Abstract
Background:
HIV Prevention Trials Network (HPTN) 083 demonstrated superiority of long-acting injectable cabotegravir (CAB-LA) compared to oral emtricitabine/tenofovir disoproxil fumarate (F/TDF) for HIV pre-exposure prophylaxis (PrEP).
Objective:
To identify the maximum price premium (i.e., greatest possible price differential) that society should be willing to accept for the additional benefits of CAB-LA over tenofovir-based PrEP among men who have sex with men and transgender women (MSM/TGW) in the United States (US).
Design:
Simulation, cost-effectiveness analysis.
Data Sources:
Trial and published data, including: estimated HIV incidence (5.32, 1.33, 0.26/100PY for off PrEP, generic F/TDF and branded emtricitabine/tenofovir alafenamide (F/TAF), and CAB-LA); 28% 6-year PrEP retention. Annual base case drug costs: $360 and $16,800 for generic F/TDF and branded F/TAF. We assumed fewer side effects with branded F/TAF versus generic F/TDF.
Target Population:
476,700 MSM/TGW at very high risk for HIV.
Time Horizon:
10 years.
Perspective:
Healthcare system.
Intervention:
CAB-LA versus generic F/TDF or branded F/TAF for HIV PrEP.
Projected Outcome Measures:
Primary transmissions, quality-adjusted life-years (QALYs), costs (2020 USD), incremental cost-effectiveness ratios (ICERs, $/QALY), maximum price premium for CAB-LA versus tenofovir-based PrEP.
Results of Base-Case Analysis:
Compared to generic F/TDF (or branded F/TAF), CAB-LA increased life expectancy by 28,000 QALYs (26,000 QALYs) among those at very high risk for HIV. Branded F/TAF cost more per QALY gained than generic F/TDF compared to no PrEP. At 10 years, CAB-LA could achieve ICER ≤$100,000/QALY compared to generic F/TDF at a maximum price premium of $3,700/year over generic F/TDF (CAB-LA price <$4,100/year).
Results of Sensitivity Analysis:
In a PrEP-eligible population at high, rather than very high, risk for HIV (n=1,906,800; off PrEP incidence: 1.54/100PY), CAB-LA could achieve an ICER ≤$100,000/QALY versus generic F/TDF at a maximum price premium of $1,100/year over generic F/TDF (CAB-LA price <$1,500/year).
Limitations:
Uncertain clinical and economic benefits of averting future transmissions.
Conclusion:
Effective oral PrEP limits the additional price society should be willing to pay for CAB-LA.
Primary funding source:
FHI 360; Eunice Kennedy Shriver National Institute for Child Health and Human Development; National Institute of Allergy and Infectious Diseases; National Hearth Lung and Blood Institute; National Institute on Drug Abuse; the Reich HIV Scholar Award and the Steve and Deborah Gorlin MGH Research Scholars Award.
Keywords: HIV, PrEP, long-acting injectable cabotegravir, men who have sex with men
INTRODUCTION
The HIV Prevention Trials Network (HPTN) 083 trial, which evaluated the efficacy of long-acting injectable cabotegravir (CAB-LA) compared to daily oral emtricitabine/tenofovir disoproxil fumarate (F/TDF) for HIV pre-exposure prophylaxis (PrEP) among men who have sex with men and transgender women (MSM/TGW), was unblinded early after demonstrating the superior effectiveness of CAB-LA (1). In this international trial, reductions in incident HIV infections were observed in the CAB-LA vs. F/TDF arm (0.41 vs. 1.22/100PY), with similar results in the subset of United States (US) participants (0.26 vs. 1.33/100PY) (1). While these results hold promise to make a long-awaited novel PrEP modality an option for patients – particularly among those who could only effectively use PrEP if it were in injectable form – the incremental value of CAB-LA in the context of its anticipated higher drug and administration costs is unknown. Concerns have also been raised about the possibility of drug resistance among those acquiring HIV while prescribed a long-acting PrEP regimen (2). Current generic F/TDF pricing has dropped far below the range of other generic antiretroviral medications (3). However the price of branded emtricitabine/tenofovir alafenamide (F/TAF) remains costly and demand has increased (4). The availability of significantly cheaper generic F/TDF options prompted us to evaluate the clinical implications of CAB-LA’s superiority and examine how much society should be willing to pay for the improved efficacy of CAB-LA over both branded and generic tenofovir-based options (5).
METHODS
Analytic Overview
We used the Cost-Effectiveness of Preventing AIDS Complications model to simulate a population prescribed PrEP with risk factors for HIV similar to those of US HPTN 083 trial participants (6). HPTN 083 enrolled MSM/TGW without HIV who self-identified as being at very high risk for HIV, based on study criteria (Appendix Methods) (1). We modeled four strategies: No PrEP, generic F/TDF, branded F/TAF, and CAB-LA. Using a 10-year planning horizon, we projected clinical benefits, including: primary transmissions, quality-adjusted life-years (QALYs) and mortality. We used the healthcare sector perspective, including the costs of HIV care and PrEP (both drug and program) and incremental cost-effectiveness ratios (ICERs: Δ costs/ Δ QALYs). Clinical outcomes and costs were discounted at 3%/year and included the health and economic benefits attributable to averted primary transmissions. We identified the highest price premium that CAB-LA could command (i.e., a ceiling), consistently choosing parameter values to portray CAB-LA as favorably as possible relative to tenofovir-based PrEP in the base case; we also chose values to portray branded F/TAF as favorably as possible relative to generic F/TDF. Given the explicit uncertainty inherent in decision analyses, if some baseline parameter values favor one strategy and some favor the other, it remains uncertain as to the influence of these conflicting baseline value choices on the optimal strategy. Choosing parameter values that consistently favor one strategy over another, then, becomes a common method used in decision analysis. When, despite those parameter value assumptions (biases) in favor of that strategy, it remains inferior or is expensive (based on a high ICER or a low price premium), the analysis provides greater confidence in the modeling results. There is no single accepted willingness-to-pay threshold; we therefore varied the threshold from $50,000-$300,000/QALY (7). We identified the maximum price premium compared to oral PrEP at which CAB-LA would achieve an ICER at or below a specific willingness-to-pay threshold i.e., the greatest possible price differential that society should be willing to accept for the additional benefits of CAB-LA over the current price of oral PrEP (generic F/TDF or branded F/TAF), varying assumptions about CAB-LA PrEP efficacy and retention (8). We repeated this exercise in a scenario analysis examining use of CAB-LA among all potential MSM/TGW PrEP users in the US because the general PrEP user may be at lower risk for HIV than those who enrolled in HPTN 083.
Model Structure
The Cost-Effectiveness of Preventing AIDS Complications model is a validated state-transition microsimulation model of HIV prevention and treatment. Individuals entered the model without HIV, and their possible HIV outcomes were simulated in monthly cycles (6).
HIV PrEP program and screening strategies
At model start, all individuals enrolled in one of the four PrEP programs. Thereafter, they faced monthly probabilities of HIV acquisition – governed by the estimated HIV incidence rate – which were attenuated while enrollees were prescribed PrEP, depending on PrEP efficacy. They also faced monthly probabilities of discontinuing the PrEP program. In addition to background HIV testing while off PrEP, all PrEP programs included interval HIV screening at program visits. Those who screened positive were linked to HIV care. Individuals who discontinued participation in a PrEP program faced the “No PrEP” monthly probability of HIV acquisition.
HIV incidence, diagnosis, and disease
We defined HIV incidence as new HIV acquired by modeled individuals. People with incident HIV infection were assigned characteristics, including initial CD4 count and HIV RNA level. In the absence of effective antiretroviral therapy (ART), CD4 count declined monthly. Those with HIV faced risks of developing opportunistic infections and death, determined by current age and CD4 count. HIV infection was detected by a background testing rate (9,10). Additionally, undiagnosed people could develop an opportunistic infection, leading to HIV diagnosis. Once diagnosed, patients who linked to HIV care were prescribed ART. On ART, patients faced a probability of virologic suppression, loss to follow up, and subsequent return to care, stratified by baseline adherence level.
Primary HIV transmissions
Incident HIV cases produced primary HIV transmissions, defined as the first generation of incident infections that would be transmitted from individuals in the initial modeled cohort to people outside this group (defined as “partners,” Appendix Figure 1). In any given PrEP strategy, partners subscribed to the same PrEP strategy as those from whom they acquired the infection, with its defined efficacy, retention, and costs; PrEP programs thus reduced both incident infections and primary transmissions. Individuals who acquired HIV through primary transmission had similar testing, treatment, and cost structures as the index modeled individuals with HIV from whom their infection was acquired.
Model Inputs
Modeled cohort: HPTN 083 trial cohort and population sizes
We defined the base case population as MSM/TGW at very high risk for HIV (VHR, incidence 5.32/100PY in HPTN 083) to be representative of the subset of US HPTN 083 trial participants (mean age 30.1 years) (1). Using US Centers for Disease Control and Prevention data, we estimated this population size at approximately 476,700 (Table 1, Appendix Methods) (11,12).
Table 1.
Input parameters for a cost-effectiveness analysis of CAB-LA vs. generic F/TDF and branded F/TAF for HIV PrEP among MSM/TGW in the US
| Parameter | Value | Range | Source |
|---|---|---|---|
|
| |||
| Initial MSM/TGW cohort characteristics | |||
| Age, mean (years) | 30.1 | 20–40 | (1) |
| Sex assigned at birth, male (%) | 100 | (1) | |
| MSM/TGW at VHR, without HIV (n) * | 476,700 | Der. From (11,12) | |
| MSM/TGW at HR, without HIV (n) | 1,906,800 | Der. From (11,12) | |
| Primary transmissions, annual (n) | |||
| Attributable to MSM/TGW at VHR | 17,770 | 1,970–19,740 | Der. From (12) |
| Attributable to MSM/TGW at HR | 19,740 | 2,800–28,200 | Der. From (12) |
| PrEP program characteristics | |||
| Initial PrEP uptake (%) | 100 | 15–100 | Modeled population |
| Estimated HIV incidence, VHR (rate/100PY) | (1) | ||
| No PrEP | 5.32 | 2.66–10.64 | |
| Generic F/TDF and branded F/TAF | 1.33 | 0.67–2.66 | |
| CAB-LA | 0.26 | 0.13–0.52 | |
| Estimated HIV incidence, HR (rate/100PY) | Der. from (1,11,12) | ||
| No PrEP | 1.54 | 0.77–3.08 | |
| Generic F/TDF and branded F/TAF | 0.39 | 0.19–0.78 | |
| CAB-LA | 0.08 | 0.04–0.16 | |
| PrEP retention (annual probability) † # | |||
| Generic F/TDF and branded F/TAF | 0.809 | (14) | |
| CAB-LA | 0.809 | 0.809–0.997 | (14) |
| PrEP program characteristics, continued | |||
| Quality-of-life on F/TDF, monthly | |||
| End-stage renal disease, lifetime | 0.53 | (17) | |
| Fracture, 12 months | 0.7 | (18) | |
| PrEP costs ($) ‡ | |||
| Generic F/TDF, annual § | 790 | ||
| Program | 430 | (25,26) | |
| Drug | 360 | Der. from (23) | |
| Branded F/TAF, annual § | 17,230 | ||
| Program | 430 | (25,26) | |
| Drug | 16,800 | Der. from (23) | |
| CAB-LA, annual || | 750 + drug price | 3,050–26,650 | |
| Program | 750 | (25,26) | |
| Drug | -- | 2,300–25,850 | |
| Characteristics of those acquiring HIV | |||
| Mean CD4 at infection (cells/µL) | 667 | (44) | |
| Quality-of-life, HIV-related | |||
| Range by CD4 count | 0.83–0.87 | (45) | |
| Opportunistic infection | 0.69–1 | (46) | |
| Upon incident HIV infection, baseline ART adherence and virologic suppression | |||
| Adherence to ART ≥90% (% of cohort) | 71 | 50–100 | (47) |
| Upon incident HIV infection, baseline ART adherence and virologic suppression, continued | |||
| Integrase inhibitor-based ART efficacy (%) ¶ | 93.0 | 85.8–100 | (19,43) |
| Late virologic failure, range by adherence level (annual probability) # | 0.0012–1 | 0.0011–1 | (48,49) |
| Retention in HIV treatment | |||
| Loss to HIV care, range by adherence level (annual probability) # | 0.0012–0.57 | 0.0012–1 | (50) |
| Return to HIV care (annual probability) # | 0.999 | 0.968–1 | (51) |
| HIV-related costs ($) ‡ | |||
| Routine care costs, range by CD4 count and ART status, annual | 3,280–32,580 | 1,640–65,510 | (29–31) |
| ART, annual | 31,560–68,680 | 15,780–137,360 | (22,31) |
ART, antiretroviral therapy; CAB-LA, long-acting injectable cabotegravir; Der., derived; F/TAF, emtricitabine/tenofovir alafenamide fumarate; F/TDF, emtricitabine/tenofovir disoproxil fumarate; HIV, human immunodeficiency virus; HR, high risk for HIV; MSM, men who have sex with men; PY, person-year; PrEP, pre-exposure prophylaxis; TGW, transgender women; VHR, very high risk for HIV.
The MSM/TGW at VHR population is a subset of the MSM/TGW at HR population.
Corresponds to 28% of the initial modeled cohort retained in a PrEP program at 6 years (sensitivity analysis range of 14–98% retention at 6 years).
Costs adjusted to 2020 US dollars.
Annual program costs for generic F/TDF or branded F/TAF: level 3–4 visit ($104/visit) 4x/year (25), HIV testing 4x/year (antigen/antibody screen and additional reactive test: $40/test and $80/test (27,28)), labs including serum creatinine and urinalysis 2x/year (total $15/year) (26) and drug cost.
Annual program costs for CAB-LA: level 3–4 visit ($104) 6x/year (25), HIV testing 6x/year (antigen/antibody screen and additional reactive test: $40/test and $80/test (27,28)), labs including serum creatinine and liver function panel 2x/year (total $27/year) (26), $16/injection administration fee 6x/year (25), and drug price.
Among individuals with ≥90% adherence to an integrase-based ART regimen, 93% achieved viral suppression (VL <50 copies/mL at 48 weeks). Additional input parameters for different adherence levels may be found in Appendix Table 2.
Monthly probabilities were used in the model.
Additional details of model input parameters and derivations may be found in Appendix Tables 1 and 2 and Appendix Methods.
HIV PrEP program, screening, and disease treatment
Estimated HIV incidence in the PrEP program was governed by the efficacy of each PrEP type (off PrEP: 5.32/100PY; generic F/TDF and branded F/TAF: 1.33/100PY; CAB-LA: 0.26/100PY) (1). Generic F/TDF and branded F/TAF had similar efficacy, as demonstrated in the DISCOVER study (13). As retention in a CAB-LA program is uncertain, we assumed it was the same as other PrEP strategies (28% at 6 years) and varied this parameter in sensitivity analyses (14). To capture the potential decreased quality-of-life due to generic vs. branded tenofovir-based PrEP regimens (i.e., to portray branded F/TAF as favorably as possible), we modeled increased renal and bone adverse events among 0.04% and 2%, respectively, of individuals ever treated with generic F/TDF (15,16), leading to reductions in quality-of-life (end-stage renal disease: 47% lifetime reduction (17); fracture: 30% reduction over one year (18)) and a cost increase of $4,100/year/person experiencing an adverse event (Appendix Methods) (8). To understand the maximum value of CAB-LA, we assumed no treatment-emergent resistance to integrase inhibitor-based ART in the base case (included in scenario analyses). Among those with >90% adherence to ART, virologic suppression was 93% (19–21).
Primary HIV Transmissions
Of the estimated 28,200 annual HIV transmissions that occurred from MSM/TGW in the US, we assumed that 63% would be transmitted from MSM/TGW at VHR in the base case; 70% would be transmitted from the larger HR population, of which the VHR population was a subset (12) (Appendix Methods). We assumed the transmissions from this population were constant over a 10-year horizon and incorporated the corresponding transmissions over the modeled time horizon.
Costs
Drug prices were: generic F/TDF: $360/year (22); branded F/TAF: $16,800/year (23). The upper bound price of CAB-LA was modeled as $25,850/year (assuming parity with current pricing for HIV treatment using CAB-LA combined with long-acting rilpivirine (RPV-LA)) (22–24) (Appendix Methods). PrEP program costs included: $104/office visit (25) (F/TAF or F/TDF: 4 visits/year; CAB-LA: 6 visits/year), laboratory monitoring ($15/year for F/TDF or F/TAF; $27/year for CAB-LA) (26), HIV testing ($40/test) (27,28), and additional costs for CAB-LA ($16/injection, Table 1, Appendix Methods) (25). Routine HIV care costs ranged from $3,280 to $32,580/year (29–31), depending on CD4 count and ART status. ART costs ranged from $31,560 to $68,680/year (22).
Sensitivity Analyses
In sensitivity analyses, we examined the impact of varying parameters, including: CAB-LA preventive efficacy, CAB-LA retention, treatment-emergent HIV drug resistance, number of primary HIV transmissions, and the costs of HIV and PrEP care and drug prices. Because long-acting PrEP strategies may lead to delayed diagnosis or require higher cost diagnostic testing to diagnose HIV earlier, we also varied HIV diagnostic sensitivity and costs (32). To determine the maximum price at which CAB-LA would attain an ICER at or below a given willingness-to pay threshold, we varied the incremental price of CAB-LA over the next less costly strategy that was not strongly or weakly dominated (strongly dominated: a strategy is both less effective and more costly than another strategy; weakly dominated: a strategy costs more and delivers fewer benefits than some combination of two other strategies). We then calculated the maximum price premium that would leave the ICER of CAB-LA (compared to the next least costly strategy) at or below the willingness-to-pay threshold.
Scenario Analyses
We considered the value of CAB-LA among a population who could only effectively use PrEP if it were in injectable form, rather than oral. To generalize the findings to the US PrEP-eligible population of MSM/TGW, we also repeated the above analyses in a population of MSM/TGW at high risk for HIV (HR, rather than VHR), with an estimated HIV incidence of 1.54/100PY in the absence of PrEP. We estimated the size of this population as 1,906,800 or 50% of all US HIV-uninfected MSM/TGW (Table 1, Appendix Methods) (11,12).
Additional model input parameters are provided in the Appendix.
Role of the Funding Source
This work was supported by FHI 360 [UM1AI068619 to KAF and RPW]. Additional support included: the Eunice Kennedy Shriver National Institute for Child Health and Human Development [K08 HD094638 to AMN]; the National Institute of Allergy and Infectious Diseases [R01 AI093269 to RPW and KPR; K23 AI137121 to MEC]; the National Heart, Lung and Blood Institute [K01 HL123349 to EPH]; the National Institute on Drug Abuse [R37 DA015612 to ADP]; the Jerome and Celia Reich HIV Scholar Award [EPH], and the Steve and Deborah Gorlin MGH Research Scholars Award [RPW].
IRB Approval
Research utilizing the Cost-Effectiveness of Preventing AIDS Complications model was approved by the Mass General Brigham Human Research Committee (Protocol 2014P002708).
RESULTS
10-year clinical outcomes
Among the 4 strategies for VHR individuals, total primary transmissions were highest for No PrEP (178,000), lower for generic F/TDF and branded F/TAF (122,000), and lowest for CAB-LA (107,000, Table 2). Averted HIV infections and primary transmissions led to the highest QALYs for CAB-LA (4,654,000). Branded F/TAF led to 2,000 more QALYs than generic F/TDF due to high F/TDF-associated bone and renal toxicity risks (inputs chosen to favor branded F/TAF).
Table 2.
Model-projected 10-year clinical, cost and cost effectiveness outcomes of CAB-LA vs. generic F/TDF and branded F/TAF for HIV PrEP among MSM/TGW in the US
| Strategy | Total Transmissions, n | Total QALY | Incremental QALY | Total cost, billion* | Incremental cost, billion* | ICER, $/QALY* |
|---|---|---|---|---|---|---|
|
| ||||||
| MSM/TGW at VHR (n=476,700) | ||||||
|
| ||||||
| Generic F/TDF | 122,000 | 4,626,000 | -- | 30.67 | -- | -- |
| No PrEP | 178,000 | 4,529,000 | (97,000) | 33.48 | 2.81 | Excluded |
| Branded F/TAF | 122,000 | 4,628,000 | 99,000 | 60.42 | 29.75 | Excluded† |
| CAB-LA | 107,000 | 4,654,000 | 26,000 | 75.84 | 15.42 | 1,582,000 |
|
| ||||||
| MSM/TGW at HR (n=1,906,800) | ||||||
|
| ||||||
| No PrEP | 197,000 | 16,864,000 | -- | 39.57 | -- | -- |
| Generic F/TDF | 135,000 | 16,982,000 | 118,000 | 44.71 | 5.14 | 43,000 |
| Branded F/TAF | 135,000 | 16,991,000 | 9,000 | 160.09 | 115.37 | Excluded‡ |
| CAB-LA | 119,000 | 17,022,000 | 31,000 | 226.32 | 66.24 | 4,571,000 |
CAB-LA, long-acting injectable cabotegravir; F/TDF, tenofovir disoproxil fumarate-emtricitabine; HR, high risk for HIV; ICER, incremental cost-effectiveness ratio; MSM, men who have sex with men; PrEP, pre-exposure prophylaxis; QALY, quality-adjusted life-year; TGW, transgender women; 2020 USD, 2020 US dollars; VHR, very high risk for HIV.
All economic outcomes are reported in 2020 US dollars with a 3% annual discount rate. CAB-LA economic outcomes (in italics) were modeled using the upper bound drug price for CAB-LA (price $25,850).
Compared to branded F/TAF, CAB-LA would have an incremental cost-effectiveness ratio of $589,000/QALY among MSM/TGW at VHR. Compared to generic F/TDF, branded F/TAF would have an ICER of $12,513,000/QALY.
Compared to branded F/TAF, CAB-LA would have an incremental cost-effectiveness ratio of $2,136,000/QALY among MSM/TGW at HR. Compared to generic F/TDF, branded F/TAF would have an ICER of $13,221,000/QALY.
Strategies are listed in order of increasing cost per cost-effectiveness convention, and increments are expressed compared to the next less costly strategy; the order of strategies may differ throughout the analysis. Results are discounted at 3 percent per year and rounded to the nearest thousand. The ICER is the difference in cost divided by the difference in life expectancy for each strategy compared with the next less costly strategy. Strategies which are “Excluded” represent either a scenario where the strategy costs more and accrues fewer QALYs (as in No PrEP for the VHR analysis) or is a less efficient use of resources than the combination of two other strategies (as in branded F/TAF, also called “extended dominance”). The ICER for CAB-LA in italics represents the comparison to generic F/TDF. CAB-LA would be cost-saving compared to generic F/TDF only at a maximum price premium to generic F/TDF of <$1,900 (CAB-LA price $2,300).
Cost, cost-effectiveness, and price premium
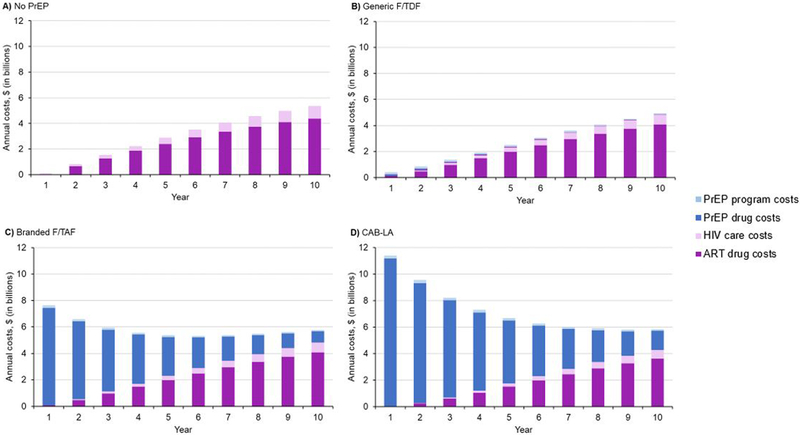
Over 10 years, costs (in 2020 dollars) would total $33.48 billion (B) for No PrEP, $30.67B for generic F/TDF, $60.42B for branded F/TAF, and, assuming the upper bound of CAB-LA price, $75.84B for CAB-LA (inclusive of primary transmissions). In the PrEP strategies, PrEP drug costs exceeded ART costs initially (Year 1 PrEP drug vs. ART: generic F/TDF: $150.03 million (M) vs. $85.65M; branded F/TAF: $7.38B vs. $85.65M; CAB-LA: $11.15B vs. $38.42M); by year 10, in all strategies, ART costs exceeded PrEP costs (Year 10: PrEP drug vs. ART: generic F/TDF: $17.57M vs. $4.09B; branded F/TAF: $862.24M vs. $4.09B; CAB-LA: $1.44B vs. $3.64B), and were highest in No PrEP (Year 10 ART cost: $4.39B) (Figure 1). When the impact of primary transmissions was excluded, annual costs followed a similar pattern (Appendix Figure 2, solid lines).
Figure 1.
Annual costs reported over 10 years for MSM/TGW at VHR in the US (n=476,700)
Panels depict the projected total annual component costs for each PrEP strategy (Panel A: No PrEP; Panel B: generic F/TDF; Panel C: branded F/TAF; and Panel D: CAB-LA). Time 1 on the horizontal axis represents the first year since the start of the model simulation. The left vertical axis shows annual total cost in billion 2020 US dollars. Annual component costs are given by the solid colors (ART drug: dark purple; HIV care: light purple; PrEP drug: dark blue; PrEP program: light blue) at any given yearly timepoint in the model simulation period (horizontal axis). For example, during Year 5 in Panel B, total annual cost was $2.47B (ART drug: $2.00B, HIV care: $326.38M, PrEP drug: $59.83M, PrEP program: $84.13M). Component costs for the cohort of MSM/TGW at high risk for HIV follow a similar pattern and are presented in Appendix Figure 6.
Abbreviations: ART, antiretroviral therapy; CAB-LA, long-acting injectable cabotegravir; F/TAF, emtricitabine/tenofovir alafenamide fumarate; F/TDF, emtricitabine/tenofovir disoproxil fumarate; MSM/TGW, men who have sex with men / transgender women; PrEP, pre-exposure prophylaxis; VHR, very high risk for HIV.
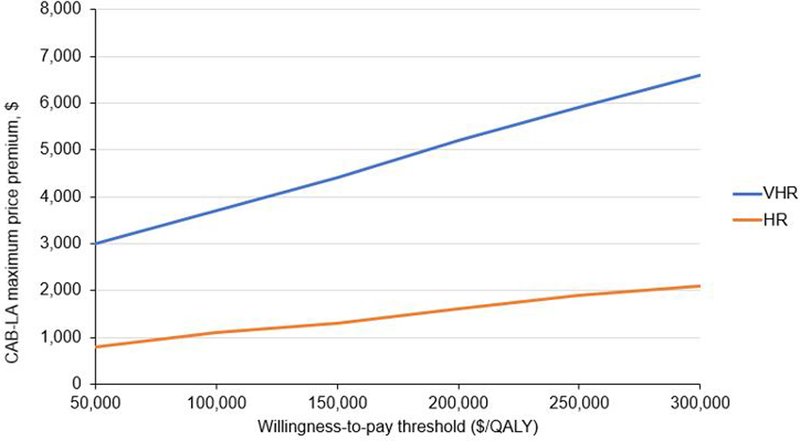
Compared to No PrEP, generic F/TDF would be cost-saving (Table 2). Assuming a price of $360/year for generic F/TDF and varying the willingness-to-pay threshold from $50,000/QALY to $300,000/QALY, the maximum price premium of CAB-LA would range from $3,000 to $6,600 (CAB-LA price $3,400 to $7,000, Table 2, Figure 2). If CAB-LA were priced to be cost-saving compared to generic F/TDF, the maximum price premium of CAB-LA would be $1,900 (CAB-LA price $2,300). Despite fewer associated adverse events, branded F/TAF cost more per QALY gained than a combination of generic F/TDF and CAB-LA (weakly dominated). At the upper bound of annual CAB-LA drug price ($25,850), compared to generic F/TDF, the ICER of CAB-LA would be $1,582,000/QALY (Table 2).
Figure 2.
Sensitivity analysis: Maximum price premiums of CAB-LA PrEP over generic F/TDF at different willingness-to-pay thresholds for MSM/TGW at VHR (n=476,700) and HR (n=1,906,800) in the US over 10 years
This figure presents a sensitivity analysis on maximum price premiums of CAB-LA PrEP at different willingness-to-pay thresholds. The vertical axis reports the maximum price premiums of CAB-LA over generic F/TDF. The horizontal axis reports willingness-to-pay thresholds, up to $300,000/QALY. Among VHR (blue line), at generic F/TDF price of $360/year, CAB-LA would achieve an ICER ≤$100,000/QALY with a maximum price premium of $3,700/year over generic F/TDF (CAB-LA price $4,100/year). As the willingness-to-pay threshold increases, the maximum price premium increases. For the $300,000/QALY threshold, the maximum price premium of CAB-LA would be $6,600/year (CAB-LA price $7,000/year). For the HR cohort (orange line), holding constant the generic F/TDF price of $360/year, the ICER of CAB-LA would be ≤$300,000/QALY at a maximum price premium of $2,100 (CAB-LA price $2,500/year).
Abbreviations: CAB-LA, long-acting injectable cabotegravir; F/TDF, emtricitabine/tenofovir disoproxil fumarate; HR, high risk for HIV; ICER, incremental cost effectiveness ratio; MSM/TGW, men who have sex with men / transgender women; PrEP, pre-exposure prophylaxis; QALY, quality-adjusted life-year; VHR, very high risk for HIV.
One-way sensitivity analyses
Varying assumptions about PrEP program retention, efficacy, and estimated HIV incidence and transmissions had the greatest impact on the incremental QALYs gained by F/TDF (Appendix Figure 3), as well as the price premium (Appendix Figure 4). Including the uncertain impact of treatment-emergent integrase inhibitor resistance among those acquiring HIV in the CAB-LA strategy would negligibly change QALYs (200–6,990 QALYs were lost), given other reasonable effective ART options; total costs would change depending on alternative first-line ART regimens (lower with efavirenz and rilpivirine, higher with protease inhibitors, Appendix Tables 3–5). With changes in HIV diagnostic testing sensitivity and cost in the CAB-LA strategy, or under a variety of assumptions about annual transmissions attributed to VHR individuals, or excluding renal and bone fracture adverse events related to generic F/TDF, the maximum price premium of CAB-LA would be similar to the base case (Appendix Table 6 and Appendix Figures 4 and 5).
Multiway sensitivity analyses
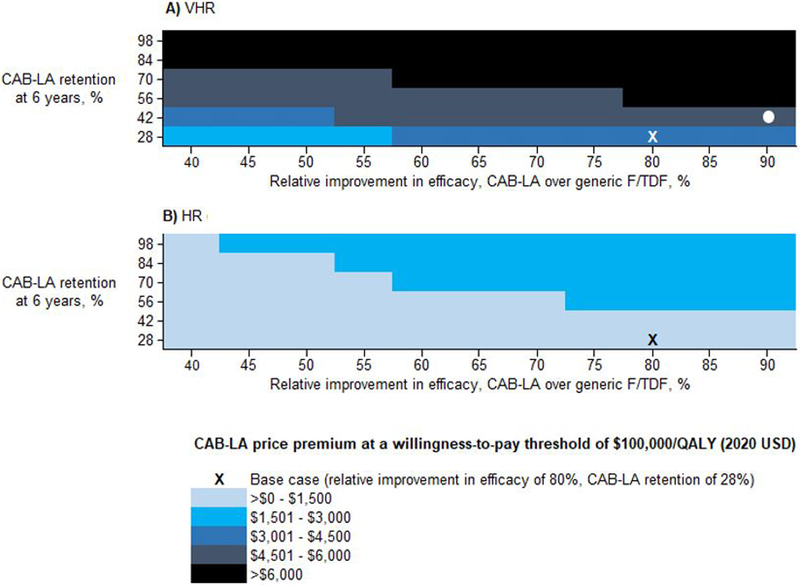
As efficacy of CAB-LA compared to generic F/TDF improved, the maximum price premium for CAB-LA would increase (to $4,000 at a $100,000/QALY willingness-to-pay threshold given a relative improvement in efficacy of CAB-LA over generic F/TDF of 90% (base case: 80%), Figure 3A, horizontal axis). As retention improved, the price premium for CAB-LA would increase (vertical axis, Figure 3A). For example, at a $100,000/QALY threshold, the price premium would be <$5,500/year when relative improvement in efficacy of CAB-LA over generic F/TDF is 90% and 6-year retention is 42% (base case: 28%, Figure 3A, white circle). Assuming a price of $16,800/year for branded F/TAF and varying the willingness-to-pay threshold from $50,000 to $300,000/QALY, the maximum price premium of CAB-LA would range from $1,800 to $5,200 over branded F/TAF (CAB-LA price $18,600 to $22,000). If individuals and their partners could not effectively engage in oral PrEP but had the base case retention for CAB-LA, CAB-LA would be cost-effective at a higher maximum price premium of $10,500 over generic F/TDF (CAB-LA price <$10,900/year, Appendix Table 7).
Figure 3.
Cost-effectiveness of CAB-LA vs. generic F/TDF sensitivity analysis: Maximum price premiums for combinations of CAB-LA efficacy and CAB-LA PrEP program retention among MSM/TGW at A) VHR (n=476,700) and B) HR (n=1,906,800) in the US
This figure shows the maximum price premium at which CAB-LA vs. generic F/TDF would be cost-effective at each combination of CAB-LA efficacy and CAB-LA PrEP program retention. Drug prices of generic F/TDF ($360) and CAB-LA ($25,850) are held constant. In the base case, for the VHR (white X, Panel A), the ICER of CAB-LA would be ≤$100,000/QALY at a maximum price premium of $3,700 (i.e., generic F/TDF price of $360 and CAB-LA price of $4,100). Although the price premium is $3,700/year in the base case, it would be <$5,500/year when relative improvement in efficacy of CAB-LA over F/TDF is 90% and 6-year retention is 42% (white circle). For the HR cohort, holding constant 28% 6-year retention and 80% relative improvement in efficacy of CAB-LA over F/TDF (black X, Panel B), the ICER of CAB-LA would be $100,000/QALY at a maximum price premium of $1,100 (i.e., generic F/TDF price of $360 and CAB-LA price of $1,500).
Abbreviations: CAB-LA, long-acting injectable cabotegravir; F/TDF, emtricitabine/tenofovir disoproxil fumarate; HR, high risk for HIV; ICER, incremental cost effectiveness ratio; MSM/TGW, men who have sex with men / transgender women; QALY, quality-adjusted life-year; VHR, very high risk for HIV.
Scenario analysis: high risk (HR) cohort
Assuming a price of $360/year for generic F/TDF and varying the willingness-to-pay threshold from $50,000/QALY to $300,000/QALY, the maximum price premium of CAB-LA in the HR cohort (PrEP-eligible and at ‘high risk’ but lower overall risk than ‘very high risk’) would range from $800 to $2,100 (CAB-LA price $1,200 to $2,500, Table 2). Branded F/TAF would also be weakly dominated by CAB-LA. Annual costs exhibited a similar pattern among individuals receiving PrEP in the HR cohort (Figure 1, Appendix Figures 2 and 6).
DISCUSSION
Although there is evidence of CAB-LA’s superior efficacy compared to oral PrEP among MSM/TGW enrolling in the HPTN 083 trial, this analysis – in which we consistently chose parameter values that would portray CAB-LA in a favorable light in order to estimate the highest possible price premium – suggests that the incremental clinical benefits of CAB-LA would not justify a large price difference compared to F/TDF. Depending on how CAB-LA is priced, the return on investment may not justify a switch from low-priced, well-tolerated, generic, oral alternatives. Using a simulation model, we found that at a range of willingness-to-pay thresholds ($50,000-$300,000/QALY), CAB-LA for PrEP among MSM/TGW at VHR for HIV would only provide good value for money if its annual price was <$3,000-$6,600 higher than generic F/TDF. This pricing is substantially less than branded F/TDF or F/TAF options and also less than half the current CAB-LA/RPV-LA pricing for HIV treatment. While the most recent pricing for generic F/TDF is $360/year, regardless of the absolute F/TDF price, the incremental value of CAB-LA would change little.
Among MSM/TGW at VHR for HIV, any PrEP strategy would lead to substantial clinical improvements – life-years gained and reductions in onward primary transmissions – compared to no PrEP and substantially reduce ART costs over time. The superior effectiveness of CAB-LA compared to oral PrEP regimens (1), thought to be due to avoiding the need to take a daily oral pill, led to the most QALYs gained and transmissions averted. Importantly, we find that compared to the modeled generic F/TDF regimen (inclusive of its potential for more side effects, and associated costs, compared to branded F/TAF), current branded F/TAF regimens would not be cost-effective. Some regulators have not found the reported differences in sensitive biomarkers of renal function and bone mineral density to reflect a compelling safety advantage (at least compared to price) of branded F/TAF over generic F/TDF (33). Simply stated, CAB-LA should be priced to compete with generic PrEP, not branded PrEP. While there are concerns that benchmarking new products to generic competitors may quash innovation, the value of an innovation from the societal (or in this analysis the healthcare payer) perspective should be measured by how much it improves upon the best available alternative use of funds.
How well CAB-LA will perform to prevent HIV infection and to retain PrEP program participants in non-trial settings is not yet known. Because non-adherence will be clearer to providers for CAB-LA compared to oral options, it may facilitate adherence support interventions. The clinical implications of resistance that may evolve if one acquires HIV while receiving CAB-LA also have not yet been fully characterized. We found, however, that potential drug resistance – while clinically important – would have negligible influence on the price premium; this is in part due to many ART regimens available for treatment in the US and many other countries as well as the small number of individuals who would be affected by resistance (Appendix Tables 3–5). Furthermore, if the improved efficacy of CAB-LA is due to improved coverage, people more adherent to oral PrEP than those who enrolled in HPTN 083 might not derive the same level of benefit from CAB-LA. Given the increased price premium when CAB-LA was used in a population which could not effectively participate in an oral PrEP program (No PrEP, as opposed to taking oral F/TDF), our conclusions might differ if failure rates on oral PrEP were assumed to be much higher, as they might be in some important sub-populations. CAB-LA in the US may have the highest value in settings where engaging in oral PrEP would be extremely challenging or impossible but PrEP use could be maintained on a bimonthly injectable regimen (e.g., people experiencing homelessness or domestic abuse or serious mental illness).
Estimated HIV incidence off PrEP in HPTN 083 was 3.78/100PY more (i.e., >300% higher) than in the population anticipated to be eligible for PrEP in the US (1,11,12). In the modeled VHR population, intended to reflect the HPTN 083 US population, the maximum price premium of CAB-LA over generic F/TDF would fall just below <$3,000-$6,600/year at willingness-to-pay thresholds ranging from $50,000-$300,000/QALY. In the modeled HR population (a population at lower risk for HIV but still meeting PrEP eligibility criteria), CAB-LA compared to generic F/TDF, would be of less value; maximum price premiums would be <$800-$2,100/year over the same willingness-to-pay thresholds.
Despite strong evidence for PrEP efficacy for over a decade (34), uptake in the US has been slow and unequal, with only 6% and 11% of eligible Black and Latinx populations using PrEP compared to 42% of eligible White populations (35). Although PrEP uptake continues to increase, achieving higher coverage, particularly among communities of color, is needed to approach Ending the HIV Epidemic goals (36). While there are many barriers to uptake along the PrEP care continuum, cost is a major concern (37), with regional disparities in prior-authorizations (38) and insurance coverage. Use of CAB-LA, which is likely to be more expensive and require more access to clinical resources, has the potential to exacerbate existing disparities. In addition to recognizing that low cost, generic, oral PrEP has emerged, pricing for novel PrEP products should reflect the extent of public sector investments (as well as those of the private sector), in their development as HIV prevention modalities (39,40). Furthermore, the provision of an injectable medication presents a host of implementation issues that are beyond the scope of this analysis. If CAB-LA were priced to be cost-saving compared to generic F/TDF (maximum price premium: $1,900/year), additional investments could be made in innovative delivery approaches such as in pharmacies and non-medical settings (41), as well as self-administration (e.g., developing formulations permitting smaller muscle group injections). In addition to expanding the range of PrEP options available to patients, such approaches could promote more equitable access to this novel preventive therapy (37).
This analysis has several limitations. First, given the uncertainties of future HIV transmissions, we assumed a stable number of primary transmissions over the 10-year horizon. We used estimated HIV incidence rates from HPTN 083 and transmissions reported by the Centers for Disease Control and Prevention; however, the trajectories of these rates over time, as well as the costs and life expectancies associated with these infections, are uncertain. For example, community-level improvements in prevention and treatment might reduce later HIV incidence and primary HIV transmissions increasing the ICERs of PrEP strategies. However, additional benefits to populations not on PrEP (e.g., to cisgender female partners), or reducing transmission of other sexually transmitted infections due to PrEP-related sexually transmitted infection screening (42), or a longer time horizon, would all decrease the ICERs for PrEP. Whether the annual transmissions attributable to MSM/TGW at VHR were large or small, the maximum price premium of CAB-LA relative to generic F/TDF remained <$3,900/year at a willingness-to-pay threshold of $100,000/QALY. Second, we lacked data to incorporate potential changes in quality-of-life on oral or long-acting PrEP; improvements in HIV-related sexual anxiety and overall quality-of-life due to a specific strategy would lower that program’s ICERs (43); conversely, long term side effects (e.g., potential negative weight or metabolic changes due to CAB-LA) could worsen a program’s ICER. Third, we excluded an oral cabotegravir lead-in for CAB-LA, which may be challenging for those who are opting for an injectable regimen; inclusion of this lead-in would further lower the price premium of CAB-LA. Finally, the model does not consider mixing within the at-risk population. Consequently, an explicit assessment of secondary benefits is not incorporated. However, we did conduct sensitivity analysis to estimate the impact of changing transmissions over time, with and without PrEP.
Our findings, based on HPTN 083 data, project the clinical benefits and costs of CAB-LA compared to generic F/TDF and branded F/TAF PrEP regimens. The superiority of CAB-LA to generic F/TDF, notwithstanding the presence of highly effective oral PrEP alternatives, limits the additional price that payers should be willing to pay for CAB-LA.
Supplementary Material
Reproducible Research Statement.
Study protocol:
Not applicable.
Statistical code:
Not applicable. Model specifications are available at https://mpec.massgeneral.org/cepac-model/.
Data set:
Available at https://giveprepashot.org/may-2020-dsmb-result.
ACKNOWLEDGMENTS
We gratefully acknowledge Mr. Tim Horn of the National Alliance of State and Territorial AIDS Directors (NASTAD) for his insights related to drug costs. We also thank Mr. Christopher Panella, who assisted with programming, Dr. Fatma Shebl who reviewed select data derivations, and Ms. Justine Scott, Ms. Guner Ege Eskibozkurt, and Ms. Stephanie Horsfall who assisted in preparing the manuscript for submission, as well as the Cost-Effectiveness of Preventing AIDS Complications research team in the Medical Practice Evaluation Center at Massachusetts General Hospital for providing feedback on study design and interpretation. Mr. Horn did not receive compensation for his support. Mr. Panella, Dr. Shebl, Ms. Scott, Ms. Eskibozkurt, and Ms. Horsfall contributed as part of their institutional roles.
Conflict of Interest and Financial Disclosures
Dr. Landovitz has been a consultant to and received fees from Gilead Sciences, Inc., Merck & Co. Inc., Janssen, and Roche. Dr. Clement reports grants to institution from Gilead Sciences, Inc, ViiV, and Janssen and has received fees from Roche. Other authors have no conflicts of interest or financial disclosures.
Footnotes
Meeting Presentation Disclosure
An oral abstract was presented at the 2021 Conference on Retroviruses and Opportunistic Infections; the findings in this manuscript have not otherwise been previously presented.
Disclaimer
The funding sources had no role in the study design, data collection, data analysis, data interpretation, writing of the manuscript, or in the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding sources. The findings, conclusions, and views expressed in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry, the United States Department of Health and Human Services, or the United States.
REFERENCES
- 1.Landovitz RJ, Donnell D, Clement ME, Hanscom B, Cottle L, Coelho L, et al. Cabotegravir for HIV prevention in cisgender men and transgender women; including personal correspondence from study authors regarding US-specific parameters. N Engl J Med. 2021. Aug 12;385(7):595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landovitz RJ, Kofron R, McCauley M. The promise and pitfalls of long acting injectable agents for HIV prevention. Curr Opin HIV AIDS. 2016. Jan;11(1):122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horn T Drug pricing in the age of generics [Internet]. ACTHIV: The American Conference for the Treatment of HIV; 2019. Apr 13 [cited 2021 Mar 3]; Miami, Florida. Available from: http://www.acthiv.org/wp-content/uploads/2019/04/Tim_Horn_09_30.pdf [Google Scholar]
- 4.Hoover KW. Trends in Truvada and Descovy prescriptions for PrEP in the United States, 2014–2020 [Internet]. Conference on Retroviruses and Opportunistic Infections; 2021. Mar 6 [cited 2021 Oct 21]; Virtual. Available from: https://www.croiconference.org/abstract/trends-in-truvada-and-descovy-prescriptions-for-prep-in-the-united-states-2014-2020/ [Google Scholar]
- 5.U.S. House of Representatives Committee Repository. Hearing: HIV prevention drug: billions in corporate profits after millions in taxpayer investments [Internet]. 2019. [cited 2021 Mar 3]. Available from: https://docs.house.gov/Committee/Calendar/ByEvent.aspx?EventID=109486
- 6.Medical Practice Evaluation Center. Ordering of patient simulation in CEPAC [Internet]. Massachusetts General Hospital, Medical Practice Evaluation Center. [cited 2021 Oct 22]. Available from: https://mpec.massgeneral.org/wp-content/uploads/2020/07/Flowcharts-cepac50c.pdf [Google Scholar]
- 7.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014. Aug 28;371(9):796–7. [DOI] [PubMed] [Google Scholar]
- 8.Walensky RP, Horn T, McCann NC. Comparative pricing of branded tenofovir alafenamide-emtricitabine relative to generic tenofovir disoproxil fumarate-emtricitabine for HIV preexposure prophylaxis: A cost-effectiveness analysis. Ann Intern Med. 2020. May 5;172(9):583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crepaz N, Song R, Lyss S. Estimated time from HIV infection to diagnosis, 50 U.S. states and the District of Columbia, 2014–2018. Abstract OAC0206 presented at: AIDS 2020: 23rd International AIDS Conference; 2020. Jul 6; Virtual. [Google Scholar]
- 10.Neilan AM, Bulteel AJB, Hosek SG. Cost-effectiveness of frequent HIV screening among high-risk young men who have sex with men in the United States. Clin Infect Dis [Internet]. 2020. Jul 30 [cited 2021 Mar 3]; Available from: 10.1093/cid/ciaa1061/5879019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grey JA, Bernstein KT, Sullivan PS. Estimating the population sizes of men who have sex with men in US states and counties using data from the American Community Survey. JMIR Public Health Surveill. 2016. Jun;2(1):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh S, Song R, Johnson AS. HIV incidence, prevalence, and undiagnosed infections in U.S. men who have sex with men. Ann Intern Med. 2018. May 15;168(10):685–94. [DOI] [PubMed] [Google Scholar]
- 13.Mayer KH, Molina J-M, Thompson MA, Anderson PL, Mounzer KC, De Wet JJ, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. The Lancet. 2020. Jul;396(10246):239–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams R, Flanagan T, Bazerman L. Who does not show up for follow-up in an HIV PrEP clinic? Oral Abstract 101 presented at: IDWeek 2020; 2020. Oct 21; Virtual. [Google Scholar]
- 15.Komatsu A, Ikeda A, Kikuchi A. Osteoporosis-related fractures in HIV-infected patients receiving long-term tenofovir disoproxil fumarate: An observational cohort study. Drug Saf. 2018. Sep;41(9):843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Indicator details: Incidence of individual CKD stages/eGFR categories [Internet]. 2019. [cited 2021 Mar 3]. Available from: https://nccd.cdc.gov/CKD/detail.aspx?Qnum=Q89
- 17.Laupacis A, Keown P, Pus N, Krueger H, Ferguson B, Wong C, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney International. 1996. Jun 1;50(1):235–42. [DOI] [PubMed] [Google Scholar]
- 18.Borgström F, Zethraeus N, Johnell O, Lidgren L, Ponzer S, Svensson O, et al. Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int. 2006. May 1;17(5):637–50. [DOI] [PubMed] [Google Scholar]
- 19.Walmsley SL, Antela A, Clumeck N. Dolutegravir plus abacavir–lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013. Nov 6;369(19):1807–18. [DOI] [PubMed] [Google Scholar]
- 20.Cahn P, Fourie J, Grinsztejn B. Week 48 analysis of once-daily vs. twice-daily darunavir/ritonavir in treatment-experienced HIV-1-infected patients. AIDS. 2011. Apr 24;25(7):929–39. [DOI] [PubMed] [Google Scholar]
- 21.Yazdanpanah Y, Fagard C, Descamps D. High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS 139 TRIO trial. Clin Infect Dis. 2009. Nov 1;49(9):1441–9. [DOI] [PubMed] [Google Scholar]
- 22.Redbook OnlineTM from Micromedex Solutions® [Internet]. Truven Health Analytics, Ann Arbor (MI). 2021. [cited 2021 Mar 3]. Available from: http://micromedex.com/redbook. [Google Scholar]
- 23.National Acquisition Center (CCST): pharmaceutical catalog search [Internet]. U.S. Department of Veterans Affairs. 2020. [cited 2021 Mar 3]. Available from: www.vendorportal.ecms.va.gov/NAC/Pharma/List
- 24.Bernstein L FDA approves breakthrough injectable HIV medication. The Washington Post [Internet]. 2021. Jan 21 [cited 2021 Mar 3]; Available from: https://www.washingtonpost.com/health/hiv-injectable-drug/2021/01/22/56a8d8c4-5ce7-11eb-8bcf-3877871c819d_story.html
- 25.Centers for Medicare & Medicaid Services, Department of Health and Human Services. 2020 physician fee schedule [Internet]. 2021. [cited 2021 Mar 3]. Available from: https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx [Google Scholar]
- 26.Centers for Medicare & Medicaid Services. Clinical laboratory fee schedule [Internet]. CMS. 2020. [cited 2021 Mar 3]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/index?redirect=/ClinicalLabFeeSched/01_overview.asp [Google Scholar]
- 27.Eggman AA, Feaster DJ, Leff JA. The cost of implementing rapid HIV testing in sexually transmitted disease clinics in the United States. Sex Transm Dis. 2014. Sep;41(9):545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schackman BR, Eggman AA, Leff JA. Costs of expanded rapid HIV testing in four emergency departments. Public Health Rep. 2016;131 Suppl 1(Suppl 1):71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bozzette SA, Berry SH, Duan N. The care of HIV-infected adults in the United States. HIV Cost and Services Utilization Study Consortium. N Engl J Med. 1998. Dec 24;339(26):1897–904. [DOI] [PubMed] [Google Scholar]
- 30.Bamezai A, Melnick G, Nawathe A. The cost of an emergency department visit and its relationship to emergency department volume. Ann Emerg Med. 2005. May;45(5):483–90. [DOI] [PubMed] [Google Scholar]
- 31.Levinson DR. Medicaid drug price comparisons: Average manufacturer price to published prices [Internet]. Department of Health and Human Services; 2005. Jun [cited 2021 Mar 3]. Available from: https://oig.hhs.gov/oei/reports/oei-05-05-00240.pdf [Google Scholar]
- 32.Marzinke M, Grinsztejn B, Fogel J, Piwowar-Manning E. Laboratory analysis of HIV infections in HPTN 083: Injectable CAB for PrEP [Internet]. Abstract 153 presented at: Conference on Retroviruses and Opportunistic Infections 2021; 2021 Mar 6 [cited 2021 Mar 9]; Virtual. Available from: https://www.croiconference.org/wp-content/uploads/sites/2/resources/2021/vcroi2021-program-and-information-guide.pdf [Google Scholar]
- 33.Gilead Announces Decision Not to Pursue Marketing Authorization for Descovy® for Pre-Exposure Prophylaxis in the European Union [Internet]. [cited 2021 Oct 29]. Available from: https://www.gilead.com/news-and-press/company-statements/gilead-announces-decision-not-to-pursue-marketing-authorization-for-descovy-for-pre-exposure-prophylaxis-in-the-european-union
- 34.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. New Engl J Med. 2010. Dec 30;363(27):2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris NS. Vital Signs: Status of human immunodeficiency virus testing, viral suppression, and HIV preexposure prophylaxis — United States, 2013–2018. MMWR Morb Mortal Wkly Rep [Internet]. 2019. [cited 2021 Mar 3];68. Available from: https://www.cdc.gov/mmwr/volumes/68/wr/mm6848e1.htm [DOI] [PMC free article] [PubMed]
- 36.Finlayson T Changes in HIV preexposure prophylaxis awareness and use among men who have sex with men — 20 urban areas, 2014 and 2017. MMWR Morb Mortal Wkly Rep [Internet]. 2019. [cited 2021 Mar 3];68. Available from: https://www.cdc.gov/mmwr/volumes/68/wr/mm6827a1.htm [DOI] [PMC free article] [PubMed]
- 37.Furukawa N, Zhu W, Huang Y. National trends in drug payments for HIV preexposure prophylaxis in the United States, 2014 to 2018. A retrospective cohort study. Ann Intern Med. 2020;173:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McManus KA, Powers S, Killelea A. Regional disparities in qualified health plans’ prior authorization requirements for HIV pre-exposure prophylaxis in the United States. JAMA Netw Open. 2020. Jun 1;3(6):e207445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nayak RK, Avorn J, Kesselheim AS. Public sector financial support for late stage discovery of new drugs in the United States: cohort study. BMJ [Internet]. 2019. Oct 23 [cited 2021 Mar 3];367. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6812612/ [DOI] [PMC free article] [PubMed]
- 40.Conti RM, David FS. Public research funding and pharmaceutical prices: do Americans pay twice for drugs? F1000Res. 2020;9:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Common drug review: vocabria (cabotegravir) and cabenuva (cabotegravir/rilpivirine) injection [Internet]. Ottawa: The Canadian Agency for Drugs and Technologies in Health (CADTH). ViiV Healthcare ULC. 2020. [cited 2021 Mar 3]. Available from: https://www.cadth.ca/sites/default/files/cdr/pharmacoeconomic/sr0628-vocabria%2Bcabenuva-pharmacoeconomic-review-report.pdf [Google Scholar]
- 42.Jenness SM, Weiss KM, Goodreau SM. Incidence of gonorrhea and chlamydia following human immunodeficiency virus preexposure prophylaxis among men who have sex with men: A modeling study. Clin Infect Dis. 2017. Sep 1;65(5):712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keen P, Hammoud MA, Bourne A. Use of HIV pre-exposure prophylaxis (PrEP) associated with lower HIV anxiety among gay and bisexual men in Australia who are at high risk of HIV infection: results from the Flux Study. J Acquir Immune Defic Syndr. 2020. Feb 1;83(2):119–25. [DOI] [PubMed] [Google Scholar]
- 44.Lundgren JD, Babiker AG, Gordin F. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015. Aug 27;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schackman BR, Goldie SJ, Freedberg KA. Comparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDS. Med Decis Making. 2002. Feb;22(1):27–38. [DOI] [PubMed] [Google Scholar]
- 46.Paltiel AD, Scharfstein JA, Seage GR 3rd. A Monte Carlo simulation of advanced HIV disease: application to prevention of CMV infection. Med Decis Making. 1998. Jun;18(2 Suppl):S93–105. [DOI] [PubMed] [Google Scholar]
- 47.Sax PE, Meyers JL, Mugavero M. Adherence to antiretroviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United States. PLoS One. 2012;7(2):e31591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallant JE, DeJesus E, Arribas JR. Tenofovir df, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354(3):251–60. [DOI] [PubMed] [Google Scholar]
- 49.Raffi F, Rachlis A, Stellbrink H-J. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013. Mar 2;381(9868):735–43. [DOI] [PubMed] [Google Scholar]
- 50.Fleishman JA, Yehia BR, Moore RD. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr. 2012. Jul 1;60(3):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Helleberg M, Engsig FN, Kronborg G. Retention in a public healthcare system with free access to treatment: a Danish nationwide HIV cohort study. AIDS. 2012. Mar 27;26(6):741–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.