Abstract
Objectives:
This study investigates the therapeutic effect of vitamin C on the development of endometrial lesions and fecundity disorders in the ovarian induction model of mouse endometriosis.
Methods:
Ovarian endometriosis was surgically induced in 14 NMRI female mice (treatment group, N = 7) and (control group, N = 7). Three days after the second surgery (to assess endometriotic implant), the mice were randomized into two intervention groups: control (placebo) and treatment (50 mg/kg vitamin C every two days orally for four weeks) groups. In the oestrus phase, the mice were sacrificed. In macroscopic assessment, endometriotic implants were evaluated in size, volume, weight, growth score and adhesion score. The microscopic assessment examined the ovarian tissue (the number of antral follicles, corpus luteum and atretic follicles) and endometriotic lesion (histologic and trichrome fibrosis scores).
Results:
Post-treatment implant volume, growth score, adhesion extent score and adhesion severity score were significantly lower in the treatment group (vitamin C) in comparison with the control group (placebo) (p < 0.0001). The difference between the median weight of endometriotic implants, epithelialization of implant tissue, trichrome fibrosis scores and follicle number in the two groups (treatment and control) was statistically significant (p < 0.05). Atretic follicles were significantly decreased after vitamin C therapy (p < 0.05). Although the numbers of corpus luteum seemed to be more preserved in specimens from the control group, there was no statistical significance between the two groups’ histological scores.
Conclusion:
As a result, we may imply that vitamin C has a significant effect on reducing the induction and growth of endometrial implants, improving the fecundity function of ovaries, and consequently prevention of endometriosis-associated cancers. Further research is needed to improve targeted interventions resulting in the prevention and treatment of human endometriosis.
Keywords: antioxidant therapy, endometriosis, mouse model, ovarian induction, vitamin C
Introduction
Endometriosis is a common gynaecological reproductive age disorder characterized by the ectopic presence of endometrial-like tissue (gland and/or stroma). The familiar classic symptoms of endometriosis are pain and infertility. Endometriosis is also seen in women aged 12–80 years (average 28 years).1,2 The prevalence of endometriosis is assumed to be more than 10% in reproductive-age women. A high prevalence ( 20% to 90%) of the disease has been reported in women with a history of pelvic pain (chronic pelvic pain, dysmenorrhea and dyspareunia) and infertility. The economic burden of endometriosis equals diseases, such as diabetes, Crohn’s and rheumatoid arthritis. 1
Endometriosis lesions were divided into three groups: peritoneal, ovarian and deep infiltrating, based on the number, location and appearance. Endometriosis is an oestrogen-dependent disease with three theories regarding its pathogenesis: ectopic endometrial tissue implantation, coelomic metaplasia and induction theory. No single theory can explain the situation of endometriosis in all cases.1,3 Over the past 20 years, numerous studies on the pathogenesis and pathophysiology of endometriosis have enhanced our knowledge of the role of steroid hormones, genetics, environment, immune system, peripheral and central nervous system, inflammatory mediators and oxidative stress in the establishment, progress/regression, signs, symptoms and complications are associated with the disease.4,5
Endometriotic cysts create a toxic environment for ovarian tissue, including high levels of proteolytic enzymes, inflammatory cytokines and reactive oxygen species. As a result, increased oxidative stress levels cause oocyte apoptosis and consequently reduced fertility. 3 Activating macrophages and neutrophils in response to ectopic endometrial tissue and retrograde menstruation increases oxidative stress levels in women with endometriosis. Immune cells produce reactive oxygen species. Therefore, in oxidative stress conditions, it is crucial to have enough neutralizing antioxidants to prevent damage to the immune cells themselves. 6
The antioxidants eliminate the overproduction of ROS (reactive oxygen species) during oxidative stress and thus have a protective role in the body. Imbalance in the antioxidant defence system is associated with reproductive disorders such as oestrous cycle defects, impaired follicogenesis, follicular atresia and endometriosis, which may cause adverse effects on fertility and reproductive physiology.7–9 The results of a study by Bhardwaj and Saraf 10 showed a negative correlation between the frequency of apoptosis and the activity of antioxidant enzymes. In patients with endometriosis, oxidative stress is responsible for local tissue destruction and aggressive disease. 11
Vitamin C prevents germ cell apoptosis by reducing oxidative stress and scavenging free radicals as a potent water-soluble antioxidant.7,12 Reducing oxidative stress in the peritoneal cavity can prevent the onset and recurrence of endometriosis. Vitamin C has effective anti-inflammatory, anti-angiogenic and immune stimulator roles, which are influential factors in preventing the development of endometriosis. 13
Although endometriosis is non-malignant and not marked by uncontrolled lesion growth, it shares similar features with cancer. Among those features are increasing pelvic and distal cysts, resistance to apoptosis and invasion of other tissues with subsequent damage to the target organs are among those features. 14
Endometriosis has been reported to be associated with increased risk factors that are related to several types of cancer. Recently, it has been estimated that 20% of ovarian and deep endometriosis lesions contain cancer-causing gene alterations. Cancer risk assessment of women with endometriosis is crucial in screening, prevention and disease management. According to meta-analysis studies, women with endometriosis are more likely to develop ovarian, endometrial and thyroid cancers, and endometriosis itself appears to be a risk factor for ovarian cancer.14–16
Based on ethical considerations and the risk and burden of experimental research on human patients, animal models have been used to develop non-invasive diagnostic methods, classification systems, novel therapeutic approaches and even prevention methods in managing endometriosis. Using rodent models is cost-effective, easily accessible and provides a way to examine multiple aspects of the disease. 2
Since endometriosis is a very complex disease with a high impact on women’s quality of life, the need for a broader range of medical treatments is essential. Studies show that common pharmacological treatments for the disease encompass hormonal agents that cause fertility problems, while newer therapies focus on oxidative stress responses. Due to the prevalence of endometriosis and the limited drug therapies, there is an increasing need for further research and development of non-hormonal drugs to treat endometriosis. Based on this background, we investigated the therapeutic effect of vitamin C on endometrial lesions and fecundity disorders of experimentally induced ovarian endometriosis in a mouse model. A few studies have investigated the effect of vitamin C on endometriosis before, but they have used the peritoneal induction method, which makes this study a novelty.
Material and methods
A double-blind placebo-controlled randomized experimental study was conducted on fourteen mature, virgin female NMRI mice (25–35 g, 6–8 weeks) provided by Kurdistan Medical Sciences University’s experimental Animal Center (Sanandaj, Iran). The mice were housed in polypropylene cages (3 per cage) in a well-ventilated room and under the standard condition (12‑h light and 12‑h dark periods, the temperature range of 22–25°C, the humidity of 55%–60%). The mice had ad libitum access to standard dry pellets and water throughout the study. They were maintained under standard conditions to observe their health conditions before the experiment for one week. Our study design was based on the requirements of ARRIVE (Animal Research: Reporting of In Vivo Experiments).
The following formula (comparison between two groups in animal study) was used for the calculation of sample size: 17
From previous studies, standard deviation = 5.2, Za/2 = 1.96 (from Z table) at type 1 error of 0.05, Zβ = 0.842 (from Z table) at 80% power, effect size = difference between mean values from previous studies = 7.7; sample size was calculated = 6. For 10% attrition, seven mice per group were chosen.
The oestrous cycle (including pro-oestrus, oestrus, metoestrus and dioestrus) was assessed by observing vaginal smears, according to protocol paper. 18 All procedures, including surgery, endometriosis induction and tissue collection, were performed at the oestrous phase.
First surgery: induction of endometriosis
The mice were administrated 50 mg/kg ketamine and 7 mg/kg xylazine intraperitoneally for anaesthesia. The endometriosis induction surgery was performed in the aseptic situation according to protocol paper. 18 After shaving and disinfecting the surgical field, a vertical incision (1.5–2 cm) was briefly made below the left kidney. An approximate 1-cm segment of the middle part of the left uterine horn was clamped with 5-0 vicryl sutures (Ethicon, Denmark) to the utero-tubal and uterocervical junction excised. The uterine tissue was placed in a petri dish containing PBS (phosphate-buffered saline) supplemented with penicillin (100 U/mL) and streptomycin (100 mg/mL) and split longitudinally with the blade of scissors. Using a 3 mm punch biopsy (Sklar, Tru-Unch, India), the tissue was divided into two 3×3 mm2 sections. Each piece of the uterine horn was implanted to one of the ovary sides using a single suture with 6-0 black silk and 6-0 blue nylon. The implants were washed with 0.5–1 ml PBS supplemented with penicillin and streptomycin to prevent adhesions and dryness. The abdomen wall (peritoneum and fascia) and the skin were closed by suturing with 5-0 separate vicryl and 5-0 nylon, respectively. After the operations and recovery, the mice were situated individually, and 0.2 mg/kg buprenorphine was administrated subcutaneously to relieve the pain.
Second surgery: determination of the groups
Four weeks after the induction of endometriosis, a second surgery was directed to assess the endometriotic implants. Each implant’s volume was calculated by measuring the maximum diameter of each side to one-tenth of a millimetre with a Vernier calliper. The volume was calculated using the ellipsoid formula (V mm3 = 0.52 × A × B × C, where A: width, B: length and C: height). 19 The implants were photographed, and their clinical adhesion and size were measured (Figure 1).
Figure 1.

Measurement of an implant (length, width and height) using the calliper at the second surgery (4 weeks after induction of endometriosis).
The implants were successfully developed in all the mice. All environmental and animal preparation stages were executed with the first surgery’s procedure (anaesthesia, cleaning, left lower back incision, suturing and recovery steps).
Three days after the second surgery, the mice were randomly divided into two intervention groups of control (placebo) and treatment with seven mice in each group. The treatment group was given 50 mg/kg (0.5 mL) vitamin C (500 mg Nature Made, American, Health Code Certificate 310009077035) every two days orally for four weeks. The control group was given a 0.5 mL mix of water and starch. The given dosages of vitamin C were calculated based on the human dose equivalent as follows:
AED (animal equivalent dose) (mg/kg) = Human dose (mg/kg)× Km (Kunming mice) ratio
Km ratio for mice = 12.3, human dose = 2 mg/kg (120 mg/d)
AED (Animal equivalent dose) = 24.6/kg/dIs almost equal to 50 mg/kg every 2 days
The researcher was blinded to the groups at all stages.
Third surgery: sacrifice and collection of samples
In the oestrus phase, the mice were sacrificed using isoflurane asphyxiation and were dissected. The endometriosis lesions were evaluated and scored based on macroscopic findings (Table 1). The endometriotic implants were excised and weighed accurately.
Table 1.
Scoring macroscopic and microscopic evaluations.
| Macroscopic evaluation | |
|---|---|
| 1 The growth of the implant | |
| Definition | Score |
| The implant vanished, or it was visible without the vesicle | 1 |
| The implant fashioned a vesicle with the major dimension less than 2 mm | 2 |
| The implant fashioned a cyst containing fluid, with the major dimension ⩾ 2 mm, but < 4.5 mm | 3 |
| The major dimension of the vesicle was ⩾ 4.5, but < 6 | 4 |
| Implant size ⩾ 6 mm | 5 |
| 2 The adhesion severity | |
| Definition | Score |
| Without resistance to separation | 0 |
| Partially resistant to separation | 0.5 |
| Sharp dissection | 1 |
| 3 The adhesion extent | |
| Definition | Score |
| No adhesions points | 0 |
| Points 25% of traumatized area | 1 |
| Points 50.0% of traumatized area | 2 |
| The whole points of traumatized area involvement | 3 |
| Microscopic evaluation | |
| 1 Implants Histologically were scored based on a semi-quantitative assessment | |
| Definition | Score |
| No epithelium | 0 |
| Extremely little (insufficient) to remain in the epithelial layers | 1 |
| Maintaining the average epithelial layer with leukocyte infiltration | 2 |
| The epithelial layers are well maintained and remain | 3 |
| 2 Masson’s trichrome staining evaluated the presence of stroke and fibrous elements | |
| Definition | Score |
| No fibrosis | 0 |
| Minimal growth of fibrous tissue | 1 |
| Irregular fibrous tissue growth | 2 |
| Integrated and hyalinized accumulated fibrosis | 3 |
Histological analysis
Collected samples (the ovary, the remaining implant and the uterine horn) were fixed in a 10% formalin solution. Specimens were dehydrated in a graded series of ethanol rinses, cleared in xylene, embedded in paraffin and cut into 4-micrometre sections (three sections for each specimen). The sections were deparaffinized by xylene and then rehydrated using ethanol and decreasing degree. The sections were stained with haematoxylin–eosin (HE) and trichrome (Masson’s stain).
Macroscopic Assessment: Endometriotic implants (the size, volume and weight of endometriotic implants, growth score and adhesion score); 20 microscopic Assessment: The ovarian tissue (the number of follicles, corpus luteum and atretic follicles) and endometriotic lesion (histologic and trichrome fibrosis scores) (Table 1 and Figures 2–4).21–23 The follicles, corpus luteum and atretic follicles on each three ovary sections were counted blindly twice with a two-week interval by an investigator (pathologist). The final result for each ovary was achieved by the average slide count of the two observations.
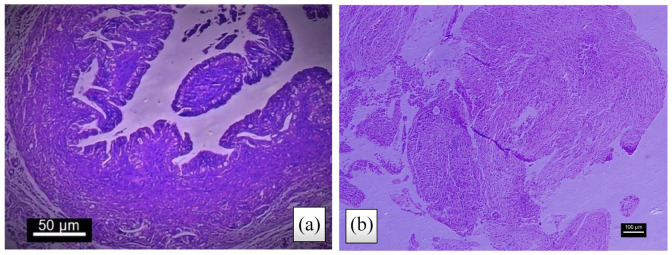
Figure 2.
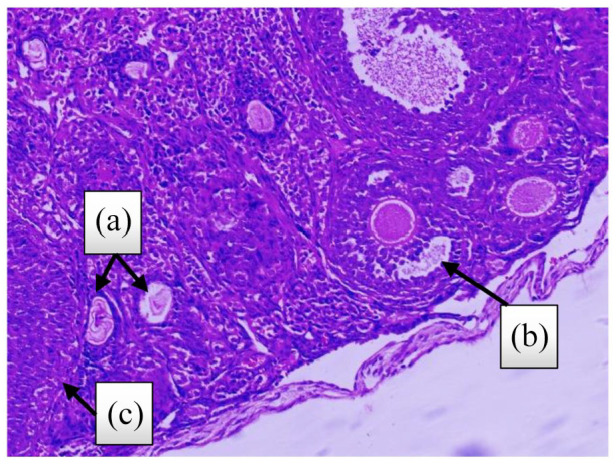
Ovarian tissue stained with H&E ((a): atretic follicle, (b): antral follicle and (c): corpus luteum) (4 weeks after treatment, control group).
Figure 3.
Implant stained with H&E, 4 weeks after treatment: (a) The epithelial layers are well maintained and remain (control group); (b) endometriotic implant with defective epithelium after vitamin C treatment (treatment group).
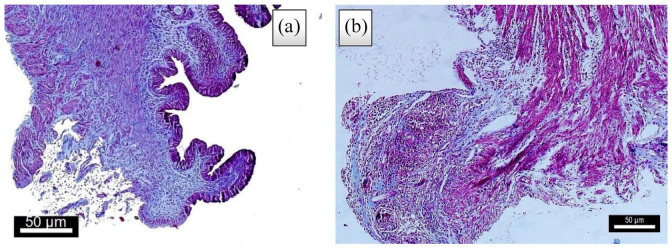
Figure 4.
Comparison of the degree of fibrosis between the two groups by Masson’s staining. (4 weeks after treatment, (a) control group, (b) treatment group).
Definition of atretic follicles: The follicle is characterized by a fragmented egg, rupture of the plasma membrane and the shedding of granulosa cells into the antrum.
Statistical analysis
The Stata (version 13) and GraphPad Prism (version 8) were used for the statistical analysis and graph generations. The Shapiro–Wilk test, measured dispersion, central tendency and histogram graph were used to check the continuous variable’s normality.
The obtained results were expressed as median and quadrant and the effect size (i.e. mean difference, standardized mean difference and mean ratio). The mean volume and scores of the extent and adhesion of the endometriotic lesions and fecundity status of the two groups (7 mice in each group) were compared.
The metric variables were compared by t-test, one-way ANOVA test and Bonferroni post hoc tests. Further, the non-metric variables were examined by the Kruskal–Wallis and Mann–Whitney U-tests.
The study groups evaluated metric (preceding and after the medical treatment) and non-metric variables, respectively, by repeated-measures ANOVA and the generalized estimating equation model. p values of < 0.05 were considered significant.
Ethics
This experimental study was carried out in an animal laboratory, and its approval was received from the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (ethics code IR.SBMU.PHARMACY.REC.1399.136). All animal experiments were performed under the Animals (Scientific Procedures) Act 1986 Amendment Regulations 2012. 24
Results
Implant assessment
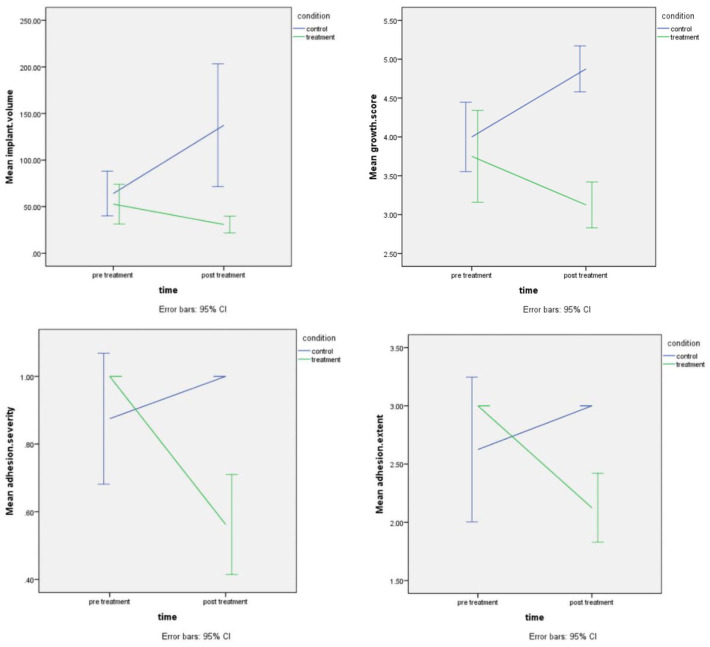
The pre-treatment volume (p = 0.521), growth score (p = 0.259), adhesion extent score (p = 0.129) and adhesion severity score (p = 0.069) of endometriotic implants were similar between the two groups. The overall result of fitting the model GEE (generalized estimating equation model) showed that post-treatment implant volume, growth score, adhesion extent score and adhesion severity score of the treatment group (vitamin C) were significantly lower than those of the control group (placebo) (p < 0.0001) (Figures 5–7).
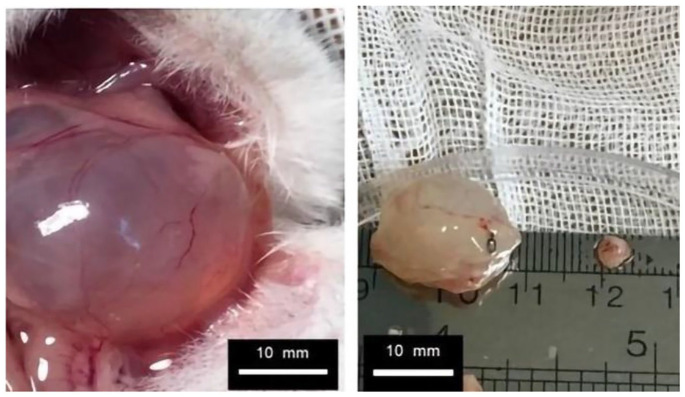
Figure 5.
Evaluation and measurement of endometriotic lesions 4 weeks after treatment in the control group (placebo).
Figure 6.
Evaluation and measurement of endometriotic lesions 4 weeks after treatment in the treatment group (vitamin C).
Figure 7.
The comparison chart of implant volume (mm3), growth score, adhesion extent and severity before and after treatment (control and treatment).
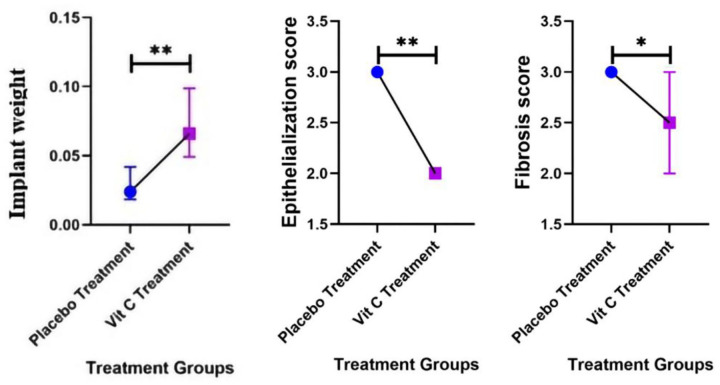
The difference between the median weight of endometriotic implants, epithelialization of implant tissue and trichrome fibrosis scores in the two groups (treatment and control) were statistically significant (p < 0.05; Figure 8, Table 2). The mean ratio in this test shows that the weight of endometriotic implants (77%), epithelialization of implant tissue score (33%) and trichrome fibrosis scores (16%) in the treatment group have decreased compared with the control group.
Figure 8.
Comparison charts of implant weights, epithelialization and fibrosis scores in two groups (4 weeks after treatment).
*p < 0.05, **p < 0.01.
Table 2.
Comparison of implant weights, epithelialization of implant tissue score, trichrome fibrosis scores in two groups (7 in each group).
| Variable | Treatment group (vit C)7 | Control group (placebo)7 | Test result | Effect size | |||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Percentile | Median | Percentile | (Mann-Whitney) | Mean ratio (95% CI) | ||||
| 25 | 75 | 25 | 75 | ||||||
| Weight of endometriotic implants (g) | 0.026 | 0.02 | 0.046 | 0.073 | 0.049 | 0.133 | Z =−2.89 | *p = 0.004 | *0.23(0.16–0.66) |
| Epithelialization of implant tissue score | 2 | 2 | 2 | 3 | 3 | 3 | Z = 3.04 | *p = .002 | *0.67(0.53–0.85) |
| Trichrome fibrosis scores | 2.5 | 2 | 3 | 3 | 3 | 3 | Z = 2.22 | *p = 0.03 | *0.84(0.72–0.98) |
statistically significant.
Reproductive assessment
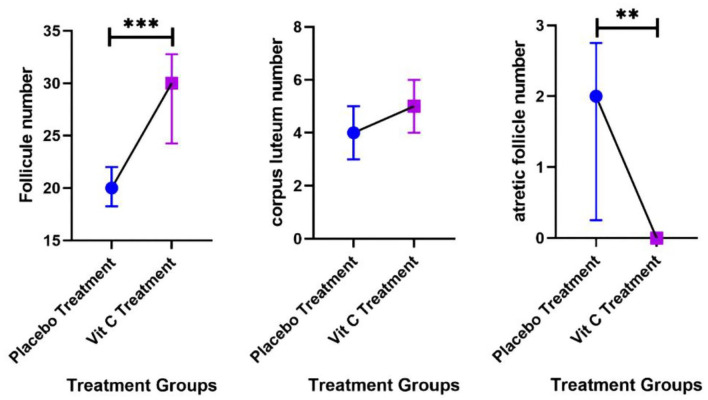
The statistical test results concerning fecundity changes showed that the number of follicles was significantly increased, and atretic follicles number were decreased considerably after vitamin C therapy (p < 0.05). Although corpus luteum number seemed to be more preserved in specimens from the treatment group, there was no statistical significance between the histological scores of the groups (Table 3; Figure 9). The sample weighted mean for the standardized mean difference of follicle number and atretic follicle number was 0.88 (0.84–0.93) and 0.54 (0.17–1.78), large and medium, respectively. The mean ratio in this test shows that the weight of the corpus luteum number (15%) in the treatment group has increased compared with the control group.
Table 3.
Comparison of follicle, atretic follicle number and corpus luteum number in two groups (7 in each group).
| Variable | Treatment group (Vit C)7 | Control group (placebo)7 | Test result | Effect size | |||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Percentile | Median | Percentile | (Mann-Whitney) | Mean ratio (95% CI) | ||||
| 25 | 75 | 25 | 75 | ||||||
| Follicle number | 30 | 24 | 32 | 20 | 18.25 | 22 | Z =−3.47 | *p = 0.0005 | *0.88(0.84–0.93) |
| Atretic follicle number | 0 | 0 | 0 | 2 | 0.25 | 2.75 | Z =−2.73 | *p = .006 | *0.54(0.17–1.78) |
| Variable | Mean | SD | Mean | SD | t-test | SMD (95% CI) |
|||
| Corpus luteum number | 5.13 | 1.13 | 4.11 | 1.27 | t = 1.73 | p = 0.10 | 0.85(–0.15–1.84) | ||
SMD: standardized mean difference.
statistically significant.
Figure 9.
Comparison charts of follicle, atretic follicle and corpus luteum number in two groups (4 weeks after treatment).
**p < 0.01, ***p < 0.001.
Discussion
Endometriosis is a complicated disease, and no animal model can fully demonstrate human conditions. This study is the first one evaluating the effect of vitamin C therapy on ovarian implant growing in an experimental endometriosis model. In the current study, the treatment group showed significant decreases in the lesion’s volume, weight, adhesion, extent, severity and histopathologic scores (epithelialization of implant tissue score and trichrome fibrosis Scores). In contrast to the treatment group, the implants volumes and weights continued to increase during the study in the control group.
In comparing the two groups of vitamin C treatment and placebo before treatment in terms of volume, intensity, extent, adhesion score and growth score of implants, no significant difference was observed between the two groups, which indicated a similar condition between the two groups before treatment.
But the result of fitting the generalized estimating equation (GEE) model in terms of comparison of volume, intensity, proliferation and adhesion score and growth score of implants in the two groups of vitamin C treatment and placebo after treatment, according to the pre-treatment results, the reduction of the mentioned cases after the treatment with vitamin C compared to the placebo group showed that it was statistically significant in all cases.
One study has reported that the endometriotic implants in rat models in all treatment groups (antioxidant herbs supplementation) were lower than the control group. 25
The study results of Erten et al. 4 showed a significant difference in the volume of the implants before and after the treatment with vitamin C. In one study, 1,200 units of vitamin E and 1,000 mg of vitamin C were prescribed for eight weeks before endometriosis surgery. That study showed a 43% reduction in pain compared to placebo recipients. 26 Durak et al. investigated the effect of vitamin C at doses of 0.5, 1.25 and 2.5 mg on induced endometriosis cysts in rats. The weight and volume of cysts treated at a dose of 2.5 mg were reduced significantly. 6
In agreement with our results, Yavuz et al. 27 revealed that antioxidant treatment had significantly reduced the histological score compared to the control group. In a study by Bakacak et al., 28 significant decreases in the treatment group’s implant volume and histopathologic scores were observed, unlike the control group. Tissue fibrosis results from a chronic inflammation following tissue damage and invasion thought to be associated with an inadequate immune response. 29
It was well estimated that by allowing free radicals to remove a hydrogen atom from the antioxidant molecule instead of polyunsaturated fatty acids, vitamins C and E effectively enhanced SOD, GST and CAT activity within granulosa cells, so, breaking free radical chain reactions and producing relatively inactive radicals. 30
Although it was a difference between the type of induction of endometriosis in the present study (ovarian induction) and other studies (peritoneal and subcutaneous induction), similar results are observed regarding the effect of vitamin C on reducing the volume, intensity, proliferation, adhesion and growth score of implants.
Follicles are a key component of reproduction biology in terms of development and function. 31 In this study, to investigate the effect of treatment on fertility, the number of follicles, corpus luteum and atretic follicles were compared in the two groups of vitamin C treatment and placebo treatment. The number of follicles and corpus luteum in the vitamin C treatment group increased compared to the placebo group. This difference in the number of follicles was statistically significant.
The number of atretic follicles in the vitamin C treatment group showed a statistically significant decrease compared to the placebo group. One study has found that treatment with antioxidants (microginone and African antada root extract) reduced the number of atretic follicles and increased follicle development, puberty and ovulation. 32
Cummings and Metcalf 33 observed that the mice with surgically induced endometriosis did not exhibit the severe fertility reduction seen in women with endometriosis.
Surgically induced endometriosis in mice conduces fecundity dysfunction, anomalous oocyte quality, embryo growth and early lost pregnancy. Balancing between reactive oxygen species and antioxidants is essential for oocyte maturation. Researchers have shown that insufficient or excessive levels of reactive oxygen species in endometriosis have adverse effects on oocyte and embryo development, a lower implantation rate, and pregnancy outcomes.34,35 It is in line with the present study’s results that the treatment group had significantly higher follicles and decreased atretic follicles than the control group. This study validates the work of Moon et al., 36 who found more luteinized unruptured follicles and fewer follicles in Endo rats compared to the controls. Recently, the number of ovulated oocytes does not decrease in endometriosis-induced mice, but the quality of the oocytes and the number of embryos decreases. 37 The type of endometriosis induction can be one of the reasons for the differences in the results of studies.
A novel, long-term treatment of endometriosis, must affect the disease initiation and progression stages, including proinflammatory environment, increased angiogenesis, resistance to apoptosis, structural and epigenetic changes, and oxidative stress, with minimum adverse effects on fertility.
There is an urgent need for a new treatment that eliminates the lesions without any side effects. Our findings showed that vitamin C affects multiple fundamental processes in the pathogenesis of endometriosis, which may help treat this common gynaecological disorder. Furthermore, vitamin C significantly inhibited the growth and cyst formation of endometriotic lesions compared to vehicle-treated controls. All these findings imply that vitamin C may play a protective or therapeutic role in endometriosis.
There were several limitations to the present study. It was an experimental study with a small number of mice per group. The second limitation of this study was the lack of using various doses in groups. Although this study’s overall results show that vitamin C has an excellent effect on endometriosis, further investigation is needed to assess its potential as an alternative therapy for endometriosis. The third limitation is that the current study does not compare the effects of a well-known antioxidant agent or a gonadotropin-releasing hormone agonist (GnRH agonist), which are proven therapies used in humans.
Conclusion
As a result, we may imply that vitamin C has a significant effect on reducing the induction and growth of endometrial implants, improving the fecundity function of ovaries, and consequently prevention of endometriosis-associated cancers. Further research is needed to improve targeted interventions aimed at preventing and treating human endometriosis. Various observations from studies support the role of oxidative stress in the development and progression of endometriosis. This evidence may pave the way for evaluating treatment approaches targeting oxidative imbalance: Oxidative stress can be the key to treatment and ultimately prevent endometriosis. In particular, clinical trial studies will help better explain the effect of antioxidants as potential treatments for endometriosis in the future.
Supplemental Material
Supplemental material, sj-docx-1-whe-10.1177_17455057221096218 for The effectiveness of antioxidant therapy (vitamin C) in an experimentally induced mouse model of ovarian endometriosis by Hayedeh Hoorsan, Masoumeh Simbar, Fahimeh Ramezani Tehrani, Fardin Fathi, Nariman Mosaffa, Hedyeh Riazi, Loghman Akradi, Sherko Nasseri and Shayan Bazrafkan in Women’s Health
Acknowledgments
The authors would like to thank members of the Cellular & Molecular Research Center, Kurdistan University of Medical Sciences (Dr Zareie, Dr Hassanzadeh, Ms P. Khalvatian, Ms F. Zamani and Mr J. Hossaini) and the Reproductive Endocrinology Research Center staff (Ms M. Noroozzadeh and Dr R. Bidhendi) for their contribution to this research.
Footnotes
Author contribution(s): Hayedeh Hoorsan: Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Visualization; Writing – original draft.
Masoumeh Simbar: Conceptualization; Project administration; Supervision; Validation; Visualization; Writing – review & editing.
Fahimeh Ramezani Tehrani: Conceptualization; Methodology; Project administration; Resources; Supervision; Visualization; Writing – review & editing.
Fardin Fathi: Conceptualization; Project administration; Resources; Supervision; Validation; Writing – review & editing.
Nariman Mosaffa: Conceptualization; Methodology; Supervision; Validation; Visualization; Writing – review & editing.
Hedyeh Riazi: Investigation; Validation; Writing – review & editing.
Loghman Akradi: Data curation; Formal analysis; Validation; Visualization; Writing – review & editing.
Sherko Nasseri: Data curation; Formal analysis; Software; Writing – original draft.
Shayan Bazrafkan: Data curation; Formal analysis; Software; Writing – original draft.
Availability of data and materials: Data for this study were extracted from a Research project at the Shahid Beheshti University of Medical Sciences. All data generated and/or analysed during the study are available by the correspondent author on official and reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Hayedeh Hoorsan  https://orcid.org/0000-0003-2960-8821
https://orcid.org/0000-0003-2960-8821
Supplemental material: Supplemental material for this article is available online.
References
- 1. Berek JS. Berek & Novak’s gynecology. 16th ed. Philadelphia, PA: Wolters Kluwer Health, 2019. [Google Scholar]
- 2. Sharpe-Timms KL. Animal models for endometriosis. Columbia, MI: Springer, 2020. [Google Scholar]
- 3. Genazzani AR, Nisolle M, Petraglia F, et al. Endometriosis pathogenesis, clinical impact and management. Cham: Springer, 2021. [Google Scholar]
- 4. Erten OU, Ensari TA, Dilbaz B, et al. Vitamin C is effective for the prevention and regression of endometriotic implants in an experimentally induced rat model of endometriosis. Taiwan J Obstet Gynecol 2016; 55(2): 251–257. [DOI] [PubMed] [Google Scholar]
- 5. Giudice LC, Evers JL, Healy DL. Endometriosis: science and practice. 1st ed. Hoboken, NJ: Wiley-Blackwell, 2012. [Google Scholar]
- 6. Durak Y, Kokcu A, Kefeli M, et al. Effect of vitamin C on the growth of experimentally induced endometriotic cysts. J Obstet Gynaecol Res 2013; 39(7): 1253–1258. [DOI] [PubMed] [Google Scholar]
- 7. Bhardwaj JK, Kumari P, Saraf P, et al. Antiapoptotic effects of vitamins C and E against cypermethrin-induced oxidative stress and spermatogonial germ cell apoptosis. J Biochem Mol Toxicol 2018; 32(8): e22174. [DOI] [PubMed] [Google Scholar]
- 8. Bhardwaj JK, Mittal M, Saraf P, et al. Pesticides induced oxidative stress and female infertility: a review. Toxin Rev 2020; 39: 1–13. [Google Scholar]
- 9. Bhardwaj JK, Saraf P. Granulosa cell apoptosis by impairing antioxidant defense system and cellular integrity in caprine antral follicles post malathion exposure. Environ Toxicol 2016; 31(12): 1944–1954. [DOI] [PubMed] [Google Scholar]
- 10. Bhardwaj JK, Saraf P. N-acetyl cysteine-mediated effective attenuation of methoxychlor-induced granulosa cell apoptosis by counteracting reactive oxygen species generation in caprine ovary. Environ Toxicol 2017; 32(1): 156–166. [DOI] [PubMed] [Google Scholar]
- 11. Carvalho LF, Samadder AN, Agarwal A, et al. Oxidative stress biomarkers in patients with endometriosis: systematic review. Arch Gynecol Obstet 2012; 286(4): 1033–1040. [DOI] [PubMed] [Google Scholar]
- 12. Bhardwaj JK, Paliwal A, Saraf P. Effects of heavy metals on reproduction owing to infertility. J Biochem Mol Toxicol 2021; 35(8): e22823. [DOI] [PubMed] [Google Scholar]
- 13. Nezhat CH. Endometriosis in adolescents: a comprehensive guide to diagnosis and management. Cham: Springer, 2020. [Google Scholar]
- 14. Kvaskoff M, Mahamat-Saleh Y, Farland LV, et al. Endometriosis and cancer: a systematic review and meta-analysis. Hum Reprod Update 2021; 27: 393–420. [DOI] [PubMed] [Google Scholar]
- 15. Guidozzi F. Endometriosis-associated cancer. Climacteric 2021; 24: 587–592. [DOI] [PubMed] [Google Scholar]
- 16. Králíčková M, Losan P, Vetvicka V. Endometriosis and cancer. Womens Health 2014; 10: 591–597. [DOI] [PubMed] [Google Scholar]
- 17. Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother 2013; 4(4): 303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoorsan H, Simbar M, Tehrani FR, et al. Induction of the mice model regarding the endometriosis and assessment of antioxidant treatment effectiveness: an experimental protocol. Int J Women Health Reprod Sci 2020; 8: 133–141. [Google Scholar]
- 19. Anğın AD, Gün İ, Alan Y, et al. The effects of leukocyte- and platelet-rich plasma (L-Prp) and pure platelet-rich plasma (P-Prp) in a rat endometriosis model. Trop J Obstet Gynaecol 2020; 37: 132–139. [Google Scholar]
- 20. Soysal D, Kızıldağ S, Saatlı B, et al. A novel angiogenesis inhibitor bevacizumab induces apoptosis in the rat endometriosis model. Balkan J Med Genet 2014; 17(2): 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gerstein AS. Molecular biology problem solver: a laboratory guide. New York: John Wiley & Sons, 2004. [Google Scholar]
- 22. Kiani K, Movahedin M, Malekafzali H, et al. Effect of the estrus cycle stage on the establishment of murine endometriosis lesions. Int J Reprod Biomed 2018; 16(5): 305–314. [PMC free article] [PubMed] [Google Scholar]
- 23. Wimmer I, Tröscher AR, Brunner F, et al. Systematic evaluation of RNA quality, microarray data reliability and pathway analysis in fresh, fresh frozen and formalin-fixed paraffin-embedded tissue samples. Sci Rep 2018; 8: 6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hollands C. The animals (scientific procedures) act 1986. Lancet 1986; 2: 32–33. [DOI] [PubMed] [Google Scholar]
- 25. Trisetiyono Y, Widjiati W, Hidayat ST, et al. Antioxidant herbs supplementation inhibits endometriosis extension in mice. J Biomed Transl Res 2019; 5: 53–61. [Google Scholar]
- 26. Brunty S, Santanam N. Current assessment of the (dys)function of macrophages in endometriosis and its associated pain. Ann Transl Med 2019; 7(Suppl. 8): S381–S385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yavuz S, Aydin NE, Celik O, et al. Resveratrol successfully treats experimental endometriosis through modulation of oxidative stress and lipid peroxidation. J Cancer Res Ther 2014; 10(2): 324–329. [DOI] [PubMed] [Google Scholar]
- 28. Bakacak M, Ercan Ö, Köstü B, et al. The effects of thalidomide in a rat model of surgically-induced endometriosis. Turk J Obstet Gynecol 2015; 12(3): 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marcellin L, Santulli P, Chouzenoux S, et al. Alteration of Nrf2 and glutamate cysteine ligase expression contribute to lesions growth and fibrogenesis in ectopic endometriosis. Free Radic Biol Med 2017; 110: 1–10. [DOI] [PubMed] [Google Scholar]
- 30. Bhardwaj JK, Mittal M, Saraf P. Effective attenuation of glyphosate-induced oxidative stress and granulosa cell apoptosis by vitamins C and E in caprines. Mol Reprod Dev 2019; 86(1): 42–52. [DOI] [PubMed] [Google Scholar]
- 31. Bhardwaj JK, Saraf P. Morphological attributes of granulosa cells perpetuating functional integrity of an ovarian follicle. J Adv Microsc 2017; 12: 92–96. [Google Scholar]
- 32. Mvondo MA, Minko Essono S, Bomba Tatsinkou FD, et al. The root aqueous extract of Entada Africana Guill. et Perr. (Mimosaceae) inhibits implant growth, alleviates dysmenorrhea, and restores ovarian dynamic in a rat model of endometriosis. Evid Based Complement Altern Med 2017; 2017: 8563909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cummings AM, Metcalf JL. Effect of surgically induced endometriosis on pregnancy and effect of pregnancy and lactation on endometriosis in mice. Proc Soc Exp Biol Med 1996; 212(4): 332–337. [DOI] [PubMed] [Google Scholar]
- 34. Lee YH, Yang JX, Allen JC, et al. Elevated peritoneal fluid ceramides in human endometriosis-associated infertility and their effects on mouse oocyte maturation. Fertil Steril 2018; 110(4): 767–777. [DOI] [PubMed] [Google Scholar]
- 35. Sönmezer M, Taşkin S. Fertility preservation in women with ovarian endometriosis. Womens Health 2015; 11: 625–631. [DOI] [PubMed] [Google Scholar]
- 36. Moon CEM, Bertero MC, Curry TE, et al. The presence of luteinized unruptured follicle syndrome and altered folliculogenesis in rats with surgically induced endometriosis. Am J Obstet Gynecol 1993; 169(3): 676–682. [DOI] [PubMed] [Google Scholar]
- 37. Cohen J, Ziyyat A, Naoura I, et al. Effect of induced peritoneal endometriosis on oocyte and embryo quality in a mouse model. J Assist Reprod Genet 2015; 32(2): 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-whe-10.1177_17455057221096218 for The effectiveness of antioxidant therapy (vitamin C) in an experimentally induced mouse model of ovarian endometriosis by Hayedeh Hoorsan, Masoumeh Simbar, Fahimeh Ramezani Tehrani, Fardin Fathi, Nariman Mosaffa, Hedyeh Riazi, Loghman Akradi, Sherko Nasseri and Shayan Bazrafkan in Women’s Health