Abstract
Objectives
Colorectal cancer (CRC) screening with a faecal immunochemical test (FIT) has been disrupted in many countries during the COVID-19 pandemic. Performing catch-up of missed screens while maintaining regular screening services requires additional colonoscopy capacity that may not be available. This study aimed to compare strategies that clear the screening backlog using limited colonoscopy resources.
Methods
A range of strategies were simulated using four country-specific CRC natural-history models: Adenoma and Serrated pathway to Colorectal CAncer (ASCCA) and MIcrosimulation SCreening ANalysis for CRC (MISCAN-Colon) (both in the Netherlands), Policy1-Bowel (Australia) and OncoSim (Canada). Strategies assumed a 3-month screening disruption with varying recovery period lengths (6, 12, and 24 months) and varying FIT thresholds for diagnostic colonoscopy. Increasing the FIT threshold reduces the number of referrals to diagnostic colonoscopy. Outcomes for each strategy were colonoscopy demand and excess CRC-related deaths due to the disruption.
Results
Performing catch-up using the regular FIT threshold in 6, 12 and 24 months could prevent most excess CRC-related deaths, but required 50%, 25% and 12.5% additional colonoscopy demand, respectively. Without exceeding usual colonoscopy demand, up to 60% of excess CRC-related deaths can be prevented by increasing the FIT threshold for 12 or 24 months. Large increases in FIT threshold could lead to additional deaths rather than preventing them.
Conclusions
Clearing the screening backlog in 24 months could avert most excess CRC-related deaths due to a 3-month disruption but would require a small increase in colonoscopy demand. Increasing the FIT threshold slightly over 24 months could ease the pressure on colonoscopy resources.
Keywords: Colorectal cancer, Screening, Colonoscopy, FIT, COVID-19
Introduction
In the first wave of the COVID-19 pandemic, many non-COVID-19-related health services were suspended, including several organized colorectal cancer (CRC) screening programmes.1 Many organized CRC screening programmes include faecal immunochemical testing (FIT), followed by a diagnostic colonoscopy after a positive FIT result.2 In some countries, only diagnostic follow-up colonoscopy services have been paused due to the COVID-19 pandemic.1 For example, in Australia, the invitation to FIT screening continued while colonoscopy services decreased by 55% from March to April 2020.3 In other countries, such as the Netherlands and Canada, both primary FIT screening and diagnostic colonoscopy have been disrupted.1
A disruption in primary FIT screening or delay in diagnostic colonoscopy after a positive FIT will hamper screening effectiveness leading to a stage-shift in CRC and worse health outcomes.4,5 This effect was illustrated in a retrospective cohort study that identified a significant increase in the number of patients with obstructive CRCs during the pandemic.6 Furthermore, modelling studies have predicted that screening disruption and diagnostic delays due to the pandemic will likely increase avoidable CRC-related deaths.7–9
Colonoscopy capacity is slowly recovering in multiple countries and efforts have been made to catch-up missed screens.1 However, the COVID-19 pandemic is unpredictable, with new variants of the virus emerging and differences in access to vaccines around the world. Thus, pressure on health services will likely persist and constraints in colonoscopy capacity can be expected. Accordingly, it is important to explore prioritisation approaches for referral to diagnostic colonoscopy to minimise excess CRC deaths.
Given the lack of empirical evidence, modelling provides a unique opportunity to generate policy-relevant information to balance the short-term impact on resource use against the long-term health impact of prioritisation strategies. The COVID-19 and Cancer Global Modelling Consortium (CCGMC; www.ccgmc.org) was established to support decision making in cancer control both during and after the COVID-19 crisis. This study's objective, conducted as part of the CCGMC, focussed on two example approaches to colonoscopy prioritisation: increasing the FIT threshold for referral to diagnostic colonoscopy, so capacity is directed towards those at higher risk,10,11 and extending the recovery period in which missed individuals are caught up, resulting in extension of the screening interval for a larger proportion of the population. A combination of both approaches would also be possible, allowing for a careful trade-off between managing colonoscopy demand and preventing COVID-19-related excess CRC-related deaths.
Methods
This analysis used four models, participating in CCGMC: the Adenoma and Serrated pathway to Colorectal CAncer (ASCCA) model, the MIcrosimulation SCreening ANalysis for CRC (MISCAN-Colon) model, the Policy1-Bowel model and the OncoSim model. The models simulate the development of CRC and the country-specific CRC screening programmes in the Netherlands (ASCCA and MISCAN-Colon), Australia (Policy1-Bowel) and Canada (OncoSim) (Table 1). Model assumptions are described in Appendix A. The modelled countries have population-based FIT CRC screening programmes for average-risk individuals and a similar burden of disease (Appendix Table A1). Models’ design, calibration and validation have been extensively described elsewhere.12–16
Table 1.
CRC burden and screening programmes for the Netherlands, Australia and Canada
| The Netherlands | Australiaa | Canadab | |
|---|---|---|---|
| Population size17 | 17.3 million | 25.4 million | 37.6 million |
| Individuals eligible for screening in 2020 | 2,260,000 | 3,360,000 | 2,020,000 |
| CRC incidence (per 100,000 people)c | 30.7 | 33.1 | 31.2 |
| CRC mortality (per 100,000 people)c | 12.4 | 8.9 | 9.9 |
| Screening test | FIT (FOB-Gold) | Two-sample FIT (Magstream HemSp) | FIT (Polymedco and Alfresa Pharma) |
| FIT threshold (μg Hb/g faeces) | 47 | 20 | 20 |
| Screening interval | Biennial | Biennial | Biennial |
| Screening age range, years | 55-75 | 50-74 | 50-74 |
| Adherence to screening | 73%18 | 41%19 | 42.3%20 |
CRC: colorectal cancer; FIT, faecal immunochemical test; µg Hb/g: microgram haemoglobin per gram faeces.
In Australia there is known to be significant out-of-programme screening, which cannot be quantified. Therefore, the included data only refer to the official national screening programme (NBCSP).
In Canada, screening varies across the country in for example screening frequency, number of samples used for FIT, and FIT threshold. OncoSim models the most common screening practice.
Age-standardized rate using WHO world standardized population per 100,000 in 2020.21
In Australia, individuals are invited to complete a two-sample FIT, a positive result on either test is sufficient for diagnostic follow-up.19
Modelled strategies
We simulated CRC screening during the period 2020-2050 under the following strategies:
No disruption.
3-month disruption without catch-up.
3-month disruption with catch-up at regular FIT threshold during a recovery period of various lengths.
3-month disruption with catch-up at increased FIT threshold during a recovery period of various lengths.
The first strategy allows for comparison with a counterfactual baseline in absence of the COVID-19 pandemic. In the second strategy, we assumed that missed screens were not caught-up and that the regular screening programme resumed immediately after the disruption, meaning that individuals due for screening during the disruption period skipped a screening round. This strategy indicates the maximum expected impact of a disruption on excess CRC-related deaths and was used to compare the benefits of each of the catch-up strategies.
For the second, third and fourth set of strategies we assumed a 3-month disruption where no primary screening nor diagnostic follow-up was performed in April, May and June 2020, corresponding to the average duration that CRC screening programmes were paused in the Netherlands, Australia and Canada (Appendix B).1
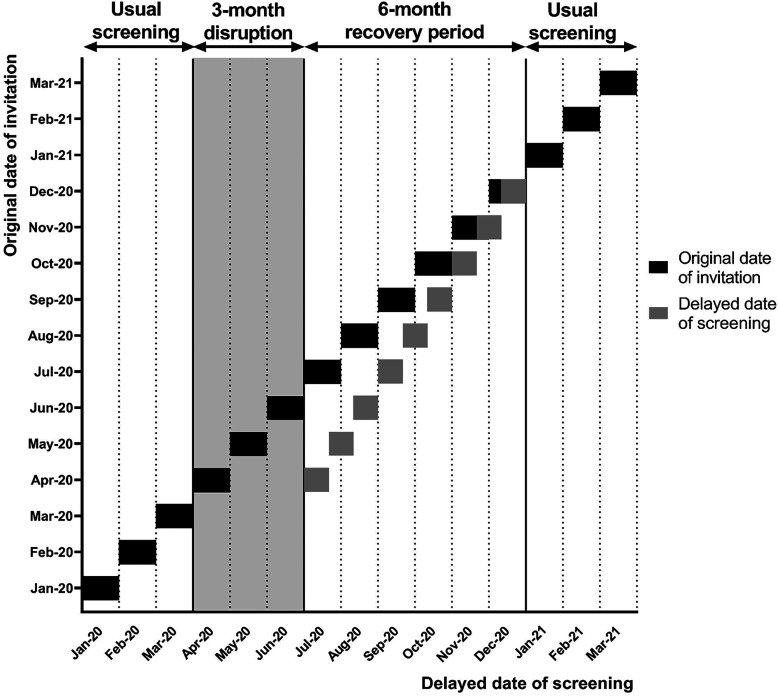
In the third and fourth set of strategies, we assumed that the disruption period was followed by a recovery period in which individuals who missed screening were caught up. FIT participation rates and compliance to diagnostic follow-up in the recovery period were set equal to pre-pandemic levels.22,23 We assumed that the order in which individuals were screened during the recovery period was based on the original date of invitation. On average, individuals were delayed for primary screening by 1.5 months (Figure 1). Details on the implementation of the screening delay can be found in Appendix B. After the recovery period, the backlog in screening was cleared and screening proceeded as usual (Figure 1).
Figure 1.
Delay in screening throughout a 6-month recovery period after a 3-month disruption period (April-June 2020)
The third set consisted of three strategies, i.e. 3-month disruption and catch-up at regular FIT threshold during a 6-, 12- and 24-month recovery period.
In the fourth set of strategies, catch-up screening was performed at increased FIT threshold for referral to diagnostic colonoscopy during the recovery period. Each model simulated a country-specific set of five increased thresholds (Appendix Table C1) because the regular FIT threshold differs between countries (Appendix Table 1). All five thresholds were simulated for the three considered recovery periods. A range of FIT thresholds were evaluated to provide insight for policy makers on appropriate FIT threshold to balance colonoscopy capacity and health outcomes.
FIT screening at increased threshold required calibration of lesion-specific FIT characteristics used in the models. This calibration was informed by data on the performance of OC-Sensor and FOB-Gold at different thresholds from a Dutch study performed in 2016.24 A detailed explanation of the calibration procedure and test characteristics at each threshold is presented in Appendix C.
Targeted prioritisation strategies
We have simulated strategies, using part of the models included in this study, where we focussed on a more targeted approach to catch-up, by increasing the FIT threshold for specific subgroups based on age and sex only. Details about these strategies are given in Appendix D.
Delayed screening strategy
In addition, as an alternative approach to catch-up, we evaluated the impact of delaying screening for all individuals, except for individuals receiving their first screening invitation after the disruption. This strategy was evaluated by part of the models and is explained in more detail in Appendix D.
Sensitivity analysis
Because screening programmes might face reductions in adherence after the disruption period, we simulated one set of strategies with catch-up at the regular and five increased FIT thresholds while assuming a relative reduction of 25% in diagnostic colonoscopy compliance during the 12-month recovery period. This sensitivity analysis was evaluated by ASCCA, MISCAN-Colon and Policy1-Bowel.
Outcomes
Outcomes of each strategy were the number of CRC cases, CRC-related deaths, FIT positivity rates, and the number of diagnostic follow-up colonoscopies, as absolute numbers. In addition, for the 3-month disruption strategies with catch-up, outcomes were given as relative changes compared to the 3-month disruption strategy without catch-up. Outcomes were calculated in individuals aged 50 years and older, because of the target age range for CRC screening.
Note that the diagnostic follow-up colonoscopy demand per strategy is a model output and therefore we did not explicitly model colonoscopy capacity limits in all strategies.
Model-predicted positivity rates and diagnostic colonoscopies performed were reported for the periods July – December 2020 (6-month recovery period), July 2020 – June 2021 (12-month recovery period), and July 2020 – June 2022 (24-month recovery period). Furthermore, predictions were obtained for the total number of CRC cases and CRC-related deaths over the period 2020-2050. The cumulative excess CRC cases and deaths compared to the no-disruption strategy were reported for the 3-month disruption strategy without catch-up and the 3-month disruption strategies with catch-up.
Results
ASCCA predicted that approximately 4,850 colonoscopies per month were required when no catch-up was performed after a 3-month disruption (Table 2). This strategy would result in 446 and 923 excess CRC-related deaths and cases in 2020-2050, respectively, which is an additional 0.32% and 0.23% of what would have been expected without a disruption. For MISCAN-Colon, Policy1-Bowel, and OncoSim the predicted monthly colonoscopy demand after a 3-month disruption without catch-up was approximately 4,160, 6,250, and 7,960, respectively. This is predicted to lead to 339 (0.21%), 978 (0.48%), and 436 (0.13%) excess CRC-related deaths in 2020-2050, respectively. MISCAN-Colon and Policy-Bowel predicted 416 (0.09%) and 1,657 (0.26%) excess CRC cases, respectively. Excess CRC cases were not available for OncoSim.
Table 2.
Outcomes for the strategies with 3 months’ disruption and 6, 12, and 24 months’ recovery period: 3-month disruption without catch-up, 3-month disruption with non-prioritised catch-up screening at regular FIT threshold and 3-month disruption with prioritised catch-up screening at increased FIT thresholds; outcomes of the strategies with catch-up (both non-prioritised and prioritised) are compared with the strategy without catch-up.
| Length of recovery period | 3-month disruption | |||||||
|---|---|---|---|---|---|---|---|---|
| No catch-up screeninga | Catch-up screening at regular FIT threshold | Catch-up screening at increased FIT threshold | ||||||
| 47 μg Hb/g | 50 μg Hb/g | 55 μg Hb/g | 60 μg Hb/g | 70 μg Hb/g | 80 μg Hb/g | |||
| ASCCA | ||||||||
| Positivity rate | 6 months | 4.7% | 4.7% | 4.5% | 4.3% | 4.0% | 3.5% | 3.1% |
| 12 months | 4.6% | 4.7% | 4.5% | 4.3% | 4.0% | 3.6% | 3.2% | |
| 24 months | 4.6% | 4.6% | 4.5% | 4.2% | 4.0% | 3.5% | 3.2% | |
| Monthly colonoscopy demand during recovery period | 6 months | 4910 | 7401 (151%) | 7198 (147%) | 6842 (139%) | 6448 (131%) | 5755 (117%) | 5232 (107%) |
| 12 months | 4846 | 6103 (126%) | 5925 (122%) | 5633 (116%) | 5309 (110%) | 4738 (98%) | 4309 (89%) | |
| 24 months | 4848 | 5445 (112%) | 5289 (109%) | 5031 (104%) | 4745 (98%) | 4242 (87%) | 3864 (80%) | |
| Cumulative excess CRC cases in 2020-2050 | 6 months | 923 | 21 (2%) | 96 (10%) | 189 (20%) | 272 (29%) | 424 (46%) | 615 (67%) |
| 12 months | 923 | 33 (4%) | 142 (15%) | 303 (33%) | 446 (48%) | 688 (75%) | 995 (108%) | |
| 24 months | 923 | 55 (6%) | 237 (26%) | 517 (56%) | 796 (86%) | 1218 (132%) | 1739 (188%) | |
| Cumulative excess CRC-related deaths in 2020-2050 | 6 months | 446 | 11 (2%) | 39 (9%) | 54 (12%) | 85 (19%) | 157 (35%) | 212 (47%) |
| 12 months | 446 | 17 (4%) | 55 (12%) | 87 (19%) | 141 (32%) | 243 (54%) | 336 (75%) | |
| 24 months | 446 | 27 (6%) | 89 (20%) | 148 (33%) | 260 (58%) | 420 (94%) | 583 (131%) | |
| MISCAN-Colon | ||||||||
| Positivity rate | 6 months | 3.7% | 3.8% | 3.6% | 3.4% | 3.1% | 2.7% | 2.5% |
| 12 months | 3.7% | 3.8% | 3.6% | 3.4% | 3.1% | 2.7% | 2.5% | |
| 24 months | 3.7% | 3.7% | 3.5% | 3.4% | 3.1% | 2.7% | 2.5% | |
| Monthly colonoscopy demand during recovery period | 6 months | 4110 | 6199 (151%) | 5915 (144%) | 5677 (138%) | 5221 (127%) | 4618 (112%) | 4282 (104%) |
| 12 months | 4178 | 5235 (125%) | 4993 (120%) | 4793 (115%) | 4404 (105%) | 3893 (93%) | 3608 (86%) | |
| 24 months | 4195 | 4684 (112%) | 4469 (107%) | 4293 (102%) | 3950 (94%) | 3502 (83%) | 3248 (67%) | |
| Cumulative excess CRC cases in 2020-2050 | 6 months | 416 | -8 (-2%) | 49 (12%) | 84 (20%) | 153 (37%) | 277 (67%) | 332 (80%) |
| 12 months | 416 | -23 (-6%) | 63 (15%) | 152 (36%) | 276 (66%) | 465 (112%) | 527 (127%) | |
| 24 months | 416 | 9 (2%) | 109 (26%) | 267 (64%) | 497 (120%) | 862 (207%) | 963 (232%) | |
| Cumulative excess CRC-related deaths in 2020-2050 | 6 months | 339 | 6 (2%) | 37 (11%) | 45 (13%) | 103 (30%) | 144 (43%) | 193 (57%) |
| 12 months | 339 | -22 (-6%) | 41 (12%) | 92 (27%) | 168 (50%) | 267 (79%) | 320 (94%) | |
| 24 months | 339 | 11 (3%) | 73 (21%) | 177 (52%) | 293 (86%) | 485 (143%) | 564 (166%) | |
| Length of recovery period | 3-month disruption | |||||||
| No catch-up screeninga | Catch-up screening at regular FIT threshold | Catch-up screening at increased FIT threshold | ||||||
| 20 μg Hb/g | 25 μg Hb/g | 30 μg Hb/g | 40 μg Hb/g | 50 μg Hb/g | 60 μg Hb/g | |||
| Policy1-Bowel | ||||||||
| Positivity rate | 6 months | 8.3% | 8.3% | 7.0% | 6.1% | 4.8% | 3.8% | 3.3% |
| 12 months | 8.2% | 8.3% | 7.0% | 6.1% | 4.9% | 4.0% | 3.4% | |
| 24 months | 8.2% | 8.3% | 7.0% | 6.1% | 4.8% | 3.9% | 3.3% | |
| Monthly colonoscopy demand during recovery period | 6 months | 6220 | 9352 (150%) | 8105 (130%) | 7128 (115%) | 5827 (94%) | 4884 (79%) | 4299 (69%) |
| 12 months | 6262 | 7829 (125%) | 6781 (108%) | 5967 (95%) | 4877 (78%) | 4087 (65%) | 3595 (57%) | |
| 24 months | 6263 | 7063 (113%) | 6072 (97%) | 5301 (85%) | 4266 (68%) | 3516 (56%) | 3047 (49%) | |
| Cumulative excess CRC cases in 2020-2050 | 6 months | 1657 | 0 (0%) | 464 (28%) | 649 (39%) | 956 (58%) | 1260 (76%) | 1361 (82%) |
| 12 months | 1657 | 24 (1%) | 843 (51%) | 1213 (73%) | 1750 (106%) | 2207 (133%) | 2558 (154%) | |
| 24 months | 1657 | 34 (2%) | 1310 (79%) | 1985 (120%) | 2944 (179%) | 3656 (221%) | 4391 (265%) | |
| Cumulative excess CRC-related deaths in 2020-2050 | 6 months | 978 | 0 (0%) | 188 (19%) | 225 (23%) | 379 (39%) | 400 (41%) | 517 (53%) |
| 12 months | 978 | 8 (1%) | 322 (33%) | 406 (42%) | 621 (63%) | 739 (76%) | 945 (97%) | |
| 24 months | 978 | 11 (1%) | 474 (48%) | 632 (65%) | 1004 (103%) | 1278 (131%) | 1620 (166%) | |
| OncoSim | ||||||||
| Positivity rate | 6 months | NA | NA | NA | NA | NA | NA | NA |
| 12 months | NA | NA | NA | NA | NA | NA | NA | |
| 24 months | NA | NA | NA | NA | NA | NA | NA | |
| Monthly colonoscopy demand during recovery period | 6 months | 7929 | 11904 (150%) | 10320 (130%) | 8820 (111%) | 7011 (88%) | 5729 (72%) | 4997 (63%) |
| 12 months | 7995 | 9960 (125%) | 8652 (108%) | 7403 (93%) | 5903 (74%) | 4845 (61%) | 4250 (53%) | |
| 24 months | 7995 | 8277 (104%) | 7205 (91%) | 6169 (74%) | 4927 (60%) | 4054 (50%) | 3563 (45%) | |
| Cumulative excess CRC cases in 2020-2050 | 6 months | NA | NA | NA | NA | NA | NA | NA |
| 12 months | NA | NA | NA | NA | NA | NA | NA | |
| 24 months | NA | NA | NA | NA | NA | NA | NA | |
| Cumulative excess CRC-related deaths in 2020-2050 | 6 months | 436 | 51 (12%) | 183 (42%) | 288 (66%) | 493 (113%) | 560 (128%) | 633 (145%) |
| 12 months | 436 | 70 (16%) | 275 (63%) | 433 (99%) | 762 (175%) | 899 (206%) | 1031 (236%) | |
| 24 monthsb | 436 | 27 (6%) | 347 (80%) | 646 (148%) | 1198 (275%) | 1443 (331%) | 1661 (381%) | |
FIT: faecal immunochemical test; CRC: colorectal cancer; µg Hb/g: microgram haemoglobin per gram faeces; NA: not available.
This is only 1 strategy, but the outcomes differed per recovery period, because the recovery periods include different months: the 6-month recovery period runs from July 2020-December 2020, the 12-month from July 2020-June 2021, and the 24-month from July 2020-June 2022.
OncoSim assumed that the 2-year screening interval was maintained in individuals delayed for screening, rather than returning to the original date of screening after the recovery period, which was assumed by the other models.
Note: For the period 2020-2050 without disruption, a total of 403,076 CRC cases and 141,400 CRC-related deaths are predicted with ASCCA, 441,960 CRC cases and 164,851 CRC-related deaths with MISCAN-Colon, 633,152 CRC cases and 204,377 CRC-related deaths with Policy1-Bowel, and 332,850 CRC deaths with OncoSim.
Catch-up at regular FIT threshold during a recovery period of various lengths
Colonoscopy demand
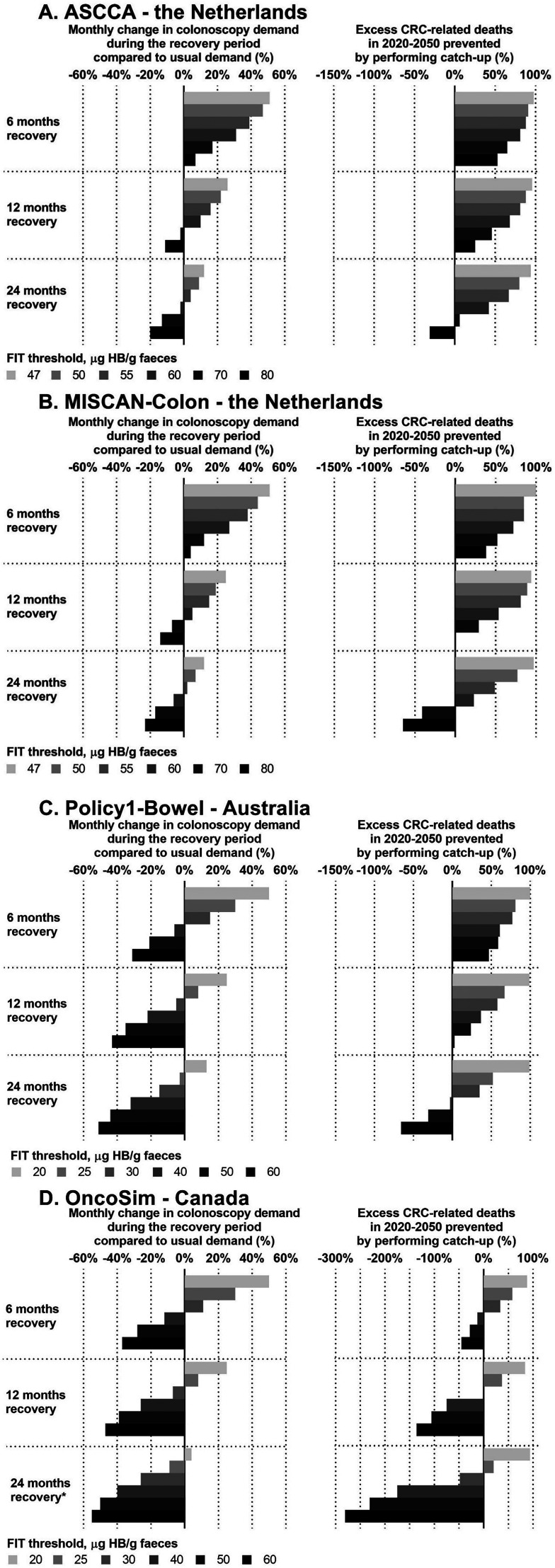
All models predicted a 50% increase in colonoscopy demand using the country-specific regular FIT threshold (Figure 2, Table 2) for a 6-month recovery period compared to the usual colonoscopy demand. Longer recovery periods required smaller increases in colonoscopy demand: 25% in 12 months and 12.5% in 24 months for ASCCA, MISCAN-Colon and Policy1-Bowel, and 4% for OncoSim which used a somewhat different assumption for this strategy (Figure 2).
Figure 2.
The change in colonoscopy demand compared to the usual colonoscopy demand and the cumulative excess CRC-related deaths in 2020-2050 that can be prevented by performing catch-up screening.
CRC: colorectal cancer; FIT: faecal immunochemical test; µg Hb/g: microgram haemoglobin per gram faeces.
* OncoSim assumed that the 2-year screening interval was maintained in individuals delayed for screening, rather than returning to the original date of screening after the recovery period, which was assumed by the other models.
Note 2: A 0% monthly change in colonoscopy demand refers to the colonoscopy demand that would have been expected without any disruption. A 0% excess CRC-related deaths in 2020-2050 prevented refers to the number of excess CRC-related deaths that would have been expected without performing any catch-up, e.g. the impact of a 3-month disruption to CRC screening.
Note 3: The scale of the x-axis in panel D deviates from the scale in panels A-C.
Excess CRC-related deaths
All models predicted that catch-up screening using the regular FIT threshold could avoid almost all excess CRC-related deaths for all lengths of recovery period (Figure 2, Table 2).
Catch-up at increased FIT threshold during a recovery period of various lengths
Colonoscopy demand
Given that catch-up at increased FIT threshold reduces the FIT positivity rate during the recovery period (Table 2), colonoscopy demand was projected to decrease, although the magnitude varied by country. ASCCA and MISCAN-Colon predicted that almost doubling the regular FIT threshold (from 47 to 80 μg Hb/g faeces) for 6 months still exceeded usual colonoscopy demand. In contrast, Policy1-Bowel and OncoSim predicted that doubling the threshold (from 20 to 40 μg Hb/g faeces) for 6 months would be sufficient to stay below the usual colonoscopy demand.
ASCCA and MISCAN-Colon projected that with a prolonged recovery period of 12 months, increasing the FIT threshold from 47 to 70 μg Hb/g faeces resulted in 2% and 7% fewer colonoscopies than usual, respectively. For a 24-month recovery period, the colonoscopy demand was below the usual demand at a threshold of 60 μg Hb/g faeces (decrease of 2% [ASCCA] and 6% [MISCAN-Colon]). Policy1-Bowel predicted 5% fewer colonoscopies than usual for increasing the threshold from 20 to 30 μg Hb/g faeces for 12 months, and 3% fewer colonoscopies at a threshold of 25 μg Hb/g faeces given a 24-month recovery period. OncoSim predicted 7% fewer colonoscopies than usual at a threshold of 30 μg Hb/g faeces for 12 months and 9% fewer colonoscopies at a threshold of 25 μg Hb/g faeces given a 24-month recovery period.
Excess CRC-related deaths
ASCCA, MISCAN-Colon and Policy1-Bowel predicted that performing catch-up screening for 6 months at the highest FIT threshold evaluated could prevent approximately 40%-50% of the excess CRC-related deaths (Figure 2, Table 2). Performing catch-up screening for 24 months at the highest threshold was predicted to lead to more excess CRC-related deaths than the 3-month disruption strategy without catch-up. In contrast, OncoSim projected that doubling the FIT threshold would lead to additional excess CRC-related deaths for any of the considered recovery periods.
Trade-off between colonoscopy demand and excess CRC-related deaths
When the usual colonoscopy demand cannot be exceeded, all models predicted that 30%-60% of excess CRC-related deaths due to the disruption could still be prevented by providing catch-up screening at an increased FIT threshold over an extended recovery period (12 or 24 months) (Appendix Figure E1). In addition, all models show that to prevent excess CRC-related deaths it is more efficient to lengthen the recovery period than to increase FIT threshold. Nevertheless, colonoscopy demand only falls below pre-pandemic demand at increased FIT thresholds.
Targeted prioritisation strategies
Both MISCAN-Colon and Policy1-Bowel predicted that it was more efficient to increase the FIT threshold for younger ages than for older ages because, at a similar additional colonoscopy demand, more excess CRC-related deaths were prevented (Appendix Figure E3). ASCCA predicted no difference in efficiency when adjusting the FIT threshold based on age. All three models showed only small differences in efficiency when adjusting the FIT threshold based on sex. Detailed results are given in Appendix E.
Delayed screening strategy
ASSCA predicted that delaying screening by 3 months for all individuals, except first-time invitees, could prevent 91% of the excess CRC-related deaths at 3% additional colonoscopy demand (Appendix Figure E4). MISCAN predicted that this strategy would lead to 7% fewer excess CRC-related deaths at 4% additional colonoscopy, compared with no disruption.
Sensitivity analysis
Under the assumption of a 25% reduced colonoscopy compliance during the recovery period, the excess CRC-related deaths due to the pandemic would increase (Appendix Figure E5). However, performing catch-up at an increased FIT threshold could still prevent a proportion of excess CRC-related deaths without exceeding the usual colonoscopy demand.
Discussion
This study compared two approaches to manage a screening backlog after a 3-month disruption period to minimise excess CRC-related deaths within available colonoscopy capacity by 1) increasing the FIT threshold for referral and 2) considering different recovery periods during which missed individuals are caught up.
The first key finding of this study is that if referral services can operate at substantially higher levels than pre-pandemic colonoscopy capacity, the impact of a disruption in CRC screening can be mitigated by performing catch-up screening at the regular FIT threshold over a recovery period of 6 months. Extending the recovery period to 12 or 24 months, resulting in short screening delays for more individuals, would greatly reduce the required additional colonoscopy demand, while still avoiding most excess CRC-related deaths.
Secondly, all models show that when colonoscopy capacity is under pressure to meet demands, catch-up screening could be performed at an increased FIT threshold over an extended period while some excess CRC-related deaths can still be prevented. As lengthening the recovery period prevents more excess CRC-related than increasing the FIT threshold, longer recovery periods should be considered while limiting the increase in FIT threshold. Large increases in the FIT threshold for an extended recovery period are predicted to increase the excess CRC-related deaths rather than preventing them.
Our third key finding is that delaying screening at the regular threshold for the duration of the disruption for all individuals except first-time invitees does not lead to additional colonoscopy demand and is as effective as catch-up at regular threshold in 24 months. In this strategy, the duration of the catch-up period is essentially equal to the length of the screening age range. A screening programme that provides screening between the ages of 55 and 75 years would thus have cleared the screening backlog in 20 years.
Policy1-Bowel and OncoSim predicted larger effects of increasing the FIT threshold on colonoscopy demand compared to ASCCA and MISCAN-Colon. This is likely because in Canada and Australia a lower threshold for colonoscopy referrals is used (20 μg Hb/g faeces) compared to the Netherlands (47 μg Hb/g faeces) and screening is performed for wider age ranges. Studies evaluating the performance of FIT at different thresholds showed that increases starting from lower thresholds affect the FIT positivity rate more than increases from higher thresholds.11 OncoSim predicted a larger impact of increasing the FIT threshold on excess CRC-related deaths compared with Policy1-Bowel. This difference in outcomes might be driven by the differences in FIT sensitivities modelled in the two settings, as Policy1-Bowel simulated 2-sample FIT and OncoSim simulated 1-sample FIT, both at 20 μg Hb/g faeces. Therefore, sensitivities for adenomas assumed by Policy1-Bowel are higher compared with OncoSim. Adenomas that have been missed due to increasing the FIT threshold are more likely to be found at subsequent rounds in Policy1-Bowel. Therefore, undetected adenomas in one screening round were projected to have smaller consequences in Policy1-Bowel than in OncoSim. This is confirmed by an exploratory analysis performed by OncoSim, in which test characteristics were assumed to be equal to those assumed by Policy1-Bowel. This led to similar results for both models (data not shown).
Overall, the impact of a short, i.e. 3 months, disruption to screening on excess CRC-related deaths was predicted to be relatively limited (0.13%-0.48% increase). Many CRC screening organisations have successfully limited the impact of the pandemic on CRC burden by expeditiously resuming screening services after the disruption.22,23 However, note that the predicted impact still amounts to 339-987 excess CRC-related deaths in each country simulated. As many countries all over the world have faced a disruption to CRC screening programmes1, globally a considerable number of potentially excess CRC-related deaths can be expected if countries lack colonoscopy capacity to overcome the screening backlog.
We have shown that modification of the FIT threshold is one possible approach to mitigate the impact of the disruption given colonoscopy constraints, as it would prioritise individuals at higher CRC risk.10,11 As with any risk-based approach to screening, especially within an established, organised population screening programme, careful consideration of the local context is required. In the Netherlands, the FIT threshold has previously been modified within the national programme25 and this could thus be considered a relatively easy option to ensure the continuity of CRC screening in the case of colonoscopy constraints. However, programmes in other countries may not be in a position to implement changes of this nature. Although beyond the scope of the current analysis, other possible ways to handle a backlog in screening, such as temporarily extending screening intervals, and novel approaches to risk-based screening should be critically explored. When considering a strategy to recover from a disruption in CRC screening, it may also be worthwhile to re-evaluate colonoscopies performed for other indications than positive FIT. In some countries, colonoscopy demand may be largely driven by surveillance colonoscopies.26 Literature suggests that the CRC risk was low for a large proportion of individuals who underwent polypectomy27 and, compared to FIT screening only, the additional health benefit of surveillance is predicted to be limited.28 Thus, more efficient use of colonoscopy resources may be possible by modifying surveillance programmes.
Our prediction that not providing any form of catch-up screening results in excess CRC-related deaths is supported by studies evaluating the impact of delay in colonoscopy for individuals with a positive FIT.29,30 Although the latter is not the same as a delay in screening, both are expected to increase CRC risk. These studies showed significant increases in CRC risk when colonoscopy was delayed by over 10 months. Our study predicted that providing catch-up screening, i.e. a maximum delay of 3 months, could prevent almost all excess CRC-related deaths. This is supported by data from Corley et al. and Lee et al. showing no significant increases in CRC risk for delays of less than 6 months.29,30 The real-world impact of the COVID-19 pandemic on excess CRC-related deaths may be larger than predicted in this study because longer disruption periods have been observed than our simulated 3-month disruption, and some screening programmes did not restart immediately at full capacity after the disruption.22
Previous studies have shown that CRC risk is dependent on sex and age.31 In addition, studies observed an increasing FIT positivity rate with age and higher positivity rates for men compared with women.32,33 In our study, both Policy1-Bowel and MISCAN predicted that increasing the FIT threshold for younger ages was more efficient than for older ages. Data from the Danish CRC screening programme support this finding (personal communication: S.H. Njor). However, this difference in efficiency was not seen in ASCCA. We hypothesize this is due to differences between the models in the time it takes to develop CRC and the assumption of age-independent screening test characteristics in ASCCA. All models showed only small differences in the efficiency of adjusting the FIT threshold for men vs women. This reflects earlier work that demonstrated a limited benefit of tailored screening for men and women.34
This study has some limitations. ASCCA and Policy1-Bowel use annual transition cycles and can thereby not directly simulate an average delay of 1.5 months. Therefore, a simulation assuming a 1-year delay and a simulation assuming no delay were weighted to reproduce an on-average 1.5-month delay. However, studies show that the relation between the length of delay and impact on CRC burden is not linear.29,30 Thereby, the impact of the delay on CRC burden may be slightly overestimated by ASCCA and Policy1-Bowel. Moreover, the COVID-19 pandemic has also restrained referrals from primary into secondary care of patients with symptoms suggestive of cancer and may also have increased the probability of death due to other causes than CRC, but this is outside the scope of the current analysis. Including this impact of the pandemic would result in more excess CRC-related deaths.35
To our knowledge, this is one of the first modelling studies exploring the impact of a short-term increase in the FIT threshold to address constraints in the health system. As the results of the four models are in line with each other, our findings could be used for FIT-based CRC screening programmes in other high-income countries. Based on the range of simulated FIT thresholds, policy makers can decide on appropriate FIT thresholds to balance colonoscopy capacity and health outcomes for their local setting. This would also depend on the local screening demand, and the extent to which it has been disrupted by the COVID-19 pandemic. Although all strategies only focus on the first COVID-19 wave, the results can be used as an illustrative example to guide decision-makers in how to manage colonoscopy resources to limit the excess CRC-related deaths due to potential subsequent waves. This remains of importance, because the COVID-19 crisis persists as new virus variants emerge and vaccines are not equally available globally. The study findings are also informative for other screening programme disruptions, such as bushfires in Australia in 2019-2020, an unanticipated interruption to test supply, or reductions in financial budgets of screening programmes. In addition, some countries have already faced challenges handling colonoscopy demand before COVID-19.36
Conclusion
In conclusion, the worldwide COVID-19 pandemic continues and therefore the health service pressure remains, causing constraints in colonoscopy capacity. If there is excessive pressure on colonoscopy capacity, catch-up screening can be performed at an increased FIT threshold over 24 months, preventing at least a proportion of excess CRC-related deaths. By adjusting FIT thresholds, screening programs can balance colonoscopy demand and long-term health outcomes when providing catch-up screening after a disruption. If a limited increase in colonoscopy services is feasible, catch-up screening can be performed at the regular FIT threshold over 24 months. This, would prevent the majority of excess CRC-related deaths. Other approaches to manage colonoscopy capacity, such as a redistribution of the capacity between surveillance and screening indications, deserve attention in future studies.
Supplementary Material
Acknowledgements
The authors thank the other members of the COVID-19 and Cancer Global Modelling Consortium (CCGMC) working group 2 (see Collaborators).
Collaborators
L. Anderson, M. Besó Delgado, G. Binefa, A.E. Cust, E. Dekker, V.A. Dell’Anna, B. Essue, J.A. Espinas, L. Flander, M. Garcia, A. Hahn, I. Idigoras, K. Katanoda, L. Laghi, F. Lamrock, E. McFerran, O. Majek, A. Molina-Barceló, M. Ledger, O.A. Musa, S. Njor, K. O’Connor, I. Portillo, D. Salas, C. Senore, H. Smith, E.L. Symonds, I. Tachecí, G. Taksler, M.A. Tolani, M. Treby, A. Zauber, Y. Zheng.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Cancer Council New South Wales, Health Canada, and Dutch National Institute for Public Health and Environment.
Competing Interests: KC is coprincipal investigator of an unrelated investigator-initiated trial of cervical screening in Australia (Compass; ACTRN12613001207707 and NCT02328872), which is done and funded by the VCS Foundation, a government-funded health promotion charity. The VCS Foundation received equipment and a funding contribution from Roche Molecular Systems USA. However, neither KC nor her institution on her behalf (Cancer Council NSW) receives direct funding from industry for this trial or any other project. All other authors declare no competing interests.
Institutional Review Board: Not seeking Institutional Review Board review was in accordance with the policy of our institutions.
Authors’ contributions: FvW: conceptualization, methodology, analysis, writing draft
LdJ: conceptualization, methodology, analysis, writing draft
JW: conceptualization, methodology, analysis, reviewing draft
MG: conceptualization, methodology, reviewing draft
JL: conceptualization, methodology, analysis reviewing draft
CN: methodology, analysis, reviewing draft
RvdP: methodology, reviewing draft
EF: conceptualization, methodology, reviewing draft
JY: conceptualization, methodology, analysis, reviewing draft
I.L-V: conceptualization, methodology, reviewing draft
KC: conceptualization, methodology, reviewing draft
VC: conceptualization, supervision, methodology, reviewing draft
All members of the COVID-19 and Cancer Global Modelling Consortium: reviewing draft
All authors had full access to the data in the study and accept responsibility to submit for publication.
ORCID iDs: Francine van Wifferen https://orcid.org/0000-0002-6891-6678
Marjolein J.E. Greuter https://orcid.org/0000-0002-6151-3382
References
- 1.Dekker E, Chiu H-M, Lansdorp-Vogelaar I, et al. Colorectal Cancer Screening in the Novel Coronavirus Disease-2019 Era. Gastroenterology 2020; 159: 1998–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: A global overview of existing programmes. Gut 2015; 64: 1637–1649. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Australia. Review of the impact of COVID-19 on medical services and procedures in Australia utilising MBS data: Skin, breast and colorectal cancers, and telehealth services. Surry Hills, NSW, September 2020. [Google Scholar]
- 4.Meester RGS, Zauber AG, Doubeni CA, et al. Consequences of Increasing Time to Colonoscopy Examination After Positive Result From Fecal Colorectal Cancer Screening Test. Clinical Gastroenterology and Hepatology 2016; 14: 1445–1451.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutter CM, Kim JJ, Meester RGS, et al. Effect of time to diagnostic testing for breast, cervical, and colorectal cancer screening abnormalities on screening efficacy: A modeling study. Cancer Epidemiology Biomarkers and Prevention 2018; 27: 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizuno R, Ganeko R, Takeuchi G, et al. The number of obstructive colorectal cancers in Japan has increased during the COVID-19 pandemic: A retrospective single-center cohort study. Annals of Medicine and Surgery 2020; 60: 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degeling K, Baxter N, Emery J, et al. An inverse stage-shift model to estimate the excess mortality and health economic impact of delayed access to cancer services due to the COVID-19 pandemic. medRxiv 2020; 30: 20117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. The Lancet Oncology 2020; 21: 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jonge L, Worthington J, van Wifferen F, et al. Impact of the COVID-19 pandemic on faecal immunochemical test-based colorectal cancer screening programmes in Australia, Canada, and the Netherlands: a comparative modelling study. The Lancet Gastroenterology & Hepatology 2021; 6: 304–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young GP, Woodman RJ, Symonds E. Detection of advanced colorectal neoplasia and relative colonoscopy workloads using quantitative faecal immunochemical tests: an observational study exploring the effects of simultaneous adjustment of both sample number and test positivity threshold. BMJ Open Gastro 2020; 7: 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selby K, Levine EH, Doan C, et al. Effect of Sex, Age and Positivity Threshold on Fecal Immunochemical Test Accuracy: a Systematic Review and Meta-Analysis. Gastroenterology 2019; 157: 1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loeve F, Boer R, van Oortmarssen GJ, et al. The MISCAN-COLON simulation model for the evaluation of colorectal cancer screening. Computers and Biomedical Research 1999; 32: 13–33. [DOI] [PubMed] [Google Scholar]
- 13.Greuter MJE, Xu XM, Lew Jb, et al. Modeling the adenoma and serrated pathway to colorectal cancer (ASCCA). Risk Analysis 2014; 34: 889–910. [DOI] [PubMed] [Google Scholar]
- 14.Lew Jb, St John DJB, Xu XM, et al. Long-term evaluation of benefits, harms, and cost-effectiveness of the National Bowel Cancer Screening Program in Australia: a modelling study. The Lancet Public Health 2017; 2: e331–e340. [DOI] [PubMed] [Google Scholar]
- 15.Lew Jb, Greuter MJE, Caruana M, et al. Validation of Microsimulation Models against Alternative Model Predictions and Long-Term Colorectal Cancer Incidence and Mortality Outcomes of Randomized Controlled Trials. Medical Decision Making 2020; 40: 815–829. [DOI] [PubMed] [Google Scholar]
- 16.Coldman A, Pader J, Gauvreau C, et al. Simulating results from trials of sigmoidoscopy screening using the OncoSim microsimulation model. Journal of Cancer Policy 2018; 15: 52–58. [Google Scholar]
- 17.World Bank Open Data. The World Bank, https://data.worldbank.org/ (accessed January 12, 2021).
- 18.National Institute for Public Health and the Environment (RIVM), Netherlands Comprehensive Cancer Organisation (IKNL). Monitor bevolkingsonderzoek darmkanker 2018. 2019.
- 19.Australian Institute of Health and Welfare. National Bowel Cancer Screening Program: monitoring report 2019. Cancer series no. 125. Cat. no. CAN 125. Canberra, 2019.
- 20.Statistics Canada. Cancer Screening, 2017, https://www150.statcan.gc.ca/n1/pub/82-625-x/2018001/article/54977-eng.htm (accessed March 1, 2021).
- 21.World Health Organization: International Agency for Research on Cancer. GLOBOCAN 2020., https://gco.iarc.fr/ (accessed January 7, 2021).
- 22.Walker MJ, Meggetto O, Gao J, et al. Measuring the impact of the COVID-19 pandemic on organized cancer screening and diagnostic follow-up care in Ontario, Canada: A provincial, population-based study. Preventive Medicine 2021; 151: 106586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kortlever TL, Jonge Ld, Wisse PHA, et al. The national FIT-based colorectal cancer screening program in the Netherlands during the COVID-19 pandemic. Preventive Medicine 2021; 151: 106643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Klerk CM, Wieten E, Lansdorp-Vogelaar I, et al. Performance of two faecal immunochemical tests for the detection of advanced neoplasia at different positivity thresholds: a cross-sectional study of the Dutch national colorectal cancer screening programme. The Lancet Gastroenterology and Hepatology 2019; 4: 111–118. [DOI] [PubMed] [Google Scholar]
- 25.Toes-Zoutendijk E, van Leerdam ME, Dekker E, et al. Real-Time Monitoring of Results During First Year of Dutch Colorectal Cancer Screening Program and Optimization by Altering Fecal Immunochemical Test Cut-Off Levels. Gastroenterology 2017; 152: 767–775.e2. [DOI] [PubMed] [Google Scholar]
- 26.de Neree tot Babberich MPM, Ledeboer M, van Leerdam ME, et al. Dutch Gastrointestinal Endoscopy Audit: automated extraction of colonoscopy data for quality assessment and improvement. Gastrointestinal Endoscopy 2020; 92: 154–162.e1. [DOI] [PubMed] [Google Scholar]
- 27.Click B, Pinsky PF, Hickey T, et al. Association of colonoscopy adenoma findings with long-term colorectal cancer incidence. JAMA - Journal of the American Medical Association 2018; 319: 2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greuter MJE, de Klerk CM, Meijer GA, et al. Screening for colorectal cancer with fecal immunochemical testing with and without postpolypectomy surveillance colonoscopy: A cost-effectiveness analysis. Annals of Internal Medicine 2017; 167: 544–554. [DOI] [PubMed] [Google Scholar]
- 29.Corley DA, Jensen CD, Quinn VP, et al. Association Between Time to Colonoscopy After a Positive Fecal Test and Risk of Colorectal Cancer Stage at Diagnosis HHS Public Access. JAMA 2017; 317: 1631–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee YC, Fann JCY, Chiang TH, et al. Time to Colonoscopy and Risk of Colorectal Cancer in Patients With Positive Results From Fecal Immunochemical Tests. Clinical Gastroenterology and Hepatology 2019; 17: 1332–1340. e3. [DOI] [PubMed] [Google Scholar]
- 31.White A, Ironmonger L, Steele RJC, et al. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer 2018; 18: 906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arana-Arri E, Idigoras I, Uranga B, et al. Population-based colorectal cancer screening programmes using a faecal immunochemical test: Should faecal haemoglobin cut-offs differ by age and sex? BMC Cancer Epub ahead of printAugust 29, 2017; 17. DOI: 10.1186/s12885-017-3555-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blom J, Löwbeer C, Elfström KM, et al. Gender-specific cut-offs in colorectal cancer screening with FIT: Increased compliance and equal positivity rate. Journal of Medical Screening 2018; 26: 92–97. [DOI] [PubMed] [Google Scholar]
- 34.van der Meulen MP, Kapidzic A, van Leerdam ME, et al. Do Men and Women Need to Be Screened Differently with Fecal Immunochemical Testing? A Cost-Effectiveness Analysis. Cancer Epidemiol Biomarkers Prev 2017; 26: 1328–1336. [DOI] [PubMed] [Google Scholar]
- 35.Loveday C, Sud A, Jones ME, et al. Prioritisation by FIT to mitigate the impact of delays in the 2-week wait colorectal cancer referral pathway during the COVID-19 pandemic: a UK modelling study. Gut 2020; 0: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.A Colonoscopy Wait-time and Performance Guarantee - Early Detection - Bowel Cancer Australia, https://www.bowelcanceraustralia.org/a-colonoscopy-wait-time-and-performance-guarantee (accessed February 22, 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.