of Picrorhiza kurroa have the maximum antioxidant, α-amylase, and α-glucosidase inhibitory activities. A positive correlation of TPC, TFC with antioxidant, and α-amylase and α-glucosidase inhibition activities of the P.K roots were also shown. The plant has capability to diminish the oxidative stress and can be used to treat diabetes by inhibiting α-amylase and α-glucosidase actions.
Keywords: Picrorhiza kurroa, total phenolic content, total flavonoids content, antioxidants, alpha-amylase inhibitory activity, alpha-glucosidase inhibitory activity
Introduction
Oxidative stress can be brought about by the abundance of reactive oxygen species (ROS) and reactive nitrogen species (RNS). 1 ROS and RNS are the expressions altogether used to depict free radicals and other non-radical reactive derived known as oxidants. ROS incorporate oxygen-containing dioxygen (O2•−), hydrogen peroxide (H2O2), and hydroxide (•OH). In RNS, nitrogen-containing oxidants such as nitrogen Dioxide (NO2), nitric oxide (NO•), and peroxynitrite (ONOO−) are included.2,3 Free radical’s high concentrations then bring about malicious cycles that can harm cell structures because of oxidative stress. 4 ROS and RNS accumulation prompts oxidative damage to essentially all particles. Such groups are not really a danger to the human body makeup in typical physiological circumstances.5,6 However, when the body neglects to eliminate them somewhat, oxidative stress invigorates the atherosclerotic plaques formation. This plaques formation may build the danger of atherosclerosis, malignancy, and Type 2 diabetes mellitus.7-9
α-Amylase are produced as hydrolytic enzymes in humans, animals, fungi, bacteria, and plants. In the salivary glands, α-amylase are originated in human that emit the enzyme into the pancreas that release it inside the small digestive tract. 10 α-amylases function is to prompt the starch hydrolysis. α-amylase separate the α-(1,4)-glycosidic linkage in starch particle prompting the creation of glucose, maltodextrins, maltotetraose, maltose, and maltotriose. 11 While α-glucosidase is present in the enterocytes luminal surface and is discharged inside the small digestive tract, 12 α-Glucosidase is an important protein that prompting the disaccharides (sucrose and maltose) hydrolytic cleavage into monosaccharides (fructose and glucose). Hence, α-amylases and α-glucosidase inhibitory activities can impede the rise of glucose and stifle postprandial hyperglycemiae.13,14
Various diseases such as diabetes, cancer, neurodegenerative, and cardiovascular diseases are associated oxidative stress. 15 Diabetes-associated cardiovascular diseases also arise by a variety of mechanisms including oxidative stress. Therefore, it is important to maintain the oxidative stress and sugar levels in the body. Nature has consistently existed abundant source of important compounds which are associated to valuable possessions for individual health. 16 There is an abundance of proof which shows that natural plants and other food stuffs are major source of antioxidants; have recognized α-amylase and α-glucosidase inhibitory activities.17-23
Picrorhiza kurroa (Family Scrophulariaceae), also recognized as kutki, is one of the therapeutic plant occurred in the alpine Himalayan area. kutkoside and iridoid glycosides (Picroside I and II) are dynamic components of the plant. 24 Over than 2000 herbal products, Picroside-I and II are utilized. 25 The plant’s economic components are its dried roots and rhizomes, and are utilized to cure different afflictions, for example, spleen disorder, liver diseases, and allergy problems. 24 P. kurroa showed β-cell recovery with upgraded insulin creation and antihyperglycemic impacts. 26 The current study is conducted to expose the antioxidant and inhibition activity of P. kurroa against α-amylases and α-glucosidase.
Material and Methods
Plant Procurement
P. kurroa was procured from local market Lahore, Pakistan. Then, recognized by the professional botanist from the Botany Department, Government College University Faisalabad, Pakistan a voucher specimen numbered 136-A-2021.
Extract Preparation
Extracts were prepared by the solvent extraction method as illustrated by Mustafa et. al. 17 The plant following rinsing with distilled water (D.W) was dried in the shade and crushed into well powder form. Then, powder (50 g) was soaked for 72 hours in distilled water, methanol, and ethanol (each 250 mL) with occasionally stirring and mixing. Mixture was filtered by utilizing filter paper (Whatman No. 1). In rotary evaporator (SCI100-Pro; SCILOGEX, USA) at 40°C, filtrates were concerted and transferred in petri dish. The petri dish was placed in incubator at 40°C dried out appropriately. Extract was stored at 4°C up to more investigation.
Qualitative Phytochemical Analysis
Phytochemical analyses of extracts were performed qualitatively by using standard techniques to detect the main phytochemical ingredients as mentioned by Singh and Bag. 27
Quantitative Phytochemical Estimation
Total Phenolic contents (TPC); mg GAE/g)
The 10 µl of plant extract (1 mg/mL) was dissolved in 100 µl of Folin–Ciocalteau reagent and 200 µl of 2.5% Na2CO3. Using the gallic acid (GA) standard curve, TPC in the extracts was evaluated as explained by Bajalan et al. 28 Absorbance (A) was noted at 760 nm by using biochemistry analyzer (Biolab-310) after 60 minutes incubation at room temperature. TPC value was expressed as mg gallic acid equivalent (GAE)/g.
Total Flavonoid contents (TFC); mg QE/g)
TFC were determined by utilizing Quercetin (Q) as a standard according to the process formerly adopted by. 28 Briefly, 100 µl of plant extract (1 mg/mL) was added to 1 mL of D.W. After 5 minutes incubation at room temperature, 125 µl of aluminum chloride (AlCl3) and 75 µl of 5% sodium nitrite (NaNo2) was added and incubated again for 6 minutes at room temperature. At the end, 1M sodium hydroxide (NaOH; 125 µl) was added and the final volume was prepared upto 2.5 mL with D.W. Absorbance was calculated at 540 nm utilizing chemistry analyzer (Biolab-310).
In Vitro Antioxidant Evaluation
Scavenging Activity Assay
2,2-Diphenyl-1-picrylhydrazyl (DPPH) was dissolved in methanol (.025 g/L). The plant extracts diluted with dimethyl sulfoxide (DMSO) at 1 mg/mL concentration. Sample solution (5 µl) was mixed with 585 µl DPPH working solution. Absorbance was evaluated at 515 nm after 20 mint incubation at room temperature by utilizing chemistry analyzer (Biolab-310). Percentage DPPH scavenging activity was measured by the following equation:
| (1) |
A0 is the control absorbance (sample was replaced with distilled water (D.W)) and A1 is the sample absorbance. 29
The ferric reducing antioxidant potential (FRAP) was assessed by the means as demonstrated by Sethi et al. 30 A volume of 3.995 mL of the working solution [300 mM acetate buffer (10 volumes), 1 volume of 2, 4, 6-tri {2-pyridyl}-s-triazine (TPTZ; 10 mM) in HCl (40 mM), and 1 volume of ferric chloride (FeCl3; 20 mM)] was assorted with the sample (5 µl). Absorbance was taken at 593 nm to monitor the reduction.
2,2'-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS; Trolox Equivalent Antioxidant Capacity) assay was performed as demonstrated by 31 with negligible modification. ABTS mixture was organized by addition of 1:1 ratio of 7 mM solution of ABTS in distilled water and solution of K2S2O8 (2.5 mM). The prepared mixture was more diluted with methanol to attain absorbance of .7 at 734 nm. Then 5 µl of each plant extract solution was mixed with 3.995 mL of ABTS solution. Absorbance was taken at 734 nm subsequent to 30 min incubation at room temperature. Results were indicated as mg of 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) equivalent per gram of dry weight of the plant.
α-Amylase Inhibitory Activity
Five hundred (500) µl of plant extract (25, 50, 75, and 100 mg/mL dH2O) and 500 µl α -amylase solution (0.5 mg/mL in phosphate buffer; pH 7.4) was set aside at room temperature for 10 mints. Then 1% starch solution (500 µl) was added in .02 M sodium phosphate buffer (pH 7.4). Reaction was ended by adding 1 mL of 3,5 dinitrosallicylic acid (DNSA) color reagent after 10 minutes incubation at room temperature. The mixture was kept in a boiling water bath for 10 minutes and then diluted with 10 mL D.W when cooled to room temperature. Absorbance was taken at 540 nm by biochemistry analyzer (Biolab-310).
The % of inhibition for α-amylase was deliberated as follows
| (2) |
A0 is the control absorbance (extract sample was replaced with D.W) and A1 is the sample absorbance. 17
α - Glucosidase Inhibitory Activity
Five hundred (500) µl of plant extract (25, 50, 75 and 100 mg/mL dH2O), 1% starch solution (500 µl) in 0.2 M Tris buffer (pH 8), and 500 µl α-glucosidase solution (1U/ml in tris buffer; pH 8) was kept at 37°C for 10 minutes. The mixture was positioned in boiling water bath for 2 minutes to terminate the reaction. The quantity of glucose liberated is measured. A blank sample not including test sample stands for 100% enzyme activity. Acarbose (α -glucosidase inhibitor) was operated as a positive control. Absorbance was measured at 540 nm by biochemistry analyzer (Biolab-310), and percent inhibition activity for α-glucosidase was deliberated as follows
| (3) |
where A0 is the control absorbance (extract sample was replaced with DW) and A1 is the absorbance of the sample. 17
Statistical Analysis
All the quantification was measured in triplicates. Obtained data was evaluated by one-way analysis of variance (ANOVA), following Tukey’s post hoc test for comparing mean values by using SPSS-23. All the results were demonstrated as mean±standard deviation.
Results
Qualitative Phytochemical Examination
Results of the qualitative examination of all extracts type of the P. kurroa are shown in the Table 1, which shows presence or absence of various phytochemicals like, carbohydrates, alkaloids, phenols, flavonoids, saponins, steroids, terpenoids, tannins, and reducing sugar in MthPk, EthPk, and AqPk.
Table 1.
Qualitative Analysis of Picrorhiza kurroa.
| Compounds | Test | MthPk | EthPk | AqPk |
|---|---|---|---|---|
| Carbohydrates | Benedict’s test | − | − | − |
| Fehling’s test | − | − | − | |
| Reducing sugar | Fehling’s test | − | − | − |
| Alkaloids | Hager’s test | − | − | − |
| Proteins | Xanthopeoteic test | − | − | − |
| Flavonoids | Alkaline reagent test | ++ | +++ | ++ |
| Phenols | Lead acetate test | +++ | +++ | ++ |
| Tannins | Lead acetate test | − | − | − |
| Steroids | Salkowski’s test | ++ | ++ | ++ |
| Terpenoids | Salkowski’s test | ++ | ++ | + |
(+): present; (−): not detected
MthPk, Methanol extract of Picrorhiza kurroa; EthPk, Ethanol extract of Picrorhiza kurroa; AqPk, Aqueous extract of Picrorhiza kurroa.
Total Phenolic and Flavonoid Contents
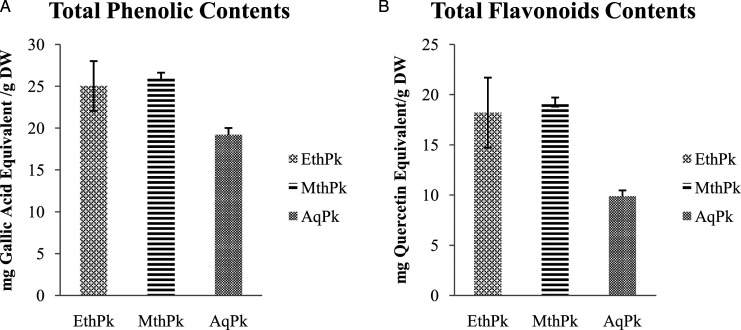
Both the TPC and TFC were significantly (P = .05) maximum in MthPk (26.18 ±.44 mg GAE/g and 19.26 ±.45 mg QE/g, respectively) than AqPk (19.18 ±.83 mg GAE/g and 9.86 ±.6 mg QE/g, respectively) and EthPk (25.03 ±2.98 mg GAE/g and 18.2 ±3.49 mg QE/g, respectively) as shown in Figure.1.
Figure 1.
A) Total Phenolic contents of Picrorhiza kurroa different root extracts. B) Total Flavonoid contents of Picrorhiza kurroa different root extracts. Results are demonstrated as Mean±Standard deviation of 3-replicates of every extract, that is, MthPk (Methanolic extract of Picrorhiza kurroa), EthPk (Ethanolic extract of Picrorhiza kurroa), and AqPk (aqueous extract of Picrorhiza kurroa). DW; Dry Weight of Picrorhiza kurroa extract.
In Vitro Antioxidant Evaluation
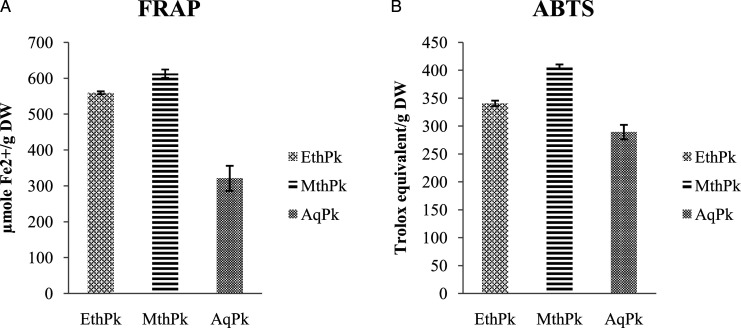
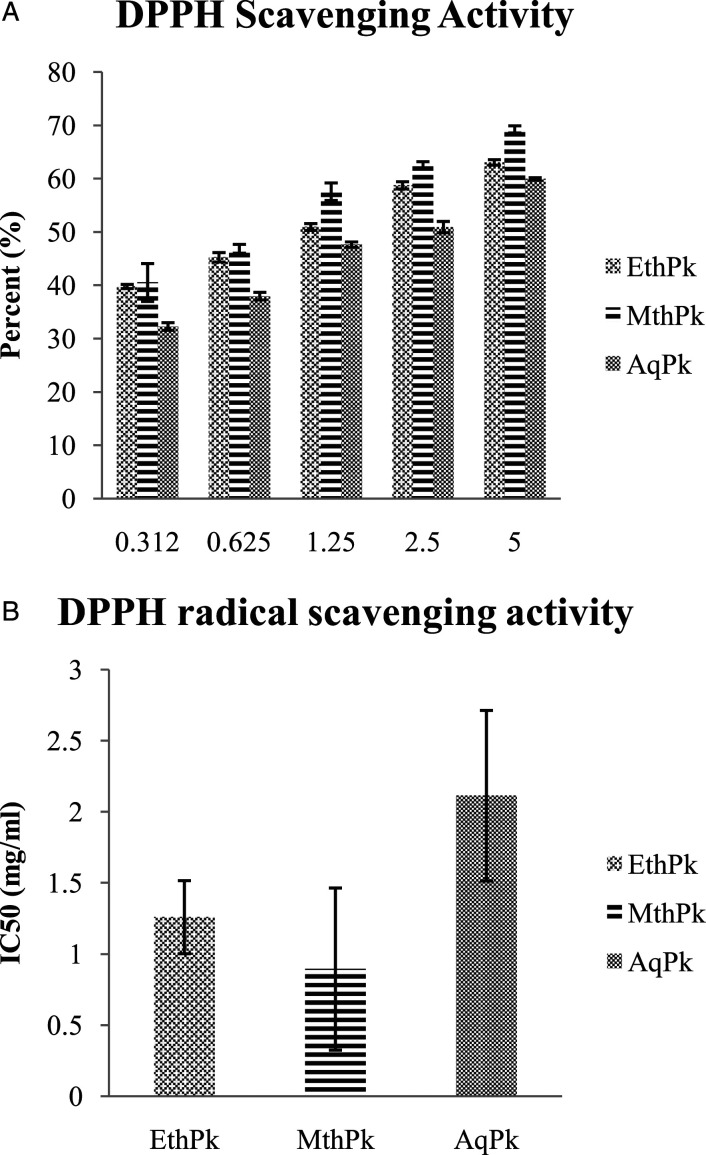
Results of DPPH• (Percent Inhibition), FRAP (FeSo4 (µmole Fe2+/g DW), and ABTS (µM Trolox Equivalent/g DW) assay are expressed in the Figures 2 and 3 that illustrated a concentration reliant raise in DPPH scavenging activity in the MthPk (Figure.3) with lowest half maximal inhibitory concentration (IC50) value (.8942 mg/mL) as compared to IC50 value of AqPk (2.11 mg/mL) and EthPk (1.25 mg/mL). The result showed MthPk has the greatest reducing potential of Fe 3+ into Fe 2+ (612.54 ±11.73µmole Fe2+/g) and similar tendency was observed in scavenging ABTS radical being greatest in MthPk (406.42 ± 4.02 µM TE/g).
Figure 2.
A) Ferric Reducing Antioxidant Potential (FRAP) of different extracts of Picrorhiza kurroa. B) Trolox Equivalent Antioxidant Capacity (TEAC; ABTS Assay) of different root extracts of Picrorhiza kurroa. Results are demonstrated as Mean±Standard deviation of 3-replicates of every extract, that is, MthPk (Methanolic extract of Picrorhiza kurroa), EthPk (Ethanolic extract of Picrorhiza kurroa), and AqPk (Picrorhiza kurroa aqueous extract).DW; Dry Weight of Picrorhiza kurroa extract.
Figure 3.
A) DPPH scavenging activity of 5 different absorptions of different root extracts of Picrorhiza kurroa. B) DPPH IC50 value of different root extracts of Picrorhiza kurroa. Results are demonstrated as Mean±Standard deviation of 3-replicates of every extract, that is, MthPk (Methanolic extract of Picrorhiza kurroa), EthPk (Ethanolic extract of Picrorhiza kurroa) and AqPk (Picrorhiza kurroa aqueous extract).DW; Dry Weight of Picrorhiza kurroa extract.
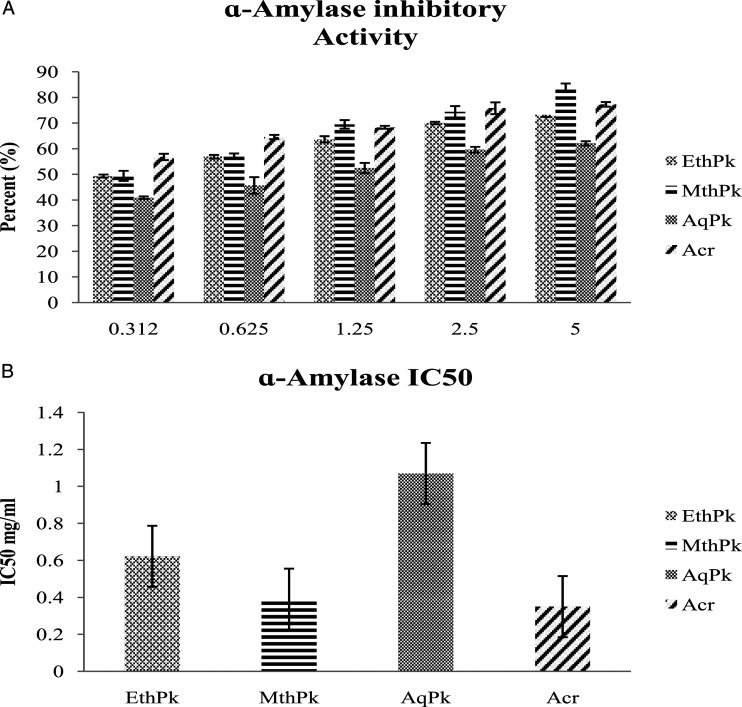
α -Amylase Inhibitory Activity
All extracts of the P. kurroa have noticeable α-amylase inhibitory activity in a concentration dependent way (Figure. 4: A). MthPk demonstrated the maximum α-amylase inhibitory activity with regard to contain lowest IC50 value as .39 ± .41 mg/mL than EthPk (.622 ± .23) and AqPk (1.07±.09).
Figure 4.
A) α-Amylase inhibitory activity of 5 different absorptions of different root extracts of Picrorhiza kurroa. B) IC50 value of α-Amylase inhibitory activity of different root extracts of Picrorhiza kurroa. Results are demonstrated as Mean±Standard deviation of 3-replicates of every extract, that is, MthPk (Methanolic extract of Picrorhiza kurroa), EthPk (Ethanolic extract of Picrorhiza kurroa), and AqPk (Picrorhiza kurroa aqueous extract).DW; Dry Weight of Picrorhiza kurroa extract.
α -Glucosidase Inhibitory Activity
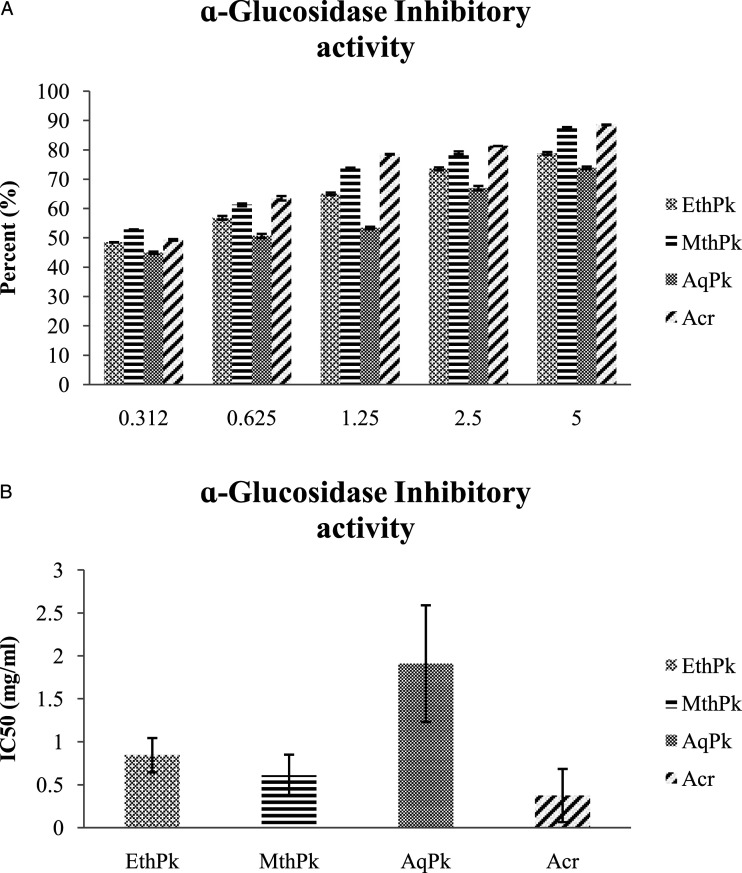
The results of the α-glucosidase inhibition activity also showed a concentration dependent increase in percent activity of the methanolic, ethanolic, aqueous extracts, and acarbose (Figure. 5: A). MthPk demonstrated the highest α-glucosidase inhibitory activity with regard to contain lowest IC50 value as .61± .24 mg/mL.
Figure 5.
A) α-Glucosidase inhibitory activity of 5 different absorptions of different root extracts of Picrorhiza kurroa. B) IC50 value of α-Glucosidase inhibitory activity of different root extracts of Picrorhiza kurroa. Results are demonstrated as Mean±Standard deviation of 3-replicates of every extract, that is, MthPk (Methanolic extract of Picrorhiza kurroa), EthPk (Ethanolic extract of Picrorhiza kurroa), and AqPk (Picrorhiza kurroa aqueous extract). DW; Dry Weight of Picrorhiza kurroa extract.
Phytochemicals and Antioxidant Activity Correlation
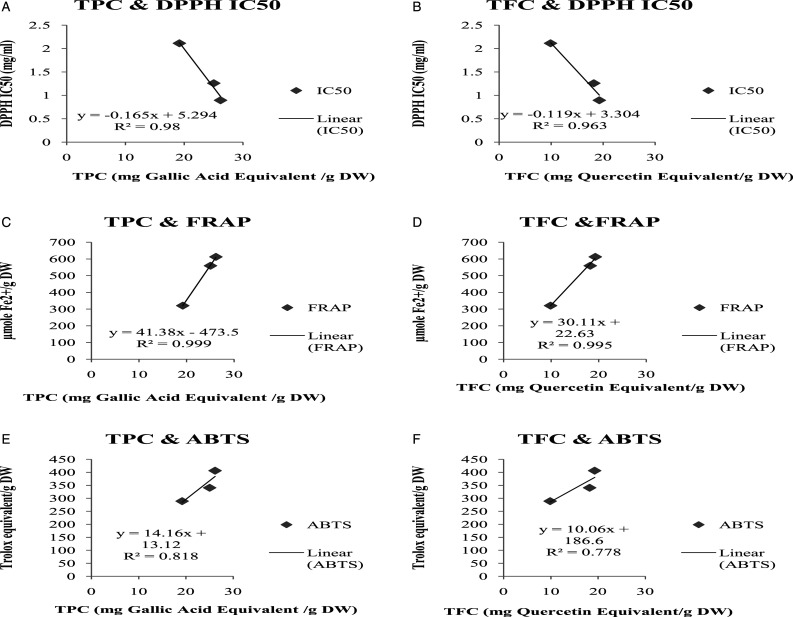
A dominant correlation was observed between TPC and antioxidant assays including FRAP (R2=.999) and DPPH IC50 value (R2=0. .98). TFC also showed strong correlation with FRAP (R2= .995) and IC50 value of DPPH (R2 = .963) (Figure 6 A, B, C, D).
Figure 6.
Total phenolic and Total Flavonoid contents of Picrorhiza kurroa correlation with different antioxidant parameters. A) Total phenolic contents correlation with DPPH IC50 value of different extracts of Picrorhiza kurroa. B) Total Flavonoid contents correlation with DPPH IC50 value of different extracts of Picrorhiza kurroa. C) Total phenolic contents correlation with FRAP of different extracts of Picrorhiza kurroa. D) Total Flavonoid contents correlation with FRAP of different extracts of Picrorhiza kurroa. E) Total phenolic contents correlation with ABTS of different extracts of Picrorhiza kurroa. F) Total Flavonoid contents correlation with ABTS of different root extracts of Picrorhiza kurroa. Results are demonstrated as Mean±Standard deviation of 3-replicates of every extract i.e. MthPk (Methanolic extract of Picrorhiza kurroa), EthPk (Ethanolic extract of Picrorhiza kurroa) and AqPk (Aqueous extract of Picrorhiza kurroa).
Phytochemicals, Antioxidants Correlation with α-Amylase and α -Glucosidase Inhibitory Activities:
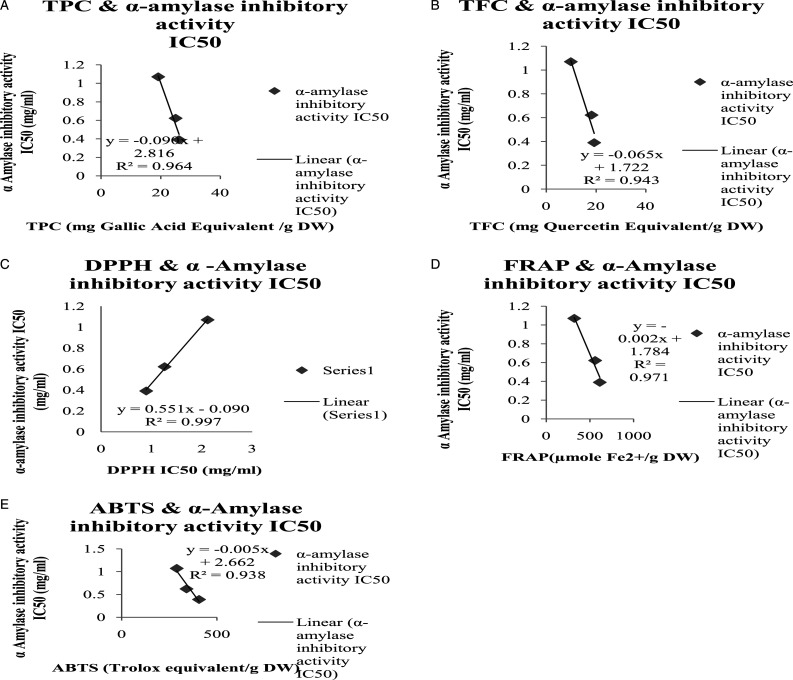
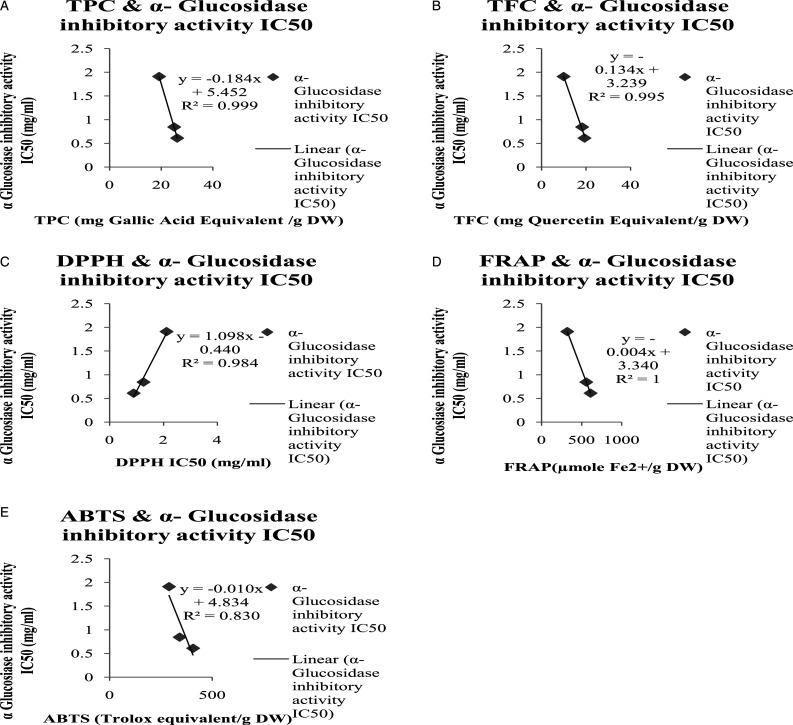
The correlation of TPC, TFC with α-amylase inhibition activity IC50 value (R2 = .964, R2= .943, respectively) showed that increase in TPC and TFC has increased the α-amylase inhibition activity (Figure 7: A: B). The correlation of α-amylase inhibitory activity IC50 value with the all antioxidant parameters also showed a highly positive correlation R2 values as demonstrated in the Figure 7C, D, E. A similar strong correlation was seen in IC50 value of α-glucosidase inhibition activity with TPC, TFC, and all antioxidant parameters as seen in the Figure.8
Figure 7.
α-Amylase inhibitory activity IC50 value of Picrorhiza kurroa correlation with Phytochemical and different antioxidant parameters. A) α-Amylase inhibitory activity IC50 value correlation with total phenolic contents of different extracts of Picrorhiza kurroa B) α- Amylase inhibitory activity IC50 value correlation with total Flavonoid contents of different extracts of Picrorhiza kurroa. C) α-Amylase inhibitory activity IC50 value correlation with DPPH IC50 value of different extracts of Picrorhiza kurroa. D) α-Amylase inhibitory activity IC50 value correlation with FRAP of different extracts of Picrorhiza kurroa. E) α-Amylase inhibitory activity IC50 value correlation with ABTS of different root extracts of Picrorhiza kurroa. Results are demonstrated as Mean±Standard deviation of 3-replicates of every extract, that is, MthPk (Methanolic extract of Picrorhiza kurroa), EthPk (Ethanolic extract of Picrorhiza kurroa), and AqPk (Aqueous extract of Picrorhiza kurroa).
Figure 8.
α-Glucosidase inhibitory activity IC50 value of Picrorhiza kurroa correlation with Phytochemical and different antioxidant parameters. A) α-Glucosidase inhibitory activity IC50 value correlation with total phenolic contents of different extracts of Picrorhiza kurroa B) α-Glucosidase inhibitory activity IC50 value correlation with total Flavonoid contents of different extracts of Picrorhiza kurroa. C) α-Glucosidase inhibitory activity IC50 value correlation with DPPH IC50 value of different extracts of Picrorhiza kurroa. D) α-Glucosidase inhibitory activity IC50 value correlation with FRAP of different extracts of Picrorhiza kurroa. E) α-Glucosidase inhibitory activity IC50 value correlation with ABTS of different root extracts of Picrorhiza kurroa. Results are demonstrated as Mean±Standard deviation of 3-replicates of every extract, that is, MthPk (Methanolic extract of Picrorhiza kurroa), EthPk (Ethanolic extract of Picrorhiza kurroa), and AqPk (Aqueous extract of Picrorhiza kurroa).
Discussion
Plants as medicines are in common use by the people specifically in less developed countries. But by time, the utilization of plants as medicine has also significantly improved in developed countries, moreover, owing to the adverse consequences and the adequacy issues of synthetic drugs.32,33 Plant extracts have numerous valuable consequences for health because of the incredible variety of free radical scavenging constituents, like phenols, flavonoids, vitamins, anthocyanins, and carotenoids. 34 Distinctive phenols contents with the antioxidant activity can assume a significant part in free radicals adsorption and neutralization. 35 These compounds contain effective biological activities36,37 as antibacterial, anticancer, antioxidant, anticholinergic, and antidiabetic.38-40 Flavonoids are secondary derivative that incorporates around 4500 recognized components. 41 The beneficial effects of flavonoids on wellbeing have been long-established for its antidiabetic, 42 anticancer, 43 antioxidant, 44 and anti-inflammatory activities. 45 A preceding study of Nepote et al 46 recommended that methanol solvent is best for the different phenolic contents extraction. This present study investigates TPC and TFC contents in methanolic, ethanolic, and aqueous extracts of the P. kurroa. Results of the study revealed that MthPk possessed the maximum phenolic contents 26.18 ± .44 GAE/g DW and TFC 19.26±.45 mg QE/g as compared to the EthPk (TPC 25.03±2.98 GAE/g DW and TFC 18.2±3.49 mg QE/g) AqPk (TPC 19.18±.83 GAE/g DW and TFC 9.86± .6 mg QE/g). Kumar et al. 47 accounted the maximum presence of flavonoids in the leaves of Picrorhiza kurroa while iridoids were present more in rhizomes. Rajkumar et al. 48 stated the presence of total phenol contents in methanolic extract of the P. kurroa. Krupashree et al. 49 also evaluated TPC and TFC in ethanolic extract of the roots of P. kurroa. Recently, Neupane and Lamichhane, 50 also showed the presence of TPC and TFC in methanolic extract of Picrorhiza kurroa.
Oxidative stress is comparative overabundance of ROS when estimated with antioxidants, has been associated to cardiovascular disease, neurodegenerative disease, diabetes mellitus, and numerous different disorders.51,52 These relations highlight that a balance should be present between the comparative overabundance of ROS and antioxidants. Antioxidants deflect or eliminate oxidative stress associated diseases by neutralizing the ROS deteriorating consequence. If the antioxidants that are produced endogenously do not prevent the reactive species production, it will be required to achieve equilibrium in redox status. Natural antioxidants, such as plants, have a significant impact in this specific situation. 53 In the current study, antioxidant activity of the methanolic extract of the P. kurroa were revealed by using DPPH, FRAP, and ABTS methods. Data that attained demonstrated the significant consequence of the plant extract as an antioxidant. FRAP results revealed that the MthPk have the maximum antioxidant ability 612.54 ± 11.73 µmole Fe2+/g as compared to EthPk 559.38 ±4.02 µmole Fe2+/g and AqPk 320.79±34.93 µmole Fe2+/g. Likewise, ABTS consequences also showed that the of MthPk possessed the maximum antioxidant ability 406.42 ±4.02 µMol Trolox as compared to EthPk 340.67±4.87 µMol Trolox and AqPk 289.19± 12.95 µMol Trolox. Antioxidant property of MthPk in terms of their capability to scavenge free radicals was also determined by most frequently used in vitro assay, the DPPH scavenging property, the results of which demonstrated that the MthPk has highest antioxidant ability with lowest IC50 (.894 ± .57 mg/mL) as compared to EthPk (1.258 ± .26 mg/mL) and AqPk (2.11 ± 0.6 mg/mL). Similarly, Kant et al. 54 revealed the antioxidant effect P. kurroa leaves in term of DPPH, and ABTS methods. Thakur et al. 55 reported antioxidant effect of the peptide of the P. kurroa. Methanolic and aqueous extracts of Picrorhiza kurroa rhizome revealed promising antioxidant potentials in term of DPPH, FRAP, and thiobarbituric acid (TBA) assays. 48
Krupashree et al. 49 also revealed P. kurroa antioxidant property in term of DPPH radical scavenging (IC50 =75.16 ± 3.2 μg/mL) and metal chelating activities (IC50 =55.5 ± 4.8 μg/mL).
A number of studies verify the close connection of TPC and TFC with antioxidant property. 17 The present study results also expose a close association of TPC and TFC with the antioxidant activities including DPPH, ABTS, and FRAP Assays. It also reveals that increased DPPH activity of MthPk is because of the increased in TPC and TFC MthPk. A strong correlation (R2= .98) was shown between TPC and DPPH IC50 that demonstrates raise in the TPC has raised the DPPH scavenging property (Figure 6A). A parallel correlation (R2= .963) was also shown between TFC and DPPH radical scavenging property (Figure 6B). Similar strong correlation between TPC and FRAP (R2 = .999) and TFC and FRAP (R2= .995) of different extracts of P. kurroa was seen in the present study. (Figure 6: C: D). TPC and TFC also showed positive correlation with ABTS (R2 = .818; .778 respectively). Mustafa et al. 17 described the correlation of phenolic and flavonoids with antioxidant activity. In a prior study, Chandra et al. 56 illustrated that TPC and TFC contribute about 75% and 30% for the antioxidant possessions in the field grown crops respectively.
Inhibition of the enzymes associated with starch hydrolysis is an elective method to modify the starch digestion rate. A diversity of digestive enzymes concerned with starch hydrolysis are available in the small intestine and oral cavity.57,58 Along with them, α-amylase and α-glucosidase are key enzymes in the starch and glycogen digestion 59 and assume significant parts in controlling the glucose concentration. 60 Generally, dietary starch is processed by α-amylase into maltose and dextrin, which might be thusly changed over by α -glucosidase into glucose, expanding the blood glucose level. Subsequently, inhibition of one or the other or both α-amylase and α -glucosidase is a powerful method to ease postprandial glycemia. In the present study, α-amylase and α-glucosidase inhibitory activities of the methanolic extract of the P. kurroa were revealed. The result of the present study showed that MthPk contained the greatest α -amylase inhibitory activity with lowest IC50 value0.39±.41 mg/mL as compared to EthPk (.622 ± .23 mg/mL) and AqPk (1.07 ± .09 mg/mL). In the same way, the results showed that MthPk contained the greatest α -glucosidase inhibitory activity as to comprising lowest IC50 value .61 ± .24 mg/mL as compared to EthPk (.844 ± 0.2 mg/mL) and AqPk (1.91±.68 mg/mL). Sanjay et al. 61 reported that protein extract (60%) of the P. kurroa inhibited rat pancreatic α -amylase 41.62±22.3%. P. kurroa also possess the inhibition activities of other enzymes like angiotensin-converting enzyme and dipeptidyl peptidase-IV. 55 Finding of a previous study shows that P. kurroa has the β-cell regeneration capacity.26,62 On the other hand, our study reported that inhibitory activities of the methanolic extract of the P. kurroa against α-amylase and α-glucosidase that is helpful to treat the diabetes. TPC and TFC correlation showed that if higher the TPC and TFC, α-amylase inhibitory activity (R2 = .964 and R2 = .943, respectively), and α-glucosidase inhibitory activity (R2 = .999 and R2 = .995, respectively) also be higher.
Conclusion
Methanolic extract of the Picrorhiza kurroa has the high TPC and TFC contents and also has highest antioxidant potential as it contains the high scavenging capability in terms of the DPPH, ABTS, and FRAP. MthPk also showed highest α-amylase and α-glucosidase inhibitory activities, shows a close connection of TPC and TFC with antioxidant, α-amylase, and α-glucosidase inhibitory activities. So, it is concluded that Picrorhiza kurroa has the potential to balance the oxidative stress and to treat the diabetes by inhibiting α-amylase and α-glucosidase enzymes activities.
Appendix. Abbreviations
- P.K
Picrorhiza kurroa
- TPC
total phenolics content
- TFC
total flavonoids content
- DPPH
Diphenyl-1-picrylhydrazyl
- ABTS
2, 2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)
- FRAP
ferric reducing antioxidant potential
- ROS
reactive oxygen species.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Jaweria Nisar https://orcid.org/0000-0002-3649-2612
Muhammad Akram https://orcid.org/0000-0002-7457-8572
References
- 1.Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer cell. 2020;38:167-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. International journal of physiology, pathophysiology and pharmacology. 2019;11(3):45-63. [PMC free article] [PubMed] [Google Scholar]
- 3.Radi R. Peroxynitrite, a stealthy biological oxidant. J Biol Chem. 2013;288(37):26464-26472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halliwell B. The wanderings of a free radical. Free Radic Biol Med. 2009;46(5):531-542. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Tan H-Y, Wang N, et al. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 2015;16(11):26087-26124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCord JM. The evolution of free radicals and oxidative stress. Am J Med. 2000;108(8):652-659. [DOI] [PubMed] [Google Scholar]
- 7.Poznyak AV, Grechko AV, Orekhova VA, Chegodaev YS, Wu W-K, Orekhov AN. Oxidative stress and antioxidants in atherosclerosis development and treatment. Biology. 2020;9(3):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang P, Li T, Wu X, Nice EC, Huang C, Zhang Y. Oxidative stress and diabetes: antioxidative strategies. Front Med. 2020;2020:1-12. [DOI] [PubMed] [Google Scholar]
- 9.Lubrano V, Pingitore A, Traghella I, et al. Emerging biomarkers of oxidative stress in acute and stable coronary artery disease: levels and determinants. Antioxidants. 2019;8(5):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim K-T, Rioux L-E, Turgeon SL. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry. 2014;98:27-33. [DOI] [PubMed] [Google Scholar]
- 11.Robyt JF. Starch: structure, properties, chemistry, and enzymology. In: Glycoscience. Berlin, Heidelberg: Springer; 2008:1437-1472. [Google Scholar]
- 12.Wang Y, Xiang L, Wang C, Tang C, He X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS One. 2013;8(7):e71144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong L, Feng D, Wang T, Ren Y, Liu Y, Wang J. Inhibitors of α‐amylase and α‐glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Science & Nutrition. 2020;8(12):6320-6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocha S, Sousa A, Ribeiro D, et al. A study towards drug discovery for the management of type 2 diabetes mellitus through inhibition of the carbohydrate-hydrolyzing enzymes α-amylase and α-glucosidase by chalcone derivatives. Food & function. 2019;10(9):5510-5520. [DOI] [PubMed] [Google Scholar]
- 15.de Araújo RF, Martins DBG, Borba MAC. Oxidative stress and disease. In: A master regulator of oxidative stress-the transcription factor nrf2. London, UK: IntechOpen; 2016. [Google Scholar]
- 16.Beidokhti MN, Jäger AK. Review of antidiabetic fruits, vegetables, beverages, oils and spices commonly consumed in the diet. J Ethnopharmacol. 2017;201:26-41. [DOI] [PubMed] [Google Scholar]
- 17.Mustafa I, Faisal MN, Hussain G, et al. Efficacy of Euphorbia helioscopia in context to a possible connection between antioxidant and antidiabetic activities: a comparative study of different extracts. BMC Complementary Medicine and Therapies. 2021;21(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agada R, Usman WA, Shehu S, Thagariki D. In vitro and in vivo inhibitory effects of Carica papaya seed on α-amylase and α-glucosidase enzymes. Heliyon. 2020;6(3):e03618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashaolu TJ. Antioxidative peptides derived from plants for human nutrition: their production, mechanisms and applications. European Food Research and Technology. 2020;246(5):853-865. [Google Scholar]
- 20.Bhagyawant SS, Narvekar DT, Gupta N, Bhadkaria A, Gautam AK, Srivastava N. Chickpea (Cicer arietinum L.) Lectin Exhibit Inhibition of ACE-I, α-amylase and α-glucosidase Activity. Protein Pept Lett. 2019;26(7):494-501. [DOI] [PubMed] [Google Scholar]
- 21.Quan N, Xuan T, Tran H-D, et al. Antioxidant, α-Amylase and α-Glucosidase Inhibitory Activities and Potential Constituents of Canarium tramdenum Bark. Molecules. 2019;24(3):605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolupaev YE, Karpets YV, Kabashnikova LF. Antioxidative System of Plants: Cellular Compartmentalization, Protective and Signaling Functions, Mechanisms of Regulation (Review). Appl Biochem Microbiol. 2019;55(5):441-459. [Google Scholar]
- 23.Nisar J, Mustafa I, Anwar H, et al. Shiitake culinary-medicinal mushroom, Lentinus edodes (Agaricomycetes): a species with antioxidant, immunomodulatory, and hepatoprotective activities in hypercholesterolemic rats. Int J Med Mushrooms. 2017;19(11):981-990. [DOI] [PubMed] [Google Scholar]
- 24.Debnath P, Rathore S, Walia S, Kumar M, Devi R, Kumar R. Picrorhiza kurroa: a promising traditional therapeutic herb from higher altitude of western Himalayas. J Herb Med. 2020;23:100358. [Google Scholar]
- 25.Sharma N. Picrorhiza kurroa. In: Himalayan Medicinal Plants. Cambridge, MA: Academic Press; 2021:67-83. [Google Scholar]
- 26.Kumar S, Patial V, Soni S, et al. Picrorhiza kurroa Enhances β-Cell Mass Proliferation and Insulin Secretion in Streptozotocin Evoked β-Cell Damage in Rats. Frontiers in pharmacology. 2017;8:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh KL, Bag Bag G. Phytochemical analysis and determination of total phenolics content in water extracts of three species of Hedychium. Phytochemical Analysis. 2013;5(4):1516-1521. [Google Scholar]
- 28.Bajalan I, Mohammadi M, Alaei M, Pirbalouti AG. Total phenolic and flavonoid contents and antioxidant activity of extracts from different populations of lavandin. Ind Crop Prod. 2016;87:255-260. [Google Scholar]
- 29.Adebiyi OE, Olayemi FO, Ning-Hua T, Guang-Zhi Z. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Beni-Suef univ j basic appl sci. 2017;6(1):10-14. [Google Scholar]
- 30.Sethi S, Joshi A, Arora B, Bhowmik A, Sharma RR, Kumar P. Significance of FRAP, DPPH, and CUPRAC assays for antioxidant activity determination in apple fruit extracts. European Food Research and Technology. 2020;246(3):591-598. [Google Scholar]
- 31.Asem N, Abdul Gapar NA, Abd Hapit NH, Omar EA. Correlation between total phenolic and flavonoid contents with antioxidant activity of Malaysian stingless bee propolis extract. J Apicult Res. 2020;59(4):437-442. [Google Scholar]
- 32.Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in pharmacology. 2014;4:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunathilake KDPP, Ranaweera KKDS, Rupasinghe HPV. Change of phenolics, carotenoids, and antioxidant capacity following simulated gastrointestinal digestion and dialysis of selected edible green leaves. Food Chemistry. 2018;245:371-379. [DOI] [PubMed] [Google Scholar]
- 35.Tlili N, Elfalleh W, Hannachi H, et al. Screening of natural antioxidants from selected medicinal plants. Int J Food Prop. 2013;16(5):1117-1126. [Google Scholar]
- 36.Rahman MJ, Ambigaipalan P, Shahidi F. Biological Activities of Camelina and Sophia Seeds Phenolics: Inhibition of LDL Oxidation, DNA Damage, and Pancreatic Lipase and α-Glucosidase Activities. Journal of food science. 2018;83(1):237-245. [DOI] [PubMed] [Google Scholar]
- 37.Granato D, Shahidi F, Wrolstad R, et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chemistry. 2018;264:471-475. [DOI] [PubMed] [Google Scholar]
- 38.Khalil R, Ali Q, Hafeez MM, Malik A. Phenolic acid profiling by RP-HPLC: evaluation of antibacterial and anticancer activities of Conocarpus erectus plant extracts. Biological and Clinical Sciences Research Journal. 2020;2020(1):e010-e010. [Google Scholar]
- 39.Bursal E, Taslimi P, Gören AC, Gülçin İ. Assessments of anticholinergic, antidiabetic, antioxidant activities and phenolic content of Stachys annua. Biocatal Agric Biotechnol. 2020;28:101711. [Google Scholar]
- 40.Aruwa CE, Amoo S, Kudanga T. Phenolic compound profile and biological activities of Southern African Opuntia ficus-indica fruit pulp and peels. LWT (Lebensm-Wiss & Technol). 2019;111:337-344. [Google Scholar]
- 41.Croteau R, Kutchan TM, Lewis NG. Natural products (secondary metabolites). Biochemistry and molecular biology of plants. 2000;24:1250-1319. [Google Scholar]
- 42.Vu NK, Kim CS, Ha MT, et al. Antioxidant and antidiabetic activities of flavonoid derivatives from the outer skins of Allium cepa L. J Agric Food Chem. 2020;68(33):8797-8811. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z, Yang L, Hou J, Tian S, Liu Y. Molecular mechanisms underlying the anticancer activities of licorice flavonoids. J Ethnopharmacol. 2020;267:113635. [DOI] [PubMed] [Google Scholar]
- 44.Zeng Y, Song J, Zhang M, Wang H, Zhang Y, Suo H. Comparison of in vitro and in vivo antioxidant activities of six flavonoids with similar structures. Antioxidants. 2020;9(8):732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Y-B, Chen G-L, Guo M-Q. Antioxidant and anti-inflammatory activities of the crude extracts of Moringa oleifera from Kenya and their correlations with flavonoids. Antioxidants. 2019;8(8):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nepote V, Grosso NR, Guzmán CA. Optimization of extraction of phenolic antioxidants from peanut skins. J Sci Food Agric. 2005;85(1):33-38. [Google Scholar]
- 47.Kumar D, Kumar R, Singh B, Singh Ahuja P. Comprehensive chemical profiling of Picrorhiza kurroa Royle ex Benth using NMR, HPTLC and LC-MS/MS techniques. Comb Chem High Throughput Screen. 2016;19(3):200-215. [DOI] [PubMed] [Google Scholar]
- 48.Rajkumar V, Guha G, Ashok Kumar R. Antioxidant and anti-neoplastic activities of Picrorhiza kurroa extracts. Food Chem Toxicol. 2011;49(2):363-369. [DOI] [PubMed] [Google Scholar]
- 49.Krupashree K, Hemanth Kumar K, Rachitha P, Jayashree GV, Khanum F. Chemical composition, antioxidant and macromolecule damage protective effects of Picrorhiza kurroa Royle ex Benth. South Afr J Bot. 2014;94:249-254. [Google Scholar]
- 50.Neupane P, Lamichhane J. Estimation of total phenolic content, total flavonoid content and antioxidant capacities of five medicinal plants from Nepal. Vegetos. 2020;33(2):360-366. [Google Scholar]
- 51.Forman HJ, Zhang H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 2021:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rekatsina M, Paladini A, Piroli A, Zis P, Pergolizzi JV, Varrassi G. Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: a narrative review. Adv Ther. 2020;37(1):113-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jamshidi-Kia F, Wibowo JP, Elachouri M, et al. Battle between plants as antioxidants with free radicals in human body. Journal of Herbmed Pharmacology. 2020;9(3):191-199. [Google Scholar]
- 54.Kant K, Walia M, Agnihotri VK, Pathania V, Singh B. Evaluation of antioxidant activity of Picrorhiza kurroa (leaves) extracts. Indian Journal of Pharmaceutical Sciences. 2013;75(3):324-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thakur S, Chhimwal J, Joshi R, Kumari M, Padwad Y, Kumar R. Evaluating Peptides of Picrorhiza kurroa and Their Inhibitory Potential against ACE, DPP-IV, and Oxidative Stress. J Proteome Res. 2021. [DOI] [PubMed] [Google Scholar]
- 56.Chandra S, Khan S, Avula B, et al. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evid base Compl Alternative Med. 2014;2014:253875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rasouli H, Hosseini-Ghazvini SM-B, Adibi H, Khodarahmi R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: a virtual screening perspective for the treatment of obesity and diabetes. Food & function. 2017;8(5):1942-1954. [DOI] [PubMed] [Google Scholar]
- 58.Taylor JR, Emmambux MN, Kruger J. Developments in modulating glycaemic response in starchy cereal foods. Starch‐Stärke. 2015;67(1-2):79-89. [Google Scholar]
- 59.Sales PM, Souza PM, Simeoni LA, Magalhães PO, Silveira D. α-Amylase Inhibitors: A Review of Raw Material and Isolated Compounds from Plant Source. J Pharm Pharmaceut Sci. 2012;15(1):141-183. [DOI] [PubMed] [Google Scholar]
- 60.Tan Y, Chang SKC, Zhang Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chemistry. 2017;214:259-268. [DOI] [PubMed] [Google Scholar]
- 61.Sanjay S, Banu SH, Chethankumar M. The study of potentiality of picrorhiza kurroa root proteins to inhibit free radicals and α-amylase enzyme. Asian J Pharm Clin Res. 2015;8(2):220-225. [Google Scholar]
- 62.Husain GM, Rai R, Rai G, Singh HB, Thakur AK, Kumar V. Potential mechanism of anti-diabetic act.ivity of Picrorhiza kurroa. Cell Med. 2014;4(4):27-31. [Google Scholar]