Abstract
We evaluated the pharmacokinetics of stavudine (d4T) and didanosine (ddI) in neonates. Eight neonates born to human immunodeficiency virus-infected mothers were enrolled to receive 1 mg of d4T per kg of body weight twice daily and 100 mg of ddI per m2 once daily in combination with nelfinavir for 4 weeks after birth. Pharmacokinetic evaluations were performed at 14 and 28 days of age. For d4T, on days 14 and 28, the median areas under the concentration-time curves from 0 to 12 h (AUC0–12s) were 1,866 and 1,603, ng · h/ml, respectively, and the median peak concentrations (Cmaxs) were 463 and 507 ng/ml, respectively. For ddI, on days 14 and 28, the median AUC0–10s were 1,573 and 1,562 h · ng/ml, respectively, and the median Cmaxs were 627 and 687 ng/ml, respectively. Systemic levels of exposure to d4T were comparable to those seen in children, suggesting that the pediatric dose of 1 mg/kg twice daily is appropriate for neonates at 2 to 4 weeks of age. Levels of exposure to ddI were modestly higher than those seen in children. Whether this observation warrants a reduction of the ddI dose in neonates is unclear.
Human immunodeficiency virus (HIV) infection has emerged as a worldwide public health problem among women and children. The Pediatric AIDS Clinical Trial Group 076 study demonstrated that zidovudine (ZDV) reduced the risk for vertical transmission by approximately 70% (5). Thereafter, several interventions to decrease the risk for vertical transmission have been developed. These include the use of short-course ZDV, short-course nevirapine, antiretroviral drug combinations, elective delivery by cesarean section, and the avoidance of breast-feeding (7, 9, 10, 14, 17, 18; G. Gray, J. McIntyre, B. Jivkov, M. Schorn, S. Lala, L. Reynolds, J.-M. Ledeine, A. Van Beek, and S. Schnittman, Abstr. 13th World AIDS Conf., abstr. TuOrB355, 2000; J. Saba, 6th Conf. Retroviruses Opportunistic Infections, 1999). However, there remains a need to explore other treatment strategies to further decrease the risk for vertical transmission. The efficacies of triple-drug combination regimens for occupational postexposure prophylaxis are well established. Thus, it is likely that the administration of a combination regimen to neonates could further reduce the risk for perinatal HIV infection. In addition, disease progression in infected infants is generally rapid (3, 19). Initiation of combined antiretroviral therapy as soon as the diagnosis of HIV infection is made early in life may reduce the risk for disease progression and improve the quality of life (6). Nevertheless, the information on the pharmacokinetics of antiretroviral drugs during early infancy is limited. We therefore evaluated the pharmacokinetics of two antiretroviral drugs, stavudine (d4T) and didanosine (ddI), used in combination with nelfinavir (NFV) in HIV-exposed neonates.
(This study was presented in part at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 16 to 19 December 2001 [Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1742, 2001].)
The study was conducted by the HIV Netherlands-Australia-Thailand Research Collaboration (HIV-NAT), the Faculty of Medicine at Chulalongkorn University, and the King Chulalongkorn Memorial Hospital in Bangkok, Thailand. Prior to study initiation, the protocol and the informed consent were reviewed and approved by the human subjects review board of the Faculty of Medicine at Chulalongkorn University. Written informed consents were obtained from the parents or guardians before study entry. Infants were eligible for the study if they were born to HIV-seropositive mothers who were at least 18 years of age. At birth, the infants had to weigh at least 2.8 kg and their gestational age had to be at least 37 weeks. Infants with a congenital anomaly or a medical condition that would compromise their safety or those with a family history of phenylketonuria were excluded from the study. All mothers received prenatal voluntary counseling and testing for HIV infection. Antepartum and intrapartum ZDV prophylaxis was strongly encouraged, and ZDV could be requested at no cost through the Thai Red Cross donation program (21). However, ZDV was not given to infants in the present study. Administration of d4T, ddI, and NFV to the infants was started within 12 h after birth and was continued for 4 weeks. All infants received 1 mg of d4T per kg of body weight every 12 h and 100 mg of ddI per m2 once daily. The dosages of NFV, however, were different for each cohort; the infants in the first, second, and third cohorts received 15, 30, and 45 mg of NFV per kg every 12 h, respectively. The study was primarily designed to evaluate the pharmacokinetics of NFV. However, the stored plasma samples obtained during the study allowed us to assess the pharmacokinetic characteristics of d4T and ddI in the study population. Because of the availability of plasma samples from eight infants in the first two cohorts, these samples were used for the pharmacokinetic analyses in the present study.
Both d4T and ddI liquid formulations were prepared according to the package inserts. The concentrations of d4T and ddI, once they were constituted, were 1 and 10 mg/ml, respectively. The doses were rounded to the nearest 0.1 ml. NFV was supplied as oral powder in its original bottle (50 mg of free base or 1 g of bulk powder per scoop). In addition, due to the infants' low weights and the small dose required, NFV was also supplied in a small pack. This was prepared by the study pharmacist as 200- and 800-mg bulk powder packs. The doses of all three drugs dispensed were calculated on the basis of anthropometric measurements obtained at birth and were subsequently recalculated and adjusted according to the measurements obtained at 14 days of age. d4T and NFV were given during formula feeding. ddI was given at 1 h before feeding, and at least 2 h must have passed since the prior feeding. The infants were observed in the hospital for 1 week after birth while receiving the study drugs. Detailed information on drug administration, drug storage, and adverse events was provided to the parents or guardians; and they had to be fully capable of giving the drugs to their infants before the infants were discharged. All families had access to a refrigerator, and d4T and ddI were kept on ice in a container which was provided for transport home. The parents and guardians were provided a study booklet that contained a calendar in which the times of study drug administration and other essential information could be recorded. Compliance was strictly monitored by measuring the amounts of drugs returned, evaluating the drug calendar in the study booklet, and questioning the parents or guardians at each visit.
Study visits were at birth; on days 4, 7, 14, and 28; and at weeks 8 and 16. Medical history, physical examination, weight, length, and head circumference were assessed at every visit. Amylase, lipase, aspartate aminotransferase (AST), alanine aminotransferase, and creatinine levels were monitored at every visit during the first 4 weeks of life. The serum bilirubin level was monitored at every visit during the first 2 weeks. Serum cholesterol and triglyceride levels were determined on days 1, 7, 14, and 28. Serum electrolyte concentrations were determined on days 14 and 28. A complete blood count was done on day 1. Hematologic and biochemical evaluations on day 1 were performed with umbilical cord blood. Adverse events were reported according to the Division of Acquired Immunodeficiency Syndrome, National Institute of Allergy and Infectious Diseases toxicity tables for children ≤3 months of age and, if applicable, for children >3 months of age. For infants who experienced grade III toxicity, the study drugs were discontinued temporarily. For those with any grade IV toxicity, the study drugs were discontinued permanently. If the study drugs were discontinued for any reason before 4 weeks of age, ZDV was given until 6 weeks of age as part of the standard postnatal prophylaxis.
On days 14 and 28, when the full pharmacokinetic study took place, d4T and NFV were given during formula feeding. ddI was given at 2 h after the feeding, and the next feeding was not given until 1 h after ddI dosing. Blood samples of 1 ml were obtained in EDTA-containing tubes at 0, 1, 2.5, 4, 8, and 12 h after d4T and NFV administration; these were equivalent to −2, −1, 0.5, 2, 6, and 10 h after ddI administration. No blood samples were obtained after 10 h following ddI administration. Plasma was immediately separated and stored in two 150-μl aliquots at −70°C until analysis. All samples were sent frozen via an overnight courier to the MDS Pharma Services Laboratory, Sunnyvale, Calif., where d4T and ddI bioanalyses were performed.
The plasma drug concentrations were determined by a validated radioimmunoassay methodology, with the lower limits of quantitation being 10 and 3 ng/ml for d4T and ddI, respectively (11; C. Knupp, B. Damle, P. Nichola, and S. Kaul, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr 1664, 2000). The mean predicted concentrations of the quality controls were within 10% of their nominal values, and the between-run and within-run variabilities, expressed as the coefficient of variation, were within 15% for both drugs. Plasma concentration-versus-time data were analyzed by the noncompartmental methods. The peak concentration (Cmax), the corresponding sampling time to Cmax (Tmax), and the trough concentration (Cmin) were obtained from direct inspection of the plasma concentration-time profile. Without the use of a weighting factor, the terminal log-linear phase of the plasma concentration-time curve was identified by least-squares linear regression of at least 3 datum points, which yielded a minimum mean square error. The half-life of the terminal log-linear phase was calculated as 0.693/K, where K is the absolute value of the slope of the terminal log-linear phase. The area under the plasma concentration-time curve from time zero to time T (AUC0–T), where T is the time point for the last measurable concentration, was calculated by using the trapezoidal rule. The area under the plasma concentration-time curve from time zero to infinity (AUC0–∞) was calculated by summing AUC0–T and the extrapolated area, which was determined by dividing the final plasma drug concentration by K.
Blood for detection of HIV DNA by PCR assay (Amplicor HIV-1 test; Roche Diagnostic Systems) was obtained within 24 h after birth, at week 8, and at week 16. If the PCRs at weeks 8 and 16 yielded discrepant results, an additional blood sample for HIV DNA detection by PCR was obtained to determine the infection status. Infants were considered HIV infected if they had at least two positive PCR results after 4 weeks of age. They were considered uninfected if all PCR results were negative. Poststudy follow-up was encouraged until 18 months of age, when the HIV antibody assay was performed to confirm the infection status.
Statistic analysis was done with the SPSS (version 9.0) program (SPSS, Chicago, Ill.). Demographic data and pharmacokinetic parameters are presented as medians and ranges unless specified otherwise. Comparisons of pharmacokinetic parameter estimates between days 14 and 28 were tested for statistical significance by paired t test or Wilcoxon's ranked sum test. A two-sided P value of 0.05 was considered significant for all tests.
Twelve newborn infants were enrolled into the first two cohorts, with six infants in each cohort, between August 1999 and July 2000. The plasma samples from all six infants in the first cohort and from the first two infants in the second cohort were used for pharmacokinetic analysis of d4T and ddI. The characteristics of the mothers and the infants are presented in Table 1. All mothers received ZDV antepartum, and all but one received ZDV intrapartum. The intrapartum ZDV regimen was modified to be given orally, as described previously (21). None of the mothers received other antiretroviral drugs before or during pregnancy. All infants received d4T, ddI, and NFV within 12 h after birth.
TABLE 1.
Baseline characteristics of mothers and infants
| Characteristic | Value(s) |
|---|---|
| Maternal age (yr) | 25.6 ± 4.7a |
| Maternal CDCb classification for HIV (no. of subjects) | |
| A | 7 |
| B | 1 |
| Duration of prenatal ZDV use, days | 75.5 (49.0–157.0)c |
| No. of ZDV doses during labor | 2 (0–7)c |
| Duration of membrane rupture before birth (min) | 18 (0–412)c |
| Mode of delivery (no. of subjects) | |
| Vaginal delivery | 6 |
| Cesarean delivery | 2 |
| Obstetric complication, fetal distress (no. of subjects) | 1 |
| Infant's gender (no. of infants) | |
| Male | 4 |
| Female | 4 |
| Infant's birth wt (kg) | 3.3 ± 0.3a |
| Infant's gestational age (wk) | 38.9 ± 2.0a |
Values are means ± standard deviations.
CDC, Centers for Disease Control and Prevention.
Values are medians (ranges).
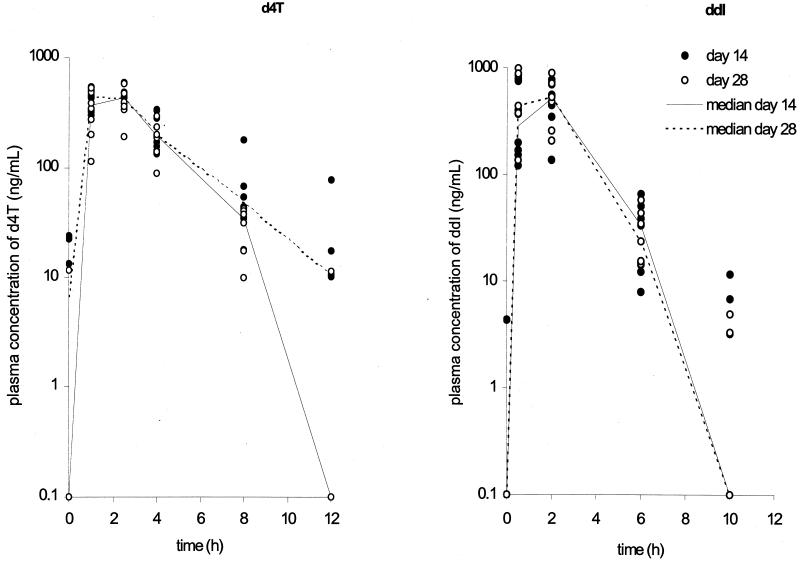
Samples for pharmacokinetic analysis retrieved on days 14 and 28 were obtained at all time points from all infants. The median plasma concentration-time curves for d4T and ddI on days 14 and 28 are shown in Fig. 1. The pharmacokinetic characteristics of d4T and ddI are presented in Table 2. There were no significant differences between the pharmacokinetic parameters at the age of 14 days and those at the age of 28 days for either d4T or ddI. The median Cmin of d4T at 28 days of age was lower than that at 14 days of age, but this did not reach statistical significance (0 versus 10.3 ng/ml [P > 0.5]). The actual Cmin of ddI could not be assessed because no plasma samples were obtained at 24 h after ddI administration. Nevertheless, plasma ddI concentrations were below the limit of detection (3 ng/ml) at 10 h after administration in five infants each on days 14 and 28. No significant correlation between the demographic parameters (age, birth weight, and gestational age) and pharmacokinetic parameters was detected.
FIG. 1.
Logarithmic plots of the median plasma concentration-time profiles of d4T and ddI in infants at 14 and 28 days of age. Each datum point represents the plasma drug concentration at a specific time point for each infant.
TABLE 2.
Pharmacokinetic parameters of d4T and ddI in newborn infantsa
| Drug and age (days) | Cmax (ng/ml) | Tmax (h) | AUC0–12 (ng · h/ml) | AUC0–∞ (ng · h/ml) | Half-life (h) |
|---|---|---|---|---|---|
| d4T | |||||
| 14 | 463 (335–582, 17.5) | 2.5 (1.0–2.5, 40.1) | 1,866 (1,286–2,420, 22.6) | 1,930 (1,369–2,461, 21.9) | 1.8 (1.2–2.0, 17.6) |
| 28 | 507 (337–589, 20.6) | 2.5 (1.0–2.5, 32.7) | 1,603 (1,107–1,986, 24.5) | 1,890 (1,126–2,010, 23.2)b | 1.4 (1.2–1.8, 13.5)b |
| ddI | |||||
| 14 | 627 (168–786, 38.9) | 1.3 (0.5–2.0, 64.1) | 1,573 (451–2,327, 39.3)d | 1,595 (464–2,332, 43.8)c | 1.2 (0.9–1.4, 14.8)c |
| 28 | 687 (208–982, 46.3) | 1.0 (0.5–2.0, 63.4) | 1,562 (776–2,270, 41.1)d | 1,856 (782–2,327, 43.6)b | 1.1 (0.5–1.5, 30.8)b |
The values are medians (ranges, coefficients of variation, with coefficients of variation presented as percentages).
The value was undetermined in an infant due to insufficient datum points in the terminal elimination phase.
The values were undetermined in two infants due to insufficient datum points in the terminal elimination phase.
For ddI, values are AUC0–10.
There were no study withdrawals due to adverse events. The following adverse events were observed: diarrhea (one infant), dry skin (two infants), delayed umbilical cord separation after 14 days of age (three infants), emesis (four infants), and elevation of AST levels (seven infants). All adverse events were mild and transient and could not be attributed solely to the study drugs. Mild hypertriglyceridemia was noted in four infants, but this resolved spontaneously in all four infants while the infants were receiving study drugs or shortly after completion of the 4-week course of treatment with the study drugs. No hypercholesterolemia or other manifestations of lipodystrophy were observed. All infants had negative PCR tests for HIV DNA at birth and at 8 and 16 weeks of age. All achieved normal growth and neurodevelopment. They are being monitored and will have an HIV antibody test at 18 months of age.
The present study shows that the pharmacokinetics of d4T or ddI at the ages of 14 and 28 days are not different. In conjunction with the short half-lives, these data suggest that for both drugs the steady-state conditions are reached in infants by day 14 of administration. Previous studies showed that the mean AUC values for d4T after administration of a dose of 1 mg/kg twice daily in children were 1,629 to 2,509 ng · h/ml (8, 12, 13), which are comparable to the values obtained in the present study (1,866 and 1,603 ng · h/ml). The Cmax values obtained for d4T in the present study (463 and 507 ng/ml) are in proximity to the Cmax values of 510 to 1,099 ng/ml obtained in previous studies (8, 12). Furthermore, the half-lives in the present study (1.8 and 1.4 h) are similar to those reported in previous studies (1.1 to 1.4 h) (8, 12, 13). Since the dose of d4T in the present study resulted in similar drug exposures in children receiving the recommended dose of 1 mg/kg twice daily, a d4T dose of 1 mg/kg given twice daily may be appropriate for infants at 14 to 28 days of age. As the kinetics of d4T were not determined prior to 14 days of age, it is unclear if the kinetics observed on days 14 and 28 would represent those before 14 days of age.
Evaluation of the pharmacokinetics of ddI in infants has been very limited. Furthermore, a wide range of ddI doses have been used, thus making cross-study comparisons difficult. Capparelli et al. evaluated the pharmacokinetics of ddI in HIV-infected infants older than 14 days of age (E. Capparelli, A. Kovacs, R. Husson, J. Connor, and C. McLaren, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A63, 1998). The mean Cmax in those receiving a 50-mg/m2 dose at 14 to 28 days of age was 307 ng/ml. In another study, Wang et al. (22) demonstrated that, in infants taking ddI at 60 mg/m2 twice daily for 6 weeks, the mean Cmax values at 1 day and 6 weeks of age were 309 and 215 ng/ml, respectively. The Cmax values of ddI in our study (627 and 687 ng/ml) are comparable to those in children (425 to 1,110 ng/ml) (1, 2), but they are approximately twice as high as the values noted in infants in the aforementioned studies. This is probably due to the different ddI doses used. The half-life of ddI in the present study is not different from those observed in other studies (2, 8, 20, 22).
It should be noted that the plasma ddI concentrations at 10 h after dosing were undetectable or were close to the limit of detection in most infants and that the AUC0–10 was in approximate to the AUC0–∞. Hence, the AUC0–10 reported in the present study should reasonably account for the AUC over the dosing interval of 24 h, as ddI was given once daily. Capparelli et al. reported that the mean AUC0–12 values were 378 and 1,394 ng · h/ml in infants at 14 to 28 days of age following the administration of ddI doses of 25 mg/m2 (n = 5 infants) and 50 mg/m2 (n = 50 infants), respectively (Capparelli et al., 38th ICAAC). The increment in AUC between the two doses is surprising since the kinetics of ddI are basically linear across this dose range, at least in adult subjects. In older infants (age 29 to 120 days; n = 7 infants) given a dose of 50 mg/m2, the AUC of ddI was 638 ng · h/ml. Wang et al. (22) described mean AUC0–12 values of 1,818 and 573 ng · h/ml at 1 day and 6 weeks of age, respectively, following the administration of a ddI dose of 60 mg/m2. However, the investigators refrained from making any conclusions due to the small sample size and the observed variability in the kinetics of ddI. The AUC0–10 values observed in the present study were 1,573 and 1,562 ng · h/ml on days 14 and 28, respectively, following administration of a 100-mg/m2 dose of ddI. In children, a 90- to 100-mg/m2 dose of ddI yielded AUC0–12 values of 402 to 968 ng · h/ml (2, 8, 15, 16, 20). Overall, the reported kinetics of ddI in pediatric patients are quite variable, but a trend toward lower systemic clearance (or higher levels of drug exposure) in infants compared to that in older children seems apparent. This is not unexpected given the significant renal clearance of ddI and the immature renal physiology in infants. Whether this observation warrants dose reduction in infants is unclear, especially in view of the small sample size in the present study and the observed variability in the kinetics of ddI. Integrated analyses of all available pharmacokinetic data across pediatric studies in relevant age groups need to be conducted to guide the dosing of ddI in infants.
The adverse effects of the combination of d4T, ddI, and NFV are a concern. These include pancreatitis, peripheral neuropathy, hepatotoxicity, diarrhea, lipodystrophy, and mitochondrial toxicity (4). Nevertheless, no significant adverse effects were encountered in the present study. Transient mild hypertriglyceridemia was noted; however, the serum samples were not obtained after fasting. The long-term effects of transient hypertriglyceridemia in these infants are unknown. Despite no clinical peripheral neuropathy in the present study, we realize that the assessment of the peripheral nervous system in infants is difficult. The development of lipodystrophy and mitochondrial toxicity is generally associated with prolonged exposure to antiretroviral drugs. Thus, they were probably unlikely to have developed in the present study. However, a long-term follow-up is essential to determine these late adverse events.
Some issues concerning the pharmacological and clinical aspects of d4T and ddI still need to be addressed. First, measurement of the levels of the intracellular active phosphorylated forms is certainly of interest, especially for ddI. Second, it is unknown whether the pharmacokinetics of d4T and ddI in premature infants or infants with low birth weights will follow those observed in term infants or infants with normal birth weights in the present study. Third, it is unclear if the findings for Thai infants are generalizable to other infants of different ethnic backgrounds. Fourth, the intersubject variability in actual drug exposure observed in the present study necessitates more exploration of the application of individual therapeutic drug monitoring in the pediatric setting. Fifth, we acknowledge the small sample size in the present study; therefore, the safety and efficacy of this regimen for the prevention of perinatal HIV transmission cannot be properly assessed. In conclusion, the results of the present study suggest that the systemic levels of exposure to d4T in infants were comparable to those seen in older pediatric populations, indicating that the d4T dose of 1 mg/kg given twice daily may be appropriate for infants at 14 to 28 days of age. The levels of exposure to ddI after administration of a dose of 100 mg/m2 once daily to infants at 14 to 28 days of age were somewhat higher than those in older members of the pediatric population. Whether this observation warrants a reduction of the ddI dose in infants is not clear.
Acknowledgments
This study was supported by grants from Roche Thailand and Bristol-Myers Squibb Thailand. The following authors have association with Bristol-Myers Squibb and/or Hoffmann-La Roche: Bharat D. Damle is an employee of Bristol-Myers Squibb and owns Bristol-Myers Squibb stocks, Aeumporn Srigritsanapol is an employee (medical director) of Roche Thailand Ltd., David A. Cooper is an advisor and clinical investigator for Bristol-Myers Squibb and Hoffmann-La Roche, and Joep M. A. Lange is a clinical investigator for Bristol-Myers Squibb and Hoffmann-La Roche.
We appreciate the contributions of the parents and their infants, the pediatric and obstetric residents and nurses at King Chulalongkorn Memorial Hospital, and HIV-NAT staff.
REFERENCES
- 1.Abreu T, Plaisance K, Rexroad V, Nogueira S, Oliveira R H, Evangelista L A, Rangel R, Silva I S, Knupp C, Lambert J S. Bioavailability of once- and twice-daily regimens of didanosine in human immunodeficiency virus-infected children. Antimicrob Agents Chemother. 2000;44:1375–1376. doi: 10.1128/aac.44.5.1375-1376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balis F M, Pizzo P A, Butler K M, Hawkins M E, Brouwers P, Husson R N, Jacobsen F, Blaney S M, Gress J, Jarosinski P, Poplack D G. Clinical pharmacology of 2′,3′-dideoxyinosine in human immunodeficiency virus-infected children. J Infect Dis. 1992;165:99–104. doi: 10.1093/infdis/165.1.99. [DOI] [PubMed] [Google Scholar]
- 3.Blanche S, Rouzioux C, Moscato M G, Veber F, Mayaux M J, Jacomet C, Tricoire J, Deville A, Vial M, Firtion G, De Crepy A, Douard D, Robin M, Courpotin C, Ciraru-Vigneron N, Le Deist F, Griscelli C. A prospective study of infants born to women seropositive for human immunodeficiency virus type 1. N Engl J Med. 1989;320:1643–1648. doi: 10.1056/NEJM198906223202502. [DOI] [PubMed] [Google Scholar]
- 4.Carr A, Cooper D A. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–1430. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 5.Connor E M, Sperling R S, Gelber R, Kiselev P, Scott G, O'Sullivan M J, VanDyke R, Bey M, Shearer W, Jacobson R L, Jimenez E, O'Neill E, Bazin B, Delfraissy J-F, Culnane M, Coombs R, Elkins M, Moye J, Stratton P, Balsley J. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med. 1994;331:73–80. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 6.de Martino M, Tovo P-A, Balducci M, Galli L, Gabiano C, Rezza G, Pezzotti P. Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV-1 infection. JAMA. 2000;284:190–197. doi: 10.1001/jama.284.2.190. [DOI] [PubMed] [Google Scholar]
- 7.The European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353:1035–1039. doi: 10.1016/s0140-6736(98)08084-2. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher C V, Brundage R C, Remmel R P, Page L M, Weller D, Calles N R, Simon C, Kline M W. Pharmacologic characteristics of indinavir, didanosine, and stavudine in human immunodeficiency virus-infected children receiving combination therapy. Antimicrob Agents Chemother. 2000;44:1029–1034. doi: 10.1128/aac.44.4.1029-1034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guay L A, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, Sherman J, Bakaki P, Ducar C, Deseyve M, Emel L, Mirochnick M, Fowler M G, Mofenson L, Miotti P, Dransfield K, Bray D, Mmiro F, Jackson J B. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIV-NET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 10.The International Perinatal HIV Group. The mode of delivery and risk of vertical transmission of human immunodeficiency virus type 1. N Engl J Med. 1999;340:977–987. doi: 10.1056/NEJM199904013401301. [DOI] [PubMed] [Google Scholar]
- 11.Kaul S, Stouffer B, Mummaneni V, Turabi N, Mantha S, Jayatilak P, Barbhaiya R. Specific radioimmunoassays for the measurement of stavudine in human plasma and urine. J Pharm Biomed Anal. 1996;15:165–174. doi: 10.1016/0731-7085(96)01839-0. [DOI] [PubMed] [Google Scholar]
- 12.Kline M W, Dunkle L M, Church J A, Goldsmith J C, Harris A T, Federici M E, Schultze M E, Woods L, Loewen D F, Kaul S, Cross A, Rutkiewicz V L, Rosenblatt H M, Hanson C, Shearer W T. A phase I/II evaluation of stavudine (d4T) in children with human immunodeficiency virus infection. Pediatrics. 1995;96:247–252. [PubMed] [Google Scholar]
- 13.Kline M W, Fletcher C V, Federici M E, Harris A T, Evans K D, Rutkiewicz V L, Shearer W T, Dunkle L M. Combination therapy with stavudine and didanosine in children with advanced human immunodeficiency virus infection: pharmacokinetic properties, safety, and immunologic and virologic effects. Pediatrics. 1996;97:886–890. [PubMed] [Google Scholar]
- 14.Lallemant M, Jourdain G, Le Coeur S, Kim S, Koetsawang S, Comeau A M, Phoolcharoen W, Essex M, McIntosh K, Vithayasai V. A trial of shortened zidovudine regimens to prevent mother-to-child transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;343:982–991. doi: 10.1056/NEJM200010053431401. [DOI] [PubMed] [Google Scholar]
- 15.Mueller B U, Butler K M, Stocker V L, Balis F M, Brouwers P, Jarosinski P, Husson R N, Lewis L L, Venzon D, Pizzo P A. Clinical and pharmacokinetic evaluation of long-term therapy with didanosine in children with HIV infection. Pediatrics. 1994;94:724–731. [PubMed] [Google Scholar]
- 16.Mueller B U, Pizzo P A, Farley M, Husson R N, Goldsmith J, Kovacs A, Woods L, Ono J, Church J A, Brouwers P, Jarosinski P, Venzon D, Balis F M. Pharmacokinetic evaluation of the combination of zidovudine and didanosine in children with human immunodeficiency virus infection. J Pediatr. 1994;125:142–146. doi: 10.1016/s0022-3476(94)70141-5. [DOI] [PubMed] [Google Scholar]
- 17.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, Ndinya-Achola J, Bwayo J, Onyango F E, Hughes J, Kreiss J. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 18.Shaffer N, Chuachoowong R, Mock P A, Bhadrakom C, Siriwasin W, Young N L, Chotpitayasunondh T, Chearskul S, Roongpisuthipong A, Chinayon P, Karon J, Mastro T D, Simonds R J. Short course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Lancet. 1999;353:773–780. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 19.Shearer W T, Quinn T C, LaRussa P, Lew J F, Mofenson L, Almy S, Rich K, Handelsman E, Diaz C, Pagano M, Smeriglio V, Kalish L A. Viral load and disease progression of infants infected with human immunodeficiency virus type 1. N Engl J Med. 1997;336:1337–1342. doi: 10.1056/NEJM199705083361901. [DOI] [PubMed] [Google Scholar]
- 20.Stevens R C, Rodman J H, Yong F H, Carey V, Knupp C A, Frenkel L M. Effect of food and pharmacokinetic variability on didanosine systemic exposure in HIV-infected children. Pediatric AIDS Clinical Trials Group Protocol 144 Study Team. AIDS Res Hum Retrovir. 2000;16:415–421. doi: 10.1089/088922200309070. [DOI] [PubMed] [Google Scholar]
- 21.Thisyakorn U, Khongphatthanayothin M, Sirivichayakul S, Rongkavilit C, Poolcharoen W, Kunanusont C, Bien D D, Phanuphak P. Thai Red Cross zidovudine donation program to prevent vertical transmission of HIV: the effect of the modified ACTG 076 regimen. AIDS. 2000;14:2921–2927. doi: 10.1097/00002030-200012220-00014. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Livingstone E, Patil S, McKinney R E, Bardeguez A D, Gandia J, O'Sullivan M J, Clax P, Huang S, Unadkat J D. Pharmacokinetics of didanosine in antepartum and postpartum human immunodeficiency virus-infected pregnant women and their neonates: an AIDS Clinical Trial Group Study. J Infect Dis. 1999;180:1536–1541. doi: 10.1086/315067. [DOI] [PubMed] [Google Scholar]