Abstract
The nature of the immune responses associated with COVID‐19 pathogenesis and disease severity, as well as the breadth of vaccine coverage and duration of immunity, is still unclear. Given the unpredictability for developing a severe/complicated disease, there is an urgent need in the field for predictive biomarkers of COVID‐19. We have analyzed IgG Fc N‐glycan traits of 82 SARS‐CoV‐2+ unvaccinated patients, at diagnosis, by nano‐LC‐ESI‐MS. We determined the impact of IgG Fc glyco‐variations in the induction of NK cells activation, further evaluating the association between IgG Fc N‐glycans and disease severity/prognosis. We found that SARS‐CoV‐2+ individuals display, at diagnosis, variations in the glycans composition of circulating IgGs. Importantly, levels of galactose and sialic acid structures on IgGs are able to predict the development of a poor COVID‐19 disease. Mechanistically, we demonstrated that a deficiency on galactose structures on IgG Fc in COVID‐19 patients appears to induce NK cells activation associated with increased release of IFN‐γ and TNF‐α, which indicates the presence of pro‐inflammatory immunoglobulins and higher immune activation, associated with a poor disease course. This study brings to light a novel blood biomarker based on IgG Fc glycome composition with capacity to stratify patients at diagnosis.
Keywords: Inflammation, agalactosylation, asialylation, COVID‐19, IgG Fc glycosylation, SARS‐CoV‐2
SARS‐CoV‐2 infection activates several branches of the immune response, including differential serum IgG glycosylation. Glycan alterations, namely galactosylation and sialylation, detected in circulating IgGs can vary according to COVID‐19 severity and have prognostic predictive potential. IgG glycovariants from poor prognosis patients were found to drive increased NK cell activation, when compared with those from patients who developed a good prognosis.
Introduction
Coronavirus SARS‐CoV‐2 is the responsible agent for the global pandemic of Coronavirus disease 2019 (COVID‐19), that, as of the March 8, 2022, has infected over 448 million people worldwide [1, 2]. As it is a highly selective disease, only some SARS‐CoV‐2‐infected individuals display symptoms and an unpredictable percentage of patients may develop a severe pathology. Over the last year, the knowledge regarding this disease has risen in an unprecedented rate. However, there is still an urgent need to improve the understanding of COVID‐19's pathophysiology, foreseeing better clinical and therapeutic strategies for patients, as well as an optimized management of health care resources and vaccination strategy improvements. The available vaccines against SARS‐CoV‐2 pose a great promise for the resolution of the pandemic, but its long‐term protection is still on trial, as well as its distribution strategies [3, 4, 5]. A complete and thorough understanding of immune responses to SARS‐CoV‐2 is still lacking. Therefore, there is an urgent unmet need to identify and characterize the immune responses associated with COVID‐19 pathogenesis and severity, envisioning the identification of predictive/prognostic biomarkers of this disease.
SARS‐CoV‐2 infection most often results in a mild DC, associated with heterogeneous symptoms [6]. Research regarding peripheral blood mononuclear cells (PBMCs) immune phenotyping of infected patients consistently revealed an immunological dysregulation that characterizes the disease.
Antibodies play a central role in humoral immunity against pathogens, as they are able to mediate immune responses to viral infections, being Immunoglobulin G (IgG) the prevalent isotype taking part in systemic antiviral immunity being a key effector of the humoral immune system by triggering leukocyte activation and inflammation [7].
IgG isolated from human serum is composed of multiple glycoforms, owing to the addition of a diverse type of glycans structures in the IgG crystallizable fragment (Fc) and fragment antigen‐binding (Fab) region. Fc domain is responsible for modulating cellular response through interaction with FcγRs in effector immune cells. All IgG Fc domains contain a single, highly conserved, glycosylation site in Asn297 that carries complex N‐glycans [8].
A single complex biantennary N‐linked glycan is attached to each heavy chain in the Fc portion, being required for Fc receptor binding. Although the IgG Fc N‐glycans are restricted to two antennae, the structures attached to the Fc domain of IgG are known to be present in a multitude of forms, being essential for the regulation of antibody effector functions [9]. Over 30 different glycan variations have been detected on circulating IgG in healthy individuals [10], which reflects a tremendous heterogeneity in IgG Fc N‐glycome. In fact, the type of glycan attached to IgGs Fc is critical for the proper effector functions of all IgGs, regulating the binding to FcγRs and instructing either a pro‐inflammatory (through binding to activating FcγRs) or an anti‐inflammatory (through binding to inhibitory FcγRs) response, associated with the pathogenesis of many inflammatory diseases. For instance, increased levels of IgG fucosylation have been observed in rheumatoid arthritis patients [11], which has been associated with the fact that IgG‐containing N‐glycans that lack core fucose have been implicated in enhanced antibody‐dependent cell‐mediated cytotoxicity due to increase affinity for FcγRIIIa [12]. Terminal sialylation of Fc glycans also modulates FcγR binding. The presence of α2,6 sialic acid on the Fc glycan significantly reduces FcγR binding affinity and is associated with anti‐inflammatory activity [10]. Accordingly, the glycoengineering of intravenous immunoglobulin (IVIg) with increased sialylation of Fc showed a 100‐fold increase in anti‐inflammatory activity in a mouse model of arthritis [10], which further supports the immunoregulatory potential of IgG Fc glycan structures. The mechanistic role of specific glycans modifying Fc and effector functions of IgG either through FcγRs binding or through other mechanism are still controversial [13, 14, 15]. Recently, using the glycoengineering of monoclonal IgG1 antibodies, further insights into the regulatory role of IgG glycan moieties in the affinity for FcγRIIIa in more complex scenarios have been generated [16, 17]. Moreover, NK cells also express glycan binding receptors, such as sialic acid‐binding receptors (siglecs) and others, that can modulate their functions [18].
Loss of terminal galactose (agalactosylation) on IgG1 Fc glycans was also observed in patients suffering from rheumatoid arthritis and appearing in circulation preceding disease onset [12]. More recently, the glycosylation profile of IgG was found to be different between ulcerative colitis and Crohn's disease patients and was associated with clinical severity of the disease [19, 20]. In systemic lupus erythematosus, IgG fucosylation was associated with kidney damage [21]. The variability of IgG glycome composition among human population appears to be influenced by genetic and environmental factors as well as age [22, 23].
In summary, this compelling body of evidence showing a tremendous variation on IgG glycome among population that have been linked to differential disease severities led us to hypothesize that IgG glycome heterogeneity among SARS‐CoV‐2 infected patients can be used as a reliable and minimally invasive biomarker to early identify those at risk of serious illness from those who might be protected.
The glycoprofile of serum IgGs in COVID‐19 patients has received some attention. Chakraborty et al. showed that afucosylated Fc N‐glycans are enriched in anti‐SARS‐CoV‐2 receptor binding domain IgG1 from severe patients, which appears to contribute to the enhancement in the production of pro‐inflammatory cytokines [24]. Consistently, Larsen et al. found that afucosylated Fc N‐glycans in SARS‐CoV‐2‐spike protein‐specific IgGs are more prevalent in critically ill patients [25]. This afucosylated anti‐spike IgGs was demonstrated to induced a hyper‐inflammatory profile of human macrophages [25, 26]. Moreover, total IgG N‐glycome (Fc and Fab glycans) analysis was also characterized by us in three European cohorts of plasma samples from COVID‐19 patients, showing that IgG glycome composition is different when comparing mild versus severe disease patients [27]. However, whether variations in circulating IgG Fc glycome composition among SARS‐CoV‐2‐infected individuals, at diagnosis, are able to predict the development of a severe/complicated disease remains unexplored. Therefore, in this study, the serum IgG N‐glycome composition was characterized in a longitudinal and multicentric cohort of SARS‐CoV‐2‐infected individuals, assessing whether a serum IgG antibody glycosignature, analyzed at diagnosis, confers prognostic value as a predictive biomarker for the development of a poor DC after 14 days postdiagnosis. In addition, we also demonstrated, at a mechanistic level, the biological effects of a differential IgG glycome composition in the activation of effector NK cells and in FcγIIIaR‐Fc binding.
Results
Glycans variations in IgG Fc among COVID‐19 patients at diagnosis: Loss of galactosylation and sialylation in total IgG Fc are observed in patients with a more severe disease at diagnosis
Taking into consideration that there is still a gap in the knowledge of humoral immune response in SARS‐CoV‐2 infection at early timepoints, we set out to characterize the glycosylation profile of serum total IgGs at time of diagnosis of SARS‐CoV‐2 infection (up to 3 days upon PCR+ test) comparing with noninfected (non‐IF) controls. A total of 82 patients with PCR+ COVID‐19 were selected for plasma collection at diagnosis. Patients were classified at diagnosis as asymptomatic (n = 8), mild disease (n = 40), and moderate/severe disease (n = 34), accordingly with WHO clinical guidelines [28]. As controls, four non‐IF individuals were included (Supporting Information Table S1).
Total serum IgG was isolated and the glycosylation profile of the Fc portion was determined by mass spectrometry (MS). Glycan structures were grouped by their traits in terms of galactosylation, sialylation, bisecting GlcNAc (Bis‐GlcNAc), and fucosylation (Fig. 1A). Specifically, total IgG Fc agalactosylation was found to be significantly increased in the moderate/severe disease patients when compared with non‐IF and with mild ones (Fig. 1B), namely in IgG1 (only between moderate/severe and mild severities) and IgG4 isotypes (Fig. 1C). Given the fact that age might influence IgG glycome composition [22, 23], the data were adjusted for age and the results showed that the increased IgG Fc agalactosylation observed in moderate/severe disease patients compared with mild disease was statistically significant after age correction (indicated with §). Accordingly, digalactosylation is significantly decreased in moderate disease patients compared with mild disease group and to non‐IF subjects (Fig. 1B, Supporting Information Fig. S1B), specifically for IgG1 and IgG2&3 (only between moderate/severe and mild severities) (Fig. 1C). The decrease in total IgG Fc digalactosylation, and specifically in IgG2&3 sub‐type, in moderate disease patients was statistically significant after age correction, when compared to the mild group (indicated with §). Moreover, asymptomatic patients present a decrease in IgG1 digalactosylation (G2) relatively to non‐IF and mild disease group (Fig. 1C). Regarding terminal sialyation of IgGs, known to be widely associated with anti‐inflammatory properties of IgG, we observed a significant decrease of this glycan trait, predominantly in IgG2&3 sub‐type, at diagnosis in the moderate disease group, compared to the mild group (Fig. 1C), that remained statistically significant after age correction (indicated with §). Distinctively, the presence of Bis‐GlcNAc is significantly increased in total IgG Fc in moderate patients when compared to mild patients, even after age correction (Fig. 1C). No differences were detected in total IgG monogalactosylation and fucosylation at diagnosis between groups (Supporting Information Fig. S1A). The glycan traits that showed an association with severity, independently of the age, have 24–26% power of explaining the overall differences between mild and moderate patients.
Figure 1.
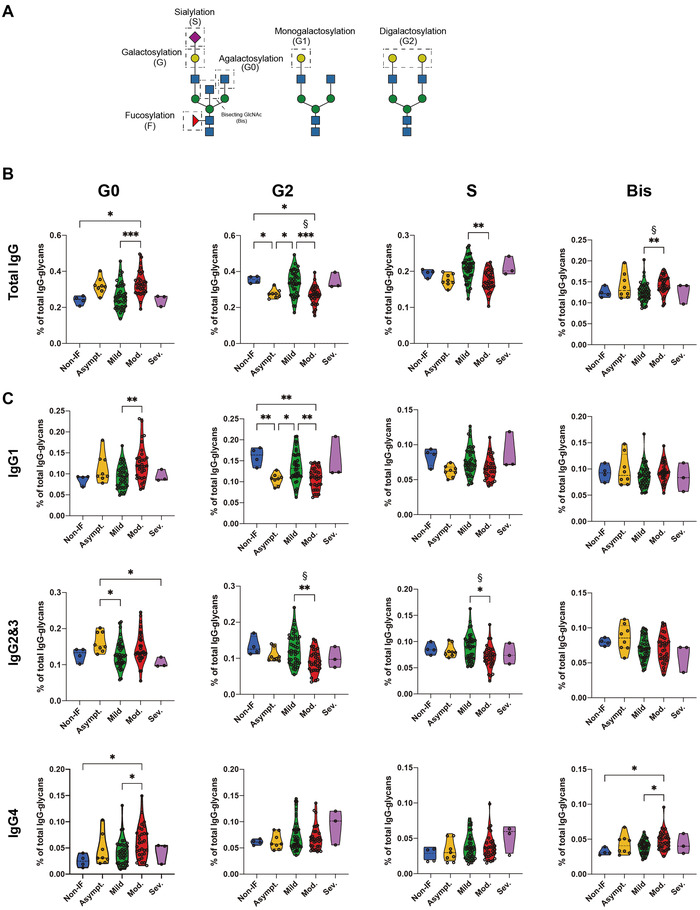
SARS‐CoV‐2 infection drives IgG Fc glycosylation alterations. (A) Schematic representation of analyzed IgG glycan traits. (B) Relative abundance of total IgG Fc agalactosylation (G0), digalatosylation (G2), sialylation (S), and Bis‐GlcNAc (Bis) in different COVID‐19 severities (asymptomatic n = 8, mild n = 40, moderate n = 31, and severe n = 3) and non‐IF individuals (n = 4). (C) Relative abundance of isotype‐specific IgG Fc glycan traits in different COVID‐19 severities and non‐IF individuals. Each data point represents the data from a single patient/subject isolated IgGs in a single LC‐MS analysis (one replicate). Kruskal–Wallis test, *p‐value < 0.05; **p‐value < 0.005; ***p‐value < 0.001. The data were corrected for age effect and linear regression model for age correction was used, § p‐value < 0.05.
In order to exclude the eventual confounder effect of anti‐SARS‐CoV‐2‐specific IgGs in this analysis, we analyzed the levels of anti‐Spike IgGs. We showed that seropositivity in our cohort is very low compared with vaccinated individuals, in which only 40% of mild patients and 33% of moderate/severe groups display presence of anti‐Spike IgG with similar levels (Supporting Information Fig. S1C). This is in accordance with the fact that serum was collected as early as up to 72‐h postdiagnosis and therefore a complete antibody response to the virus had not occurred yet in the majority of the patients. To rule out the contribution of anti‐Spike IgG glycosylation in our findings, we performed an ELISA using the ECA lectin to determine if the galactosylation pattern of these antigen‐specific IgGs was following the total IgG behavior. We observed that in symptomatic seropositive patients (mild, moderate, and severe) the galactosylation levels of anti‐Spike IgG decreased when compared to asymptomatic ones, with no differences between mild, moderate, and severe (Supporting Information Fig. S1D). Moreover, total IgG ECA binding, from the same set of seropositive patients, followed the same decrease in terms of galactosylation levels (Supporting Information Fig. S1E), as previously observed in the bigger cohort of samples (Fig. 1B). Overall, these results suggest that the galactosylation pattern of anti‐Spike IgG is not a confounder in this study.
Overall, our data show that total IgG glycome composition is correlated with disease severity at diagnosis. A pro‐inflammatory IgG glycosignature characterized by loss of galactosylation and loss of sialylation was correlated with patients that exhibit a moderate disease at diagnosis when compared with those presenting a mild disease.
Serum IgG glycosylation profile at diagnosis predicts COVID‐19 prognosis
In order to explore the prognostic potential of serum IgG glycosylation profile as a biomarker for COVID‐19 disease course (DC) prediction, we have tested for a correlation between IgG Fc N‐glycome composition at diagnosis and a worse DC at day 14 postdiagnosis. Briefly, patients were grouped, according to their disease severity, 14 days postdiagnosis in terms of the presence of symptoms (no symptoms > good prognosis; presence of at least one of the criteria: pneumonia and/or need for oxygen therapy > poor prognosis) (Supporting Information Table S2). Our data showed that patients with a poor DC were those who displayed a pro‐inflammatory IgG glycoprofile at diagnosis. Specifically, patients that, at day 14 postdiagnosis, did not improve the symptomatology (having thereby a poor disease prognosis) exhibited, at diagnosis, an increased agalactosylation (mainly in IgG1) and Bis‐GlcNAc in total IgGs, together with a decrease of digalactosylation (mainly in IgG2) and decreased sialylation (in IgG2), with no major differences in monogalactosylation and fucosylation (Fig. 2A and B, Supporting Information Fig. S2A). Considering the age of patients as a possible confounder effect, the results were adjusted for age (indicated with § in Fig. 2A and B). We showed that changes in IgG Fc galactosylation have survived, highlighting the biological relevance of total IgG Fc digalactosylation (mainly IgG2&3 subtypes) as well as IgG1 Fc agalactosylation as independent predictors of COVID‐19 prognosis.
Figure 2.
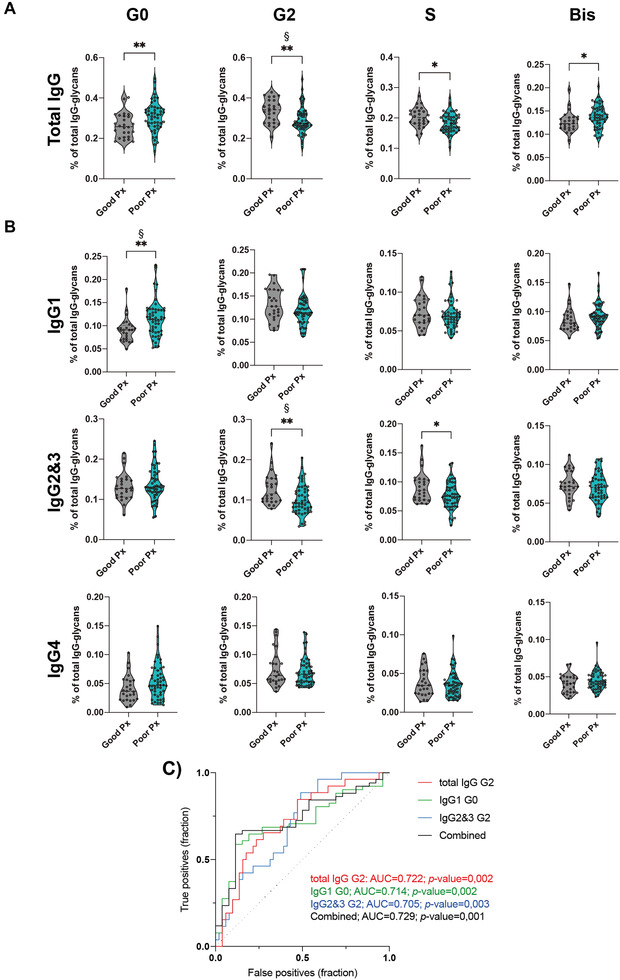
Specific glycosignature of IgG Fc associates with COVD‐19 prognosis. (A) Relative abundance of total IgG Fc agalactosylation (G0), digalatosylation (G2), sialylation (S), and Bis‐GlcNAc (Bis) in good (asymptomatic at day 14, n = 51) and poor (symptomatic at day 14, n = 26) prognosis (Px) of COVID‐19 disease. (B) Relative abundance of isotype‐specific IgG Fc glycan traits (GT) in good and poor prognosis. (C) Significant associations between IgG glycan traits and disease outcome (FDR < 0.1). (D) Receiver operating characteristic (ROC) curve plotted for the IgG Fc glycan traits levels of COVID‐19 patients (n = 77), either separated or combined. Each data point represents the data from a single patient/subject isolated IgGs in a single LC‐MS analysis (one replicate). Kruskal–Wallis test, *p‐value < 0.05; **p‐value < 0.005. Binomial logistic regression model for age correction, § p‐value < 0.05.
In order to further determine the predictive performance of serum IgG glycosignature in predicting COVID‐19 prognosis, and to validate the predictive potential of serum IgG glycan traits in stratifying patients at diagnosis regarding good versus poor disease prognosis, receiver operating characteristic curve analysis was performed. Total IgG digalactosylation (AUC = 0.722; p‐value = 0.002), IgG1 agalactosylation (AUC = 0.714; p‐value = 0.002), and IgG2&3 digalactosylation (AUC = 0.705; p‐value = 0.003) were found to, independently, be able to stratify patients according with prognosis. Remarkably, the combined values (age‐corrected) of these IgG glycan traits (AUC = 0.729; p‐value < 0.001) significantly improved the prediction capacity (at diagnosis) in terms of likelihood of developing a good versus a poor COVID‐19 DC (Fig. 2C). As IgG Fc N‐glycosylation has been associated with several health conditions, we tested their association with the presence of comorbidities in the patients of our cohort (Supporting Information Fig. S2B). We detected no significant associations, concluding that IgG Fc N‐glycan traits are able to predict good and poor prognosis of COVID‐19, independently of the presence of comorbidities.
Taken together, we demonstrate that the composition of glycans of total IgG, in terms of presence/absence of galactose, sialic acid, and Bis‐GlcNAc structures in IgG Fc domain, analyzed at diagnosis of COVID‐19, has prognostic capacity in predicting a poor DC (up to 14 days postdiagnosis).
Loss of galactosylation in total IgGs from SARS‐CoV‐2‐infected patients promotes increased FcγRIIIa binding and NK cell activation associated with poor prognosis
In order to gain mechanistic insights on the impact of a differential IgG glycoprofile, detected at diagnosis, in immune cell activation and function, we evaluated the potential of isolated IgGs to activate NK cells in vitro. We started by assessing the binding capacity of isolated total IgGs to FcγRIIIa, one of the major targets for IgG‐mediated NK cell activation; thus, we used a subset of patients and analyzed the binding potential by surface plasmon resonance (SPR). Our results showed a 30% increase in FcγRIIIa binding of the IgGs from SARS‐CoV‐2‐infected subjects, compared to non‐IF (Supporting Information Fig. S3A). Accordingly, patients that developed a poor prognosis revealed an increased binding of IgG Fc to FcγRIIIa when compared to patients with good prognosis (Supporting Information Fig. S3B).
NK cells recognize a multitude of targets with a vast repertoire of membrane‐expressed receptors. Among them, there is the FcγRIIIa, as mentioned, but also glycan‐binding receptors, such as siglecs. We asked if the increased binding between FcγRIIIa and patient‐derived IgG, and/or their differential glycosylation profiles could have an impact in NK cell activation. To tackle this hypothesis, NK cells were cultured with purified IgGs from patients with different disease severities, and NK cells’ activation were evaluated through the expression of the activation marker CD69 (Supporting Information Fig. S3C). Interestingly, we observed that NK cell activation was increased in infected and asymptomatic patients, when compared to non‐IF and mild ones. Moreover, in symptomatic patients, we have observed that moderate patients‐derived IgGs (low galactosylation and sialylation) induced increased CD69 expression when compared to mild ones (high galactosylation and sialylation) (Fig. 3A).
Figure 3.
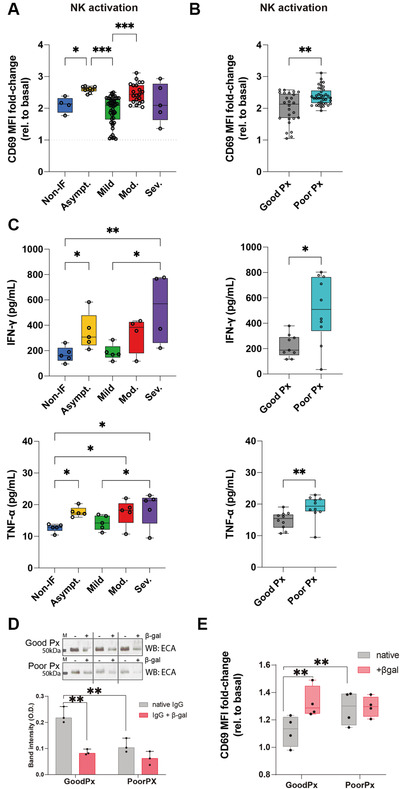
Loss of IgG galactosylation in patients with poor prognosis leads to increased NK cell activation. (A) NK cell activation quantified by the fold change of CD69 expression (compared to basal NK cells) after culture with coated IgGs from COVID‐19 patients with different severities (asymptomatic n = 9; mild n = 46; moderate n = 21; severe n = 5) and (B) prognosis (good prognosis n = 26; poor prognosis n = 39). (C) Quantification of pro‐inflammatory cytokine, IFN‐γ and TNF‐α, production by IgG‐activated NK cells. (D) ECA lectin (recognizing terminal galactose) blot and band intensity quantification of different patients’ IgGs (each patient is exhibited in paired lanes) upon β1‐4‐galactosidase S digestion (+βgal) or not (‐βgal, native), n = 8. (E) NK cell activation quantified by the fold change of CD69 expression (compared to basal NK cells) after culture with coated IgGs digested with β‐galactosidase S (+βgal) or not (native) from COVID‐19 patients. Results shown from at least two independent experiments. Each data point represents data from a single patient/subject. Mann–Whitney t‐test *p‐value < 0.05; **p‐value < 0.005.
Importantly, from the clinical point of view, we found that IgG, isolated at diagnosis, from patients that developed a poor prognosis, enriched in agalactosylated and asialylated N‐glycans, induced a higher activation of NK cells, when compared to IgG from patients with a good prognosis (Fig. 3B). To further characterize isolated IgG‐mediated NK cell activation, we have analyzed the production of pro‐inflammatory cytokines (IFN‐γ and TNF‐α), which showed increased production, in line with CD69 expression between the severity groups and prognosis (Fig. 3C). These evidence highlight the pro‐inflammatory role of agalactosylated total IgG in COVID‐19 disease.
To further validate the role of the IgGs Fc glycosylation composition, specifically the galactosylation levels, in defining immune cell activation and disease prognosis, we have glycoengineered the galactose levels of IgGs to assess its impact in NK cell activation. In fact, IgG agalactosylation has been widely described as imposing a pro‐inflammatory function to IgGs in different immune‐mediated diseases [20, 29, 30]. In accordance with our results on COVID‐19, lack of galactosylation in the Fc portion has been shown to promote FcγR binding and activation of innate cellular response [31]. Total IgGs isolated from patients that displayed a good versus poor prognosis were digested with β‐galactosidase (β‐gal; which removes the terminal galactose residues). The removal of galactose from IgGs was confirmed by lectin blot analysis with Erythrina cristagalli agglutinin (ECA) lectin, which recognizes terminal galactose, showing a reduction in the reactivity to this lectin in IgG treated with β‐gal (Fig. 3D). This reduction in ECA staining was more pronounced in IgGs from patients with a good prognosis compared to poor prognosis, as the latter have already low levels of galactosylation (Fig. 3D). The glycoengineered IgGs were tested for NK cell activation, where the removal of galactose from IgG isolated from patients who displayed a good DC (and high IgG Fc galactosylation levels) increased NK cells activation, which was not observed in patients with poor prognosis that already display low levels of IgG Fc galactosylation (Fig. 3E). These results support the specific role of IgG galactosylation as a chief glyco‐determinant for the modulation of NK cells activation, associated with COVID‐19 prognosis.
Discussion
In this study, we have demonstrated that IgGs Fc domain from SARS‐CoV‐2‐infected patients display different glycosylation profiles (glycan variations) that can be detected at diagnosis and with potential to predict COVID‐19 prognosis. We found that COVID‐19 patients that display a poor DC at day 14 post‐PCR+ test (exhibiting either pneumonia and/or need for oxygen therapy), had at diagnosis, IgGs with a deficiency in galactosylation and sialylation, and increased levels of Bis‐GlcNAc structures. This specific plasma IgG glycosignature can be detected within the first 72 h upon diagnosis. Despite having a small fraction of seropositive patients (anti‐Spike IgG) in our cohort, we have shown that the glycosylation pattern of anti‐Spike IgG does not constitute a confounding issue. Some mechanisms have been pointed out as possible drivers of IgG glycosylation changes such as those occurring through the action of extracellular glycosidases/glycosyltransferases enzymes [32]. The presence of extracellular sialidases, responsible for the removal of terminal sialic acid, secreted by endothelial cells may also represent a player for the extracellular modulation of sialylation in circulating IgGs [33]. Recently, we also proposed that SARS‐CoV‐2 infection appears to impose a glycan switch on T cells associated with COVID‐19 severity [34]. Moreover, age, gender, lifestyle, and pathologies may implicate in the steady state of an inherited IgG glycosylation profile [23]. Importantly, several studies have shown that afucosylation of SARS‐CoV‐2‐specific IgGs was associated with overall poor prognosis, namely in hospitalized versus outpatients [24], infected subjects with or without acute respiratory distress syndrome [25], and patients that progressed to a severe disease [35]. Therefore, many causes have been proposed to explain glycan‐variations of IgG Fc among population, however the mechanisms underlying the glycoalterations in IgGs associated with SARS‐CoV‐2 infection is a topic that needs to be further investigated.
Levels of IgG galactosylation are one of the most prominent glycosylation alteration observed in several chronic inflammatory and autoimmune diseases [36]. In fact, galactosylation of total IgGs has been described to affect activation thresholds of immune cells. Karsten et al. showed in mice that the anti‐inflammatory property of high galactosylation of IgG immune complexes is due to the promotion of its association with FcγRIIB and dectin‐1, which blocks the pro‐inflammatory effector functions [37]. Therefore, this suggests that individuals with higher levels of immune activation would have more pro‐inflammatory antibodies, as they have lower levels of IgG galactosylation/sialylation. In accordance with our study, a seminal report from Larsen et al., where the N‐glycome of total IgG and SARS‐CoV‐2 specific IgGs were studied, from patients with or without acute respiratory distress syndrome, it was described that the levels of galactosylation and sialylation are decreased in the higher severity group [25]. Our results showing the loss of galactose in IgGs from COVID‐19 patients can be thereby the reflection of a general pro‐inflammatory humoral immune response that we found to be associated with a poor prognosis. This decrease of galactosylated IgGs was independent of the age of the subjects, and was accompanied by decreased sialylation. In fact, we demonstrated that patients with low galactosylation of IgGs display increased activation potential of NK cells, with production of pro‐inflammatory cytokines such as IFN‐γ and TNF‐α. These basal pro‐inflammatory antibodies defined by glycans composition appear to underlie an exaggerated immune response that may foster the “cytokine storm” associated with poor prognosis of COVID‐19. The mechanism underlying NK cell activation by IgG Fc‐glycosylation still needs further research, namely the involvement of other molecules besides FcγRs, such as the case of glycan binding receptors.
In accordance, previous studies on IgG Fc glycosylation demonstrated that terminal sialylation also reduces FcγR binding [10]. Our results suggest that loss of sialylation and decreased galactosylation in IgG Fc N‐glycans from patients diagnosed with COVID‐19 is consistent with an immunological misfiring, associated with NK cell activation, that defines severity and poor prognosis of COVID‐19. These evidence suggest that the degree of plasma IgG galactosylation and sialyation can be used as a biomarker for immune activation and thus as a biomarker for COVID‐19 prognosis. Moreover, we cannot exclude the potential contribution of increased Bis‐GlcNAc in the immunological misfiring we propose, given that it has been suggested that Bis‐GlcNAc in the Fc domain increases the affinity of IgG1 for FcγRIIIa binding, independent of fucosylation and galactosylation [17].
Our study was conducted during the first wave of COVID‐19 (March–July 2020) and had a limitation in terms of sample size, undefined day 0 of infection, and limited biological material that explain the fact that differential FcγR binding between the groups did not present major differences, indicating the need to validate these promising observations in larger and well‐characterized multicentric cohorts as well as analyzing the impact of other SARS‐CoV‐2 variants in total IgG N‐glycosylation. Moreover, NK cell‐IgG interactions are far from being fully understood, and IgG‐glycans should be a major focus of research. Therefore, the identification of the precise molecular mechanism of IgG‐glycan‐mediated NK cell activation needs to be further explored.
One aspect that needs to be explored should be the potential effect of COVID‐19 vaccination in the levels of total IgG galactosylation and in IgG glycome composition in general. Curiously, influenza and tetanus vaccination modulates the galactosylation of antigen‐specific IgG1 associated with increase on antibody titers [38]. Whether COVID‐19 vaccine modulates levels of galactosylation in total (non‐ and antigen‐specific) IgGs as a way of assessing vaccine efficacy and development of neutralizing antibodies needs to be investigated.
Taken together, this study brings to light a novel risk screening system based on the serum IgG Fc N‐glycome signature (as a minimally invasive blood biomarker) able to discriminate at diagnosis high‐risk individuals of progressing to a severe/complicated disease from those with low/no risk. This new knowledge has the potential to improve and optimize the allocation and management of health care resources and vaccination strategies in COVID‐19.
Materials and Methods
Cohort description and patient's selection criteria
The present study integrates a total of 82 patients diagnosed with SARS‐CoV‐2 between from three individual Portuguese cohorts (May to July 2020): 20 patients from Infectious Disease Department, Centro Hospitalar Universitário do Porto (CHUP), Porto, Portugal; 17 patients from Infectious Disease Department, Centro Hospitalar de Vila Nova de Gaia/Espinho (CHVNG), Vila Nova de Gaia, Portugal; and 45 patients from Internal Medicine Department of Hospital Beatriz Ângelo (HBA), Loures, Portugal. The total cohort includes patients of both genders and age between 19 and 99 years old. Blood was collected for plasma at the time of diagnosis, within 72 h of the PCR SARS‐CoV‐2+ test. The eligibility criteria for inclusion in this study were a confirmed SARS‐CoV‐2 infection (by RT‐PCR) in a nasopharyngeal swab, without evidence of previous infection, irrespectively of symptoms (asymptomatic or symptomatic) or disease severity (mild, moderate/severe). Asymptomatic patients (n = 8) were defined as positive for the SARS‐CoV‐2 PCR test, but with no signs of disease. The following criteria were used to stratify symptomatic SARS‐CoV‐2 patients in terms of severity, accordingly with WHO guidelines [28]; 1‐MILD (n = 40): individuals without evidence of pneumonia; 2‐MODERATE/SEVERE (n = 34): individuals with evidence of pneumonia and need of supplemental oxygen and/or individuals with need of invasive mechanical ventilation and of admission to hospital intensive care unit. Patients’ demographic and relevant clinical data are summarized in Supporting Information Table S1. Additionally, disease progression was evaluated at day 14 postdiagnosis and patients were grouped according to their severity: presence of symptoms at day 14 postdiagnosis (poor prognosis) (n = 51) versus absence of symptoms (good prognosis) (n = 26) (Supporting Information Table S2). Diagnosis of pneumonia and need of oxygen therapy were the clinical criteria used to define presence/absence of symptomatology at day 14 postdiagnosis.
Healthy controls (n = 7) are represented by individuals with no history of recent or active infectious diseases.
Informed consent was obtained from all included patients and the research protocols were previously approved by the institutional ethics committee of CHUP, CHVNG, and HBA.
Isolation of IgG from human plasma
All human plasma samples (100 μL) were defrosted and centrifuged at 1620 g for 10 min. Afterwards, the plasma samples were diluted 8× with PBS (1×) and filtered through 0.45 μm Supor AcroPrep 96‐well filter plates. Plasma samples were applied to the protein G plate and instantly washed three times with 10 column volume (CV)× PBS (1×) to remove unbound proteins. IgG was eluted from the protein G using 5 CV of 0.1 M formic acid (pH 2.5), into a 96‐deep‐well plate (Waters, Milford, MA) and immediately neutralized to pH 7.0 with 1 M ammonium bicarbonate to maintain the IgG stability. Preparation and analysis of Fc N‐glycans is described in the Supporting Information.
Glycopeptide preparation
To 20 mg of IgG sample, 0.2 μg of TPCK‐treated trypsin (Promega, Madison, WI) was added, followed by an overnight incubation at 37°C. Tryptic digests were purified using a solid‐phase extraction on C18 beads (Chromabond, MACHEREY‐NAGEL, Düren, Germany). Note that 10 μg of C18 beads was applied to each well of an OF1100 96‐well polypropylene filter plate (Orochem, Naperville, IL). The RP stationary phase was activated three times with 200 μL of 80% ACN and conditioned with three times with 200 μL 0.1 % TFA (Sigma‐Aldrich/Merck, Darmstadt, Germany). The IgG digests were diluted with 0.1% TFA (Sigma‐Aldrich/Merck, Darmstadt, Germany), loaded onto the C18 beads, and washed with three times 200 μL 0.1% TFA. The entire procedure was performed on a vacuum manifold under pressure reduction of 2 inches of mercury. IgG glycopeptides were eluted into an ABgene PCR 96 well plate (Thermo, Waltham, MA) with 200 μL of 20% ACN by centrifugation at 105 g for 5 min. Eluates were dried by vacuum centrifugation and stored at –20°C until analysis by MS.
nano‐LC‐ESI‐MS of IgG N‐glycopeptides
Purified tryptic IgG glycopeptides were analyzed on Waters ACQUITY M‐Class UPLC system (Waters, Milford, MA), consisting of binary pump, auxiliary pump, autosampler maintained at 10°C, and column oven compartment set at 30°C. Note that 10 mL of sample was applied to a PepMap 100 C18 (5 mm × 300 μm id, 5 μm; Thermo, Waltham, MA) solid‐phase extraction trap column conditioned with 0.1% TFA (mobile phase A) for 1 min at 40 μL/min. After sample loading, the trap column was switched in‐line with the gradient and C18 nano‐LC column (150 mm x 100 μm id, 2.7 μm HALO fused core particles; Advanced Materials Technology, Wilmington, DE) for 9.5 min. Trap column was cleaned with two full loop injections containing 20 μL of 95% ACN and 50% ACN in IPA, respectively. C18 nano‐LC column was equilibrated with 100% mobile phase A (0.1% TFA) for 2 min. IgG glycopeptides were reconstituted in 80 μL of ultrapure water before injection. Separation was achieved at 1 mL/min using the following gradient of mobile phase A and mobile phase B (80% ACN and 20% 0.1% TFA, respectively): 0.5 min 12% B, 0.5–4 min 12% B–17% B, and 4–5 min 17% B. The ACQUITY M‐Class system was coupled with a Compact mass spectrometer (Bruker Daltonics, Bremen, Germany) equipped with Captive Spray ion source and nanoBooster for introduction of acetonitrile vapor into the source. A nitrogen was used as a drying gas (4 L/min) and nebulizer (0.4 bars). Quadrupole and collision energies were set to 4 eV. Spectra were recorded from m/z 800 to 2000 with two averaged scans at a frequency of 0.5 Hz. Acquity M‐class UPLC system was operated under MassLynx software 4.1 while Bruker Compact Q‐TOF‐MS was operated under HyStar software version 4.1.
Glycan traits were grouped accordingly: agalactosylated—G0 (H3N4, H3N4F1, H3N5F1, H3N5), monogalactosylated—G1 (H4N4, H4N4F1, H4N4F1S1, H4N5F1, H4N5F1S1, H4N5), digalactosylated—G2 (H5N4, H5N4F1, H5N5F1, H5N5F1S1, H5N4F1S1, H5N4S1), sialylated—S (H4N4F1S1, H4N5F1S1, H5N5F1S1, H5N4F1S1, H5N4S1), bisecting—Bis (H3N5F1, H3N5, H4N5F1, H4N5F1S1, H4N5, H5N5F1, H5N5F1S1), and fucosylated—F (H3N4F1, H3N5F1, H4N4F1, H4N4F1S1, H4N5F1, H4N5F1S1, H5N4F1, H5N5F1, H5N5F1S1, H5N4F1S1).
NK cells isolation
Peripheral blood mononuclear cells (PMBCs) were obtained from buffy coats from healthy donors. The separation of PMBCs was achieved by density centrifugation using Lymphoprep (density 1.077 g/mL) (Stem Cell Technologies, Vancouver, Canada). NK cells were purified by negative selection from freshly PBMCs using EasySep Human NK Cell Isolation Kit, according to the manufacturer's instructions (Stem Cell Technologies). The purity (>94%) of NK cells was determined by flow cytometry analysis on FACSCanto II system (BD Biosciences, San Jose, CA). Monoclonal antibodies used for staining of NK cells were Pacific Blue anti‐human CD3 (OKT3), PE anti‐human CD56 (HCD56), and Brilliant Violet 421 anti‐human CD16 (3G8) (all from BioLegend, San Diego, CA). Cells were cryopreserved in freeze medium (90% of fetal bovine serum [FBS] and 10% of dimethyl sulfoxide [DMSO] [Scienova, Jena, Thuringia, Germany]) at –80°C. After defrosting, cryo‐preserved PBMCs and cryo‐preserved NK cells were maintained in complete culture medium, RPMI 1640 Medium, GlutaMAX Supplement, HEPES (Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 10% of FBS, 1% penicillin/streptomycin, and 0.1% gentamicin at 37°C in an incubator with a humidified atmosphere with 5% of CO2, overnight in order to recover and settle.
SPR analysis for FcγRIIIa‐IgG Fc binding analysis
Due to the limited amount of total IgG available, for each sample of patients and healthy controls, a traditional affinity assay was not feasible, hence we opted for the binding assay format.
SPR interaction analysis was performed on a Biacore X100 system (Cytiva). For interaction analysis of FcγRIIIa and the Fc fragment of IgG isolated from COVID‐19 patients and healthy controls, FcγRIIIa (R&D Systems) was immobilized with standard amine coupling (Cytiva) on a CM5 chip (Cytiva) at a density of 233 resonance units (RU) using a solution of 10 μg/mL in sodium acetate buffer 10 mM at pH 4.5. IgG samples were diafiltered for buffer exchange into the SPR running buffer (HBS‐EP buffer: 10 mM HEPES, pH 7.4, 150 mM NaCl, 3 mM EDTA, and 0.005% Tween‐20) using Amicon Ultra‐2 Centrifugal Filter Units (Merck) and then were injected at a concentration of 250 nM in triplicate and a flow rate of 30 μL/min for 240 s (association time). The dissociation phase was monitored for 600 s. Regeneration was carried out with two 30 s‐injections of 3.6 M MgCl2, 20 mM 2‐(N‐morpholino)ethanesulfonic acid (MES), pH 6.6. For the evaluation (Biacore X100 evaluation software 2.0.3), a report point has been set before injection of the analyte and another at the end of the association phase. The differences between these two report points were used for the evaluation of relative differences in binding.
Antibody‐dependent NK activation
In order to evaluate the impact on IgG effector functions, the activation of NK cells was evaluated. Briefly, a pre‐coating of 96‐wells cell culture plate, flat bottom, tissue culture treated, polystyrene (Orange Scientific) was performed with 3 μg/mL of native IgGs and 3 μg/mL of IgGs treated with β‐galactosidase. Coated 96‐well plate was maintained at 37°C in an incubator with a humidified atmosphere with 5% of CO2 overnight. Afterwards, purified NK cells were washed once with culture medium, suspended in complete RPMI medium supplemented with IL‐2 (100 U/mL) and adjusted to a final concentration of 2 × 105 cells per well in a coated 96‐well plate. Cells were maintained at 37°C in an incubator with a humidified atmosphere with 5% of CO2. Following 24 h of incubation, cells were stained with PE anti‐human CD69 (FN50) (Thermo Fisher Scientific), as marker of activated NK cells (Supporting Information Fig. S2). Posteriorly, cells were analyzed by flow cytometry on a FACSCanto II (BD, Biosciences) using the FACSDiva software (BD), compensated and analyzed in FlowJo v10.4. (Tree Star, Inc.). Data acquisition and analysis adhered to the general guidelines for flow cytometry [39].
Degalactosylation of human IgG
Total IgGs from each patient (1 μg) were incubated for 1 h at 37°C with 1 μL β1‐4 Galactosidase S (New England BioLabs). The efficiency of sugar removal was confirmed by lectin blotting. Briefly, 100 ng of IgG was subjected to 10% SDS‐PAGE electrophoresis and membranes were blocked with BSA 4% before incubation with Erythrina cristagalli (ECA) lectin, which recognizes terminal galactose residue. Bands were then visualized using the Vectorstain Elite ABC kit (Vector Labs) and the detection was performed using ECL reagent (GE Healthcare, Life Sciences).
Anti‐Spike and total IgG ELISA and ECA lectin ELISA
Detection of lectin‐binding motifs in the serum total IgG and anti‐Spike IgG from COVID‐19 seropositive patients was performed by a lectin ELISA adapted from Ref. [40]. Briefly, serum anti‐Spike IgG was captured using SARS‐CoV‐2 ELISA kit plates (Immunostep). Blocking was performed with Carbo‐free blocking solution (Vector Labs) for 1 h at RT. Plates were washed five times with PBS + 0.05% Tween 20 (PBST) before COVID‐19 samples, diluted 1:50 in PBS containing 1% Carbo‐free blocking solution (diluent solution) (Vector Labs), were added, and incubated by shaking at 200 rpm for 2 h at RT. After washing as described above, galactosylation by incubating captured IgG with biotinylated ECA lectin (Vector Labs) diluted 1:2000 in diluent solution (Vector Labs) and incubated for 1 h shaking at 200 rpm at RT. Bound lectin was detected using an HRP‐conjugated streptavidin (DuoSet R&D Systems) incubated for 20 min, and tetramethylbenzidine substrate (DuoSet R&D Systems) was incubated for 20 min protected from dark. Reaction was stopped using H2SO4 and the amount of bound lectin was measured at 450 nm using a μQuant Microplate Reader (BioTek, Agilent).
Total IgG galactosylation was detected by coating microtiter plates (Maxisorp, Nunc) with 1:50 isolated IgGs (as described above) from seropositive patients. The procedure afterwards was performed as described above for anti‐Spike IgG.
Serum IgG antibodies against recombinant SARS‐CoV‐2 spike protein (S) were analyzed from vaccinated healthy donors (n = 4), noninfected individuals (n = 4), asymptomatic‐infected individuals (n = 8), mild disease (n = 10), and moderate/severe disease patients (n = 12) using a chemiluminescence method (Immunostep) according to the manufacturer's instructions. An antibody level of ≥1 signal was considered positive and a level of <0.8 was considered negative.
Cytokine quantification
Supernatants from 24‐h‐cultured NK cells were analyzed by flow cytometry using the BD Cytometric Bead Array Human Th1/Th2/Th17 Cytokine Kit (BD Biosciences) following the manufacturer's instructions.
Statistical analysis
Data visualization and statistical analysis were done using GraphPad Prism 9 and SPSS software. For the age correction analysis between the association of IgG glycan traits and disease severity, logarithm of IgG glycan traits abundance was used in a linear regression model. For the age correction analysis between IgG glycan traits and prognosis, logarithm of IgG glycan traits abundance was used in a binary logistics regression model.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
MMV, IA, and SSP designed research. MMV, IA, JG, CSR, ÂF, AMD, JS, TP and FFS performed research. SSP and GL contributed new reagents/analytical tools. AS, NS, TT, LM, MMA, and RSC provided and characterized clinical samples. MMV and IA analyzed data. MMV, IA, and PO performed statistical adjustments. MMV, IA and SSP wrote the manuscript with contributions from all authors.
Ethics approvals
Informed consent was obtained from all included patients and the research protocols were previously approved by the institutional ethics committee of CHUP, CHVNG, and HBA, Portugal.
Peer review
The peer review history for this article is available at https://publons.com/publon/10.1002/eji.202149491.
Abbreviations
- DC
disease course
- ECA
Erythrina cristagalli agglutinin
- SPR
surface plasmon resonance
Supporting information
Supporting Information
Acknowledgements
We would like also to thank to all the Portuguese clinicians involved in COVID‐19 patients' care, with a special thanks to those from CHUP, CHVNG, and HBA that directly or indirectly contributed to this work and to all the patients that accepted to participate in this study. We also acknowledge the nurses and technicians that collaborated in the collection of the samples, especially Nurse Teresa Cruz from CHUP. The Institute of Molecular Pathology and Immunology of the University of Porto integrates the Institute for Research and Innovation in Health (i3S) research unit, which is partially supported by the Portuguese Foundation for Science and Technology (FCT). This article was partially funded by the FCT, in collaboration with the Portuguese Agency for Clinical Research and Biomedical Innovation (AICIB) under the special funding, “RESEARCH4COVID‐19”_Project#006 granted to the PI Salomé S. Pinho. IA [SFRH/BD/128874/2017] and MMV [PD/BD/135452/2017] received funding from the FCT.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. WHO . Dashboard | WHO coronavirus disease (COVID‐19).
- 2. Dong, E. , Du, H. , Gardner, L. , An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect. Dis. 2020. 20: 533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bloom, B. R. , Nowak, G. J. , Orenstein, W. , “When will we have a vaccine?”—Understanding questions and answers about Covid‐19 vaccination. N. Engl. J. Med. 2020. 8: 2202–2204. [DOI] [PubMed] [Google Scholar]
- 4. Krammer, F. , SARS‐CoV‐2 vaccines in development. Nature 2020. 586: 516–527. [DOI] [PubMed] [Google Scholar]
- 5. Lederer, K. , Castaño, D. , Atria, D. G. , Oguin, T. H. , Wang, S. , Manzoni, T. B. , Lei, T. et al., SARS‐CoV‐2 mRNA vaccines foster potent antigen‐specific germinal center responses associated with neutralizing antibody generation. Immunity 2020. 53: 1281–1295.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan, W. , Ni, Z. , Hu, Y. , Liang, W. , Ou, C. , He, J. , Li, P. et al., Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020. 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bournazos, S. , Gupta, A. , Ravetch, J. V. , The role of IgG Fc receptors in antibody‐dependent enhancement. Nat Rev Immunol. 2020. 20: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cobb, B. A. , The history of IgG glycosylation and where we are now. Glycobiology. 2020, 30: 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson, J. L. , Jones, M. B. , Ryan, S. O. , Cobb, B. A. , The regulatory power of glycans and their binding partners in immunity. Trends Immunol. 2013. 34: 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaneko, Y. , Nimmerjahn, F. , Ravetch, J. V. , Anti‐inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006. 313: 670–673. [DOI] [PubMed] [Google Scholar]
- 11. Gornik, I. , Maravić, G. , Dumić, J. , Flögel, M. , Lauc, G. , Fucosylation of IgG heavy chains is increased in rheumatoid arthritis. Clin Biochem. 1999. 32: 605–608. [DOI] [PubMed] [Google Scholar]
- 12. Shields, R. L. , Lai, J. , Keck, R. , O'Connell, L. Y. , Hong, K. , Meng, Y. G. , Weikert, S. H. A. et al., Lack of fucose on human IgG1 N‐linked oligosaccharide improves binding to human Fcgamma RIII and antibody‐dependent cellular toxicity. J Biol Chem. 2002. 277: 26733–26740. [DOI] [PubMed] [Google Scholar]
- 13. Thomann, M. , Reckermann, K. , Reusch, D. , Prasser, J. , Tejada, M. L. , Fc‐galactosylation modulates antibody‐dependent cellular cytotoxicity of therapeutic antibodies. Mol Immunol. 2016. 73: 69–75. [DOI] [PubMed] [Google Scholar]
- 14. Thomann, M. , Schlothauer, T. , Dashivets, T. , Malik, S. , Avenal, C. , Bulau, P. , Rüger, P. , Reusch, D. , In vitro glycoengineering of IgG1 and its effect on Fc receptor binding and ADCC activity. PLoS One. 2015. 10: e0134949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Taeye, S. W. , Bentlage, A. E. H. , Mebius, M. M. , Meesters, J. I. , Lissenberg‐Thunnissen, S. , Falck, D. , Sénard, T. et al., FcγR Binding and ADCC Activity of Human IgG Allotypes. Front Immunol. 2020. 11: 740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dekkers, G. , Treffers, L. , Plomp, R. , Bentlage, A. E. H. , de Boer, M. , Koeleman, C. A. M. , Lissenberg‐Thunnissen, S. N. et al., Decoding the human immunoglobulin G‐glycan repertoire reveals a spectrum of Fc‐receptor‐ and complement‐mediated‐effector activities. Front Immunol. 2017. 8: 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lippold, S. , Nicolardi, S. , Domínguez‐Vega, E. , Heidenreich, A.‐.K. , Vidarsson, G. , Reusch, D. , Haberger, M. et al., Glycoform‐resolved FcɣRIIIa affinity chromatography‐mass spectrometry. MAbs. 2019. 11: 1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosenstock, P. , Kaufmann, T. , Sialic acids and their influence on human NK cell function. Cells 2021. 10: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pereira, M. S. , Durães, C. , Catarino, T. A. , Costa, J. L. , Cleynen, I. , Novokmet, M. , Krištić, J. et al., Genetic variants of the MGAT5 gene are functionally implicated in the modulation of T cells glycosylation and plasma IgG glycome composition in ulcerative colitis. Clin Transl Gastroenterol. 2020. 11: e00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Šimurina, M. , de Haan, N. , Vučković, F. , Kennedy, N. A. , Štambuk, J. , Falck, D. , Trbojević‐Akmačić, I. et al., Glycosylation of immunoglobulin G associates with clinical features of inflammatory bowel diseases. Gastroenterology 2018. 154: 1320–1333.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhargava, R. , Lehoux, S. , Maeda, K. , Tsokos, M. G. , Krishfield, S. , Ellezian, L. Y. , Pollak, M. et al., Aberrantly glycosylated IgG elicits pathogenic signaling in podocytes and signifies lupus nephritis. JCI Insight 2021. 6: e147789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klarić, L. , Tsepilov, Y. A. , Stanton, C. M. , Mangino, M. , Sikka, T. T. , Esko, T. , Pakhomov, E. et al., Glycosylation of immunoglobulin G is regulated by a large network of genes pleiotropic with inflammatory diseases. Sci Adv. 2020. 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gudelj, I. , Lauc, G. , Pezer, M. , Immunoglobulin G glycosylation in aging and diseases. Cell Immunol. 2018. 333: 65–79. [DOI] [PubMed] [Google Scholar]
- 24. Chakraborty, S. , Gonzalez, J. , Edwards, K. , Mallajosyula, V. , Buzzanco, A. S. , Sherwood, R. , Buffone, C. et al., Proinflammatory IgG Fc structures in patients with severe COVID‐19. Nat Immunol. 2021. 22: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larsen, M. D. , Graaf, E.L.d , Sonneveld, M. E. , Plomp, H. R. , Nouta, J. , Hoepel, W. , Chen, H. J. et al., Afucosylated IgG characterizes enveloped viral responses and correlates with COVID‐19 severity. Science (80‐). 2021. 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoepel, W. , Chen, H.‐J. , Geyer, C. E. , Allahverdiyeva, S. , Manz, X. D. , Taeye, SWd. , Aman, J. et al., High titers and low fucosylation of early human anti‐SARS‐CoV‐2 IgG promote inflammation by alveolar macrophages. Sci Transl Med. 2021. 13: eabf8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petrović, T. , Alves, I. , Bugada, D. , Pascual, J. , Vučković, F. , Skelin, A. , Gaifem, J. et al., Composition of the immunoglobulin G glycome associates with the severity of COVID‐19. Glycobiology 2020. 31: 372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. COVID‐19 Clinical management: living guidance [Internet]. https://www.who.int/publications/i/item/WHO‐2019‐nCoV‐clinical‐2021–1
- 29. Gudelj, I. , Salo, P. P. , Trbojević‐Akmačić, I. , Albers, M. , Primorac, D. , Perola, M. , Lauc, G. et al., Low galactosylation of IgG associates with higher risk for future diagnosis of rheumatoid arthritis during 10 years of follow‐up. Biochim Biophys Acta ‐ Mol Basis Dis. 2018. 1864: 2034–2039. [DOI] [PubMed] [Google Scholar]
- 30. Rombouts, Y. , Ewing, E. , van de Stadt, L. A. , Selman, M. H. J. , Trouw, L. A. , Deelder, A. M. et al., Anti‐citrullinated protein antibodies acquire a pro‐inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann Rheum Dis. 2015. 74: 234–241. [DOI] [PubMed] [Google Scholar]
- 31. Nimmerjahn, F. , Anthony, R. M. , Ravetch, J. V. , Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc Natl Acad Sci USA. 2007. 104: 8433–8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee‐Sundlov, M. M. , Ashline, D. J. , Hanneman, A. J. , Grozovsky, R. , Reinhold, V. N. , Hoffmeister, K. M. , Lau, J. T. et al., Circulating blood and platelets supply glycosyltransferases that enable extrinsic extracellular glycosylation. Glycobiology 2017. 27: 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cross, A. S. , Hyun, S. W. , Miranda‐Ribera, A. , Feng, C. , Liu, A. , Nguyen, C. , Zhang, L. et al., NEU1 and NEU3 sialidase activity expressed in human lung microvascular endothelia: NEU1 restrains endothelial cell migration, whereas NEU3 does not. J Biol Chem. 2012. 287: 15966–15980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alves, I. , Vicente, M. M. , Gaifem, J. , Fernandes, Â. , Dias, A. M. , Rodrigues, C. S. , Oliveira, J. C. et al., SARS‐CoV‐2 infection drives a glycan switch of peripheral T cells at diagnosis. J. Immunol. 2021. 207: 1591–1598. [DOI] [PubMed] [Google Scholar]
- 35. Chakraborty, S. , Gonzalez, J. C. , Sievers, B. L. , Mallajosyula, V. , Chakraborty, S. , Dubey, M. , Ashraf, U. et al., Early non‐neutralizing, afucosylated antibody responses are associated with COVID‐19 severity. Sci Transl Med. 2022. 14: eabm7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seeling, M. , Brückner, C. , Nimmerjahn, F. , Differential antibody glycosylation in autoimmunity: sweet biomarker or modulator of disease activity? Nat Rev Rheumatol. 2017. 13: 621–630. [DOI] [PubMed] [Google Scholar]
- 37. Karsten, C. M. , Pandey, M. K. , Figge, J. , Kilchenstein, R. , Taylor, P. R. , Rosas, M. , McDonald, J. U. et al., Anti‐inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin‐1. Nat Med. 2012. 18: 1401–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Selman, M. H. J. , de Jong, S. E. , Soonawala, D. , Kroon, F. P. , Adegnika, A. A. , Deelder, A. M. , Hokke, C. H. et al., Changes in antigen‐specific IgG1 Fc N‐glycosylation upon influenza and tetanus vaccination. Mol Cell Proteomics. 2012. 11: M111.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cossarizza, A. , Chang, H.‐D. , Radbruch, A. , Acs, A. , Adam, D. , Adam‐Klages, S. , Agace, W. W. et al., Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur J Immunol. 2019. 49: 1457–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Åström, E. , Stål, P. , Zenlander, R. , Edenvik, P. , Alexandersson, C. , Haglund, M. , Rydén, I. et al., Reverse lectin ELISA for detecting fucosylated forms of α1‐acid glycoprotein associated with hepatocellular carcinoma. PLoS One. 2017. 12. 10.1371/journal.pone.0173897 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


