Abstract
The current study aimed to explore active metabolites of locally recognized and high yielding cultivar cluster bean (BR-99) with a wide range of adaptability having antioxidant, antidiabetic, antimicrobial, and cytotoxic potential. Six solvents were used (crude methanol, n-hexane, chloroform, ethyl acetate, butanol, and aqueous) with escalating polarity for colorimetric determination of antioxidants such as total phenolic contents (TPC), total flavonoid contents (TFC), and free radical scavenging activity (FRSA) by DPPH (2, 2-diphenyl-1-picrylhydrazyl) assay. Moreover, an antidiabetic and anticancer study was conducted by α-amylase inhibition and MTT (3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-tetrazolium bromide) assay. Biological investigations were carried out against the most commonly found infectious microbial strains. The significant results (P ≤ .001) of each activity were seen among six tested solvent extracts. The ethyl acetate and methanol extract have more antioxidant potential with the highest TPC (16.38 ± .13 mg GAE/g) and TFC (8.15 ± .24 mg CE/g), respectively. Similarly, methanol extract presented the highest free radical scavenging activity (46.31 ± .91%), followed by ethyl acetate, butanol, chloroform, aqueous, and n-hexane extract. However, the maximum α-amylase inhibition (62.54 ± 1.47%) and anticancer activity against human lung cancer cells were congregated (78.31 ± 1.46%) in butanol and chloroform, respectively. A positive correlation was seen between TPC with TFC (R2= .8356), FRSA (R2= .8381), and anti-diabetic activity (R2= .8082), which highlights the phenolic contents as strong anti-oxidant agents especially flavonoids. Each extract of cluster bean (BR-99) showed significant antimicrobial activities for all tested bacterial strains except B. cereus and E. coli. The profound results of maximum antibacterial activity were witnessed by chloroform extract while ethyl acetate extracts showed great antifungal potential against all tested fungal strains. The HPLC quantitative analysis results of cluster bean (BR-99) revealed the presence of active phytochemicals such as gallic acid, HB acid, vanillic acid, kaempferol, sinapic acid, ferulic acid, salicylic acid, coumarins, quercetin, rutin, p-coumaric acid, and catechin, and the variation in both phytochemical and biological spectrums envisioned the cluster bean (BR-99) used in future as a cheap, safer, and potent source of bioactive drugs.
Keywords: cluster bean, anti-oxidants, anti-diabetic potential, cytotoxic assay, anti-bacterial activity, anti-fungal activity
Introduction
Plants are continuously exploitive as a source of diverse chemical compounds used for natural drugs and related products. The plants produce these natural compounds in bulk amounts, used in herbicides, executioners, chemotherapeutics, or different medications (Pandey et al, 2013). These plant-driven natural bio products possess anti-microbial and anti-diabetic properties (Davidson and Naidu, 2000; Alexander, 2005; Singhal et al, 2017). Natural drugs have received preference owing to their safety than failure in the advancement of modern treatment of chronic diseases, unavoidable side effects, and microorganism resistance (Escosteguy, 2014; Li et al, 2016; Saleem et al, 2019; Kuralkar and Kuralkar, 2021). So, photo-therapy programs are encouraged nowadays and supported by governments with allopathic medicine in numerous countries, such as Pakistan, India, Russia, China, Mexico, and others (Kadian et al, 2013). World Health Organization (WHO) concluded that about eighty percent of the total world’s population lies on plant-dependent medicines (Adki et al, 2020). Currently, numerous plant-based secondary compounds (PSC), Phyto-biotics, phytogenic feed additives (PFA), or drugs considering anti-microbial, anti-oxidants, and osmoprotectants owed significant consideration due to strong positive influence on the health and well-being of living organisms (Stevanović et al, 2018; Singh and Gaikwad, 2020).
Cluster bean is known as folklore medicine, a highly potent source of phytochemicals or natural compounds (Daniel, 1989; Morris and Wang, 2007; Badr et al, 2013; Kaushik et al, 2020), and cultivated for its multiple uses in tropical Africa and Asia (Ashraf et al, 2002; Gresta et al, 2013; Mubarak et al, 2015). Pakistan is the second producer of cluster beans after India (Yadav et al, 2013; Anonymous, 2018; Rajaprakasam et al, 2021). It is well-known as the heart of the farmer fields or cash crop with broad economic benefits in food, bakery, paper, textile, explosives, mining, cosmetic, pharmaceutical, nutraceutical, well drilling industries (Whistler and Hymowitz, 1979; Ashraf et al, 2005; Abidi et al, 2015; Gresta et al, 2017) and also used for chicken, buffalos, and cattle food (Salehpour et al, 2012; Rao et al, 2014; Saeed et al, 2017). This crop is profitably contributing to crop rotation systems (Jukanti et al, 2019; Deng et al, 2019; Jerine Peter et al, 2019) with an improved soil-plant nutritional profile (Elsheikh and Ibrahim, 1999; Hinson and Adams, 2020).
Cluster bean plant has received tremendous attention as functional foods based on their high nutritional profile like 28.3 to 35.0% crude protein, 4.1 to 8.0% crude fiber, 38.8 to 59.1% carbohydrate contents, 2.3% to 7.3% oil and moisture contents along with polyphenols, tannins, and phytic acid up to 25 mg, 1750 mg and, 540 mg/100 g contents, respectively, in cluster bean seed reported by Pathak et al, 2011; Bouchenak and Lamri-Senhadji, 2013. The cluster bean seeds with important Fatty acids such as linoleic acid (55.1%), palmitic acid (24.97%), and oleic acid (23.59%) were reported by Arora et al, 1985. Cluster beans enriched in tannins, flavonoids, and coumarins are used by diabetes patients in Pakistan (Mukhtar et al 2006; Ahmed et al, 2015; Majeed et al, 2021). It is considered effective in dyspepsia, anorexia, anti-secretory, hypo-lipidemic, and anti-hyperglycemic effects (Morris and Wang, 2007). Its use as a complementary medicinal plant (Badr et al, 2013; Jamshed et al, 2018; Kaushik et al, 2020) is due to the presence of several pharmaceutically active compounds such as quercetin, daidzein, and kaempferol, reported by Sharma et al, 2011; Jain and Rijhwani, 2018. These natural bioactive molecules are now a trend in the food industry due to their cost-effective and eco-friendly nature (Tripathi and Pandey, 2016; Beyene et al, 2020). The aqueous and ethanol extract of cluster bean fruits exhibited noteworthy antidiabetic potential in alloxan and streptozocin-induced diabetes among rats (Saeed et al, 2012; Quero et al, 2020). The methanolic extract of cluster bean was reported beneficial for having maximum anti-oxidants properties linked directly to its phenolic contents (Moteriya et al, 2015; Babbar et al, 2014). Additionally, the methanolic extract was found helpful in the therapeutic cure of anti-Parkinson’s activity and motor dysfunction, owing to its anti-oxidants potential (Kaur and Saxena, 2021). The significant (99.99%) anti-viral potential of this plant was reported by Kaushik et al, 2020 tested against dengue-2-virus.
Keeping in view the pharmacological potential of cluster bean, the BR-99 is the locally available cultivar of cluster bean with maximum grain yield potential that is, 1900 kg ha−1, and also provides 30 t ha−1 fodder yield in Pakistan (lqbal Saleem et al, 2002; Khan et al, 2018; Shakir et al, 2020), however, with very less phytochemical exploitation knowledge. The current study aimed with detail exploration of the phytochemical and biological potential of locally recognized with high yield and wide range of adaptation cluster bean (BR-99) cultivar.
Methodology
Experiment Design
Seeds of one cultivar named BR-99 were obtained from Arid Zone Research Institute Bhakkar, Pakistan. A pot experiment was conducted from July to October 2019 in the Botanical Garden of Botany department GCUF in the month of July. During the experiment, the average maximum (39.2 + 2°C) and minimum temperature (28.3 + 2°C) was noticed. Seeds were sterilized with 5% NaClO4 solution up to 5 min before use. The twelve seeds were surface sterilized with .5% NaClO4 solution for up to 5 min, rinsed with water, and dried before sown. Pure, washed, and dried river sand was used in the pot to avoid contamination. After emergence, the seedlings were thinned to 5 plants per pot and allowed to grow till the maturity. Weeding and watering were done at regular intervals to ensure healthy plant growth.
Preparation of the Crude Extract
The entire plant body of cluster bean cultivar was washed thoroughly, dried in the shaded area, and then grinded into a moderately coarse powder (1 kg), and soaked in 10 L methanol in the ratio of 1:10 (1 g plant powder: 10 mL methanol solvent) for a period of 20–25 days, along with regular agitation in extraction drum (Jain and Rijhwani, 2018). The extraction drum was kept at room temperature. The methanol-soluble compounds were filtered and evaporated with rotary evaporator (Buchi, Switzerland) under reduced pressure at 45°C.
Fractionation of Crude Extract
The crude methanol extract was further used to ensure the complete and efficient extraction of all active compounds with five different solvents in order of increasing polarity. A separating funnel was used for solvent extraction with the evaporation of solvents by rotary evaporator, and resulting extracts of all solvents were poured in three different Petri plates and allowed to dry. The crude methanol extract (10 g) was poured into glass vials as crude methanol extract for further phytochemical and biological study. The remaining portion was mixed with distilled water to make an aqueous fraction and then transferred to a separating funnel. Analytical grade n-hexane was used and poured into the separating funnel. The funnel was agitated till two separate layers were formed. The n-hexane soluble compounds layers were obtained and dried to a semisolid state with a rotary evaporator. The recovered aqueous layer was re-extracted with n-hexane till all soluble compounds were obtained. The semisolid n-hexane fractionation was dried in a petri dish at about 45°C and stored in sterilized vials till next used. The same process was repeated with chloroform, ethyl acetate, and butanol, respectively. In the end, different extracts (six solvents) were formed that is, crude methanol extract, n-hexane, chloroform, ethyl acetate, butanol, and aqueous (Figure 1). These extracts were placed at 2–8°C for further phytochemical and biological investigation study.
Figure 1.
Schematic representation of different extract preparation of cluster bean (BR-99) using different solvents (methanol, n-hexane, chloroform, ethyl acetate, butanol, and aqueous).
Percent Yield of Extract
The total yield of each dried extracts were weighted to find percentage yield of each extract (Fatima et al, 2015)
Phytochemical Analysis
Total Phenolic Contents (TPC) Determination
The total phenolic of each extract was assessed by the Folin-Ciocalteau reagent assay used by Pranoothi et al (2014). Reaction mixture was made with 1 mL of each test sample (.5-4 mg/mL), Folin-Ciocalteau reagent (.5 mL) and 1.5 mL 20% Na2CO3 (20%). The absorbance of the reaction was read at 765 nm with a spectrophotometer after 60 minutes of incubation. The blank reagent was made with distilled water. Analysis was performed in triplicate and the quantification of phenolics was done by using a standard curve of gallic acid and expressed in the unit of mg gallic acid equivalent (GAE)/g dry weight (DW) of the sample.
Estimation of Total Flavonoid Contents (TFC)
Total flavonoid contents were quantified with AlCl3 colorimetric assay reported by Abdel-Sattar et al, 2008; Beyene et al, 2020. Briefly, 1 mL of each test sample (.5–4 mg/mL) were mixed with .3 mL of 5% NaNO2, and .3 mL of AlCl3 (10%) with the interval of 5 min followed by reaction with 2 mL of NaOH (1M). After 5–6 minute intervals, the absorbance was determined at 510 nm using a spectrophotometer. Distilled water was used as blank. Standard calibration was done using catechin and expressed as mg of catechin equivalents (CE)/g (DW).
High-Performance Liquid Chromatography (HPLC) Quantitative Analysis
Six solvent extracts of cluster bean were used for reverse-phase HPLC to quantify the phenolic and flavonoids contents (Proestos et al, 2005). Reaction mixture of each extract was prepared in methanol and filtered through .2 μm syringe membrane filters (Saleem et al, 2020) before injecting into HPLC. The C18 column (250 × 4.6 mm internal diameter) of 5 μm film thickness was used with temperature control system set at 30°C. Chromera HPLC system (Perkin Elmer, USA.) attached with Flexer Binary LC pump, UV/Vis LC Detector (Shelton CT, 06484 USA) controlled by software V. 4.2.6410 used to analyze the data. The mobile phase consisting of solvent A (acetonitrile: methanol, 70:30) and solvent B (double distilled water with .5% glacial acetic acid). The UV spectra were adjusted at 275 nm. Each compound was identified by retention time combination and matching of spectra. HPLC separation efficiency was assessed by the separation factor and resolution.
Anti-Oxidant Potential Estimation
In Vitro Free Radical Scavenging Assay
The antioxidant potential of cluster beans was estimated by using the method of Zahra et al (2017). Test samples were prepared in DMSO (.5-4 mg/mL). Then 20 μL of the test samples were prepared in dimethyl sulfoxide (DMSO) and mixed with 180 μL of 2, 2-diphenyl-1-picrylhydrazyl (DPPH). The DPPH was prepared by 9.2 mg dissolving in 100 mL methanol. The reaction mixtures were incubated at 37°C in the dark for 60 minutes. Ascorbic acid and DMSO were used as positive and negative controls. The absorbance of samples was measured at 517 nm. This assay is based on the discoloration of DPPH purple color and inhibition or scavenging (%) was measured
Determination of Anti-Diabetic Activity
In Vitro Alpha (α)-Amylase Inhibition Assay
The assay was performed as described by Zahra et al, 2017 with slight modification to determine the antidiabetic potential in cluster bean extracts. The stock solution of each extract was prepared in DMSO (.5–4 mg/mL). The reaction mixture was prepared in designated wells of a microtiter plate with 15 μL phosphate buffer (pH 6.8), α-amylase enzyme (25 μL), test sample (10 μL), and starch solution (40 μL), 20 μL HCl (1M), and an iodine reagent (90 μL: 5 mM) were added after incubation (50°C) for 30 minutes. Blank was prepared with α-amylase enzyme and an equal amount of phosphate buffer without test samples. Acarbose (positive control) and DMSO (negative control) were used. The absorbance of the mixture was read at 540 nm wavelength in triplicate. The inhibition (%) was calculated by
Biological Activity Determination
Cytotoxicity Assay
Cytotoxicity of cluster bean solvent extracts was determined by the MTT assay as mentioned by Rasul et al (2013). Briefly, A549 cells (100 μL) were seeded in 96-well plates at a density of 1 × 103 to 104 cells/well for a period of overnight to grow in well. After that, cells were incubated and treated with each test sample (100 μL) of plant extracts prepared by dissolving the 4 mg/mL DMSO (.05%) for 24/48 hours. Following incubation, cell growth of each well was determined after a reaction with MTT (10 μL: 5 mg/mL in saline phosphate buffer) separately, and incubated for another 4 hours at 37°C. Afterward, DMSO (150 μL) was added and shaken with each well after the removal of the medium to dissolve formazan crystals. The absorbance was measured at 490 nm. Taxol was used as a positive control
Cell Culture
Dulbecco’s Modified Eagle’s medium was used to culture Human A549 lung cancer cells supplemented with (FBS) Fetal bovine serum (10%), penicillin (100 units/mL), and streptomycin (100 μg/mL) in a humidified atmosphere with carbon dioxide (5%) and air (95%) at 37°C. Cells were cultured in a culture dish (10 cm) and allowed to grow till confluent (70%) before testing.
Anti-Microbial Assay
Supplemented nutrient agar media utilized for the growth of testing microorganisms. Culture media was prepared for shaking, development, and standardization of microorganisms (Tassou et al, 2000). The respective agar media were prepared in a culture bottle of 500 mL capacity. The culture bottles were sterilized in an autoclave and kept these media bottles at a constant temperature of about 45 to 50oC to avoid contamination. After that, media (20–25 mL) shifted to Petri dishes (sterilized) and solidified into smooth and uniform thin media layers. The sterile climate was ensured during pouring. After solidification of media, the plates were used for respective microorganism growth, separately. The plates were inoculated by spreading techniques aseptically with prepared inoculums of specific fungal and bacterial culture in a laminar hood (Table 1). On each plate, eight wells (8 mm) were bored with borer into agar media, afterward, 100 μL per well of each test sample of plant extracts dissolved in DMSO were poured into the well. An equivalent volume of the reference standard (anti-biotic as a positive control), and DMSO (negative control) were filled parallel in separate wells. Inoculated plates were placed at 37°C for a period of 24 hr. The average diameter of inhibition zones was measured in mm to determine the sensitivity of microorganisms toward plant extracts. Different drugs such as azithromycin (gram-positive bacterial strain), ciprofloxacin (gram-negative bacterial strain), and clotrimazole (fungal strain) were used as positive controls. The assay was run in triplicate for every microorganism.
Table 1.
Different microbial strains tested for vulnerability to cluster bean extracts.
| Sr. No. | Microbial species | Strain type |
|---|---|---|
| 1 | Bacillus cereus | Gram positive |
| 2 | E. coli | Gram positive |
| 3 | Salmonella enterica | Gram negative |
| 4 | Staphylococcus aureus | Gram negative |
| 5 | Fusarium avenaceum | Fungi |
| 6 | Fusarium brachygibbosum | Fungi |
| 7 | Aspergillus niger | Fungi |
Statistical Analysis
The results of phytochemical as well as biological assays were statistically analyzed by one-way analysis of variance (ANOVA) to test the statistical significance of variability over the treatment followed by LSD test and P < .05, P < .01, or P < .001 was considered as significant. Data were expressed as mean ± SE. Correlation coefficients were also calculated from measured phytochemical variables of cluster bean (BR-99) extracts.
Results and Discussion
Extraction Yield
Each extract yield (%) recovered in six different solvents was depicted in Figure 2. The extraction of phytochemicals from cluster bean (BR-99) was showing good yield in methanol followed by aqueous, butanol, chloroform, and ethyl acetate extract, that is, 17.80 ± .3, 12.43 ± .09, 8.67 ± .05, 7.86 ± .09, and 5.67 ± .13%, respectively, while the low yield was obtained from a hexane extract (4.36 ± .09%). It is clear from the above results that different solvent has different extraction potential as it was observed in the current study that polar solvents have shown more potential toward phytochemical extraction in comparison to non-polar solvents; however, the plant has a diverse group of compound/drugs of varying nature that may or may not be soluble in specific solvent since compound extraction is totally dependent on nature or polarity of the solvent. So, the solvent selection is a critical factor for phytochemical extraction (Fatima et al, 2015). However, greater extract yield does not ensure the maximum biological activity or the medical potential of the extract. The activity might be more prominent or striking in low yield solvent or vice versa, independent of extract yield. However, the medicinal active potential is depending on the intrinsic nature of components or compounds either present in the crude fraction form or pure form (Zahra et al, 2017).
Figure 2.
Percent extract recovery of cluster bean (BR-99) using different extraction solvents (methanol, n-hexane, chloroform, ethyl acetate, butanol, and aqueous). The level of significant was set at P<.05 using one-way ANOVA followed by LSD test. The results are expressed as mean ± SE, n= 3. *** represents P<.001, ** represents P<.01, * represents P<.05 and ns=non-significant. Abbreviations: MeOH = methanol, CHCl3 = chloroform, and EtOAc= ethyl acetate.
Phytochemical Analysis
Total Phenolic and Total Flavonoid Contents
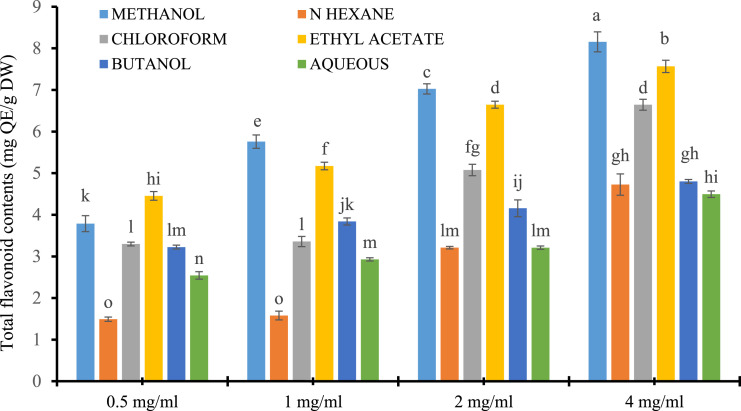
Plant polyphenols are ubiquitously found in each part of the plant. They are the metabolites of anti-oxidant potential for singlet O2 quenching, free radical scavenging, metal-ion ligation, as hydrogen donors, or for superoxide dismutation (Zahra et al, 2017). Plant-based natural bioactive molecule uses are now in trend as improved neuroprotective, anti-diabetic, hepatic-protective, anti-microbial, and nutritional stress suppressors for the better growth and development of living organisms (Kumar et al, 2014; Beyene et al, 2020). Previous literature reported cluster bean as a profound source of TPC and TFC (Kallel et al, 2014; Moteriya et al, 2014, Ammar et al, 2015) suggesting it as the phytochemically active plant. Similarly, both contents (TPC and TFC) were showing significant variation (P ≤ .001) among extracts of cluster bean prepared in different solvents as given in Figure 3 and 4, respectively. The maximum TPC ranges from the highest value of 16.38 ± .13 to the low value of 10.47 ± .084 mg GAE/g DW were produced by ethyl acetate extract followed by chloroform with the range of maximum value 16.27 ± .13 to the low value of 9.37 ± .13 mg GAE/g DW. On the whole, the phenolic contents among each extract exhibited the increasing pattern in the following order: aqueous < hexane < butanol < methanol < chloroform < ethyl acetate (Figure 3). While the flavonoids are free radical acceptors, found the diversified form of phenolics (Nijveldt et al, 2001). The order to increase in total flavonoids contents among different solvents was methanol > ethyl acetate > chloroform > butanol > hexane > aqueous with the highest value of 8.15 ± .24 to the lowest value of 3.78 ± .19 mg QE/g DW given by the methanol fraction (Figure 4). The results of the present study were supported by the findings of Moteriya et al, 2015; Tripathi and Pandey, 2016 which showed that cluster bean cultivar BR-99 has a rich source of phenolics and flavonoids.
Figure 3.
Total phenolic content (TPC) determination in different solvent extracts (methanol, n-hexane, chloroform, ethyl acetate, butanol, and aqueous) of cluster bean (BR-99). The results are expressed as mean ± SE, n= 3. *** represents P<.001, ** represents P<.01, * represents P<.05 and ns=non-significant.
Figure 4.
Total flavonoid content estimation in different solvent extracts (methanol, n-hexane, chloroform, ethyl acetate, butanol, and aqueous) of cluster bean (BR-99). The results are expressed as mean ± SE, n= 3. *** represents P<.001, ** represents P<.01, * represents P<.05 and ns=non-significant.
A strong positive correlation (correlation coefficient, R2 = .8356) was found to be present between the TPC and TFC signifying the antioxidant potential of phenols due to the presence of flavonoids. On the basis of literature survey, the phenolic contents (ferulic, caffeic, vanillic, gentisic acid, p-coumaric, and ellagic acids) and flavonoids contents (luteolin, daidzein, quercetin, 3-arabinosides, and kaempferol) reported by Sharma et al, 2011; Kobeasy et al, 2011; Badr et al, 2014; Adki et al, 2020, while gallic acid, sinapic acid, salicylic acid, coumarins, HB acid, vanillic acid, kaempferol, ferulic acid, quercetin, p-coumaric acid, and catechin were found more active in cluster bean cultivar BR-99 extract.
Quantification by HPLC
The phenolic compounds found in cluster bean extracts are given in Figure 5 and quantities are mentioned in Table 2. Out of 13 tested polyphenols, the methanol extract contained p-coumaric acid, gallic acid, kaempferol, quercetin, ferulic acid, catechin, and HB acid followed by chloroform extract with four compounds; gallic acid, kaempferol, p-coumaric acid, and rutin were, and three compounds; gallic acid, coumarins, and p-coumaric acid were noticed in the ethyl acetate extract. The aqueous and hexane extracts were found to contain few amount of flavonoids and phenolics in comparison with other extracts. The presence of all these plant metabolites was drawing a parallel correlation of plant potential with their known bioactivities, for example, rutin is usually found in the invasive plant species and shown strong antibacterial and antioxidant properties, observed in chloroform fraction with maximum yield (Dain and Mumper, 2010). The butanol extract contained a less number of testing phenols than the other extracts, but it exhibited significant FRSA, alpha-amylase inhibition assay, and biological activities. On a whole, it proposed that there are some other polyphenols along with these tested phenols which are responsible for the above-said bioactivities. However, phenolic acids, polyphenols, or flavonoids are documented for having strong potential against most common chronic diseases resulting from oxidative stress (Zhang and Tsao, 2016). Hence, the presence of bioactive polyphenols concluded this cultivar a potential source for as cheap, nontoxic, and active anticancer drugs.
Figure 5.
RP-HPLC chromatograms of (A), standard compounds (B), methanol (C), n-hexane (D) chloroform (E), ethyl acetate (F), butanol (G), and aqueous extract of cluster bean (BR-99).
Table 2.
Chemical profiling of different solvent extracts of cluster bean cultivar by using HPLC.
| Components | Methanol (MeOH) | n-Hexane | Chloroform (CHCI3) | Ethyl acetate (EtOAc) | Butanol | Aqueous | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retention time | Area (%) | Quantity (ppm) | Retention time | Area (%) | Quantity (ppm) | Retention time | Area (%) | Quantity (ppm) | Retention time | Area (%) | Quantity (ppm) | Retention time | Area (%) | Quantity (ppm) | Retention time | Area (%) | Quantity (ppm) | |
| Chlorogenic acid | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Gallic acid | 3.389 | 539 388.20 | 47.4661616 | - | - | - | 3.364 | 829 189.40 | 72.9686672 | 3.37 | 3,072,129.80 | 270.3474224 | 3.337 | 1 142 225.50 | 100.515844 | - | - | - |
| HB acid | 7.011 | 494 438.80 | 79.110208 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Caffeic acid | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Vanillic acid | - | - | - | 7.661 | 75 504.90 | 5.88673073 | - | - | - | - | - | - | - | - | - | |||
| Kaempferol | 11.061 | 1 434 588.20 | 58.53119856 | - | - | - | 11.088 | 3 760 808.20 | 153.4409746 | - | - | - | - | - | - | - | - | - |
| Sinapic acid | - | - | - | - | - | - | - | - | - | - | - | - | 12.286 | 677 438.40 | 38.41075728 | - | - | - |
| Ferulic acid | 12.92 | 1 469 042.80 | 112.6755828 | 12.432 | 140 736.00 | 10.7944512 | - | - | - | - | - | - | - | - | - | |||
| Salicylic acid | - | - | - | - | - | - | - | - | - | - | - | - | 14.94 | 330 811.20 | 124.7158224 | |||
| Coumarin | - | - | - | - | - | - | 15.851 | 519 448.70 | 623.33844 | - | - | - | 16.048 | 141 539.00 | 169.8468 | |||
| Quercetin | 24.922 | 494 438.80 | 344.1294048 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Benzoic acid | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Rutin | - | - | - | - | - | - | 29.546 | 9 899 746.40 | 1118.671682 | - | - | - | - | - | - | - | - | - |
| P-Coumaric acid | 3.161 | 289 399.60 | 2.80717612 | - | - | - | 3.172 | 203 367.10 | 1.97266087 | 3.166 | 1 142 964.50 | 11.08675565 | ||||||
| Catechin | 3.389 | 539 388.20 | 0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
Antioxidant Potential
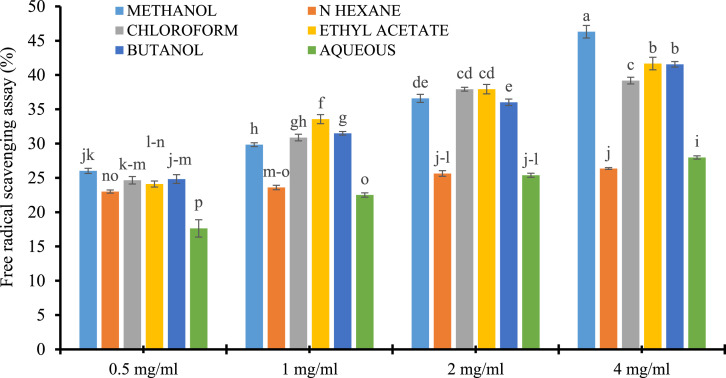
Each solvent extract of the cluster bean cultivar showed significant (P ≤ .001) antioxidant potential. The potential of each test sample was estimated by calculating the percent scavenging of free radical DPPH as shown in Figure 6. The DPPH scavenging method is independent of the polarity of sample extracts and widely accepted for the screening of many samples (Magalhães et al, 2008). Highest FRSA was shown by methanol extract, that is, 46.31 ± .91%, followed by ethyl acetate, butanol, chloroform, aqueous, and n-hexane extract, that is, 41.67 ± .92, 41.55 ± .40%, 39.19 ± .49%, 27.98 ± .24%, and 26.36 ± .13%, respectively. However, the free radical scavenging acid of the cluster bean (BR-99) was also supported by Kobeasy et al, 2011. Similarly, leaf methanol extract of cluster bean has shown significant DPPH scavenging activity studied by Moteriya et al, 2015. Antioxidant activity may be due to its phenolic and/or nonphenolic contents. The correlation results with TPC (R 2 = .8381) were shown phenolics as strong anti-oxidant agents. The positive correlation of phenolics with cluster bean anti-oxidants potential was also reported by Babbar et al, 2014. The phenolic contents or their derivatives, provide the electron or hydrogen group of free radicals, thus neutralizing the free radicals, or acting as a chain breaker in the lipid peroxidation chain (Pande and Srinivasan 2013; Quero et al, 2020). However, it seems that methanol in comparison with other solvents was found best for phenolic compound extraction based on its polarity and solubility of phenolic compounds or its derivatives (Kallel et al, 2014; Ammar et al, 2015). Even so, it was assumed by Zahra et al, 2017 that these H-donating molecules may be volatile oils, flavonoids like quercetin, naringenin, catechin, and kaempferol, or phenolic acids such as protocatechuic, gallic acid, caffeic acid, and rosmarinic acids and also reported by Brewer, 2011. While the correlation results with TPC values concluded that probably these are derivatives that could result in the free radical scavenging acid of the extracts which were further confirmed by HPLC results.
Figure 6.
Free radical scavenging activity determination in different solvent extracts of (methanol, n-hexane, chloroform, ethyl acetate, butanol, and aqueous) of cluster bean (BR-99). The results are expressed as mean ± SE, n= 3. *** represents P<.001, ** represents P<.01, * represents P<.05 and ns=non-significant.
Anti-Diabetic Potential
Diabetes is now becoming a non-curable metabolic disorder worldwide (Rehman et al, 2019; Babu et al, 2013). The prolonged exposure and poor management in the handling of this disease severely damaged the body organs and became a major threat to human life (Akbar et al, 2018; Haq et al, 2020; Sabir et al, 2019). Currently, many synthetic antidiabetic agents have been used to combat diabetes (Akbar et al, 2018) but with several unavoidable effects (Akash et al, 2012; Shafiee et al, 2012; Saleem et al, 2019). In this context, the traditional use of plants has produced a great interest among people as well as pharmaceutical chemists (Adki et al, 2020). However, traditional medicinal plants are a source for the treatment of common to life-threatening diseases since civilization (Ahmad et al 2009; Akash et al, 2014; Majeed et al, 2021; Kaushik et al, 2020). In this context, the antidiabetic potential of each test sample of cluster bean cultivar was estimated by measuring the inhibition percentage of α-amylase enzyme in test samples as shown in Figure 7. The current work shows the therapeutic potential (P ≤ .001) of these extracts against the inhibition of α-amylase enzyme. Enzymes including α-amylase are involved in carbohydrate metabolism and absorption that increase the level of glucose in the blood (Krentz et al, 2005; Arika et al, 2015; Ledda et al 2017; Akbar et al, 2018). So, inhibition of α-amylase is one of the prominent strategies in the amelioration of diabetes (Kim et al, 2008; Tundis et al, 2016; Sangeetha and Vedasree, 2012). The maximum inhibition percentage was seen in butanol extract, that is, 62.54 ± 1.47% following methanol (61.55±1.03%), ethyl acetate (59.58 ± .41%), chloroform (58.58 ± .77%), n-hexane (48.01±.31), and water extract (31.76 ± 1.01%). The results about the anti-diabetic potential of cluster bean cultivar were supported by the previous study of Mukhtar et al, 2004; Saeed et al, 2012; Singh and Bhagwati, 2016; Gandhi et al, 2014; Moteriya et al, 2015; Adki et al, 2020. The study of Saeed et al, 2012 reported the significant anti-diabetic activity of aqueous extract and ethanolic extract of cluster bean pod in alloxan and normal diabetic rats or guinea pigs which were testified by Mukhtar et al, 2006. In vivo studies emphasized the antidiabetic potential of water and methanolic extract of cluster bean seed in streptozocin diabetic rats after successfully reversing the damage in β -cells of the pancreas (Mukhtar et al, 2004; Saeed et al, 2012; Gandhi et al, 2014 as well as Quero et al, 2020). However, the current study signifies the antidiabetic potential of butanol fraction. Butanol fraction of cluster been might have a direct influence on insulin secretion or presence of flavonoids, phenolics contents, or polyphenols as these compounds are the inhibitors of α-amylase and α-glucosidase enzymes (Tundis et al, 2016; Akbar et al, 2018). ROS (reactive oxygen species) is also one of the major factors that are linked with the progression of diabetes and its comorbidities (Khullar et al, 2010; Rehman and Akash, 2017; Akbar et al, 2018; Haq et al, 2020). As a result, oxidative stress disturbed the insulin secretion, oxidization of glucose, proteinaceous enzyme, and genesis of lipid peroxides (Zhang and Tsao, 2016; Samarghandian et al, 2017; Saeed et al, 2012). Literature survey explained oxidative stress occurred when endogenous anti-oxidant levels staggered by rising in free radical species (Kaur and Saxena, 2021). Furthermore, it resulted in an increase in the complication of treatment of diabetes mellitus (DM) patients, and Alzheimer’s and Parkinson’s diseases (Rani et al, 2016; Samarghandian et al, 2016; Akhtar et al, 2021; Pande and Srinivasan, 2013; Kaur and Saxena, 2021). The antidiabetic potential of the cluster bean cultivar (BR-99) showed a strong correlation with phenolic (R 2 = .8082) and flavonoid contents (R 2 = .7685), which are the natural antioxidants found in plants.
Figure 7.
Alpha amylase inhibition assay determination in different solvent extracts (methanol, n-hexane, chloroform, ethyl acetate, butanol, and aqueous) of cluster bean (BR-99). The results are expressed as mean ± SE, n= 3. *** represents P<.001, ** represents P<.01, * represents P<.05 and ns=non-significant.
Anti-Cancer Activity
Cancer is among the most common life-threatening diseases throughout the globe. It results due to various genetic and epigenetic modifications that affected the regulatory and functionality of genes (Kumareswari and Rani, 2020). In 2008, WHO assessed a total of 12.7 million cases and deaths as 7.6 million globally (Zahra et al, 2017) were due to cancer. Out of these, 56% of cases were related to cancer in developing countries. By 2020, the global cancer mortality rate is projected to cross 10 million (Soliman et al, 2013). Chemotherapy is considered the primary mode of therapy for the treatment of various types of cancers (Fisher et al, 2005). Natural plant-based novel chemotherapeutic agents are now used as alternative anticancer agents (Widowati et al, 2013). Fruits, spices, and vegetables are used to suppress cancerous activity as a rich source of antioxidants ascorbic acids, amino acids, β-carotene, lycopene, polyphenols, and flavonoids (Siegel et al, 2016). Previous literature has presented the strong correlation exist between legume consumption and health benefits like protection from breast cancer, colon cancer, and other cancers (Mathers, 2002). Therefore, the cluster bean (BR-99) extracts were explored for in vitro anticancer activity against Human A549 lung cancer cells by MTT assay (Table 3). It is the reliable colorimetric method that measured the viability, proliferation, and activation of cells (Kumareswari and Rani, 2020). After incubation, the viability of cancer cell line was significantly (P ≤ .001) reduced by chloroform (21.68 ± 1.46%) followed by hexane (35.08 ± 1.80%), methanol (60.68 ± 3.22%), ethyl acetate (77.2 ± 2.14%), butanol (77.93 ± 3.4%), and aqueous extract (80.78 ± 4.43%). However, it was attractive to note that maximum anticancer potential was shown by a non-polar solvent fraction as compared to the polar ones. Previously the methanolic extract of guar seed has been reported for cytotoxicity activity against human breast adenocarcinoma (MCF-7), intestine carcinoma cell, colon carcinoma cell, human prostate carcinoma cell (PC3), and human hepatocellular carcinoma (Hep G2) cell lines (Shyale et al, 2006; Sharma et al, 2011; Badr et al, 2014; Kumareswari and Rani, 2020). Similarly, water extract of cluster bean leaves was also tested against oral cancer cell lines (Soni et al, 2017; Vaishnavi et al, 2019). Cytoprotective effect of ethanol extract of cluster bean pod was also reported by Rafatullah et al, 1994. The current study signifies the non-polar extract as a source of novel anti-cancer active compounds. The anti-cancer effect of cluster bean extract was reported due to the presence of dietary polyphenols (Perveen and Al-Taweel, 2017) which are the strong cardio, neuro, and caner protective supplements that reduced the risk of colorectal, gastric, lung, breast, or prostate cancer (Xie et al, 2013; Salazar-Ramiro et al, 2016; Tse et al, 2016). Soehhnlen et al, 2011 reported the cytotoxic potential of the cluster bean seeds to extract due to the presence of different flavonoids such as daidzein, genistein, quercetin, and kaempferol.
Table 3.
Cytotoxicity assay of different solvent extracts of cluster bean cultivar.
| Plant extract (mg/ml) | Cell viability (%) | Cytotoxicity (%) |
|---|---|---|
| Methanol | 60.67b ± 3.29 | 39.32c ± 3.21 |
| n-hexane | 35.08c ± 1.81 | 64.9b ± 1.81 |
| Chloroform | 21.68d ± 1.46 | 78.31a ± 1.46 |
| Ethyl acetate | 77.20a ± 2.13 | 22.79d ± 2.13 |
| Butanol | 77.93a ± 3.42 | 22.06d ± 3.42 |
| Aqueous | 80.78a ± 4.43 | 19.22d ± 4.43 |
Values are presented as mean ± SE (n = 3). The values with different superscript (a–d) letters show significantly (P < .05) different means.
Anti-Microbial Potential
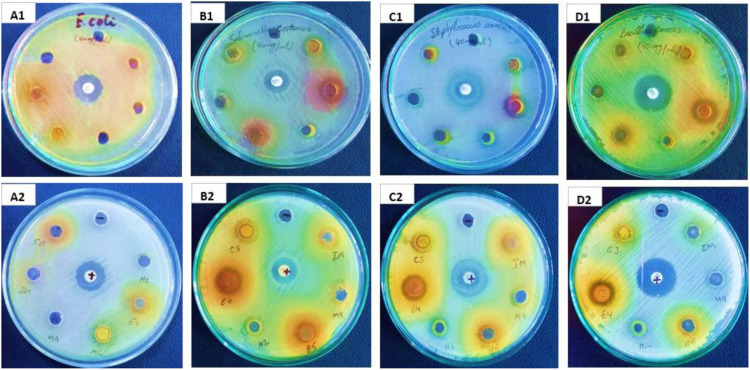
Plants are being used as a rich source of anti-microbial agents. From an early age, it is well known that before modern medicines are developed, plants are being exploited for various disease treatments (Dalavi and Patil, 2016). However, the treatment with modern synthetic drugs has several unavoidable effects and is also too costly for the people of developing countries (Escosteguy, 2014; Kuralkar and Kuralkar, 2021). The antimicrobial substance is used to be compatible with mammalian cells and only kills pathogens (Moteriya et al, 2015). Recently human pathogenic organisms have shown resistance against antibiotics due to their multiple drug resistance in human infectious microbes induced by its indiscriminate use. Despite that, bacterial groups that lack cell walls have now become a problem for researchers due to their ability to remain unaffected by cell wall targeted antibiotics like penicillin or beta-lactam (Ayling et al, 2000). So, there is an imperative need to find new antimicrobial moieties with strong newly emerging infectious diseases resistant potential (Jones et al, 2008). Hence, traditional plants are continuously being exploited to develop new leading drugs against infectious objects (Sukanya et al, 2009). A number of plant-based anti-fungal and anti-tumor drugs are available in the market for clinical uses (Mustafa et al, 2017). The antimicrobial potential of the plant was checked against different bacterial and fungal strains; the results are shown in Tables 4 and 5, with pictorial illustration in Figure 8 and 9.
Table 4.
Antibacterial activity of different solvent extracts of cluster bean cultivar tested against various bacterial strains.
| Plant extract | Diameter of inhibition zone (mm) | |||
|---|---|---|---|---|
| Bacillus cereus | Staphylococcus aureus | Salmonella enterica | E. coli | |
| Methanol | 12.5b± .29 | 13.4b ± .23 | 13.3c ± .26 | ---- |
| n-hexane | 10c ± .11 | 12.3c± .23 | 11.3d ± .17 | ---- |
| Chloroform | 12.2b ± .13 | 15.1a ± .32 | 15.56a ± .29 | 13.1b ± .32 |
| Ethyl acetate | 13.4a ± .23 | 12.27c ± .24 | 14.23b ± .15 | 12.2c ± .20 |
| Butanol | 12.56b ± .17 | 11.07d ± .06 | 10.03e ± .08 | ---- |
| Aqueous extract | 12.23b ± .15 | 11.2d ± .20 | 10.23e ± .34 | 14.2a ± .31 |
| Azithromycin | 22 ± .28 | 11 ± .32 | ---- | ---- |
| Ciprofloxacin | ---- | ---- | 15 ± .23 | 17.5 ± .32 |
| DMSO | ---- | ---- | ---- | ---- |
Values are presented as mean ± SE (n = 3). The values with different superscript (a–e) letters show significantly (P < .05) different means. ----: No activity.
Table 5.
Antifungal activity of different solvent extracts of cluster bean cultivar tested against various fungal strains.
| Plant extract | Diameter of inhibition zone (mm) | ||
|---|---|---|---|
| Fusarium avenaceum | Fusarium brachygibbosum | Aspergillus niger | |
| Methanol | 13.4d ± .20 | 21.24bc ± .12 | 14.03d ± .08 |
| n-hexane | 14.33c ± .24 | 20.47c ± .29 | 14.23d ± .28 |
| Chloroform | 18.5b ± .29 | 21.53b ± .29 | 21.03b ± .26 |
| Ethyl acetate | 20.53a ± .29 | 25.41a ± .25 | 23.36a ± .31 |
| Butanol | 14.33c ± .19 | 17.55d ± .29 | 15.17c ± .30 |
| Aqueous | 10.5e ± .28 | 15.15e ± .33 | 11.2e ± .42 |
| Clotrimazole | 16 ± .32 | 15 ± .24 | 14 ± .26 |
| DMSO | ----- | ----- | ----- |
Values are presented as mean ± SE (n = 3). The values with different superscript (a–e) letters show significantly (P < .05) different means. -----: No activity.
Figure 8.
Pictorial illustration of antibacterial activity of cluster bean (BR-99) different solvent extracts (methanol, n-hexane, chloroform, ethyl acetate, butanol, and aqueous) against E. coli (A1) front side and (A2) back side, S. enterica (B1) front side and (B2) back side, S. aureus (C1) front side and (C2) back side, and B. cereus (D1) front side and (D2) back side of cluster bean (BR-99).
Figure 9.
Pictorial illustration of antifungal activity of cluster bean (BR-99) different solvent extracts (methanol, n-hexane, chloroform, ethyl acetate, butanol, and aqueous) against F. avenaceum (A1) front side and (A2) back side, F. brachygibbosum (B1) front side and (B2) back side, and A. niger (C1) front side and (C2) back side.
Anti-Bacterial Assay
The antibacterial potential of the cluster bean various extracts was tested against (gram +ve and gram −ve) bacterial strain (Bacillus cereus: Staphylococcus aureus and Salmonella enterica: E. coli, respectively) (Table 4). Each extract showed a different level of inhibition against tested microbes. Amongst all, the chloroform, methanol, and ethyl acetate extracts were found to be more active against S. aureus (gram +ve bacterial strain) yielding the highest zones of inhibition, that is, 15.1 ± .32 mm, 13.4 ± .23 mm, and 12.67 ± .24 mm at 100 μL/disc, respectively, in comparison with azithromycin which produced 11 ± .32 mm zone of inhibition. While in the case of gram-negative bacteria, the chloroform fraction was effective with a 15.56 ± .29 mm zone of inhibition against S. enterica with respect to ciprofloxacin 15 ± .2 mm. All tested extracts showed very poor antibacterial activity against B. cereus and E. coli. However, the significant antibacterial potential of methanolic extract of cluster bean against S. aureus was also reported by Hassan et al (2010). The antibacterial activity of this plant was more intensified against gram-positive bacteria. The negative control used was DMSO which showed no inhibition. S. aureus and S. enterica both are causal agents of the wide range of diseases in all mammals, birds, reptiles, and insects but especially in poultry and dairy animals resulting in significant economic loss or reduced yield (Myint, 2004). These pathogens are rich in the production and secretion of enzyme coagulase, responsible for blood clotting after conversion of serum fibrinogen to fibrin. However, it is also reported that S. aureus caused Mastitis in dairy cows which reduced milk production or milk was thrown out due to either pathogen or antibiotic contamination. Staphylococcus and Salmonella species were shown high resistance against multiple antibiotics such as penicillin, streptomycin, ampicillin, tetracycline, and erythromycin, and more than 80 000 tons of antibiotics were used for agricultural purposes (Ungemach, 2000, Witte, 2000, Hassan, 2008; Hassan et al, 2010). However, it is mentionable that guar extract of these fractions should be used as feed for the agriculturally important domestic animals for their healthy growth and development products as the previous study recommended guar meal (rich in proteins and essential amino acid) in the diet of cattle, buffalo, sheep, and chicken (Salehpour et al, 2012; Saeed et al, 2017; Biel and Jaroszewska, 2019). The literature survey explained the predominant chemicals for most effective antibacterial activity against gram-positive bacteria are phenolics (Rios and Recio, 2005). However, the guar plant exhibited the antibacterial potential is because of galactomannan and saponin content reported by Hassan, 2008. According to Jeeshna et al, 2011, the plant-rich alkaloids, flavonoids, glycosides, steroids, phenols, tannins, saponins, and resins extracts showed maximum antimicrobial activities. Cluster bean cultivar (BR-99) is concluded as rich in aforesaid compounds which were further confirmed by the phytochemical analysis.
Anti-Fungal Assay
Fungal infections in both plants and animals have now become a major threat for having higher production or yield in this era (Kundu, et al, 2016; Tavernier et al, 2015). The fungal infection is also common in humans. Azole drugs have shown fungistatic activity by interfering in the synthesis of the fungoid ergosterol. These broad-spectrum drugs are continuously used in microbial contagious diseases (Katiraee et al, 2017). The action mechanism of these drugs involves interference with certain human functional pathways; therefore, they have important side effects on the human body (Laniado-Laborín and Cabrales-Vargas, 2009). Antifungal compounds are also scanty and are often very toxic (Kundu, et al, 2016). Medicinal plant extracts rich in biologically active compounds are endowed with the effective antimicrobial potential to replace man-made drugs (Abirami et al, 2013). Extracts of the cluster bean plant (BR-99) were used against three fungal strains (Fusarium avenaceum, Fusarium brachygibbosum, and Aspergillus niger) to evaluate its anti-fungal potential. All the test sample extract was shown significant results presented in Table 5. Among all fractions, the ethyl acetate was seen as more potent with an inhibition zone of 25.41 ± .25 mm, 23.61 ± .31 mm, and 20.53 ± .29 mm against F. brachygibbosum, A. niger, and F. avenaceum, respectively. Clotrimazole was used as the positive control and displayed the maximum zone of inhibition of 16 ± .32 mm, 15 ± .24 mm, and 14 ± .26 mm against F. avenaceum, F. brachygibbosum, and A. niger, respectively, while DMSO was used as a negative control to rule out any activity given by the solvent. Similarly, the antifungal potential of the cluster bean plant was also supported by Moteriya et al (2015), although Pawar (2013) reported no antifungal activity of cluster bean fruit extract against A. niger which contradicted the finding of our study. However, F. brachygibbosum was found more susceptible to each test extract with most prominent zones of inhibition 21.24 ± .12 (methanol extract), 20.47 ± .29 (hexane extract), 21.53 ± .29 (chloroform extract), 25.41 ± .25 (ethyl acetate extract), 17.55 ± .29 (butanol extract), and 15.15 ± .33 mm (aqueous extract) at 100 μ g/disc, respectively. Globally, Fusarium species are the highly virulent staple food or cash crop pathogens (Al-Sadi et al 2012; Al-Mahmooli et al 2013; Beukes et al, 2017; Xia et al 2020; Wang et al 2021), owing to its strong mycotoxins secondary metabolites like deoxynivalenol, zearalenone, and fumonisin B1 (Khaledi et al, 2017; Pollard, 2018). It causes the development of multiple epidemic diseases called fusarium head blight in the panicle and heads of cereals as well as seedlings blight and root rot in legumes (Chang et al, 2014) with the increase in yield, size, and quality of grain reduction (Ismaiel and Papenbrock, 2015). In addition, these mycotoxins could be easily entered and infected the whole food chain. It is worthful to note that in comparison polar extracts have shown significant antifungal potential than moderately polar extracts and nonpolar extracts.
Conclusion
As current study aimed to explore the phytochemical and biological profiling of highly adapted, locally recognized, and recommended high yielding cultivar of cluster bean to not only endorse the traditional uses but also bring to enlighten some furtive attributes of the subjected plant. The six solvent extracts were used to reveal the broad biological spectrum of cluster bean (BR-99) particularly. While the key finding of this study indicates that butanol fractions among all extracts are potent inhibitors of alpha-amylase. It must be concluded from this study that butanol extract has some phytochemical constituents that are more responsive toward α-amylase inhibition as exhibited by HPLC. On the other hand, cytotoxic activity against lung cancer cells was found to be congregated in the extract of chloroform and hexane fraction. Furthermore, chloroform and ethyl acetate extract were found more responsive toward anti-microbial activity. The chloroform fraction showed maximum antioxidant, antibacterial activity, and anticancer activity may be owed to the presence of rutin. In the current study, HPLC-based quantitative analysis of cluster bean cultivar (BR-99) revealed the presence of phytochemical active compounds such as gallic acid, HB acid, vanillic acid, kaempferol, sinapic acid, ferulic acid, salicylic acid, coumarin, quercetin, rutin, P-coumaric acid, and catechin. This study supports our prediction of cluster bean (BR-99) as a potent source of natural anti-oxidants. There is a dire need for the identification, isolation, and characterization of phytochemicals that are responsible for particular computed pharmacological activities and further in vivo investigations to certify and strengthen the in vitro findings.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researcher Dr Azhar Rasool, Department of Zoology for performing the anticancer activity.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Abdel-Sattar E, Harraz F, El-Gayed S. Antimicrobial activity of extracts of some plants collected from the Kingdom of Saudi Arabia. J King Abdulaziz Univ Med Sci. 2008;15(1):25-33. [Google Scholar]
- Abidi N, Liyanage S, Auld D, et al. Chapter 12: Challenges and Opportunities for Increasing Guar Production in the United States to Support Unconventional Oil and Gas Production. CRC Press; 2015:207-225. [Google Scholar]
- Abirami LS, Pushkala R, Srividya N. Antimicrobial activity of selected plant extracts against two important fungal pathogens isolated from papaya fruit. IJRPBS. 2013;4(1):234-238. [Google Scholar]
- Adki KM, Laddha AP, Gaikwad AB, Kulkarni YA. Potential Role of Seeds From India in Diabetes. In: Nuts and Seeds in Health and Disease Prevention. Academic Press; 2020:365-391. [Google Scholar]
- Ahmad M, Qureshi R, Arshad M, Khan MA, Zafar M. Traditional herbal remedies used for the treatment of diabetes from district Attock (Pakistan). Pakistan J Bot. 2009;41(6):2777-2782. [Google Scholar]
- Ahmed N, Mahmood A, Mahmood A, Sadeghi Z, Farman M. Ethnopharmacological importance of medicinal flora from the district of Vehari, Punjab province, Pakistan. J Ethnopharmacol. 2015;168:66-78. [DOI] [PubMed] [Google Scholar]
- Akash MSH, Rehman K, Chen S. Spice plant Allium cepa: Dietary supplement for treatment of type 2 diabetes mellitus. Nutrition. 2014;30(10):1128-1137. [DOI] [PubMed] [Google Scholar]
- Akash MSH, Shen Q, Rehman K, Chen S. Interleukin-1 receptor antagonist: a new therapy for type 2 diabetes mellitus. J Pharmaceut Sci. 2012;101(5):1647-1658. [DOI] [PubMed] [Google Scholar]
- Akbar MU, Zia KM, Akash MSH, Nazir A, Zuber M, Ibrahim M. In-vivo anti-diabetic and wound healing potential of chitosan/alginate/maltodextrin/pluronic-based mixed polymeric micelles: Curcumin therapeutic potential. Int J Biol Macromol. 2018;120:2418-2430. [DOI] [PubMed] [Google Scholar]
- Akhtar M, Saleem A, Shagufta A, et al. Tylophora hirsuta L. leaf extract attenuates alloxan-induced diabetes in mice by suppressing oxidative stress and α-amylase. Asian Pac J Trop Biomed. 2021;11(9):394. [Google Scholar]
- Alexander G. Effect of Plant Extracts on Rumen Fermentation and Nutrient Utilization in Sheep. Doctoral dissertation. IVRI; 2005. [Google Scholar]
- Al-Mahmooli IH, Al-Bahri YS, Al-Sadi AM, Deadman ML. First report of Euphorbia larica dieback caused by Fusarium brachygibbosum in Oman. Plant Dis. 2013;97(5):687-687. [DOI] [PubMed] [Google Scholar]
- Al-Sadi AM, Al-Jabri AH, Al-Mazroui SS, Al-Mahmooli IH. Characterization and pathogenicity of fungi and oomycetes associated with root diseases of date palms in Oman. Crop Protect. 2012;37:1-6. [Google Scholar]
- Ammar I, Ennouri M, Attia H. Phenolic content and antioxidant activity of cactus (Opuntia ficus-indica L.) flowers are modified according to the extraction method. Ind Crop Prod. 2015;64:97-104. [Google Scholar]
- Anonymous . Agriculture Statistics at a Glance. : Directorate of Economics & Statistics, Department of Agriculture & Cooperation; 2018. [Google Scholar]
- Arika WM, Abdirahman YA, Mawia MA, et al. In vivo antidiabetic activity of the aqueous leaf extract of Croton macrostachyus in alloxan induced diabetic mice. Pharm Anal Acta. 2015;6(11):1-5. [Google Scholar]
- Arora SK, Joshi UN, Jain V. Lipid classes and fatty acid composition of two promising varieties of Cyamopsis tetragonoloba (L.) Taub. Guar Res Ann. 1985;4:9-10. [Google Scholar]
- Ashraf MY, Akhtar K, Sarwar G, Ashraf M. Evaluation of arid and semi-arid ecotypes of guar (Cyamopsis tetragonoloba L.) for salinity (NaCl) tolerance. J Arid Environ. 2002;52(4):473-482. [Google Scholar]
- Ashraf MY, Akhtar K, Sarwar G, Ashraf M. Role of the rooting system in salt tolerance potential of different guar accessions. Agron Sustain Dev. 2005;25(2):243-249. [Google Scholar]
- Ayling RD, Baker SE, Nicholas RAJ, Peek ML, Simon AJ. Comparison of in vitro activity of danofloxacin, florfenicol, oxytetracycline, spectinomycin and tilmicosin against recent field isolates of Mycoplasma bovis. Vet Rec. 2000;146(26):745-747. [DOI] [PubMed] [Google Scholar]
- Babbar N, Oberoi HS, Sandhu SK, Bhargav VK. Influence of different solvents in extraction of phenolic compounds from vegetable residues and their evaluation as natural sources of antioxidants. J Food Sci Technol. 2014;51(10):2568-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu PVA, Liu D, Gilbert ER. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J Nutr Biochem. 2013;24(11):1777-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr SE, Abdelfattah MS, El-Sayed SH, El-Aziz AS, Sakr DM. Evaluation of anticancer, antimycoplasmal activities and chemical composition of guar (Cyamopsis tetragonoloba) seeds extract. Res J Pharmaceut Biol Chem Sci. 2014;5(3):413-423. [Google Scholar]
- Badr SE, Sakr DM, Mahfouz SA, Abdelfattah MS. Licorice (Glycyrrhiza glabra L.): Chemical composition and biological impacts. Res J Pharmaceut Biol Chem Sci. 2013;4(3):606-621. [Google Scholar]
- Beukes I, Rose LJ, Shephard GS, Flett BC, Viljoen A. Mycotoxigenic Fusarium species associated with grain crops in South Africa-A review. South Afr J Sci. 2017;113(3-4):1-2. [Google Scholar]
- Beyene BB, Alem FA, Ayana MT. Determination of antioxidant and antibacterial activities of leaf extracts of Plumbago zeylanica (Amira). Cogent Chemistry. 2020;6(1):1831715. [Google Scholar]
- Biel W, Jaroszewska A. Compositional and Nutritional Evaluation of Guar (Cyamopsis tetragonoloba L.) Meal. Anim Nutr Feed Technol. 2019;19(3):385-393. [Google Scholar]
- Bouchenak M, Lamri-Senhadji M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: a review. J Med Food. 2013;16(3):185-198. [DOI] [PubMed] [Google Scholar]
- Brewer MS. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf. 2011;10(4):221-247. [Google Scholar]
- Chang Y-M, Chen L-C, Wang H-Y, Chiang C-L, Chang C-T, Chung Y-C. Characterization of an Acidic Chitinase from Seeds of Black Soybean (Glycine max (L) Merr Tainan No. 3). PLoS One. 2014;9(12):e113596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalavi C, Patil S. FTIR spectroscopic screening of phytochemicals of two medicinally important species of solanum used in preparation of Dashmula formulation. Int J Pharmaceut Sci Rev Res. 2016;19:112-120. [Google Scholar]
- Daniel M. Polyphenols of some Indian vegetables. Curr Sci. 1989;58(23):1332-1334. [Google Scholar]
- Davidson PM, Naidu AS. Natural Food Antimicrobial Systems. A. Naidu, Phyto-Phenols. CRC Press LLC; 2000:265-294. [Google Scholar]
- Deng Y, Huang L, Zhang C, Xie P, Cheng J, Wang X. Physicochemical and functional properties of water soluble gum from Wrinkle Floweringquince (Chaenomeles Speciosa) seeds. JB (J Biochem). 2019;4(4):222-230. [Google Scholar]
- Elsheikh EA, Ibrahim KA. The effect of Bradyrhizobium inoculation on yield and seed quality of guar (Cyamopsis tetragonoloba L.). Food Chem. 1999;65(2):83-87. [Google Scholar]
- Escosteguy A. Potential use of medicinal plants in animal production: results in Brazil. Building. Org Bridg. 2014;1:81-84. [Google Scholar]
- Fatima H, Khan K, Zia M, Ur-Rehman T, Mirza B, Haq IU. Extraction optimization of medicinally important metabolites from Datura innoxia Mill.: an in vitro biological and phytochemical investigation. BMC Complementary and Alternative Medicine. 2015;15(1):376-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652-1662. [DOI] [PubMed] [Google Scholar]
- Gandhi GR, Vanlalhruaia P, Stalin A, Irudayaraj SS, Ignacimuthu S, Paulraj MG. Polyphenols-rich Cyamopsis tetragonoloba (L.) Taub. beans show hypoglycemic and β-cells protective effects in type 2 diabetic rats. Food Chem Toxicol. 2014;66:358-365. [DOI] [PubMed] [Google Scholar]
- Gresta F, Ceravolo G, Presti VL, D’Agata A, Rao R, Chiofalo B. Seed yield, galactomannan content and quality traits of different guar (Cyamopsis tetragonoloba L.) genotypes. Ind Crop Prod. 2017;107:122-129. [Google Scholar]
- Gresta F, Sortino O, Santonoceto C, Issi L, Formantici C, Galante YM. Effects of sowing times on seed yield, protein and galactomannans content of four varieties of guar (Cyamopsis tetragonoloba L.) in a Mediterranean environment. Ind Crop Prod. 2013;41:46-52. [Google Scholar]
- Haq MEU, Akash MSH, Sabir S, Mahmood MH, Rehman K. Human exposure to bisphenol A through dietary sources and development of diabetes mellitus: a cross-sectional study in Pakistani population. Environ Sci Pollut Control Ser. 2020;27(21):26262-26275. [DOI] [PubMed] [Google Scholar]
- Hassan SM, Haq AU, Byrd JA, Berhow MA, Cartwright AL, Bailey CA. Haemolytic and antimicrobial activities of saponin-rich extracts from guar meal. Food Chem. 2010;119(2):600-605. [Google Scholar]
- Hassan SM. Antimicrobial Activities of Saponin-Rich Guar Meal Extract. Texas A&M University; 2008. [Google Scholar]
- Hinson PO, Adams CB. Quantifying tradeoffs in nodulation and plant productivity with nitrogen in guar. Ind Crop Prod. 2020;153:112617. [Google Scholar]
- Ismaiel A, Papenbrock J. Mycotoxins: producing fungi and mechanisms of phytotoxicity. Agriculture. 2015;5(3):492-537. [Google Scholar]
- Jain PK, Rijhwani S. Comparative gc-ms analysis of Cyamopsis tetragonoloba fruit extracts. Int J Pharma Sci Res. 2018;9(10):4236-4242. [Google Scholar]
- Jamshed M, Ali ST, Rizwani GH, Zahid H, Asif ST. Pharmacognostic evaluation of pods of Cyamopsis tetragonoloba L. Int J Herb Med. 2018;6(1):51-53. [Google Scholar]
- Jeeshna MV, Paulsamy S, Mallikadevi T. Phytochemical constituents and antimicrobial studies of the exotic plant species, Croton bonplandianum Baill. J Life Sci. 2011;3(1):23-27. [Google Scholar]
- Jerine Peter S, Arunraj N, Ram Kumar K, Manisha P, Sangeetha N. Beneficial Binding Affinity of the Active Compound of Cyamopsis Tetragonoloba with the Receptors Responsible for Hepatotoxicity. J chem pharm. 2019;11(1):51-71. [Google Scholar]
- Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukanti AK, Pathak R, Mushyam C. Cluster Bean [Cyamopsis tetragonoloba (L.) Taub] Breeding. In: Advances in Plant Breeding Strategies: Legumes; 2019:113-149. [Google Scholar]
- Kadian N, Yadav K, Aggarwal A. Significance of bioinoculants in promoting growth, nutrient uptake and yield of Cyamopsis tetragonoloba (L.) "Taub.''. Eur J Soil Biol. 2013;58:66-72. [Google Scholar]
- Kallel F, Driss D, Chaari F, et al. Garlic (Allium sativum L.) husk waste as a potential source of phenolic compounds: Influence of extracting solvents on its antimicrobial and antioxidant properties. Ind Crop Prod. 2014;62:34-41. [Google Scholar]
- Katiraee F, Afshar SA, Ahmadi Afshar S, Rahimi Pirmahalleh SF, Shokri H. In vitro antifungal activity of essential oils extracted from plants against fluconazole-susceptible and -resistant Candida albicans. Current Medical Mycology. 2017;3(2):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur B, Saxena J. Evaluation of anti-parkinson’s activity of Cyamopsis tetragonoloba L methanol plant extract with behavioral and biochemical analysis. IJPSR. 2021;12(6):3236-3242. [Google Scholar]
- Kaushik S, Kaushik S, Kumar R, Dar L, Yadav JP. In-vitro and in silico activity of Cyamopsis tetragonoloba (Gaur) L. supercritical extract against the dengue-2 virus. VirusDisease. 2020;31(4):470-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaledi N, Taheri P, Falahati-Rastegar M. Evaluation of resistance and the role of some defense responses in wheat cultivars to Fusarium head blight. J Plant Protect Res. 2017;57(4). [Google Scholar]
- Khan D, Jahan I, Akhtar LH, Zaki MJ, Minhas R. Seed mass variation in seed lots of fifteen germplasms of guar [Cyamopsis tetragonoloba (L.) Taub.]. Int J Biol Biotechnol. 2018;15(4):711-720. [Google Scholar]
- Khullar M, Al-Shudiefat AA-RS, Ludke A, Binepal G, Singal PK. Oxidative stress: a key contributor to diabetic cardiomyopathyThis review is one of a selection of papers published in a Special Issue on Oxidative Stress in Health and Disease. Can J Physiol Pharmacol. 2010;88(3):233-240. [DOI] [PubMed] [Google Scholar]
- Kim KY, Nam KA, Kurihara H, Kim SM. Potent α-glucosidase inhibitors purified from the red alga Grateloupia elliptica. Phytochemistry. 2008;69(16):2820-2825. [DOI] [PubMed] [Google Scholar]
- Kobeasy I, Abdel-Fatah M, Abd El-Salam SM, Mohamed ZE. Biochemical studies on Plantago major L. and Cyamopsis tetragonoloba L. Int J Biodivers Conserv. 2011;3(3):83-91. [Google Scholar]
- Krentz AJ, Bailey CJ. Oral antidiabetic agents. Drugs. 2005;65(3):385-411. [DOI] [PubMed] [Google Scholar]
- Kumar K, Fateh V, Verma B, Pandey S. Some herbal drugs used for treatment of diabetes. IIJRDPL. 2014;3(5):1116-1120. [Google Scholar]
- Kumareswari T, Rani SM. Anticancer Activity Of Extract Of Ulva reticulata Grown Fruit Of Cyamopsis tetragonoloba (L.) Taub. ON MCF-7 BREAST CANCER CELL LINE. Journal for Pharmaceutical and Medical Research and Technology. 2020;9:2333-2340. [Google Scholar]
- Kundu S, Abdullah MF, Das A, et al. Antifungal ouzo nanoparticles from guar gum propionate. RSC Adv. 2016;6(108):106563-106571. [Google Scholar]
- Kuralkar P, Kuralkar SV. Role of herbal products in animal production - An updated review. J Ethnopharmacol. 2021;278:114246. [DOI] [PubMed] [Google Scholar]
- Laniado-Laborín R, Cabrales-Vargas MN. Amphotericin B: side effects and toxicity. Rev Iberoam De Micol. 2009;26(4):223-227. [DOI] [PubMed] [Google Scholar]
- Ledda C, Loreto C, Zammit C, et al. Non-infective occupational risk factors for hepatocellular carcinoma: A review. Mol Med Rep. 2017;15(2):511-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Gong F, Li F. Hypoglycemic and hypolipidemic effects of flavonoids from tatary buckwheat in type 2 diabetic rats. Biomed Res J. 2016;27(1). [Google Scholar]
- Saleem MI, Shah SA, Akhtar LH. BR-99 a new guar cultivar released for general cultivation in Punjab Province. Asian J Plant Sci. 2002;1(3):266-268. [Google Scholar]
- Magalhães LM, Segundo MA, Reis S, Lima JL. Methodological aspects about in vitro evaluation of antioxidant properties. Anal Chim Acta. 2008;613(1):1-19. [DOI] [PubMed] [Google Scholar]
- Majeed Y, Shaukat MB, Abbasi KY, Ahmad MA. Indigenous plants of Pakistan for the treatment of Diabetes: a review. Agrobiologiya. 2021;4:44-63. [Google Scholar]
- Mathers JC. Pulses and carcinogenesis: potential for the prevention of colon, breast and other cancers. Br J Nutr. 2002;88(S3):273-279. [DOI] [PubMed] [Google Scholar]
- Morris JB, Wang ML. Characterization of Guar [Cyamopsis Tetragpnoloba (L.) Taub] Genetic Resource for Their Flavonoid Traits. InAmerican Society of Plant Biologist Annual Meeting; 2007:135. [Google Scholar]
- Moteriya P, Ram J, Moradiya R, Chanda S. In vitro free radical scavenging and antimicrobial activity of Cyamopsis tetragonoloba L. J Pharmacogn Phytochem. 2015;4(2). [Google Scholar]
- Moteriya P, Ram J, Rathod T, Chanda S. In vitro antioxidant and antibacterial potential of leaf and stem of Gloriosa superba L. AJPCT. 2014;2(6):703-787. [Google Scholar]
- Mubarak AR, Salih NO, Hassabo AA. Fate of 15 N-labeled urea under a guar-wheat rotation as influenced by crop residue incorporation in a semi-arid Vertisol. Trop Agric. 2015;41(3216):30172-30212. [Google Scholar]
- Mukhtar HM, Ansari SH, Ali M, Bhat ZA, Naved T. Effect of aqueous extract of Cyamopsis tetragonoloba Linn. beans on blood glucose level in normal and alloxan-induced diabetic rats. NISCAIR-CSIR. 2004;42(12):1212-1215. [PubMed] [Google Scholar]
- Mukhtar HM, Ansari SH, Bhat ZA, Naved T. Antihyperglycemic Activity ofCyamopsis tetragonoloba. Beans on Blood Glucose Levels in Alloxan-Induced Diabetic Rats. Pharmaceut Biol. 2006;44(1):10-13. [Google Scholar]
- Myint MS. Epidemiology of Salmonella Contamination of Poultry Meat Products: Knowledge Gaps in the Farm to Store Products. Doctoral dissertation; 2004. [Google Scholar]
- Nijveldt RJ, Van Nood E, Van Hoorn DE, Boelens PG, Van Norren K, Van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74(4):418-425. [DOI] [PubMed] [Google Scholar]
- Pande S, Srinivasan K. Protective effect of dietary tender cluster beans (Cyamopsis tetragonoloba) in the gastrointestinal tract of experimental rats. Appl Physiol Nutr Metabol. 2013;38(2):169-176. [DOI] [PubMed] [Google Scholar]
- Pandey MM, Rastogi S, Rawat AK. Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid base Compl Alternative Med : eCAM. 2013;2013:376327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak R, Singh M, Henry A. Genetic diversity and interrelationship among clusterbean (Cyamopsis tetragonoloba) genotypes for qualitative traits. Indian J Agric Sci. 2011;81(5):402. [Google Scholar]
- Pawar BT. Antifungal activity of some fruit extracts against seedborne pathogenic fungi. Adv Bio Res. 2013;4(3):95-97. [Google Scholar]
- Perveen S, Al-Taweel AM. Phenolic Compounds Natural Sources. Importance and Applications; 2017.Phenolic compounds from the natural sources and their cytotoxicity [Google Scholar]
- Pollard AT. Seedsvsfungi: an enzymatic battle in the soil seedbank. Seed Sci Res. 2018;28(3):197-214. [Google Scholar]
- Pranoothi EK, Narendra K, Joshi DS, et al. Studies on qualitative, quantitative, phytochemical analysis and screening of in vitro biological activities of Leucas indica (L) VAR. Nagalapuramiana. Int J Herb Med. 2014;2(3):30-36. [Google Scholar]
- Proestos C, Chorianopoulos N, Nychas G-JE, Komaitis M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J Agric Food Chem. 2005;53(4):1190-1195. [DOI] [PubMed] [Google Scholar]
- Quero J, Mármol I, Cerrada E, Rodríguez-Yoldi MJ. Insight into the potential application of polyphenol-rich dietary intervention in degenerative disease management. Food Funct. 2020;11(4):2805-2825. [DOI] [PubMed] [Google Scholar]
- Rafatullah S, Al-Yahya MA, Al-Said MS, Taragan KUAH, Mossa JS. Gastric Anti-Ulcer and Cytoprotective Effects of Cyamopsis tetragonoloba ('Guar') in Rats. Int J Pharmacogn. 1994;32(2):163-170. [Google Scholar]
- Rajaprakasam S, Rahman H, Karunagaran S, et al. Comparative transcriptome and metabolome profiling in the maturing seeds of contrasting cluster bean (Cyamopsis tetragonoloba L. Taub) cultivars identified key molecular variations leading to increased gum accumulation. Gene. 2021;791:145727. [DOI] [PubMed] [Google Scholar]
- Rani V, Deep G, Singh RK, Palle K, Yadav UCS. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016;148:183-193. [DOI] [PubMed] [Google Scholar]
- Rama Rao SV, Prakash B, Raju MVLN, Panda AK, Murthy OK. Effect of supplementing non-starch polysaccharide hydrolyzing enzymes in guar meal based diets on performance, carcass variables and bone mineralization in Vanaraja chicken. Anim Feed Sci Technol. 2014;188:85-91. [Google Scholar]
- Rasul A, Bao R, Malhi M, et al. Induction of apoptosis by costunolide in bladder cancer cells is mediated through ROS generation and mitochondrial dysfunction. Molecules. 2013;18(2):1418-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman K, Akash MSH. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: how are they interlinked? J Cell Biochem. 2017;118(11):3577-3585. [DOI] [PubMed] [Google Scholar]
- Rehman K, Chohan TA, Waheed I, Gilani Z, Akash MSH. Taxifolin prevents postprandial hyperglycemia by regulating the activity of α‐amylase: Evidence from an in vivo and in silico studies. J Cell Biochem. 2019;120(1):425-438. [DOI] [PubMed] [Google Scholar]
- Ríos JL, Recio MC. Medicinal plants and antimicrobial activity. J Ethnopharmacol. 2005;100(1-2):80-84. [DOI] [PubMed] [Google Scholar]
- Sabir S, Akhtar MF, Saleem A. Endocrine disruption as an adverse effect of non-endocrine targeting pharmaceuticals. Environ Sci Pollut Control Ser. 2019;26(2):1277-1286. [DOI] [PubMed] [Google Scholar]
- Saeed M, Hassan FU, Shah QA, et al. Practical application of guar (cyamopsis tetragonoloba L. Taub) meal in poultry nutrition. Adv Anim Vet Sci. 2017;5(12):491-499. [Google Scholar]
- Saeed S, Mosa-Al-Reza H, Fatemeh A, Saeideh D. Antihyperglycemic and antihyperlipidemic effects of guar gum on streptozotocin-induced diabetes in male rats. Phcog Mag. 2012;8(29):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Ramiro A, Ramírez-Ortega D, Pérez de la Cruz V, et al. Role of redox status in development of glioblastoma. Front Immunol. 2016;7:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M, Sabir S, Akhtar MF, et al. Stem cell therapy for diabetes mellitus: Recent progress and hurdles. Crit Rev Eukaryot Gene Expr. 2019;29(5):471-482. [DOI] [PubMed] [Google Scholar]
- Salehpour M, Qazvinian K, Cadavez V. Effects of feeding different levels of guar meal on performance and blood metabolites in Holstein lactating cows. Scientific Papers; 2012:73-77. [Google Scholar]
- Samarghandian S, Azimi-Nezhad M, Farkhondeh T. Catechin treatment ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Dose-response : A Publication of International Hormesis Society. 2017;15(1):1559325817691158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarghandian S, Azimini-Nezhad M, Farkhondeh T. The Effects of Zataria Multiflora on Blood Glucose, Lipid Profile and Oxidative Stress Parameters in Adult Mice During Exposure to Bisphenol A. Cardiovascular & Hematological Disorders-Drug Targets. 2016;16(1):41-46. [DOI] [PubMed] [Google Scholar]
- Sangeetha R, Vedasree N. In Vitro α-Amylase Inhibitory Activity of the Leaves of Thespesia populnea. ISRN pharmacology. 2012;2012:515634-515637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiee G, Mohajeri-Tehrani M, Pajouhi M, Larijani B. The importance of hypoglycemia in diabetic patients. J Diabetes Metab Disord. 2012;11(1):17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakir M, Sadaqat HA, Farooq Q, et al. A Review on guar (cyamopsis tetragonolobA L.): A cash crop. Int Res J Pharm. 2020;11(4). [Google Scholar]
- Sharma P, Dubey G, Kaushik S. Chemical and medico-biological profile of Cyamopsis tetragonoloba (L) Taub: an overview. J Appl Pharmaceut Sci. 2011;1:32-37. [Google Scholar]
- Shyale S, Chowdary KPR, Krishnaiah YSR, Bhat NK. Pharmacokinetic evaluation and studies on the clinical efficacy of guar gum--based oral drug delivery systems of albendazole and albendazole-β-cyclodextrin for colon-targeting in human volunteers. Drug Dev Res. 2006;67(2):154-165. [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA A Cancer J Clin. 2016;66(1):7-30. [DOI] [PubMed] [Google Scholar]
- Singh J, Gaikwad DS. Phytogenic feed additives in animal nutrition. In: Natural Bioactive Products in Sustainable Agriculture; 2020:273-289. [Google Scholar]
- Singh S, Bhagwati D. Cyamopsis tetragonoloba (L). Taub.: A Phyto-Pharmacological Review. Human Journals. 2016;7(4):166-174. [Google Scholar]
- KK Singhal A, Shilpi Kerketta AKS, Kerketta S. Nutritional evaluation of indigenous plants and quantification of total saponins in plant extracts. International Journal of Current Microbiology and Applied Sciences. 2017;6(9):1368-1377. [Google Scholar]
- Soliman A, Schottenfeld D, Boffetta P, eds Cancer epidemiology: low-and middle-income countries and special populations. Oxford University Press; 2013. [Google Scholar]
- Soni A, Femida P, Sharma P. In-vitro cytotoxic activity of plant saponin extracts on breast cancer cell-line. Res J Pharmacogn Phytochem. 2017;9(1):17-22. [Google Scholar]
- Stevanović ZD, Bošnjak-Neumüller J, Pajić-Lijaković I, Raj J, Vasiljević M. Essential oils as feed additive future perspectives. Molecules. 2018;23(7):1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukanya SL, Sudisha J, Hariprasad P, Niranjana SR, Prakash HS, Fathima SK. Antimicrobial activity of leaf extracts of Indian medicinal plants against clinical and phytopathogenic bacteria. Afr J Biotechnol. 2009;8(23). [Google Scholar]
- Tassou C, Koutsoumanis K, Nychas GJ. Inhibition of Salmonella enteritidis and Staphylococcus aureus in nutrient broth by mint essential oil. Food Res Int. 2000;33(3-4):273-280. [Google Scholar]
- Tavernier E, Desnos-Ollivier M, Honeyman F, et al. Development of echinocandin resistance in Candida krusei isolates following exposure to micafungin and caspofungin in a BM transplant unit. Bone Marrow Transplant. 2015;50(1):158-160. [DOI] [PubMed] [Google Scholar]
- Tripathi P, Pandey R. Phytochemical screening of guar (Cyamopsis tetragonoloba) seeds extract. Int J Appl Res. 2016;2(10):98-100. [Google Scholar]
- Tse G, Eslick GD. Soy and isoflavone consumption and risk of gastrointestinal cancer: a systematic review and meta-analysis. Eur J Nutr. 2016;55(1):63-73. [DOI] [PubMed] [Google Scholar]
- Tundis R, Bonesi M, Sicari V, et al. Poncirus trifoliata (L.) Raf.: Chemical composition, antioxidant properties and hypoglycaemic activity via the inhibition of α-amylase and α-glucosidase enzymes. J Funct Foods. 2016;25:477-485. [Google Scholar]
- Ungemach FR. Figures on quantities of antibacterials used for different purposes in the EU countries and interpretation. Acta Veterinaria Scandinavica. Supplementum. 2000;93:89-97. [PubMed] [Google Scholar]
- Vaishnavi A, Kavitha S, Priya VV, Gayathri R. Cytotoxicity of Cyamopsis tetragonoloba leaves on oral cancer cells. Drug Invent Today. 2019;12(4). [Google Scholar]
- Wang N, Pan D, Guo Z, et al. Effects of guar gum on blood lipid levels: A systematic review and meta-analysis on randomized clinical trials. J Funct Foods. 2021;85:104605. [Google Scholar]
- Whistler RL, Hymowitz T. Guar: Agronomy, Production, Industrial Use, and Nutrition. Purdue University Press; 1979. [Google Scholar]
- Widowati W, Mozef T, Risdian C, Yellianty Y. Anticancer and free radical scavenging potency of Catharanthus roseus, Dendrophthoe petandra, Piper betle and Curcuma mangga extracts in breast cancer cell lines. Oxidants and Antioxidants in Medical Science. 2013;2(2):137-142. [Google Scholar]
- Witte W. Antimicrobial therapy in a historical perspective. Acta Veterinaria Scandinavica. Supplementum. 2000;93:7-16. [PubMed] [Google Scholar]
- Xia R, Schaafsma AW, Wu F, Hooker DC. Impact of the improvements in Fusarium head blight and agronomic management on economics of winter wheat. World Mycotoxin J. 2020;13(3):423-439. [Google Scholar]
- Xie Q, Chen ML, Qin Y, et al. Isoflavone consumption and risk of breast cancer: a dose-response meta-analysis of observational studies. Asia Pac J Clin Nutr. 2013;22(1):118-127. [DOI] [PubMed] [Google Scholar]
- Yadav H, Prasad AK, Goswami P, Pednekar S, Haque E, Shah M. Guar Industry Outlook 2015. Report Made for: National Commodity & Derivatives Exchange Limited. NIAM; 2013. [Google Scholar]
- Zahra SS, Ahmed M, Qasim M, et al. Polarity based characterization of biologically active extracts of Ajuga bracteosa Wall. ex Benth. and RP-HPLC analysis. BMC Complementary and Alternative Medicine. 2017;17(1):443-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci. 2016;8:33-42. [Google Scholar]
- Mustafa G, Arif R, Atta A, Sharif S, Jamil A. Bioactive compounds from medicinal plants and their importance in drug discovery in Pakistan. Matrix Science Pharma. 2017;1(1):17-26. [Google Scholar]
- Dai J, Mumper RJ. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules. 2010;15:7313-7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem A, Saleem M, Akhtar MF, Ashraf Baig MMF, Rasul A. HPLC analysis, cytotoxicity, and safety study of Moringa oleifera Lam. (wild type) leaf extract. J Food Biochem. 2020;44(10):e13400. [DOI] [PubMed] [Google Scholar]
- Soehnlen MK, Tran MA, Lysczek HR, Wolfgang DR, Jayarao BM. Identification of novel small molecule antimicrobials targeting Mycoplasma bovis. Journal of Antimicrobial Chemotherapy 2011;66(3):574-577. [DOI] [PubMed] [Google Scholar]