Abstract
Aims
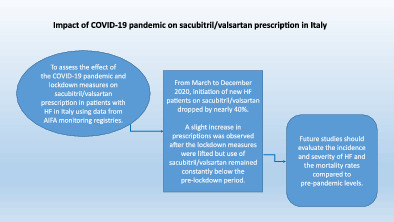
The present study sought to examine the effect of the COVID‐19 pandemic and lockdown measures on the prescription of sacubitril/valsartan in patients with heart failure (HF) in Italy.
Methods and results
Data from Italian Medicines Agency (AIFA) monitoring registries were analysed. The sacubitril/valsartan monitoring registry is based on 6‐month prescriptions. A monthly aggregation on new activations throughout the observational period was computed. From March to December 2020, the initiation of new HF patients on sacubitril/valsartan decreased by nearly 40% with prescriptions dropping to values similar to 2018 when the registry was still operated off‐line. A slight increase in prescriptions was observed after the lockdown measures were lifted, but prescriptions remained constantly below the pre‐lockdown period.
Conclusion
A marked and worrisome decline during the COVID‐19 pandemic in the activation of a life‐saving treatment such as sacubitril/valsartan was observed. This decline was clearly linked to the lockdown measures instated to counteract the COVID‐19 pandemic. Upcoming studies should analyse the occurrence of new cases of HF as well as the severity of patients admitted to hospitals and their mortality compared to pre‐pandemic levels.
Keywords: COVID‐19, Heart failure, Sacubitril/valsartan, Registry
Impact of the COVID‐19 pandemic on sacubitril/valsartan prescription in Italy. AIFA, Italian Medicines Agency; HF, heart failure.
Introduction
Despite the significant death toll of the coronavirus disease 2019 (COVID‐19) pandemic, cardiovascular diseases claim each year more deaths than COVID‐19. 1 The outbreak of COVID‐19 and containment measures and lockdown have caused social and physical isolation, psychological distress and the adoption of unhealthy lifestyle behaviours, but they have also limited access to care and decreased medication adherence. 2
Patients with cardiovascular diseases have been particularly affected by the limitations imposed by the pandemic. A significant decrease in the number of patients with cardiovascular diseases seeking medical attention for acute coronary syndromes and a parallel increase in out‐of‐hospital sudden deaths has been demonstrated during lockdown in many countries. 3 , 4 Limitations in access to care have also impacted the management of chronic non‐communicable diseases having negatively influenced lifestyle behaviours, diet, and medication adherence, 5 which are the foundations of medical management.
In Italy, several medications for chronic conditions have been restricted to hospital only dispensation or prescription with regions having the power to select centres that may prescribe life‐saving medications like proprotein convertase subtilisin/kexin type 9 inhibitors, sodium–glucose cotransporter 2 inhibitors, glucagon‐like petide‐1 receptor agonists and sacubitril/valsartan. 6
The present study sought to examine the effect of the COVID‐19 pandemic and lockdown measures on the prescription of sacubitril/valsartan in patients with heart failure in Italy.
Methods
The analysis was conducted using data from the Italian Medicines Agency (AIFA) monitoring registries. The specifics of AIFA monitoring registries, including their position within the Italian regulatory framework, have been discussed previously. 7 , 8 The inclusion in a monitoring registry is mandatory to obtain the reimbursement from the Italian National Healthcare System (NHS) for the prescription of sacubitril/valsartan to treat heart failure in Italy. Therefore, data presented in this work represent a census of patients treated with sacubitril/valsartan during the observational period.
The inclusion in the registry involves the collection of patient baseline features, like sex and age, and the monitoring of drug prescriptions in time. The sacubitril/valsartan monitoring registry is based on 6‐month prescriptions, which implies that patients are generally observed twice per year.
The sacubitril/valsartan registry has been operated off‐line from 12 March 2017 to 19 February 2019. When the registry has been switched to the on‐line platform, clinicians could decide whether upgrading ongoing treatments (maintaining the original information on patients), or starting new ones, entirely on‐line, for patients who were already treated with the drugs – in the latter case, no on‐line information is available for patients prior to the activation of the new treatment after 19 February 2019; the activation of new treatments for already in‐treatment patients is generally associated with the first prescription following this date. As a consequence, the total number of activations might be underestimated up to February 2019 and overestimated for the six following months. Starting from September 2019, new activations are considered to be unbiased, as perturbing factors are no longer present.
In order to verify the impact of the COVID‐19 lockdown period on sacubitril/valsartan treatment starts, a monthly aggregation on new activations throughout the observational period has been computed.
The measure unit is the number of new treatments, i.e. new treated patients. This study is focused only on new activations, for the following reasons: first, it is aimed at the identification and measurement of possible under‐detection of severe heart conditions during the lockdown period; second, repeated prescriptions are less likely to have been severely impacted by social and physical distancing, as a quick and automated procedure has been implemented in Italy from March 2020. Data have been analysed using a two‐step procedure.
In the first step, a linear model to account for the aforementioned registry features has been estimated on the data up to February 2020 (i.e. the last month before the COVID‐19 lockdown period, that started in Italy on 10 March 2020). Three exogenous variables have been incorporated in the model: one identifying time to account for linear increase of new treatment activations, one identifying the period of on‐line monitoring (i.e. equal to 0 up to January 2019, equal to 1 from March 2019 and equal to 0.3 in February 2019, to account for the fact that the on‐line registry was available in the last 10 days of the month), and one identifying the 6 months of extra‐activations due to the conversion of off‐line treatments.
In the second step, after rejecting the augmented Dickey–Fuller test's null of unit‐root against an alternative of stationarity (p < 0.01) on the series obtained after eliminating the effects emerged in the first step, a seasonal ARIMA has been estimated and used to compute forecasts for the following 10 months. These forecasts have then been adjusted using the first‐step coefficients; the resulting values have been compared with the observed new activations in the same period, as recorded in the registry, to evaluate the effects of the COVID‐19 pandemic‐related reduced access to cure on the treatment of cardiac diseases.
The study complies with the Declaration of Helsinki.
Results
A total of 36 682 treatments with sacubitril/valsartan have been activated in the monitoring registry between March 2017 and February 2020. Males were the large majority (77.1%); median age (Q1–Q3) at treatment start was 71 (62–78) years. Median (Q1–Q3) ventricular ejection fraction was 30% (29–35) and 48.4% of patients had an implantable cardioverter defibrillator. All patients were previously treated with at least two of the following medications: angiotensin‐converting enzyme inhibitors, mineralocorticoid receptor antagonists, beta‐blockers, angiotensin II receptor blockers, diuretics.
The first‐step linear model shows that all exogenous considered factors have a significant impact on the observed values: trends show that new prescriptions between March 2017 and February 2020 increased on average by 32.6 each month; after the switch to the on‐line platform, the number of monthly activations increased steadily by an average of 876.9; this switch also caused an effect of 418.1 extra activations per month in the first 6 months.
The observed series, along with the two‐step model fit, is reported in Figures 1 and 2 ; a strong, and partly unpredictable, decline in the month of August 2019 was compensated by unusually high values in the previous 2 months: this suggests that extra‐activations of already treated patients scheduled for August (a month typically associated with a seasonal drop in the registry activity) might have been carried out in anticipation, during June and July. Figure 3 and Table 1 report the comparisons between the forecasted values and the observed activations between March and December 2020. Both point and 95% interval forecasts are presented.
Figure 1.
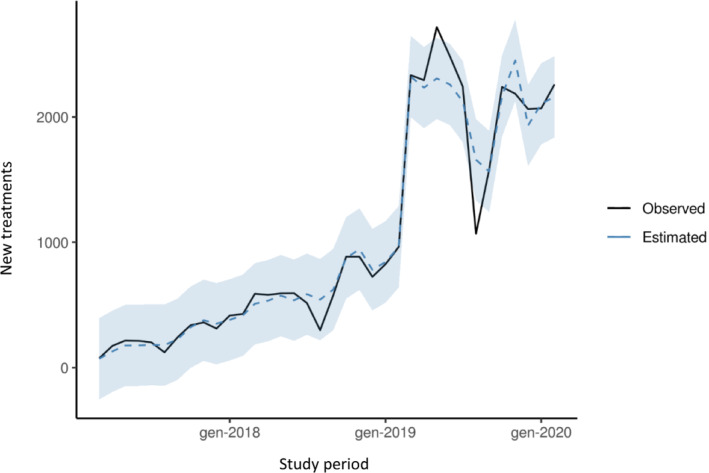
Observed series and model fitted values of new prescriptions of sacubitril/valsartan between March 2017 and February 2020.
Figure 2.
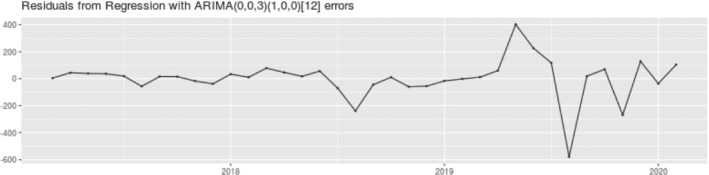
Analysis of residual distribution of the S‐ARIMA model; S‐ARIMA was estimated using the following parameters: (p = 0, I = 0, q = 3) for the main model and (p = 1, I = 0, q = 0) for the seasonal component, where p indicates the order of the AR component, q indicates the order of the MA component, and I indicates the order of integration.
Figure 3.
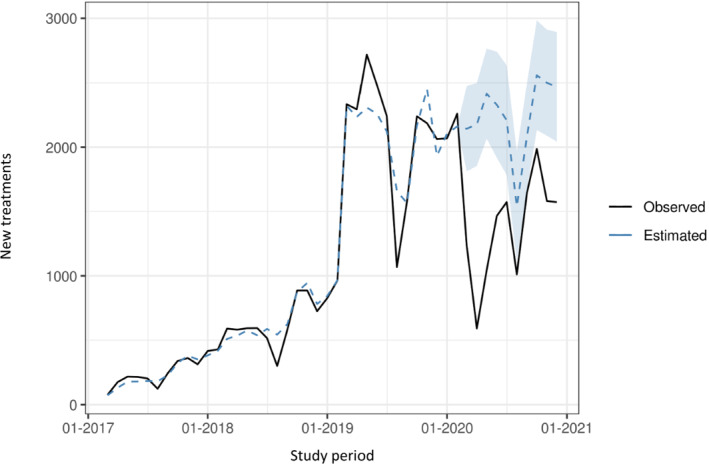
Comparison between the observed and forecasted sacubitril/valsartan new prescriptions between March and December 2020.
Table 1.
Observed and forecasted sacubitril/valsartan new prescriptions between March and December 2020; percentage differences are reported with 95% confidence intervals (CI)
| Month | Observed | Forecasts | Comparison observed/forecasted | ||||
|---|---|---|---|---|---|---|---|
| Lower CI | Forecasted | Upper CI | Lower CI | % Difference | Upper CI | ||
| 03/20 | 1235 | 1777 | 2143 | 2441 | −30.5% | −42.4% | −49.4% |
| 04/20 | 591 | 1891 | 2177 | 2535 | −68.7% | −72.9% | −76.7% |
| 05/20 | 1044 | 2028 | 2415 | 2725 | −48.5% | −56.8% | −61.7% |
| 06/20 | 1466 | 1957 | 2329 | 2779 | −25.1% | −37.1% | −47.3% |
| 07/20 | 1573 | 1749 | 2209 | 2599 | −10.0% | −28.8% | −39.5% |
| 08/20 | 1011 | 1089 | 1539 | 1939 | −7.2% | −34.3% | −47.9% |
| 09/20 | 1646 | 1709 | 2085 | 2531 | −3.7% | −21.1% | −35.0% |
| 10/20 | 1986 | 2093 | 2559 | 2942 | −5.1% | −22.4% | −32.5% |
| 11/20 | 1581 | 2130 | 2500 | 2953 | −25.8% | −36.8% | −46.5% |
| 12/20 | 1573 | 2004 | 2468 | 2854 | −21.5% | −36.3% | −44.9% |
| Total | 13 706 | 18 426 | 22 425 | 26 297 | −25.6% | −38.9% | −47.9% |
The effect of lockdown measures became immediately evident in March 2020 and turned critical in April, nearly 1 month after the first lockdown measures imposed throughout Italy. In this month, the new prescriptions of sacubitril/valsartan fell by nearly 75% with 591 prescriptions compared to 2177 forecasted (1891–2535 according to the 95% interval prediction). A slight increase in prescriptions was observed after the lockdown measures were lifted but the use remained constantly below the pre‐lockdown period; noteworthy, all observed values fall below the interval prediction.
In the period between March and December 2020, the initiation of new heart failure patients on sacubitril/valsartan fell by nearly 40% with prescriptions dropping to values similar to 2018, when the registry was still operated off‐line.
Discussion
Patients with heart failure, especially those with reduced ejection fraction, have a poor prognosis and life expectancy 9 and new drug classes like sacubitril/valsartan and sodium–glucose cotransporter 2 inhibitors have demonstrated a significant mortality and morbidity benefit in these patients. 10 The present data, covering the Italian‐wide population of heart failure patients eligible for sacubitril/valsartan treatment (Graphical Abstract), show a marked and worrisome decline during the COVID‐19 pandemic in the activation of such a life‐saving treatment. This decline was clearly linked to the lockdown measures instated to counteract the COVID‐19 pandemic but the lack of increase in the new activations to the pre‐pandemic levels could be multifactorial. 6 Italian hospitals, severely hit by the pandemic, had to reduce the access for non‐emergency procedures for several months after the end of the lockdown. Furthermore, even after the outpatient services were re‐opened, the length of the waiting lists limited patient access. Also, patients' fear of contagion reduced the seek for medical attention, even for severe conditions like acute coronary syndromes. 3
Thus, the Italian lockdown had a significant impact on patient care and access to disease‐modifying drugs. 6 This effect has been, in part, due to the lockdown restrictions and the critical issues in the organization of home drug delivery. Furthermore, sacubitril/valsartan new activations are managed by hospital physicians, working in specialized centres, identified by Italian regions. As a consequence, movement restrictions and limited access to ambulatory care significantly impacted the prescription of this drug. 5
The observed decline in prescriptions of sacubitril/valsartan will likely result in an increase in heart failure‐related mortality and hospitalizations with a significant increase in the corresponding costs. Out‐of‐hospital mortality has increased during the COVID‐19 pandemic and lockdowns, with most of this mortality being cardiovascular. 6 , 11 , 12 , 13 It will be, therefore, useful in the future to analyse the occurrence of new cases of heart failure, the severity of heart failure patients admitted to hospitals and their mortality compared to pre‐pandemic levels.
Our data also show that, despite a bounce back in the prescription of sacubitril/valsartan after the first lockdown, the current level of new prescriptions of this medication, as of September 2020, was still significantly below that forecasted on the basis of the pre‐pandemic observed values. A possible explanation of this lack of increase in new prescriptions may be related to the elevated death toll of elderly and frail patients during the COVID‐19 pandemic, a relevant number of which might have had heart failure and might have been eligible for sacubitril/valsartan treatment. However, it is impossible to verify this hypothesis since, in Italy, there are no uniform national databases that link events, hospital admissions and census.
It is possible that the late presentation of acute coronary syndrome cases together with the delay in seeking attention for heart failure and/or comorbidities aggravating or favouring heart failure will lead to an increase in more severe forms of heart failure with a consequent increase, in the medium term, in the prescription of sacubitril/valsartan.
Furthermore, PARADIGM‐HF has demonstrated that the prognostic effect of sacubitril/valsartan starts early after treatment, 14 therefore, the delay in its initiation may carry significant consequences on survival, hospitalizations and need for device therapy in the months to come. It will be, therefore, important to analyse in the future the type of hospitalisations for heart failure and the severity of new patients presenting with de novo heart failure.
The lack of a return to pre‐pandemic prescription levels of sacubitril/valsartan may be manifold and, together with the difficulties in access to care and to hospital prescription of this drug, it is likely that the high mortality of comorbid patients, most of which have been labelled as COVID deaths, has led to a premature death of many patients with heart failure, thereby reducing the population of patients that were eligible for sacubitril/valsartan treatment.
Finally, there is consistent evidence suggesting that sacubitril/valsartan titration is low worldwide. However, this does not relate only to angiotensin receptor–neprilysin inhibitors but to the majority of cardiovascular and non‐cardiovascular medicines. European real‐world data show that only 35% of heart failure patients are titrated to the highest dose of sacubitril/valsartan. 15 It is reassuring that, in PARADIGM‐HF, patients requiring dose reduction still derived benefit of sacubitril/valsartan over enalapril at lower than target dosing. 16 On the contrary, as shown by the TITRATION study, 17 up‐titration is necessary in patients with low systolic blood pressure or taking low‐dose angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers.
In conclusion, the COVID‐19 pandemic and lockdown measures have had an important and significant impact on the prescriptions of sacubitril/valsartan in Italy. Future studies will clarify whether this lack of prescriptions or the delayed prescriptions have claimed an important toll amongst patients with heart failure.
Acknowledgements
The views expressed in this work are personal and may not be understood or quoted as being made on behalf of or reflecting the position of the Italian Medicines Agency (AIFA) or of one of their committees or working parties.
Although the AIFA monitoring registries operate within a regulatory framework that provides for the mandatory collection by all public health facilities of data from real clinical practice on specific treatments reimbursed by the Italian National Healthcare System (NHS), the authors are nonetheless grateful for the contribution of the physicians who filled the registry of sacubitril/valsartan. The present work originated from the collaboration between AIFA, the Italian National Institute of Health (ISS), the Italian Association of Hospital Cardiologists (ANMCO) and representatives of the Italian clinical cardiology for the scientific development of the data extracted from AIFA monitoring registries.
Conflict of interest: none declared.
References
- 1. Sokolski M, Trenson S, Sokolska JM, D'Amario D, Meyer P, Poku NK, et al. Heart failure in COVID‐19: the multicentre, multinational PCHF‐COVICAV registry. ESC Heart Fail. 2021;8:4955–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wosik J, Fudim M, Cameron B, Gellad ZF, Cho A, Phinney D, et al. Telehealth transformation: COVID‐19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27:957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Onder G, Olimpieri PP, Celant S, Di Lenarda A, Ambrosio G, Reboldi G, et al.; AIFA Monitoring Registries Group . Under‐prescription of direct oral anticoagulants for treatment of non‐valvular atrial fibrillation and venous thromboembolism in the COVID‐19 lockdown period. Eur J Prev Cardiol. 2021. 10.1093/eurjpc/zwab09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sofi F, Dinu M, Reboldi G, Stracci F, Pedretti RFE, Valente S, et al. Worldwide differences of hospitalization for ST‐segment elevation myocardial infarction during COVID‐19: a systematic review and meta‐analysis. Int J Cardiol. 2022;347:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Degli Esposti L, Buda S, Nappi C, Paoli D, Perrone V; Network Health‐DB . Implications of COVID‐19 infection on medication adherence with chronic therapies in Italy: a proposed observational investigation by the Fail‐to‐Refill Project. Risk Manag Healthc Policy. 2020;13:3179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colivicchi F, Di Fusco SA, Magnanti M, Cipriani M, Imperoli G. The impact of the coronavirus disease‐2019 pandemic and italian lockdown measures on clinical presentation and management of acute heart failure. J Card Fail. 2020;26:464–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xoxi E, Facey KM, Cicchetti A. The evolution of AIFA registries to support managed entry agreements for orphan medicinal products in Italy. Front Pharmacol. 2021;12:699466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olimpieri PP, Di Lenarda A, Mammarella F, Gozzo L, Cirilli A, Cuomo M, et al. Non‐vitamin K antagonist oral anticoagulation agents in patients with atrial fibrillation: insights from Italian monitoring registries. Int J Cardiol Heart Vasc. 2020;26:100465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018;6:678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aimo A, Pateras K, Stamatelopoulos K, Bayes‐Genis A, Lombardi CM, Passino C, et al. Relative efficacy of sacubitril‐valsartan, vericiguat, and SGLT2 inhibitors in heart failure with reduced ejection fraction: a systematic review and network meta‐analysis. Cardiovasc Drugs Ther. 2021;35:1067–76. [DOI] [PubMed] [Google Scholar]
- 11. Chan PS, Girotra S, Tang Y, Al‐Araji R, Nallamothu BK, McNally B. Outcomes for out‐of‐hospital cardiac arrest in the United States during the coronavirus disease 2019 pandemic. JAMA Cardiol. 2021;6:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nef HM, Elsässer A, Möllmann H, Abdel‐Hadi M, Bauer T, Brück M, et al.; CoVCAD Study Group . Impact of the COVID‐19 pandemic on cardiovascular mortality and catherization activity during the lockdown in Central Germany: an observational study. Clin Res Cardiol. 2021;110:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rashid Hons M, Gale Hons CP, Curzen Hons N, Ludman Hons P, De Belder Hons M, Timmis Hons A, et al. Impact of coronavirus disease 2019 pandemic on the incidence and management of out‐of‐hospital cardiac arrest in patients presenting with acute myocardial infarction in England. J Am Heart Assoc. 2020;9:e018379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yeoh SE, Dewan P, Desai AS, Solomon SD, Rouleau JL, Lefkowitz M, et al. Relationship between duration of heart failure, patient characteristics, outcomes, and effect of therapy in PARADIGM‐HF. ESC Heart Fail. 2020;7:3355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giovinazzo S, Carmisciano L, Toma M, Benenati S, Tomasoni D, Sormani MP, et al. Sacubitril/valsartan in real‐life European patients with heart failure and reduced ejection fraction: a systematic review and meta‐analysis. ESC Heart Fail. 2021;8:3547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vardeny O, Claggett B, Packer M, Zile MR, Rouleau J, Swedberg K, et al.; Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF) Investigators . Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM‐HF trial. Eur J Heart Fail. 2016;18:1228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Senni M, McMurray JJV, Wachter R, McIntyre HF, Anand IS, Duino V, et al. Impact of systolic blood pressure on the safety and tolerability of initiating and up‐titrating sacubitril/valsartan in patients with heart failure and reduced ejection fraction: insights from the TITRATION study. Eur J Heart Fail. 2018;20:491–500. [DOI] [PubMed] [Google Scholar]


