Abstract
COVID‐19 vaccines prevent severe forms of the disease, but do not warrant complete protection against breakthrough infections. This could be due to suboptimal mucosal immunity at the site of virus entry, given that all currently approved vaccines are administered via the intramuscular route. In this study, we assessed humoral and cellular immune responses in BALB/c mice after intranasal and intramuscular immunization with adenoviral vector ChAdOx1‐S expressing full‐length Spike protein of SARS‐CoV‐2. We showed that both routes of vaccination induced a potent IgG antibody response, as well as robust neutralizing capacity, but intranasal vaccination elicited a superior IgA antibody titer in the sera and in the respiratory mucosa. Bronchoalveolar lavage from intranasally immunized mice efficiently neutralized SARS‐CoV‐2, which has not been the case in intramuscularly immunized group. Moreover, substantially higher percentages of epitope‐specific CD8 T cells exhibiting a tissue resident phenotype were found in the lungs of intranasally immunized animals. Finally, both intranasal and intramuscular vaccination with ChAdOx1‐S efficiently protected the mice after the challenge with recombinant herpesvirus expressing the Spike protein. Our results demonstrate that intranasal application of adenoviral vector ChAdOx1‐S induces superior mucosal immunity and therefore could be a promising strategy for putting the COVID‐19 pandemic under control.
Keywords: ChAdOx1‐S, mucosal immunity, SARS‐CoV‐2, vaccination, vaccine vectors
Intranasal immunization of mice with adenoviral vector ChAdOx1‐S is superior compared to intramuscular immunization in the induction of systemic and mucosal anti‐S IgA response and lung‐resident CD8 TRM. Both routes elicit systemic anti‐S IgG response and SARS‐CoV‐2‐specific cellular response and provide efficient protection of mice after challenge with MCMV‐S. Figure was created with Biorender.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is the causative agent of the COVID‐19 pandemic. Despite the asymptomatic or mild disease course for the majority of infected people, more than 5 million people have succumbed to infection [1]. The extensive morbidity and mortality associated with the COVID‐19 pandemic have made the development of SARS‐CoV‐2 vaccines an urgent global health priority.
Among the proteins encoded by SARS‐CoV‐2 genes, the S protein generated the most attention as it binds to the cell‐surface receptor angiotensin‐converting enzyme 2 (ACE2) on the epithelium in the respiratory tract, initiating viral entry and the infection of the susceptible cells [2]. Consequently, vaccine development against SARS‐CoV‐2 has primarily focused on the induction of adaptive immune response targeting this viral protein. Alongside mRNA‐based vaccines [3, 4], replication‐deficient adenoviral vector vaccines expressing full‐length S protein (e.g., ChAdOx1‐S/nCoV‐19) [5, 6] were also administered worldwide. Even though both approaches give rise to protective antibody and T‐cell responses, it is now evident that vaccination, particularly at later time points, does not warrant complete protection against reinfection. The reasons for this may not only pertain to the waning of antibody response and the appearance of new viral variants escaping recognition by neutralizing antibodies but also to the fact that the intramuscular route of vaccination might not be optimal for the induction of mucosal immunity, in particular IgA response [7, 8]. There is substantial evidence indicating that both local IgA antibodies and resident T cells (TRM) play a crucial role in rapid protection against viral entry and dissemination [9]. In a mouse model of influenza virus infection, Oh and colleagues have shown that local IgA secretion in the bronchoalveolar space was induced by intranasal immunization, but not after intraperitoneal immunization of mice with influenza virus [10]. Moreover, mucosal IgA levels correlated with superior protection against a secondary challenge with homologous and heterologous virus infection when compared with circulating antibodies alone. These and several other studies [11, 12, 13] emphasize that the intranasal route of vaccine administration may generate a superior protection against respiratory viral infections. In addition to protection against symptomatic infection, such an approach might also prevent or reduce viral spread through the population by asymptomatic individuals.
In this study, we compared intranasal and intramuscular vaccination of mice with adenoviral vaccine vector ChAdOx1‐S. Our results demonstrate that intranasal application of this vaccine vector expressing the S protein of SARS‐CoV‐2 results in a superior IgA response, both locally and systemically, compared to the intramuscular route of vaccination. Moreover, CD8 T‐cell response in the lungs was also stronger after intranasal vaccination. In a challenge experiment, using recombinant cytomegalovirus (CMV) expressing the S protein, we showed a similar protective capacity of both routes. Altogether, our results indicate that mucosal immunization against SARS‐CoV‐2 with adenoviral vectors may give a strategic advantage over the intramuscular route in the induction of immune response at the barrier mucosal tissue.
Results and Discussion
Superior systemic IgA response after intranasal vaccination with ChAdOx1‐S vaccine
Despite the development of several different COVID‐19 vaccines that have efficiently reduced viral spread, hospitalization, and mortality rates, the COVID‐19 pandemic is far from controlled. With the emergence of new variants carrying mutations in the S protein of the SARS‐CoV‐2, it has become evident that the specific immune response acquired by intramuscular vaccination will not sufficiently protect against breakthrough infections [14, 15]. Several recent studies related to respiratory pathogens, including SARS‐CoV‐2, provided strong evidence that vaccine administered via the intranasal mucosa could be more effective in inducing not only IgA antibodies, but also tissue‐resident cellular immunity [8, 13, 16]. One could wonder why new vaccine modalities, administered through the respiratory mucosa either as a primary vaccination or as a booster dose, which would likely enhance the mucosal component of systemic immunity already created by previous intramuscular vaccination, are still not available. A few experimental studies have already confirmed the validity of such an approach [17, 18].
Therefore, we decided to tackle this issue by comparing intranasal and intramuscular routes of vaccination against SARS‐CoV‐2 using the commercial adenoviral vaccine vector ChAdOx1‐S [5]. We immunized BALB/c mice with 2.5 × 10⁶ infectious units of ChAdOx1‐S, revaccinated them with the same dose 6 weeks later, and measured IgG and IgA levels in the sera at different time points (Fig. 1A). Three weeks after initial immunization, we collected sera from mice and measured SARS‐CoV‐2 S‐specific antibody response. While a single dose of ChAdOx1‐S was sufficient to induce an IgG response after both intramuscular and intranasal immunization, IgA antibodies were only detectable in intranasally immunized animals at this early time point (Fig. 1B). No differences in IgG antibody levels between immunization routes were observed neither after the second vaccine dose. However, IgA antibody levels remained significantly higher in the group of mice immunized intranasally (Fig. 1C). In addition, titers of IgA antibodies were significantly higher in the group of mice vaccinated intranasally, whereas this was less pronounced for IgG titers in sera (Fig. 1D). Finally, to evaluate the neutralization capacity of antibodies induced via different immunization routes, at 8 and 10 weeks after the first immunization we performed a SARS‐CoV‐2 neutralization assay in vitro [19]. Both intramuscular and intranasal immunization generated a robust neutralization activity of sera (Fig. 1E). Altogether, our results indicate that both immunization routes with adenoviral vector expressing the S protein induced similar IgG levels, while the intranasal route resulted in a stronger IgA antibody response. Our findings are in accordance with the results of Hassan et al. [7, 20] showing a superior IgA response after intranasal compared to intramuscular vaccination.
Figure 1.
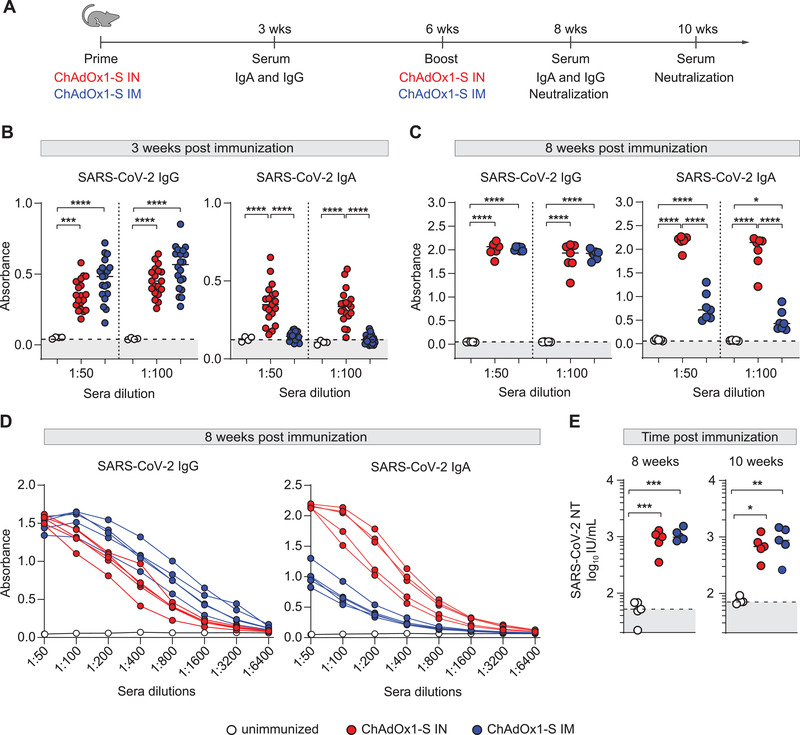
Intranasal immunization with ChAdOx1‐S adenoviral vector vaccine induces a superior IgA response and a similar IgG response to intramuscular immunization in mice. (A) BALB/c mice were vaccinated via intranasal (IN) or intramuscular (IM) route with ChAdOx1‐S and were boosted 6 weeks after the first immunization. At 3, 8, and 10 weeks after first immunization, sera were collected for analysis. (B–D) Spike‐specific IgG and IgA were assessed by ELISA (n = 5–20). Dotted lines indicate the median absorbance value of unimmunized mice sera. (E) Neutralization titers were determined by in vitro neutralization assay (all groups n = 5). Dotted lines indicate the median value of IU/mL of unimmunized mice sera. Dots represent individual data points. Data were analyzed by one‐way ANOVA followed by Tukey's multiple comparison test; p values indicate significant differences (*p < 0.05; **p < 0.01, ***p < 0.001, ****p < 0.0001). All experiments (B–E) have been repeated at least two times. IU: international units.
Intranasal immunization with ChAdOx1‐S provides a higher quality of mucosal immunity
It is well known that local antigen delivery substantially alters the pattern of mucosal adaptive immune response [9]. Therefore, we sought to investigate the differences in the development of adaptive immunity in the respiratory tract upon intranasal and intramuscular vaccination with ChAdOx1‐S. We assessed whether high levels of IgA in blood also correspond to secretory IgA in the respiratory tract. To that aim, we performed bronchoalveolar lavage (BAL) in mice vaccinated intranasally or intramuscularly with ChAdOx1‐S (Fig. 2A) and measured IgA and IgG in BAL 9 weeks after immunization (Fig. 2B, left). A significantly higher level of IgA in BAL was detected in intranasally vaccinated mice compared to animals vaccinated intramuscularly. Notably, even IgG level in BAL was higher in mice vaccinated intranasally. Importantly, we also tested the neutralizing capacity of BAL from immunized mice. BAL derived from intranasally vaccinated animals showed significant neutralizing capacity, which was completely lacking in BAL of intramuscularly vaccinated group (Fig 2B, right). Hence, intranasal immunization with the adenovirus vaccine vector induces a superior mucosal antibody response, which can efficiently neutralize SARS‐CoV‐2.
Figure 2.
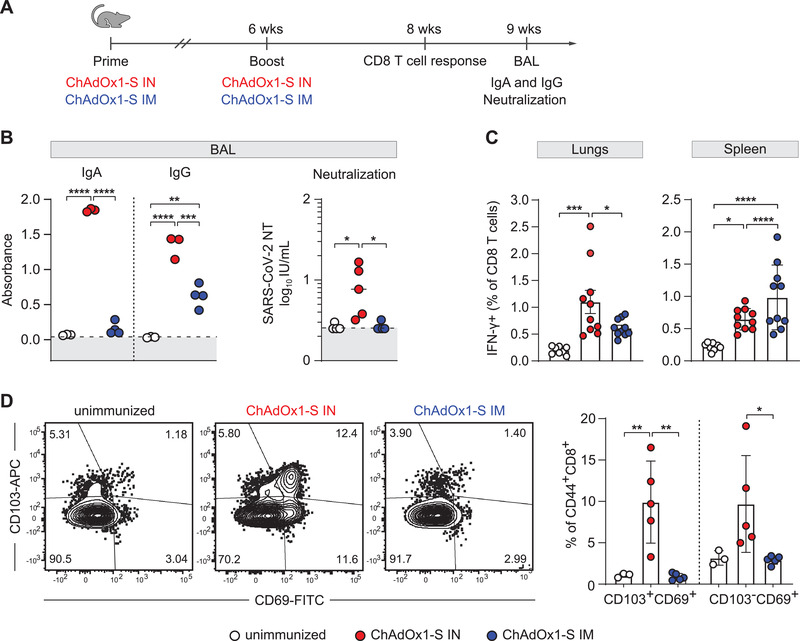
Intranasal immunization with ChAdOx1‐S induces a superior mucosal immunity in the lungs compared to intramuscular immunization. (A) BALB/c mice were vaccinated via intranasal (IN) or intramuscular (IM) route with ChAdOx1‐S. (B) At 6 weeks after the first immunization, mice were boosted, and at 9 weeks mucosal, Spike‐specific immune response in BALs was assessed by ELISA (n = 3‐4). Dotted lines indicate the median absorbance value of unimmunized mice sera. Neutralization titers were determined by in vitro neutralization assay (all groups n = 5). Dotted lines indicate the median value of IU/mL of unimmunized mice sera. Dots represent individual data points. (C) Eight weeks after the first immunization, spleen and lung homogenates were restimulated with Spike‐specific peptide KNKCVNFNF (S535‐543). The responding CD8 T cells were identified by intracellular staining for accumulated IFN‐γ. Bars represent group means overlaid with individual data points (n = 10). (D) Tissue‐resident phenotypes of antigen‐experienced CD8 T cells (CD8+CD44+) were assessed by staining for CD69 and/or CD103. Data were analyzed by one‐way ANOVA followed by Tukey's multiple comparison test; p values indicate significant differences (*p < 0.05; **p < 0.01, ***p < 0.001, ****p < 0.0001). All experiments (B–D) have been repeated two to three times. IU: international units.
Tissue resident T cells are known to play a key role in preventing severe viral pneumonia and resolving the infection [21]. It is also well established that intranasal vaccination promotes the induction of virus‐specific T lymphocytes in the lungs [7, 8]. Clinical studies have shown a good correlation between disease resolution and T lymphocyte response [22, 23]. We, therefore, compared CD8 T‐cell response in mice upon intranasal or intramuscular immunization with ChAdOx1‐S (Fig. 2A). Two weeks after the second dose, lymphocytes were isolated from spleens and lungs and stimulated with the H2‐Dd restricted S protein epitope KNKCVNFNF [24]. Epitope‐specific CD8 T cells were identified with intracellular IFN‐γ staining (gating strategy in Supporting Information Fig. S1). Although vaccination elicited CD8 T‐cell response in both groups of immunized mice, epitope‐specific CD8 T cells were more frequent in the lungs after intranasal immunization and in the spleen of the intramuscularly immunized group, indicating the importance of the immunization route for the generation of local cellular immunity (Fig. 2C). Notably, a substantially higher percentage of CD8 T cells in the lungs exhibited tissue resident phenotype upon intranasal immunization compared to the intramuscular route (Fig. 2D), which is in accordance with recently published data [7, 8] (gating strategy in Supporting information Fig. S2). We have also performed experiments using a peptide pool consisting of 158 overlapping peptides (JPT Peptide Technologies) and observed no major differences compared to the data obtained with the KNKCVNFNF peptide (Supporting information Fig. S3). Based on these results, we concluded that ChAdOx1‐S applied intranasally not only generates superior mucosal antibody response, but also potent CD8T cell response in lungs, which may be essential for efficient virus control.
Intranasal immunization with ChAdOx1‐S confers protection against recombinant CMV vector expressing the S protein
To compare the protective capacity induced with different immunization routes against viral infection, in the absence of a BSL3 animal facility, we performed a challenge experiment using mouse CMV (MCMV) engineered to express S protein of SARS‐CoV‐2 (MCMV‐S) (Fig. 3A). We have previously characterized several MCMV vectors and demonstrated their capacity to present foreign proteins [25, 26]. For this study, we generated an MCMV‐S vector, which contains the full‐length coding sequence for the S protein in place of the MCMV ie2 gene. The expression of the S protein in purified virus stock of MCMV‐S was verified by western blot (Fig. 3B). Moreover, intravenous infection of mice with MCMV‐S (Fig. 3C) induced S protein‐specific CD8 T‐cell response (Fig. 3D) (gating strategy in Supporting information Fig. S1) and SARS‐CoV‐2 neutralizing antibodies (Fig. 3E). A similar cellular and humoral response was evident in mice after intranasal infection with MCMV‐S (Supporting information Fig. S4). Mice vaccinated with ChAdOx1‐S were intraperitoneally challenged with MCMV‐S at 8 weeks after vaccination, sacrificed 3 and 7 days after the challenge (Fig. 3F) and viral titers in organs were determined (Fig. 3G). On day 3 after the challenge, mice immunized intranasally and intramuscularly had significantly lower viral titers in the spleen, lungs, and liver compared to the unimmunized group. The protection was also evident 7 days after the challenge, when once again, viral titers in the spleen and lungs of intranasally or intramuscularly immunized mice were lower compared to the unimmunized group. No virus was detected in the liver samples at day 7 after MCMV challenge. At both time‐points, there were no differences in viral titers in any of the organs between the intranasally and intramuscularly immunized groups of mice. Moreover, we performed an experiment in which mice vaccinated either intranasally or intramuscularly with ChAdOx1‐S were challenged with MCMV‐S intranasally at 8 weeks after vaccination. Five days after the challenge, virus titer in the lungs was dramatically reduced (2 to 3 log10 differences) in both groups of vaccinated mice compared to the unvaccinated group (Fig. 3H). We did not detect any plaques in spleen and liver samples at day 5 after intranasal MCMV challenge (Supporting information Fig. S5B), which is in agreement with previously published data [27]. To test if efficient immune control of MCMV‐S in the ChAdOx1‐S immunized groups depends on CD8 T cells, mice were treated with a depleting anti‐CD8α antibody 1 day prior to intranasal MCMV‐S challenge and viral titers were determined in organs 5 days after the challenge. Efficacy of CD8 T‐cell depletion in the spleen and perfused lungs was almost 100% (Supporting information Fig. S6). Although the depletion of CD8 T cells resulted in increase of viral titers, the difference between unimmunized group of mice and group of mice vaccinated intranasally was still significant (Fig. 3I). High level of S protein specific antibodies in BAL of mice vaccinated intranasally (Fig. 2B) is the most likely explanation for the control of MCMV‐S virus in mice vaccinated intranasally and depleted of CD8 T cells before challenge. All in all, the results provide strong evidence that intranasal immunization with the ChAdOx1‐S vaccine vector provides an equal or slightly better reduction of MCMV‐S titer in tissues as compared to intramuscular vaccination after viral challenge. One may wonder why a high titer of neutralizing antibodies induced by intranasal immunization failed to induce a sterilizing immunity after the intranasal challenge with MCMV‐S. MCMV uses different glycoprotein complexes for cell entry (e.g., gH/gL/gO or gH/gL/MCK‐2 [28]), and therefore, in MCMV‐S, ectopic Spike protein most likely does not participate in the infection of susceptible cells in the respiratory tract. In that case, antibodies targeting Spike protein may not be fully efficient in neutralizing MCMV‐S, which would explain the lack of differences in protection between intranasal and intramuscular route of immunization. Furthermore, it is important to emphasize the differences between CMV and SARS‐CoV‐2 infections. Although CMV can cause viral pneumonia that can manifest as ARDS [29], it primarily causes a systemic infection. After initial infection of the epithelial cells, CMV enters the blood stream, either as a free or cell‐associated virus, and, due to its wide cellular tropism, can infect virtually any tissue in the host [30]. CMV also uses a plethora of immunevasins that enable the virus to persist within the host [31]. Also, intranasal immunization with MCMV‐S failed to induce an IgA antibody response (Supporting information Fig. S4B), which implies that the IgA response is not protective in the context of CMV infection. These differences are important to consider when evaluating the data in Fig. 3, as they might explain why we observed a similar protective capacity to MCMV‐S challenge, following both immunization routes. However, the fact that intranasal immunization with ChAdOx1‐S induced neutralizing antibodies in the BAL fluid of vaccinated animals, which were completely lacking in intramuscularly immunized group (Fig. 2B, right), suggests that in the context of SARS‐CoV‐2 challenge, intranasal route of vaccination would provide superior protection.
Figure 3.
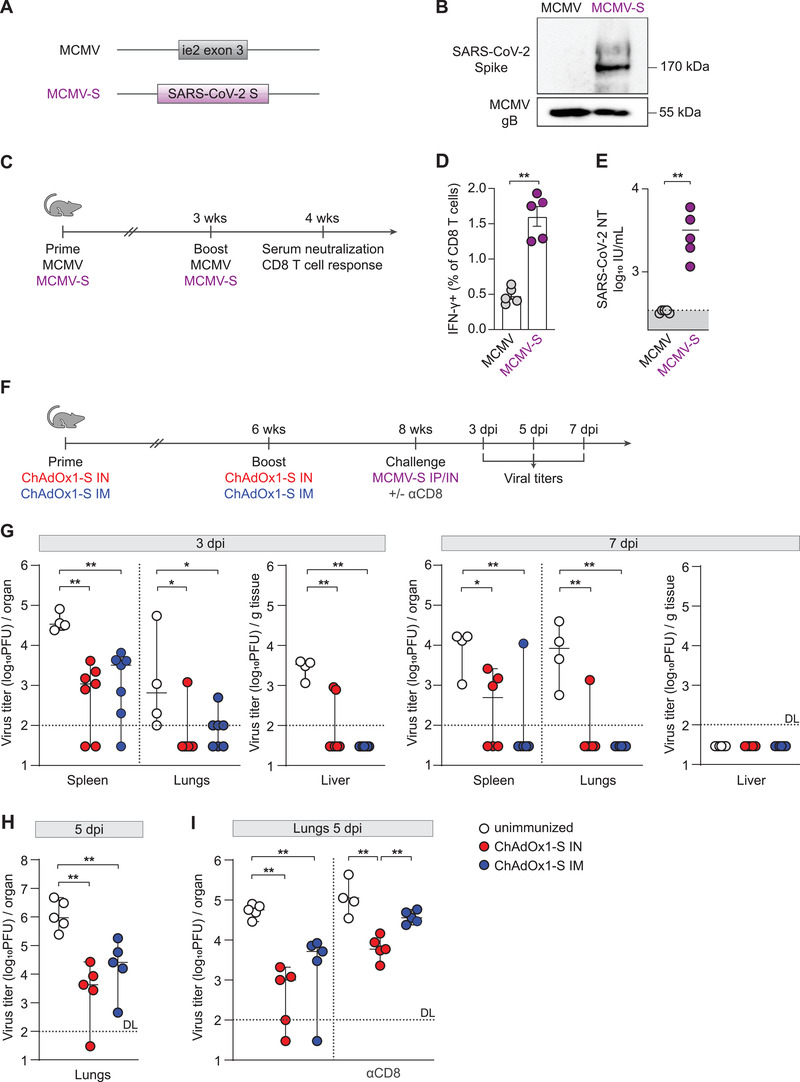
Intranasal immunization with ChAdOx1‐S confers excellent protection from challenge with a heterologous virus expressing SARS‐CoV‐2 S protein. (A) Schematic representation of the recombinant MCMV vector (MCMV‐S). The SARS‐CoV‐2 Spike ORF was inserted in the place of the MCMV ie2. (B) Western blots of purified virus stocks of MCMV‐S and MCMV were performed with an antibody against the SARS CoV‐2 S protein. Protein gB/M55 of MCMV was used as a loading control. (C) BALB/c mice were infected intravenously with MCMV or MCMV‐S (2 × 105 PFU/mouse), and 3 weeks later, mice were boosted. (D) Four weeks after the first immunization, spleen homogenates were restimulated with Spike‐specific peptide KNKCVNFNF (S535‐543). The responding CD8 T cells were identified by intracellular staining for accumulated IFN‐γ. Bars represent group means overlaid with individual data points (n = 5). (E) Neutralization titers were determined by in vitro neutralization assay (all groups n = 5). Dotted lines indicate the median value of IU/mL of unimmunized mice sera. Dots represent individual data points. (F) BALB/c mice were vaccinated via intranasal (IN) or intramuscular (IM) route with ChAdOx1‐S. Six weeks after the first immunization, mice were boosted and 2 weeks later challenged with either intraperitoneal (IP) or intranasal (IN) inoculation of MCMV‐S (2 × 105 PFU/mouse). At different time points, namely at 3 and 7 days after the intraperitoneal (G), or 5 days after the intranasal challenge (H–I), respectively, tissues were harvested, and viral titers were determined in lung, spleen, and liver homogenates (n = 4–7). (I) In some groups, CD8 T cells were depleted systemically by intraperitoneal injection of 500 µg of α‐CD8α antibody 1 day before intranasal MCMV‐S challenge. (G–I) Titers in organs of individual mice are shown (circles); horizontal bars indicate the median values. Dotted lines indicate the detection limit of the assay (DL). Data were analyzed by Mann–Whitney U test (D‐E, G‐I); p values indicate significant differences (*p < 0.05; **p < 0.01). All experiments (B, D, G‐I) have been repeated two to three times. IU: international units.
Overall, our results, together with the already published data, support the view that mucosal vaccination might have a strategic advantage in combating respiratory viruses since it provides protection at the site of virus entry [7, 20]. In a recent study, Lapuente et al. used intranasal application of adenovirus vaccine vectors as a boost strategy and showed complete protection against SARS‐CoV‐2 infection in mice primarily vaccinated with plasmid DNA [8]. However, in their case, unlike in our study, as well as in the study by Hassan et al. [11], two adenovirus vectors, Ad5 and Ad19a, expressing the SARS‐CoV‐2 S protein, failed to induce a strong immune response without prior plasmid DNA vaccination, suggesting that these vectors differ in their capacity to establish efficient mucosal immunity. It is currently unclear what could be the explanation for a differential efficacy of adenovirus vectors, but one of the possibilities could be the use of different receptors for virus entry [8].
Concluding Remarks
Although existing COVID‐19 vaccines are efficient in preventing severe disease, we are witnessing their low efficiency in protecting vaccinated people from breakthrough infection. In addition to the viral evasion of antibody control, one reason certainly lies in the fact that intramuscular administration of the vaccine does not induce strong local immunity at the site of SARS‐CoV‐2 entry into the body. Together with recently published data, our study using ChAdOx1‐S demonstrated that in addition to systemic immunity, an adenovirus vector applied intranasally can induce a superior mucosal IgA response to the S antigen, as well as CD8 T‐cell response in lungs. Antiviral antibodies also exhibited a strong capacity to neutralize SARS‐CoV‐2. In addition, intranasal vaccination appears to be equally protective as intramuscular vaccination against challenge infection with CMV expressing the SARS‐CoV‐2 S protein. Altogether, our results further emphasize the importance of mucosal immunization for combating respiratory viral infections such as SARS‐CoV‐2.
Materials and Methods
Vaccination of mice
Mice were housed and bred under specific pathogen‐free conditions at the Central Animal Facility, Faculty of Medicine, University of Rijeka. Ministry of Agriculture, Croatia, approved all experiments. Age‐matched, eight‐ to twelve‐week‐old female BALB/c mice were used in all experiments. The animals were immunized with 2.5 × 10⁶ infectious units of the commercially available ChAdOx1‐S (ChAdOx1 nCoV‐19, lot number ABW4801) diluted in PBS. The ChAdOx1‐S consisted of a replication‐deficient chimpanzee adenovirus ChAdOx1 containing the SARS‐CoV‐2 gene encoding the S protein as described elsewhere [5, 32]. For intranasal immunizations, mice were anesthetized and then inoculated using a plastic tip onto the external nares with 20 µL of the vaccine. For intramuscular immunizations, the vaccine was injected in a volume of 50 µL in the thigh muscles of the hind leg. Blood samples for ELISA and neutralization analysis were obtained from the tail vein and the retro‐orbital sinus, respectively. For BAL sampling, mice were sacrificed, and lungs were rinsed with 1 mL cold HBSS supplemented with 10 nM EDTA through the cannulated trachea. For challenge experiments, mice were inoculated intraperitoneally or intranasally with 2 × 105 PFU/mouse of MCMV‐S. Viral titers in the organs were determined by plaque assay on murine embryonic fibroblasts [33]. In vivo CD8 T‐cell depletion was performed by intraperitoneal injection of 500 µg of α‐CD8α antibody (YTS 169.4) 1 day before MCMV‐S challenge.
Detection of anti‐Spike antibodies in mouse sera
SARS‐CoV‐2‐specific IgA and IgG titers were determined by ELISA. In short, high‐binding ELISA 96‐well plates (Greiner Bio‐One) were coated overnight at 4°C with 2 µg/mL of target protein in carbonate/bicarbonate coating buffer and then blocked for 2 h at room temperature (RT). Additionally, commercially available pre‐coated plates (EUROIMMUN Medizinische Labordiagnostika AG) were used. After incubation of samples on prepared plates, plates were washed with PBS and incubated with HRP‐conjugated mouse IgA‐ or IgG‐specific antibodies for 1 h at RT. The OPD substrate was used to develop the reaction. The stop solution (1 M H2SO4) was added to stop the reaction. The absorbance of the samples was read using an optic reader at 490 nm, with 630 nm as the reference wavelength.
Virus neutralization assay
The SARS‐CoV‐2 isolate of B.1.1.1. lineage 297/20 Zagreb was used in experiments (GISAID database, accession ID GISAID EPI _ISL _3013041). Virus neutralization assay was performed as described in more detail in Ravlić et al. [19]. Briefly, serial dilutions of mouse sera were preincubated with SARS‐CoV‐2 working stock at 37°C and 5% CO2 for 90 min. Subsequently, Vero E6 cells were added to the mixture, and after 4 days of incubation at 37°C and 5% CO2, the wells with cytopathic effect were counted. The anti‐SARS‐CoV‐2 in‐house standard was calibrated to the first WHO International Standard for anti‐SARS‐CoV‐2 (NIBSC, UK), upon its availability, enabling expression of neutralization titer (NT) in IU mL−1.
T‐cell assays
Flow cytometry was performed according to the Guidelines for the use of flow cytometry and cell sorting in immunological studies [34]. Single‐cell leukocyte suspensions for in vitro stimulation and phenotype assay were prepared from perfused lungs and spleen as described before [35]. Fc receptors were blocked with 2.4G2 mAb to reduce nonspecific staining. CD8 T‐cell‐surface staining was performed for the following antigens: anti‐CD8α SB780 (clone: 53–6.7; 1:200), anti‐CD45.2 eFluor 506 (clone: 104, 1:200), anti‐CD44 A700 (clone: IM7, 1:100), anti‐CD69 FITC (clone: H12.F3; 1:100), anti‐CD103 APC (clone: 2E7, 1:100), and Fixable Viability Dye eFluor‐780 (1:1000, eBioscience) were used to exclude dead cells. IFN‐γ production by CD8 T cells was stimulated as previously described [36] and examined by intracellular staining using anti‐IFN‐γ PE or eFluor450 (XMG1.2, 1:100) antibody. All antibodies were purchased from eBioscience. In vitro stimulation assay has been performed using an overlapping pool of 158 peptides PepMix SARS‐CoV‐2 Spike (JPT Peptide Technologies) and H2‐Dd restricted S protein epitope KNKCVNFNF (GenScript). Data were acquired using FACSAriaIIu (BD Biosciences) and analyzed using FlowJo v10 (TreeStar) software.
MCMV mutagenesis
A recombinant MCMV vector expressing the SARS‐CoV‐2 Spike (MCMV‐S) was generated by inserting the full‐length Spike ORF from the Wuhan‐1 strain (GenBank: MN_908947) [26] in place of the MCMV ie2 gene. It was based on the BAC molecular clone pSM3fr. The infectious virus was reconstituted by transfection of purified BAC DNA into mouse embryonic fibroblasts.
Western blot analysis
Western blot analysis on virus preparations was performed as described elsewhere [37]. Affinity purified mAbs (Center for Proteomics, Faculty of Medicine Rijeka) used for Western blot are as follows: anti‐SARS‐CoV‐2 Spike (S1‐S2.22) and anti‐gB (M55.01).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software, Inc.). One‐way ANOVA analysis was used to compare multiple groups at single time points. Comparisons between two groups were performed using the Mann–Whitney U test. p < 0.05 was considered statistically significant. In all figures, only statistically significant differences are indicated.
Conflict of Interest
The authors declare no commercial or financial conflict of interest.
Author Contributions
MCB, JM, MŠ, IB, VJL, BL, AK, and SJ designed the study and wrote the manuscript. MCB, JM, MŠ, KM, TR, MPM, BH, and SR performed the experiments. MCB, JM, BH, SR, MŠ, ZK, LŠ, DR, AK, and SJ analyzed the data. MS, FB, LČŠ, and AM provided reagents and critically read the manuscript. SJ, BH, and AK supervised the study. All authors contributed to the manuscript and approved the submitted version.
Peer review
The peer review history for this article is available at https://publons.com/publon/10.1002/eji.202249823.
Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- MCMV
mouse CMV
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
Supporting information
Suporting information
Acknowledgements
We thank D. Rumora, A. Miše, M. Gašparević, N. Vragović, A. Šarlija, I. Nenadić, and C. Paulović for excellent technical and administrative support. This work has been supported in part by the grant “Strengthening the capacity of CerVirVac for research in virus immunology and vaccinology”, KK.01.1.1.01.0006, awarded to the Scientific Centre of Excellence for Virus Immunology and Vaccines and co‐financed by the European Regional Development Fund (SJ), by the Croatian Science Foundation under the project IP‐CORONA‐04‐2055 (AK), project IP‐CORONA‐04‐2073 (IB) and the project IP‐CORONA‐04‐2053 (BH) and by the Helmholtz Association under the Helmholtz‐EU Partnering grant PIE‐0008 (LČŠ). We are also grateful to Ivo Usmiani for his support for this study.
Stipan Jonjić and Astrid Krmpotić shared senior authorship.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. World Health Organization . WHO Coronavirus (COVID‐19) Dashboard. (2022). Available at: https://covid19.who.int/ (Accessed: 12th January 2022).
- 2. Piccoli, L. , Park, Y. J. , Tortorici, M. A. , Czudnochowski, N. , Walls, A. C. , Beltramello, M. , Silacci‐Fregni, C. et al., Mapping neutralizing and immunodominant sites on the SARS‐CoV‐2 spike receptor‐binding domain by structure‐guided high‐resolution serology. Cell 2020. 183: 1024–1042, e1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polack, F. P. , Thomas, S. J. , Kitchin, N. , Absalon, J. , Gurtman, A. , Lockhart, S. , Perez, J. L. et al., Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N. Engl. J. Med. 2020. 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baden, L. R. , El Sahly, H. M. , Essink, B. , Kotloff, K. , Frey, S. , Novak, R. , Diemert, D. et al., Efficacy and Safety of the mRNA‐1273 SARS‐CoV‐2 Vaccine. N. Engl. J. Med. 2021. 384: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voysey, M. , Clemens, S. A. C. , Madhi, S. A. , Weckx, L. Y. , Folegatti, P. M. , Aley, P. K. , Angus, B. et al., Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021. 397: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sadoff, J. , Gray, G. , Vandebosch, A. , Cardenas, V. , Shukarev, G. , Grinsztejn, B. , Goepfert, P. A. et al., Safety and efficacy of single‐dose Ad26.COV2.S vaccine against Covid‐19. N. Engl. J. Med. 2021. 384: 2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hassan, A. O. , Kafai, N. M. , Dmitriev, I. P. , Fox, J. M. , Smith, B. K. , Harvey, I. B. , Chen, R. E. et al., A single‐dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS‐CoV‐2. Cell 2020. 183: 169–184,e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lapuente, D. , Fuchs, J. , Willar, J. , Vieira Antao, A. , Eberlein, V. , Uhlig, N. , Issmail, L. et al., Protective mucosal immunity against SARS‐CoV‐2 after heterologous systemic prime‐mucosal boost immunization. Nat. Commun. 2021. 12: 6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iwasaki, A. , Exploiting mucosal immunity for antiviral vaccines. Annu. Rev. Immunol. 2016. 34: 575–608. [DOI] [PubMed] [Google Scholar]
- 10. Oh, J. E. , Song, E. , Moriyama, M. , Wong, P. , Zhang, S. , Jiang, R. , Strohmeier, S. et al., Intranasal priming induces local lung‐resident B cell populations that secrete protective mucosal antiviral IgA. Sci. Immunol. 2021. 6: eabj5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hassan, A. O. , Feldmann, F. , Zhao, H. , Curiel, D. T. , Okumura, A. , Tang‐Huau, T. L. , Case, J. B. et al., A single intranasal dose of chimpanzee adenovirus‐vectored vaccine protects against SARS‐CoV‐2 infection in rhesus macaques. Cell Rep. Med. 2021. 2: 100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trimpert, J. , Adler, J. M. , Eschke, K. , Abdelgawad, A. , Firsching, T. C. , Ebert, N. , Thao, T. T. N. et al., Live attenuated virus vaccine protects against SARS‐CoV‐2 variants of concern B.1.1.7 (Alpha) and B.1.351 (Beta). Sci. Adv. 2021. 7: eabk0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng, X. , Oduro, J. D. , Boehme, J. D. , Borkner, L. , Ebensen, T. , Heise, U. , Gereke, M. et al., Mucosal CD8+ T cell responses induced by an MCMV based vaccine vector confer protection against influenza challenge. PLoS Pathog. 2019. 15: e1008036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lipsitch, M. , Krammer, F. , Regev‐Yochay, G. , Lustig, Y. and Balicer, R. D. , SARS‐CoV‐2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nat. Rev. Immunol. 2022. 22(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hacisuleyman, E. , Hale, C. , Saito, Y. , Blachere, N. E. , Bergh, M. , Conlon, E. G. , Schaefer‐Babajew, D. J. et al., Vaccine breakthrough infections with SARS‐CoV‐2 variants. N. Engl. J. Med. 2021. 384: 2212–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morabito, K. M. , Ruckwardt, T. J. , Bar‐Haim, E. , Nair, D. , Moin, S. M. , Redwood, A. J. , Price, D. A. et al., Memory inflation drives tissue‐resident memory CD8(+) T cell maintenance in the lung after intranasal vaccination with murine cytomegalovirus. Front Immunol 2018. 9: 1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu, S. , Huang, J. , Zhang, Z. , Wu, J. , Zhang, J. , Hu, H. , Zhu, T. et al., Safety, tolerability, and immunogenicity of an aerosolised adenovirus type‐5 vector‐based COVID‐19 vaccine (Ad5‐nCoV) in adults: preliminary report of an open‐label and randomised phase 1 clinical trial. Lancet Infect. Dis. 2021. 21: 1654–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Altimmune, I. , Altimmune announces update on AdCOVIDTM phase 1 clinical trial. 2021. https://ir.altimmune.com/news‐releases/news‐release‐details/altimmune‐announces‐update‐adcovidtm‐phase‐1‐clinical‐trial
- 19. Ravlić, S. , Hećimović, A. , Kurtović, T. , Ivančić Jelečki, J. , Forčić, D. , Slović, A. , Kurolt, I. C. et al., Is better standardization of therapeutic antibody quality in emerging diseases epidemics possible? Front. Immunol. 2022. 13(816159). 10.3389/fimmu.2022.816159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hassan, A. O. , Shrihari, S. , Gorman, M. J. , Ying, B. , Yaun, D. , Raju, S. , Chen, R. E. et al., An intranasal vaccine durably protects against SARS‐CoV‐2 variants in mice. Cell Rep. 2021. 36: 109452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zheng, M. Z. M. , Wakim, L. M. , Tissue resident memory T cells in the respiratory tract. Mucosal. Immunol. 2021. 10.1038/s41385-021-00461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Notarbartolo, S. , Ranzani, V. , Bandera, A. , Gruarin, P. , Bevilacqua, V. , Putignano, A. R. , Gobbini, A. et al., Integrated longitudinal immunophenotypic, transcriptional and repertoire analyses delineate immune responses in COVID‐19 patients. Sci Immunol 2021. 6(62). [DOI] [PubMed] [Google Scholar]
- 23. Lafon, E. , Diem, G. , Witting, C. , Zaderer, V. , Bellmann‐Weiler, R. M. , Reindl, M. , Bauer, A. et al., Potent SARS‐CoV‐2‐specific t cell immunity and low anaphylatoxin levels correlate with mild disease progression in COVID‐19 patients. Front Immunol 2021. 12: 684014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhuang, Z. , Lai, X. M. , Sun, J. , Chen, Z. , Zhang, Z. Y. , Dai, J. , Liu, D. L. et al., Mapping and role of T cell response in SARS‐CoV‐2‐infected mice. J. Exp. Med. 2021. 218: e20202187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trsan, T. , Busche, A. , Abram, M. , Wensveen, F. M. , Lemmermann, N. A. , Arapovic, M. , Babic, M. et al., Superior induction and maintenance of protective CD8 T cells in mice infected with mouse cytomegalovirus vector expressing RAE‐1gamma. Proc. Natl. Acad. Sci. U S A 2013. 110: 16550–16555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim, Y. , Zheng, X. , Eschke, K. , Chaudhry, M. Z. , Bertoglio, F. , Tomic, A. , Krmpotic, A. et al., MCMV‐based vaccine vectors expressing full‐length viral proteins provide long‐term humoral immune protection upon a single‐shot vaccination. Cell. Mol. Immunol. 2022. 19(2):234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oduro, J. D. , Redeker, A. , Lemmermann, N. A. W. , Ebermann, L. , Marandu, T. F. , Dekhtiarenko, I. , Holzki, J. K. et al., Murine cytomegalovirus (CMV) infection via the intranasal route offers a robust model of immunity upon mucosal CMV infection. J. Gen. Virol. 2016. 97: 185–195. [DOI] [PubMed] [Google Scholar]
- 28. Wagner, F. M. , Brizic, I. , Prager, A. , Trsan, T. , Arapovic, M. , Lemmermann, N. A. , Podlech, J. et al., The viral chemokine MCK‐2 of murine cytomegalovirus promotes infection as part of a gH/gL/MCK‐2 complex. PLoS Pathog. 2013. 9: e1003493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luyt, C. E. , Combes, A. , Trouillet, J. L. , Nieszkowska, A. and Chastre, J. , Virus‐induced acute respiratory distress syndrome: epidemiology, management and outcome. Presse Med. 2011. 40: e561–e568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Griffiths, P. and Reeves, M. , Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat. Rev. Microbiol. 2021. 19: 759–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berry, R. , Watson, G. M. , Jonjic, S. , Degli‐Esposti, M. A. and Rossjohn, J. , Modulation of innate and adaptive immunity by cytomegaloviruses. Nat. Rev. Immunol. 2020. 20: 113–127. [DOI] [PubMed] [Google Scholar]
- 32. Folegatti, P. M. , Ewer, K. J. , Aley, P. K. , Angus, B. , Becker, S. , Belij‐Rammerstorfer, S. , Bellamy, D. et al., Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet 2020. 396: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brizic, I. , Lisnic, B. , Brune, W. , Hengel, H. and Jonjic, S. , Cytomegalovirus infection: mouse model. Curr. Protoc. Immunol. 2018. 122: e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cossarizza, A. , Chang, H. D. , Radbruch, A. , Abrignani, S. , Addo, R. , Akdis, M. , Andra, I. et al., Guidelines for the use of flow cytometry and cell sorting in immunological studies (third edition). Eur. J. Immunol. 2021. 51: 2708–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miletic, A. , Lenartic, M. , Popovic, B. , Brizic, I. , Trsan, T. , Miklic, K. , Mandelboim, O. et al., NCR1‐deficiency diminishes the generation of protective murine cytomegalovirus antibodies by limiting follicular helper T‐cell maturation. Eur. J. Immunol. 2017. 47: 1443–1456. [DOI] [PubMed] [Google Scholar]
- 36. Sustic, M. , Cokaric Brdovcak, M. , Lisnic, B. , Materljan, J. , Juranic Lisnic, V. , Rozmanic, C. , Indenbirken, D. et al., Memory CD8 T cells generated by cytomegalovirus vaccine vector expressing NKG2D ligand have effector‐like phenotype and distinct functional features. Front. Immunol. 2021. 12: 681380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ruzic, T. , Lisnic, V. J. , Lucin, H. M. , Rovis, T. L. , Zeleznjak, J. , Brdovcak, M. C. , Vrbanovic, A. et al., Characterization of M116.1p, a murine cytomegalovirus protein required for efficient infection of mononuclear phagocytes. J. Virol. 2021. 96: JVI0087621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suporting information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


