Abstract
Aims
To assess heart failure (HF) in‐hospital quality of care and outcomes before and during the COVID‐19 pandemic.
Methods and results
Patients hospitalized for HF with ejection fraction (EF) <40% in the American Heart Association Get With The Guidelines©‐HF (GWTG‐HF) registry during the COVID‐19 pandemic (3/1/2020–4/1/2021) and pre‐pandemic (2/1/2019–2/29/2020) periods were included. Adherence to HF process of care measures, in‐hospital mortality, and length of stay (LOS) were compared in pre‐pandemic vs. pandemic periods and in patients with vs. without COVID‐19. Overall, 42 004 pre‐pandemic and 37 027 pandemic period patients (median age 68, 33% women, 58% White) were included without observed differences across clinical characteristics, comorbidities, vital signs, or EF. Utilization of guideline‐directed medical therapy at discharge was comparable across both periods, with rates of implantable cardioverter defibrillator (ICD) placement or prescription lower during the pandemic (vs. pre‐pandemic period). In‐hospital mortality (3.0% vs. 2.5%, p <0.0001) and LOS (mean 5.7 vs. 5.4 days, p <0.0004) were higher during the pandemic vs. pre‐pandemic. The highest in‐hospital mortality during the pandemic was observed among patients hospitalized in the Northeast region (3.4%). Among patients concurrently diagnosed with COVID‐19 (n = 549; 1.5%), adherence to ICD placement or prescription, prescription of aldosterone antagonist or angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor–neprilysin inhibitor at discharge were lower, and in‐hospital mortality (8.2% vs. 3.0%, p <0.0001) and LOS (mean 7.7 vs. 5.7 days, p <0.0001) were higher than those without COVID‐19.
Conclusion
Among GWTG‐HF participating hospitals, patients hospitalized for HF with reduced EF during the pandemic received similar care quality but experienced higher in‐hospital mortality than the pre‐pandemic period.
Keywords: Heart failure, Quality of care, Outcomes, COVID‐19
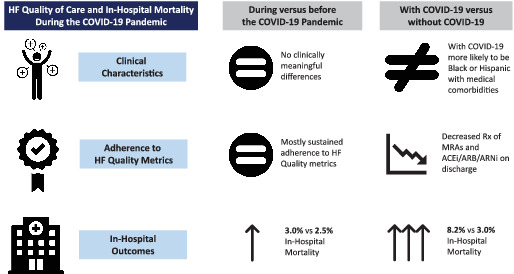
Heart failure (HF) quality of care and in‐hospital mortality during the COVID‐19 pandemic. ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor.
Introduction
The COVID‐19 pandemic has led to significant patient morbidity and mortality with over 300 million reported infections and over 5 million deaths worldwide. 1 Patients with cardiovascular risk factors and established cardiovascular diseases such as obesity, hypertension, diabetes, coronary artery disease, and heart failure (HF) have a higher risk of severe infection and worse clinical outcomes. 2 COVID‐19 infection may also impact patients with HF either as a potential cause of decompensation — similar to other respiratory viruses — or as direct viral harm in the form of myocardial injury through viral invasion, or from vascular endothelial dysfunction from systemic inflammation. 3 , 4 , 5 Overall cardiovascular death rates were higher during the initial phase of the COVID‐19 pandemic among geographic areas that experienced a surge in cases. 6 Estimates of in‐hospital mortality for patients with HF and COVID‐19 have ranged from 24% to 50% across the United States and Europe and across different time periods of the pandemic. 3 , 7 , 8
Besides the direct patient harm caused by viral infection, reports also demonstrate excess mortality not explained by COVID‐19 infection, 9 , 10 suggesting significant downstream effects of the pandemic on patient care and outcomes. 6 These include a dramatic decline in hospitalizations for cardiovascular conditions such as myocardial infarction and HF 11 , 12 , 13 with a concomitant disruption in ambulatory care as in‐person appointments were transitioned to telehealth. 14 Additionally, there were decreases in both emergent and elective procedures during the COVID‐19 pandemic, 12 further illustrating the challenges to delivering high‐quality care to patients without COVID‐19.
Given massive care disruptions and worldwide mortality from COVID‐19, understanding quality of care and in‐hospital outcomes of patients with HF are of principle importance. Accordingly, we aimed to describe and compare clinical characteristics, adherence to evidence‐based quality measures, and in‐hospital mortality among patients hospitalized for HF with reduced ejection fraction (HFrEF) during the COVID‐19 pandemic compared with the pre‐pandemic period and among patients with concurrent HF and COVID‐19 versus without COVID‐19.
Methods
Data source and patient population
This study utilized the American Heart Association's Get With The Guidelines®‐Heart Failure (GWTG‐HF) registry, and details regarding the registry have been published previously. 15 GWTG‐HF is a continuous quality improvement‐based registry of patients hospitalized for HF. The details of the registry design and data collected are reported in online supplementary Methods. Patients in the GWTG‐HF registry with a principal hospital admission diagnosis of HFrEF from 2/1/ 2019 to 4/1/ 2021 were included in this analysis. The details of the study inclusion and exclusion criteria are reported in online supplementary Table S1 . The pre‐pandemic cohort included 150 677 patients from 533 centres admitted between 2/1/2019 to 2/29/2020. The pandemic period cohort included 129 495 patients from 424 centres admitted from 3/1/ 2020 to 41/2021. The pandemic period cohort of patients with acute decompensated HFrEF included 549 patients with a concomitant COVID‐19 and 36 478 patients without COVID‐19 diagnosis (recorded in the GWTG‐HF registry as active SARS‐CoV‐2 infection at the time of admission or during hospitalization).
Outcomes of interest
The primary outcomes were in‐hospital all‐cause mortality, length of stay (LOS, in days), and discharge disposition. Secondary outcomes included rates of adherence to GWTG‐HF hospital quality measures. Adherence was presented only for patients who did not die during the HF hospitalization, and rates were calculated by dividing the number of patients who received the therapy by the number of patients eligible for the therapy, excluding patients with missing information and those who had a listed contraindication from the eligible population. GWTG‐HF quality measures included in the analysis are reported in online supplementary Table S2 .
Statistical analysis
The study population was stratified by the hospitalization period (before vs. during the pandemic) and by the presence of concurrent COVID‐19 (for the pandemic period hospitalizations). Patient‐ and hospital‐level characteristics were reported as percentages (for categorical variables) or median (interquartile range, IQR) for continuous variable. Pearson χ 2 tests were used to compare differences in categorical variables, and Kruskal–Wallis tests were used to compare differences across continuous variables. Standardized differences were reported for all variables, and a difference >10 indicated a meaningful difference between groups. P‐values were reported for the primary outcomes, with p <0.05 set as the level of significance.
Multivariable adjusted regression models were constructed for mortality and LOS outcomes with the following covariates: patient demographics, medical history, vital signs at admission, and hospital characteristics. Logistic regression and negative binomial log‐linear regression models were used for mortality and LOS, respectively, and fit using Generalized Estimating Equations with an exchangeable working correlation structure to account for within‐hospital clustering. Time‐to‐event cumulative incidence curves determined time‐dependent in‐hospital mortality rates. Patient characteristics and unadjusted outcome rates were also compared for subgroups stratified by geographic region: Northeast, Midwest, South, and West. Finally, sensitivity analyses were also conducted limiting the cohort to hospitals that were represented in both the pre‐pandemic and the pandemic cohort.
Results
The final population included 79 031 patients (median age 68 years, 33% women, 28% Black patients, median left ventricular ejection fraction 25%). Of these, 42 004 (from 386 centres) were admitted during the pre‐pandemic period, and 37 027 patients (from 373 centres) were admitted during the pandemic period (online supplementary Table S1 ). Before the pandemic, the median hospital had 10 qualifying HF patients per month (range 1–104, IQR 5–16), compared with a median of 9 qualifying HF patients per month (range 1–83, IQR 5–15) during the pandemic. Over the entire pre‐pandemic period, median hospital had 88 qualifying HF admissions (range 1–825, IQR 48–145) versus 77 qualifying HF admissions (range 1–584, IQR 40–126) during the pandemic. There was no statistically significant difference in the number of total HF admissions between the two periods (non‐parametric Wilcoxon score [rank sums] test, p = 0.1064).
There were no clinically significant differences in demographic characteristics and clinical comorbidities among patients admitted during the pre‐pandemic versus the pandemic period (Table 1 ). Patients hospitalized during the pandemic versus the pre‐pandemic period had higher average troponin levels. There was also greater incidence of cardiac ischaemia or acute coronary syndromes among hospitalized HF patients during the pandemic versus the pre‐pandemic period (11.8% vs. 8.4%). In contrast, rates of venous thromboembolism (deep vein thrombosis/pulmonary embolism) were not meaningfully different in the pre‐pandemic versus the pandemic period (Table 1 ). There were no differences in in‐hospital care measures such as the utilization of coronary angiography, cardiac device implantation, or administration of dialysis. Hospital‐level characteristics such as hospital location, teaching status, and geographic region were not different across the pre‐pandemic versus the pandemic period groups.
Table 1.
Baseline characteristics of patients hospitalized for heart failure before and during the COVID‐19 pandemic
| Overall (n = 79 031) | Before COVID‐19 pandemic (n = 42 004) | During COVID‐19 pandemic (n = 37 027) | Standardized difference a | p‐value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years), median (IQR) | 68 (57–79) | 68 (58–79) | 67 (57–78) | 5.6 | <0.001 |
| Female sex (%) | 33.2 | 33.2 | 33.1 | 0.2 | 0.761 |
| Race (%) | |||||
| White | 58.3 | 58.6 | 58.0 | 1.2 | <0.0001 |
| Black | 28.2 | 28.1 | 28.3 | 0.5 | |
| Hispanic | 8.1 | 8.1 | 8.1 | 0.1 | |
| Asian | 1.9 | 2.0 | 1.8 | 1.6 | |
| Other | 3.5 | 3.2 | 3.8 | 3.1 | |
| Insurance (%) | |||||
| None documented | 6.2 | 6.2 | 6.1 | 1.7 | <0.0001 |
| Medicare | 44.8 | 44.7 | 45.0 | 4.3 | |
| Medicaid | 21.2 | 20.3 | 22.2 | 1.7 | |
| Other | 27.8 | 28.8 | 26.7 | 7.8 | |
| Medical history | |||||
| HF admissions in past 6 months (%) | 0.5 | <0.0001 | |||
| >2 | 8.3 | 8.7 | 7.8 | ||
| 2 | 7.8 | 8.4 | 7.1 | ||
| 1 | 24.3 | 24.9 | 23.5 | ||
| 0 | 42.6 | 41.7 | 43.7 | ||
| Atrial fibrillation/flutter (%) | 36.5 | 36.8 | 36.1 | 1.5 | 0.040 |
| Coronary disease (%) | 52.8 | 52.7 | 52.9 | 0.3 | 0.649 |
| Valvular heart disease (%) | 18.7 | 18.7 | 18.7 | 0.1 | 0.879 |
| Anaemia (%) | 22.8 | 21.9 | 23.7 | 4.4 | <0.0001 |
| COPD or asthma (%) | 32.2 | 31.9 | 32.6 | 1.4 | 0.051 |
| CVA/TIA (%) | 16.1 | 16.0 | 16.2 | 0.6 | 0.383 |
| Peripheral vascular disease (%) | 11.5 | 11.2 | 11.9 | 2.2 | 0.002 |
| Hyperlipidaemia (%) | 58.4 | 56.6 | 60.6 | 8.1 | <0.0001 |
| Hypertension (%) | 83.5 | 82.0 | 85.1 | 8.4 | <0.0001 |
| Diabetes (%) | 45.7 | 44.7 | 46.8 | 4.1 | <0.0001 |
| Renal insufficiency (%) | 26.5 | 26.8 | 26.2 | 1.2 | 0.098 |
| Dialysis (%) | 4.2 | 4.1 | 4.3 | 1.2 | 0.097 |
| ICD (%) | 17.3 | 17.9 | 16.5 | 3.6 | <0.0001 |
| Smoking (%) | 24.4 | 23.9 | 25.0 | 2.7 | 0.0002 |
| DVT or PE (%) | 1.9 | 1.7 | 2.0 | 2.5 | 0.004 |
| Ischaemia/ACS | 10.0 | 8.4 | 11.8 | 11.3 | <0.0001 |
| Admission laboratory and vital signs | |||||
| Heart rate (bpm), median (IQR) | 78 (69–89) | 78 (69–88) | 79 (70–90) | 5.8 | <0.0001 |
| Lower extremity oedema (%) | 64.9 | 64.3 | 65.6 | 2.7 | 0.046 |
| Systolic BP (mmHg), median (IQR) | 132 (114–151) | 131 (114–151) | 132 (115–152) | 4.2 | <0.0001 |
| Diastolic BP (mmHg), median (IQR) | 70 (61–78) | 69 (61–78) | 70 (62–79) | 5.9 | <0.0001 |
| BMI (kg/m2), mean (SD) | 28.7 (24.5–34.3) | 28.4 (24.4–34.1) | 28.7 (24.6–34.4) | 3.8 | <0.0001 |
| Obese (BMI >30 kg/m2) (%) | 42.7 | 41.8 | 43.4 | 3.4 | 0.0002 |
| Respiration (breath/min), median (IQR) | 20 (18–22) | 20 (18–22) | 20 (18–22) | 9.4 | <0.0001 |
| BNP (pg/ml), mean (SD) | 1442 (2046) | 1458 (1983) | 2116 (4250) | 4.1 | 0.201 |
| NT‐proBNP (pg/ml), mean (SD) | 12 669 (15334) | 12 395 (15072) | 13 031 (15667) | 4.1 | 0.0007 |
| eGFR (ml/min/1.73 m2), median (IQR) b | 52.2 (34.5–71.4) | 51.9 (34.4–71.1) | 52.6 (34.6–71.7) | 6.7 | 0.016 |
| Troponin (ng/dl), mean (SD) | 3.0 (15.1) | 1.7 (8.5) | 4.5 (20.3) | 17.7 | <0.0001 |
| Troponin (%) | 0.001 | ||||
| Troponin I | 80.4 | 79.6 | 81.4 | 4.5 | |
| Troponin T | 19.6 | 20.4 | 18.6 | 6.1 | |
| Troponin abnormal (%) | 56.4 | 55.1 | 58.1 | 6.1 | <0.0001 |
| In‐hospital care | |||||
| Ejection fraction (%), median (IQR) | 25 (20–32) | 25 (20–32) | 25 (20–32) | 2.5 | 0.0002 |
| Ejection fraction <30% (%) | 66.0 | 65.4 | 66.7 | 2.8 | <0.0001 |
| Coronary angiography (%) | 16.2 | 15.7 | 16.5 | 2.3 | 0.014 |
| Intra‐aortic balloon pump (%) | 0.5 | 0.5 | 0.6 | 1.9 | 0.048 |
| Mechanical ventilation (%) | 2.9 | 3.0 | 2.8 | 1.0 | 0.264 |
| Dialysis (%) | 3.6 | 3.3 | 3.7 | 2.1 | 0.026 |
| Left ventricular assist device (%) | 0.6 | 0.4 | 0.4 | 3.4 | 0.0003 |
| Heart transplant (%) | 0.1 | 0.1 | 0.1 | 0.3 | 0.520 |
| Hospital characteristics | |||||
| No. of beds, median (IQR) | 385 (248–583) | 385 (248–581) | 387 (248–595) | 2.5 | 0.0003 |
| No. of HF admissions per year, median (IQR) | 103 (62–170) | 118 (71–190) | 86 (54–140) | 27 | <0.0001 |
| Geographic region (%) | 0.065 | ||||
| West | 15.8 | 15.8 | 15.7 | 1.3 | |
| South | 34.0 | 34.4 | 33.6 | 1.7 | |
| Midwest | 24.2 | 24.3 | 24.6 | 0.7 | |
| Northeast | 25.8 | 25.6 | 26.2 | 0.2 | |
| Rural location (%) | 2.5 | 2.7 | 2.3 | 2.6 | 0.0002 |
| Teaching hospital (%) | 79.2 | 79.5 | 79.0 | 1.4 | 0.058 |
ACS, acute coronary syndrome; BMI, body mass index; BNP, B‐type natriuretic peptide; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DVT, deep vein thrombosis; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter‐defibrillator; IQR, interquartile range; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PE, pulmonary embolism; SD, standard deviation; TIA, transient ischaemic attack.
Standardized difference represents the absolute difference in rank‐based means or proportions divided by the standard deviation and multiplied by 100.
eGFR calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) equation.
Overall, the in‐hospital death rate was 2.7%, and the mean LOS was 5.6 days (standard deviation [SD] 5.3). The in‐hospital mortality rate was significantly higher during the pandemic vs. the pre‐pandemic period (3.0% vs. 2.5%, p <0.0001) (Table 2 ). Furthermore, hospitalization during the COVID‐19 pandemic was associated with a greater risk of in‐hospital mortality (adjusted odds ratio 1.23, 95% confidence interval [CI] 1.11–1.37, p <0.0001) (Table 2 ). In time to event analysis, the time to death during hospitalization was not different between pre‐pandemic versus the pandemic period (p = 0.9550; online supplementary Figure S1 ). There were similar rates of cardiovascular and non‐cardiovascular death before and during the COVID‐19 pandemic (p = 0.582). In analysis evaluating mortality rate over time during the pandemic, the rates of in‐hospital morality were highest between March 2020 to May 2020 and December 2020 to March 2021 consistent with the COVID‐19 first and second waves. The adjusted risk of in‐hospital mortality did not change significantly during the pandemic period as compared with the first month of the COVID‐19 pandemic (online supplementary Table S3 ). Mean LOS was longer during the pandemic versus the pre‐pandemic period (5.7 vs. 5.4 days, p = 0.0004). After adjustment for relevant patient and hospital‐level differences, there was a 3% greater LOS (adjusted ratio of means 1.03, 95% CI 1.01–1.05, p = 0.0012) during the COVID‐19 pandemic versus the pre‐pandemic period. Discharge to home was modestly higher during the COVID‐19 pandemic versus the pre‐pandemic period (76.8% vs. 75.3%, p <0.0001). Discharge to other healthcare facilities was lower during the pandemic versus the pre‐pandemic period (9.3% vs. 12.5%, p <0.001). Across GWTG‐HF quality metrics, there were no significant differences in beta‐blocker, aldosterone antagonist, or angiotensin‐converting enzyme inhibitor (ACEi), angiotensin receptor blocker (ARB), or angiotensin receptor–neprilysin inhibitor (ARNi) prescription at discharge (Figure 1 ). During the pandemic, there was a modest but significant increase in discharge prescription of triple therapy (ACEi/ARB/ARNi, mineralocorticoid receptor antagonist [MRA], beta‐blocker) compared with the pre‐pandemic period (25.4% vs. 24.0%). Finally, implantable cardioverter‐defibrillator (ICD) placement or prescription at discharge (54.1% vs. 64.2%) and blood pressure control at discharge (67.8% vs. 82.6%) were significantly lower during the pandemic versus the pre‐pandemic period.
Table 2.
Outcomes of patients hospitalized for heart failure prior to the pandemic and during the pandemic and in patients with COVID‐19 and without COVID‐19
| Before COVID‐19 pandemic (n = 42 004) | During COVID‐19 pandemic (n = 37 027) | p‐value | Without COVID‐19 during hospitalization (n = 36 478) | With COVID‐19 during hospitalization (n = 549) | p‐value | |
|---|---|---|---|---|---|---|
| In‐hospital death (%) | 2.5 | 3.0 | <0.0001 | 3.0 | 8.2 | <0.0001 |
| Cause of death (%) | 0.582 | 0.0007 | ||||
| Cardiovascular | 70.7 | 68.5 | 69.8 | 37.5 | ||
| Non‐cardiovascular | 19.9 | 20.2 | 19.0 | 50.0 | ||
| Unknown | 9.4 | 11.3 | 11.2 | 12.5 | ||
| Unadjusted OR (95% CI) a | Reference | 1.22 (1.10–1.35) | <0.0001 | Reference | 3.14 (2.23–4.42) | <0.0001 |
| Adjusted OR (95% CI) a | Reference | 1.23 (1.11–1.37) | <0.0001 | Reference | 2.92 (2.14–3.99) | <0.0001 |
| Length of stay (days), mean (SD) | 5.4 (4.9) | 5.7 (5.7) | 0.0004 | 5.7 (5.7) | 7.7 (7.9) | <0.0001 |
| Unadjusted ratio of means (95% CI) b | Reference | 1.04 (1.02–1.06) | 0.0004 | Reference | 1.35 (1.22–1.49) | <0.0001 |
| Adjusted ratio of means (95% CI) b | Reference | 1.03 (1.01–1.05) | 0.0012 | Reference | 1.35 (1.22–1.50) | <0.0001 |
| Discharge disposition (%) | <0.0001 | <0.0001 | ||||
| Home | 75.3 | 76.8 | 76.9 | 68.5 | ||
| Home hospice | 2.8 | 3.4 | 3.4 | 2.9 | ||
| Healthcare facility hospice | 1.7 | 1.4 | 1.4 | 2.0 | ||
| Acute care facility | 2.8 | 2.7 | 2.8 | 1.5 | ||
| Other healthcare facility | 12.5 | 9.3 | 9.2 | 14.0 | ||
| Against medical advice | 2.4 | 3.3 | 3.4 | 2.7 |
CI, confidence interval; OR, odds ratio; SD, standard deviation.
p‐values based on Pearson chi‐square for all categorical row variables and chi‐square rank based group means score for all continuous/ordinal row variable.
Adjusted OR and ratio of means controlled for patient demographics (age, sex, race, insurance), medical history (anaemia, ischaemic heart disease, cerebrovascular accident/transient ischaemic attack, diabetes mellitus, hyperlipidaemia, hypertension, chronic obstructive pulmonary disease/asthma, peripheral vascular disease, renal insufficiency, cigarette smoking in the past year), vital signs at admission (systolic blood pressure, heart rate, left ventricular ejection fraction), and hospital characteristics (region, hospital type, number of beds, rural vs. urban location).
ORs were assessed using a logistic regression model.
Ratio of means were calculated using a negative binomial model with a log link.
Figure 1.

Adherence to guidelines of patients hospitalized for heart failure and discharged alive, with comparison before and during the COVID‐19 pandemic. Asterisks note clinically significant differences, as determined by a standardized difference of 10 or greater. Standardized differences were calculated as the absolute differences in rank‐based means or proportions divided by the standard deviation and multiplied by 100. ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; ICD, implantable cardioverter‐defibrillator.
Clinical characteristics and outcomes stratified by geographic region
Across each geographic region, there were significant differences in patient outcomes (online supplementary Table S4 ). In‐hospital mortality was highest during the pandemic for patients with HF admitted to the Northeast (3.4%) versus other regions. Changes in GWTG‐HF quality metrics during the pandemic (vs. pre‐pandemic) period were similar across all four regions, with clinically significant decreases in prescription of ACEi/ARB/ARNi, ICD placement or prescription, and blood pressure control at discharge (online supplementary Tables S5–S8 ).
Comparison of patients with heart failure and COVID‐19 versus patients without COVID‐19
The GWTG‐HF registry included records for 37 027 patients hospitalized for acute HFrEF during the COVID‐19 pandemic; 549 (1.5%) patients had concurrent COVID‐19, and 36 478 patients (98.5%) did not have concomitant COVID‐19. Overall, there were no clinically meaningful differences in age or sex between patients with COVID‐19 versus patients without COVID‐19 (Table 3 ). Patients with (vs. without) COVID‐19 were more commonly of Hispanic (14.2% vs. 8.1%) or Black race (29.5% vs. 28.3%). Patients with (vs. without) COVID‐19 had a higher prevalence of co‐existing anaemia (28.8% vs. 23.7%) and diabetes (51.9% vs. 46.7%) without other meaningful differences in clinical comorbidities or admission vital signs. Overall troponin levels and percent of patients with an abnormal troponin level were greater in patients with (vs. without) COVID‐19 (mean 7.7 ng/dl, 63.8% abnormal vs. 4.4 ng/dl, 58.0% abnormal) with no significant differences in N‐terminal pro‐B‐type natriuretic peptide levels across the two groups. There was a significant increase in venous thromboembolism in patients with (vs. without) COVID‐19 (6.1% vs. 2.0%) without significant difference in cardiac ischaemia or acute coronary syndrome. There was less lower extremity edema on admission in patients with (vs. without) COVID‐19. Utilization of mechanical ventilation and dialysis were higher, and utilization of coronary angiography was lower in patients with (vs. without) COVID‐19. At the hospital level, patients with (vs. without) COVID‐19 were admitted to smaller hospitals and were more likely to be hospitalized in the geographic northeast (31.0% vs. 26.1%).
Table 3.
Baseline characteristics of patients hospitalized for heart failure with and without COVID‐19 during the pandemic
| Overall (n = 37 027) | With COVID‐19 during hospitalization (n = 549) | Without COVID‐19 during hospitalization (n = 36 478) | Standardized difference a | p‐value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years), mean (SD) | 66.7 (15.0) | 65.9 (14.9) | 66.7 (15.0) | 5.5 | 0.204 |
| Female sex (%) | 33.1 | 33.2 | 33.1 | 4.3 | 0.311 |
| Race (%) | |||||
| White | 58.0 | 48.8 | 58.1 | 18.7 | <0.0001 |
| Black | 28.3 | 29.5 | 28.3 | 2.7 | |
| Hispanic | 8.1 | 14.2 | 8.1 | 19.7 | |
| Asian | 1.8 | 2.6 | 1.8 | 5.1 | |
| Other | 3.8 | 4.9 | 3.8 | 5.7 | |
| Insurance (%) | 0.008 | ||||
| None documented | 6.1 | 6.9 | 6.1 | 3.5 | |
| Medicare | 45.0 | 39.5 | 45.1 | 9.3 | |
| Medicaid | 22.2 | 27.9 | 22.1 | 13.9 | |
| Other | 26.7 | 25.7 | 26.7 | 1.1 | |
| Medical history | |||||
| HF admissions in past 6 months | 3.1 | 0.199 | |||
| >2 | 7.8 | 12.4 | 7.7 | ||
| 2 | 7.1 | 5.6 | 7.2 | ||
| 1 | 23.5 | 19.8 | 23.6 | ||
| 0 | 43.7 | 45.1 | 43.7 | ||
| Atrial fibrillation/flutter (%) | 36.1 | 37.2 | 36.1 | 2.3 | 0.602 |
| Coronary disease (%) | 52.9 | 52.6 | 52.9 | 0.6 | 0.892 |
| Valvular heart disease (%) | 18.7 | 19.4 | 18.7 | 1.7 | 0.698 |
| Anaemia (%) | 23.7 | 28.8 | 23.7 | 11.7 | 0.005 |
| COPD or asthma (%) | 32.6 | 31.7 | 32.6 | 1.9 | 0.669 |
| CVA/TIA (%) | 16.2 | 15.7 | 16.3 | 1.5 | 0.722 |
| Peripheral vascular disease (%) | 11.9 | 12.7 | 11.9 | 2.6 | 0.537 |
| Hyperlipidaemia (%) | 60.6 | 62.7 | 60.5 | 4.6 | 0.296 |
| Hypertension (%) | 85.1 | 84.1 | 85.2 | 2.8 | 0.506 |
| Diabetes (%) | 46.8 | 51.9 | 46.7 | 10.3 | 0.017 |
| Renal insufficiency (%) | 26.2 | 27.5 | 26.2 | 2.9 | 0.506 |
| Dialysis (%) | 4.3 | 5.5 | 4.3 | 5.7 | 0.164 |
| ICD (%) | 16.5 | 16.1 | 16.5 | 1.3 | 0.759 |
| Smoking (%) | 52.0 | 21.5 | 25.1 | 8.3 | 0.059 |
| DVT or PE (%) | 2.0 | 6.1 | 2.0 | 21.0 | <0.0001 |
| Iscahemia/ACS | 11.8 | 13.0 | 11.8 | 3.8 | 0.640 |
| Admission laboratory and vital signs | |||||
| Heart rate (bpm), median (IQR) | 79 (70–90) | 78 (68–89) | 79 (70–90) | 3.6 | 0.452 |
| Lower extremity oedema (%) | 65.6 | 57.2 | 65.7 | 17.5 | 0.033 |
| Systolic BP (mmHg), median (IQR) | 132 (115–152) | 130 (113–148) | 132 (115–152) | 9.8 | 0.716 |
| Diastolic BP (mmHg), median (IQR) | 70 (62–79) | 72 (63–80) | 70 (62–79) | 8.8 | 0.063 |
| BMI (kg/m2), mean (SD) | 30.3 (30.3) | 29.7 (29.7) | 30.3 (30.3) | 7.5 | 0.091 |
| Obese (BMI >30 kg/m2) (%) | 43.4 | 43.2 | 43.5 | 0.6 | 0.908 |
| Respiration (breath/min), median (IQR) | 20 (18–22) | 20 (18–22) | 20 (18–22) | 8.1 | 0.773 |
| BNP (pg/ml), mean (SD) | 2116 (4250) | 1801 (1870) | 2121 (4277) | 9.7 | 0.158 |
| NT‐proBNP (pg/ml), mean (SD) | 13 031 (15667) | 11 913 (13660) | 13 046 (15693) | 7.7 | 0.674 |
| eGFR (ml/min/1.73 m2), median (IQR) b | 52.6 (34.6–71.7) | 53.7 (33.1–75.4) | 52.5 (34.6–71.7) | 3.2 | 0.631 |
| Troponin (ng/dl), mean (SD) | 4.5 (20.3) | 7.7 (26.7) | 4.4 (20.2) | 14.0 | |
| Troponin (%) | 0.659 | ||||
| Troponin I | 81.4 | 80.0 | 81.4 | 3.6 | |
| Troponin T | 18.6 | 20.0 | 18.6 | 12.0 | |
| Troponin abnormal (%) | 58.1 | 63.8 | 58.0 | 12.0 | 0.162 |
| In‐hospital care | |||||
| Ejection fraction (%), median (IQR) | 25 (20–32) | 25 (20–33) | 25 (19–32) | 11.4 | 0.009 |
| Ejection fraction <30% (%) | 66.7 | 63.8 | 66.8 | 6.3 | 0.138 |
| Coronary angiography (%) | 16.5 | 11.3 | 16.6 | 15.4 | 0.003 |
| Intra‐aortic balloon pump (%) | 0.6 | 0.7 | 0.6 | 1.5 | 0.752 |
| Mechanical ventilation (%) | 2.8 | 5.2 | 2.8 | 12.4 | 0.003 |
| Dialysis (%) | 3.7 | 5.9 | 3.7 | 10.3 | 0.017 |
| Left ventricular assist device (%) | 0.7 | 0.2 | 0.7 | 6.5 | 0.269 |
| Heart transplant (%) | 0.1 | 0 | 0.1 | 3.7 | 0.594 |
| Hospital characteristics | |||||
| No. of beds, median (IQR) | 387 (248–595) | 371 (237–539) | 387 (248–595) | 13.3 | 0.014 |
| No. of HF admissions per year, median (IQR) | 86 (54–140) | 88 (51–135) | 86 (55–140) | 3.1 | 0.349 |
| Geographic region (%) | 0.013 | ||||
| West | 15.7 | 11.7 | 15.8 | 12 | |
| South | 33.6 | 33.0 | 33.6 | 1.3 | |
| Midwest | 24.6 | 24.4 | 24.6 | 0.4 | |
| Northeast | 26.2 | 31.0 | 26.1 | 10.8 | |
| Rural location (%) | 2.3 | 1.6 | 2.3 | 4.6 | 0.324 |
| Teaching hospital (%) | 78.9 | 79.5 | 78.9 | 1.4 | 0.740 |
ACS, acute coronary syndrome; BMI, body mass index; BNP, B‐type natriuretic peptide; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DVT, deep vein thrombosis; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter‐defibrillator; IQR, interquartile range; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PE, pulmonary embolism; SD, standard deviation; TIA, transient ischaemic attack.
Standardized difference represents the absolute difference in rank‐based means or proportions divided by the standard deviation and multiplied by 100.
eGFR calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) equation.
In‐hospital mortality for patients admitted with HF and concurrent COVID‐19 was 8.2% versus 3.0% in patients without COVID‐19 (p <0.0001) (Table 2 ). After adjustment for both patient‐ and hospital‐level differences, patients with concurrent COVID‐19 and HF had a nearly three‐fold higher adjusted likelihood of mortality during the COVID‐19 pandemic (adjusted odds ratio 2.92, 95% CI 2.14–3.99, p <0.0001). In time to event analysis, the time to death during hospitalization was significantly different among HF patients with versus without COVID‐19. The mortality curves between patients with (vs. without) COVID‐19 separated within 1 week, with a significantly increased hazard of in‐hospital death in COVID‐19 patients, and continued to diverge (p = 0.0002, online supplementary Figure S2 ). Among patients who died, there was greater non‐cardiovascular death among patients with COVID‐19 compared to patients without COVID‐19 (50.0% vs. 19.0%, p = 0.0007). Mean LOS was significantly longer for patients with concurrent COVID‐19 and HF (mean 7.7 days vs. 5.7 days, p <0.0001). In adjusted analysis accounting for patient‐ and hospital‐level factors, patients with COVID‐19 and HF had 35% longer LOS (adjusted ratio of means 1.35, 95% CI 1.22–1.50, p <0.0001). In the adjusted model, other factors associated with longer LOS during the pandemic were Black race, ischaemic aetiology of HF, presence of comorbidities such as kidney insufficiency, diabetes, and anaemia, lower ejection fraction, and hospitalization in teaching hospitals. Patients with concurrent COVID‐19 and HF hospitalization had greater likelihood of discharge to healthcare facilities than those without COVID‐19 (Table 2 ). Across GWTG‐HF quality metrics, HF patients with COVID‐19 (vs. those without COVID‐19) had significantly lower prescription of aldosterone antagonist (53.5% vs. 61.2%), prescription of ACEi/ARB/ARNi (82.1% vs. 85.7%), prescription of triple therapy (21.6% vs. 25.5%), and ICD placement or prescription at discharge (47.8% vs. 55.0%) during the pandemic (Figure 2 ).
Figure 2.
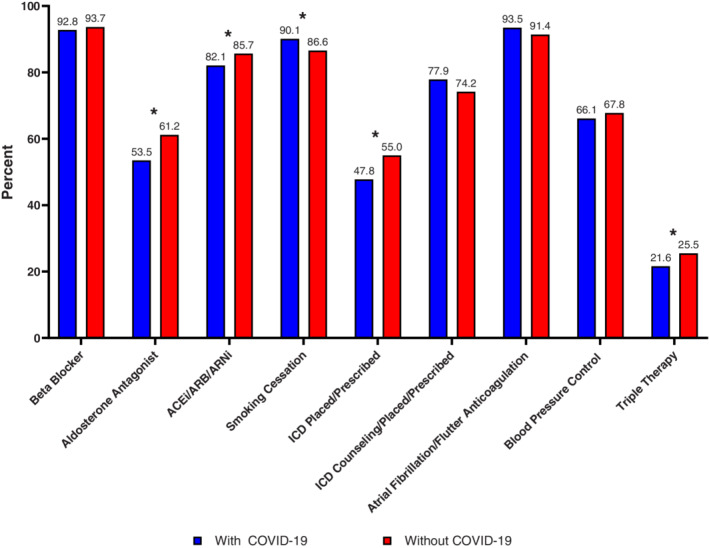
Adherence to guidelines of patients hospitalized for heart failure and discharged alive, with comparison in patients with COVID‐19 to patients without COVID‐19. Asterisks note clinically significant differences, as determined by a standardized difference of 10 or greater. Standardized differences were calculated as the absolute differences in rank‐based means or proportions divided by the standard deviation and multiplied by 100. ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; ICD, implantable cardioverter‐defibrillator.
Sensitivity analysis
A sensitivity analysis was performed limited to centres that enrolled in both the pre‐pandemic and pandemic periods. This analysis excluded 40 centres (1999 patients) before the pandemic and 27 centres (1466 patients) during the pandemic (online supplementary Table S1 ). Among 40 005 patients hospitalized during the pre‐pandemic period and 35 561 patients hospitalized during the pandemic, there were no significant differences in patient demographics, clinical comorbidities, or admission vital signs (online supplementary Table S9 ). In the sensitivity analysis cohort, the pattern of difference between the pre‐pandemic versus the pandemic period for the risk of in‐hospital death, LOS, and adherence to GWTG‐HF quality metrics was like that observed in the primary cohort (online supplementary Table S10 and Figure S3 ).
Discussion
This study has several notable findings. First, in‐hospital mortality and LOS were significantly higher during the pandemic compared with the pre‐pandemic study period, even after adjustment for patient‐ and hospital‐level differences. In‐hospital death was highest for patients admitted to the geographic Northeast. Secondly, the implementation of evidence‐based, guideline‐recommended HFrEF therapies during the COVID‐19 pandemic was comparable to that observed during the pre‐pandemic period among hospitals participating in GWTG‐HF. Finally, among patients hospitalized with HF and COVID‐19, risk of in‐hospital death and LOS were significantly higher and rates of prescription of guideline‐directed medical therapies for HFrEF at discharge were lower as compared to patients without COVID‐19 infection (Graphical Abstract).
To our knowledge, these findings represent the largest national‐level assessment of differences in in‐hospital care and adherence to quality measures in patients admitted with HFrEF before and during the COVID‐19 pandemic in the US. Prior studies evaluating the use of HF therapies focused during the pandemic period in the US have relied on claims based pharmacy data and showed a minimal decline in prescription refills for cardiometabolic and HF therapies. 16 , 17 Conversely, findings from Australia noted similar rates of beta‐blockers and MRAs with decreased prescription of ACEi/ARBs during the pandemic compared to the pre‐pandemic era. 18 Complementing these observations, our findings demonstrate sustained to minimally increased prescription of guideline‐recommended therapies across both time periods. Individual guideline‐directed medical therapy prescription at discharge was sustained, and we observed higher rate of prescription on discharge of triple therapy (ACEi/ARB/ARNi, beta‐blocker, MRA) during the pandemic versus the pre‐pandemic period. However, there were some meaningful differences in other HF care quality metrics during the pre‐pandemic vs. the pandemic period. First, blood pressure control at discharge was worse during the pandemic period. Second, the present study also observed significant declines in prescription or placement of ICD at discharge, which is consistent with reports from Italy 19 and the UK 20 in patients with HF during the pandemic. This also mimics the overall decrease in elective procedures noted during the early phase of the pandemic due to concern for care team exposure to patients during lengthy procedures, lack of pre‐procedural COVID‐19 testing capabilities, and state‐wide or institution‐wide mandates to limit elective or semi‐elective procedures to preserve hospital resources. 21 Among patients with both acute HFrEF and COVID‐19, there were significantly lower rates of prescription of evidence‐based HFrEF therapies such as ACEi/ARB/ARNi and aldosterone antagonist, and ICD placement or prescription. Decreased prescription of ACEi/ARBI/ARNi to patients with COVID‐19 and acute HFrEF on discharge may have been impacted by concerns of the involvement of the ACE2 receptor as a target for viral entry into cells 22 ; however, it is unknown if sustained concerns with regard to the ACE2 receptor led to decreased prescription later in the pandemic given no evidence of harm with ACEi/ARB therapies with COVID‐19. 23
We report increased in‐hospital mortality among all hospitalized HF patients during the pandemic compared to the pre‐pandemic period. Our findings are similar to reports early in the pandemic from Germany 24 and in London, 25 although our findings represent a sustained increased mortality past the initial months of the COVID‐19 pandemic. In contrast, reports from Australia, a country that managed to limit overall COVID‐19 infections, did not note an increase in in‐hospital mortality during the COVID‐19 pandemic. 18
We also observed a nearly three‐fold higher risk of in‐hospital death in patients with acute HFrEF with versus without concomitant COVID‐19 (8.2% vs. 3.0%). There have been several evaluations of in‐hospital mortality of patients with a history of HF admitted for COVID‐19, particularly early during the pandemic (online supplementary Table S11 ). Bhatt et al. 8 observed an in‐hospital mortality of 24.2% of patients admitted with COVID‐19 and history of HF across the United States from March through September 2020 as compared with 2.6% of patients admitted for acute HF. Similarly, for patients with history of HF and COVID‐19, Alvarez‐Garcia et al. 26 observed a mortality of 40.0% across five New York hospitals through June 2020. Chatrath et al. 7 observed a 50.0% mortality in a tertiary care center from London, United Kingdom through May 2020; Rey et al. 27 observed a 48.7% mortality from through April 20, 2020 from a tertiary care center in Madrid, Spain; and Tomasoni et al. 28 observed a 41.1% mortality through April 9, 2020 across 13 Italian centers. The observed mortality rate in the present study is substantially lower than assessments in the United States and internationally. Specifically, Tomasoni et al. 28 and Alvarez‐Garcia et al. 26 highlight findings during initial COVID‐19 surges. The reported differences in mortality rate may be related to the longer follow‐up period with stabilization in death rates over time with health systems getting better equipped at managing COVID‐19 during later stages of the pandemic. Consistent with other studies, we observed higher rates of mortality during the first and second wave of the COVID‐19 pandemic surge. Regional differences in in‐hospital mortality also suggest improvements in mortality as the pandemic endured. In the present study, highest rates of mortality during the pandemic were observed in the Northeast region, and given that the first large outbreak in the United States was predominantly in New York and New Jersey, this elevated mortality may be driven by initially overwhelmed hospital systems in this region. 29 Lastly, the differences in mortality may be due to differences in patient population. Previous studies focused on patients with primary COVID‐19‐related hospitalizations with concomitant history of HF, while the current study focuses on patients with HF‐related hospitalizations with concomitant COVID‐19. Thus, patients in the current study may have had less severe COVID‐19 infection than patients in previous studies, and this may have contributed to lower in‐hospital mortality in the present study.
Aetiologies of the observed higher mortality during the pandemic may be multifactorial. It has been theorized that patients with HF were aggressively triaged and kept out of the hospital unless critically ill in an effort to preserve hospital resources. Outpatient changes in ambulatory care such as clinic cancellations, diagnostic testing delays, and reallocation of resources may have led to worsening outpatient HF management leading to more severe patient presentations. Reports early in the pandemic from London 30 and Australia 18 note greater disease severity in patients admitted with HF compared to historical controls. However, in the present study, there were few observed differences in clinical comorbidities, presentation vital signs, or presentation laboratory values before and during the pandemic, arguing against this theory in the United States, especially as the COVID‐19 pandemic persisted over the study period. Perhaps more importantly, the COVID‐19 pandemic stressed inpatient healthcare systems that had hereto not faced vast surges of severely ill patients. 31 Within the hospital, patients may have been cared for by inpatient teams working outside of their given specialty which may have impacted patient outcomes. 32 For example, hospital medicine physicians or cardiologists may have been re‐deployed to care for patients with COVID‐19, and physician teams with less inpatient medicine experience may have been tasked with caring for less critically ill patients or patients without COVID‐19. 33
Among patients with COVID‐19 and acute HF, greater severity and mortality of COVID‐19 in patients with HF may have been due to poor physiologic reserve from coexisting HF in conjunction with greater prevalence of comorbidities in patients with HF such as hypertension, diabetes, and coronary artery disease. 34 Direct viral effects on endothelial dysfunction triggering microvascular disease and inflammatory surges may also contribute to elevated mortality risk. 35 Circulating troponin levels were overall higher during the pandemic, and myocardial injury has been a prominent component of COVID‐19 infection. However, it is not currently known if the COVID‐19 virus has direct effects on the myocardium, although recent reports suggest that myocardial injury as assessed by troponin levels are similar to other viral‐induced acute respiratory distress syndromes and appear to be a function of age, baseline comorbidities, and multi‐organ dysfunction. 36 Efforts to prevent COVID‐19 infection with vaccination and non‐pharmacologic interventions will have significant value for patients with HF given this persistent observed elevated risk of mortality.
Our study is not without limitations. Use of the GWTG‐HF registry allowed for detailed investigation with high‐quality clinical data. However, while participating centres are diverse and located across the United States, hospital participation and enrolment is voluntary. As such, findings from this study may not be generalizable to hospitals not enrolled in the registry. Inter‐period comparisons may be subject to confounding or limited by factors that were not accounted for. Data quality may have been impacted by the pandemic as hospitals were faced with overwhelming patient surges. However, in sensitivity analysis excluding centres that did not report data both before and after the pandemic, findings remained consistent. Analyses could not be stratified by the type or intensity of non‐pharmacologic policy interventions to reduce COVID‐19 spread, including date and type of COVID‐19 ‘lockdown’. Diuretic use during the hospitalization could not be reliably captured due to missingness in reporting. The GWTG‐HF registry did not contain information on the New York Heart Association functional class on admission. The GWTG‐HF registry did not include information on care team specialty, thus, we were unable to assess for disruptions in delivery of specialty care by cardiologists during the pandemic. The GWTG‐HF registry did not contain data on rehospitalization, thus, rates of 30‐day readmission could not be compared across the pandemic. Lastly, there was overall low prevalence of patients with concurrent COVID‐19 and HF in the population (1.5%), which limits the generalizability of the outcomes assessed in this group.
Conclusion
In conclusion, in‐hospital mortality for patients hospitalized for HFrEF was higher during the COVID‐19 pandemic compared to the pre‐pandemic period, but adherence to HF quality measures was mostly maintained through the pandemic. Among patients hospitalized for acute HFrEF and COVID‐19, in‐hospital mortality was significantly higher with observed worse performance across a variety of HF quality measures as compared to patients hospitalized with acute HFrEF without COVID‐19.
Funding
The Get With The Guidelines®‐Heart Failure (GWTG‐HF) program is provided by the American Heart Association. GWTG‐HF is sponsored, in part, by Novartis, Boehringer Ingelheim, Boehringer Ingelheim and Eli Lilly Diabetes Alliance, Novo Nordisk, Sanofi, AstraZeneca, Bayer, Tylenol and Alnylam Pharmaceuticals.
Conflict of interest: S.J.G. has received research support from the Duke University Department of Medicine Chair's Research Award, American Heart Association, Amgen, AstraZeneca, Bristol Myers Squibb, Cytokinetics, Merck, Novartis, Pfizer, and Sanofi; has served on advisory boards for Amgen, AstraZeneca, Bristol Myers Squibb, Cytokinetics, Roche Diagnostics, and Sanofi; and serves as a consultant for Amgen, Bayer, Bristol Myers Squibb, Merck, Sanofi and Vifor. A.D.D. has received research funding through his institution from the American Heart Association, Amgen, AstraZeneca, Bayer, Intra‐Cellular Therapies, American Regent, the National Heart, Lung, and Blood Institute, Novartis, and the Patient‐Centered Outcomes Research Institute; consults for Amgen, AstraZeneca, Bayer, CareDx, InnaMed, LivaNova, Mardil Medical, Novartis, Procyrion, scPharmaceuticals, Story Health, and Zoll; and has received nonfinancial support from Abbott for educational activities. G.C.F. has received consulting with Abbott, Amgen, AstraZeneca, Bayer, Edwards, Janssen, Merck, Medtronic, and Novartis. A.P. has received research funding from the Texas Health Resources Clinical Scholarship, Gilead Sciences Research Scholar Program, Applied Therapeutics (investigator‐initiated grant), and National Institute of Aging (GEMSSTAR grant 1R03AG067960–01); and serves on the advisory board of Roche Diagnostics. All other authors have nothing to disclose.
Supporting information
Appendix S1. Table S1. Cohort derivation.
Table S2. Get With The Guidelines‐Heart Failure quality measures.
Table S3. Adjusted odds of mortality in each quarter during the COVID‐19 period, compared to March 2020.
Table S4. Outcomes of patients hospitalized for heart failure prior to the pandemic and during the pandemic across regions of the United States.
Table S5. Adherence to guidelines of patients hospitalized for heart failure and discharged alive, with comparison before and during the COVID‐19 pandemic, admitted to the Northeast region.
Table S6. Adherence to guidelines of patients hospitalized for heart failure and discharged alive, with comparison before and during the COVID‐19 pandemic, admitted to the Midwest region.
Table S7. Adherence to guidelines of patients hospitalized for heart failure and discharged alive, with comparison before and during the COVID‐19 pandemic, admitted to the South region.
Table S8. Adherence to guidelines of patients hospitalized for HF and discharged alive, with comparison before and during the COVID‐19 pandemic, admitted to the West region.
Table S9. Baseline patient and hospital characteristics before and during the COVID‐19 pandemic excluding centres that did not report both before and during the pandemic.
Table S10. Outcomes of patients hospitalized for heart failure prior to the pandemic and during the pandemic excluding centres that did not report both before and during the pandemic.
Table S11. Review of previously published evaluations of the association of heart failure and COVID‐19 and in‐hospital mortality.
Figure S1. Time‐to‐event analysis for in‐hospital mortality among patients hospitalized before the pandemic versus patients hospitalized during the pandemic.
Figure S2. Time‐to‐event analysis for in‐hospital mortality among patients hospitalized with COVID‐19 versus patients hospitalized without COVID‐19.
Figure S3. Adherence to guidelines of patients hospitalized for heart failure and discharged alive, with comparison before and during the COVID‐19 pandemic, excluding centres that did not report both before and during the pandemic.
References
- 1. World Health Organization . WHO Coronavirus (COVID‐19) Dashboard. https://covid19.who.int/ (accessed 15 March 2022).
- 2. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tomasoni D, Italia L, Adamo M, Inciardi RM, Lombardi CM, Solomon SD, et al. COVID‐19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail. 2020;22:957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okada H, Yoshida S, Hara A, Ogura S, Tomita H. Vascular endothelial injury exacerbates coronavirus disease 2019: the role of endothelial glycocalyx protection. Microcirculation. 2021;28:e12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kytomaa S, Hegde S, Claggett B, Udell JA, Rosamond W, Temte J, et al. Association of influenza‐like illness activity with hospitalizations for heart failure: the Atherosclerosis Risk in Communities study. JAMA Cardiol. 2019;4:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wadhera RK, Shen C, Gondi S, Chen S, Kazi DS, Yeh RW. Cardiovascular deaths during the COVID‐19 pandemic in the United States. J Am Coll Cardiol. 2021;77:159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chatrath N, Kaza N, Pabari PA, Fox K, Mayet J, Barton C, et al. The effect of concomitant COVID‐19 infection on outcomes in patients hospitalized with heart failure. ESC Heart Fail. 2020;7:4443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhatt AS, Jering KS, Vaduganathan M, Claggett BL, Cunningham JW, Rosenthal N, et al. Clinical outcomes in patients with heart failure hospitalized with COVID‐19. JACC Heart Fail. 2021;9:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Faust JS, Krumholz HM, Du C, Mayes KD, Lin Z, Gilman C, et al. All‐cause excess mortality and COVID‐19‐related mortality among US adults aged 25‐44 years, March‐July 2020. JAMA. 2021;325:785–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinberger DM, Chen J, Cohen T, Crawford FW, Mostashari F, Olson D, et al. Estimation of excess deaths associated with the COVID‐19 pandemic in the United States, March to May 2020. JAMA Intern Med. 2020;180:1336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhatt AS, Moscone A, McElrath EE, Varshney AS, Claggett BL, Bhatt DL, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID‐19 pandemic. J Am Coll Cardiol. 2020;76:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer FA, et al. Reduction in ST‐segment elevation cardiac catheterization laboratory activations in the United States during COVID‐19 pandemic. J Am Coll Cardiol. 2020;75:2871–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baum A, Schwartz MD. Admissions to Veterans Affairs hospitals for emergency conditions during the COVID‐19 pandemic. JAMA. 2020;324:96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verma S. Early impact of CMS expansion of Medicare telehealth during COVID‐19. In Health Affairs Blog. 2020, https://www.healthaffairs.org/do/10.1377/forefront.20200715.454789/full/ (accessed March 15, 2022).
- 15. Smaha LA; American Heart Association . The American Heart Association Get With The Guidelines program. Am Heart J. 2004;148(5 Suppl):S46–8. [DOI] [PubMed] [Google Scholar]
- 16. Vaduganathan M, van Meijgaard J, Mehra MR, Joseph J, O'Donnell CJ, Warraich HJ. Prescription fill patterns for commonly used drugs during the COVID‐19 pandemic in the United States. JAMA. 2020;323:2524–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vaduganathan M, Li D, Van Meijgaard J, Warraich HJ. Prescription fill patterns of evidence‐based medical therapies for heart failure during the COVID‐19 pandemic in the United States. J Card Fail. 2021;27:1280–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toner L, Koshy AN, Ko J, Driscoll A, Farouque O. Clinical characteristics and trends in heart failure hospitalizations: an Australian experience during the COVID‐19 lockdown. JACC Heart Fail. 2020;8:872–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boriani G, Palmisano P, Guerra F, Bertini M, Zanotto G, Lavalle C, et al.; AIAC Ricerca Network Investigators . Impact of COVID‐19 pandemic on the clinical activities related to arrhythmias and electrophysiology in Italy: results of a survey promoted by AIAC (Italian Association of Arrhythmology and Cardiac Pacing). Intern Emerg Med. 2020;15:1445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leyva F, Zegard A, Okafor O, Stegemann B, Ludman P, Qiu T. Cardiac operations and interventions during the COVID‐19 pandemic: a nationwide perspective. Europace. 2021;23:928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poulin MF, Pinto DS. Strategies for successful catheterization laboratory recovery from the COVID‐19 pandemic. JACC Cardiovasc Interv. 2020;13:1951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fosbol EL, Butt JH, Ostergaard L, Andersson C, Selmer C, Kragholm K, et al. Association of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker use with COVID‐19 diagnosis and mortality. JAMA. 2020;324:168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Konig S, Hohenstein S, Meier‐Hellmann A, Kuhlen R, Hindricks G, Bollmann A. In‐hospital care in acute heart failure during the COVID‐19 pandemic: insights from the German‐wide Helios hospital network. Eur J Heart Fail. 2020;22:2190–201. [DOI] [PubMed] [Google Scholar]
- 25. Cannatà A, Bromage DI, Rind IA, Gregorio C, Bannister C, Albarjas M, et al. Temporal trends in decompensated heart failure and outcomes during COVID‐19: a multisite report from heart failure referral centres in London. Eur J Heart Fail. 2020;22:2219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alvarez‐Garcia J, Lee S, Gupta A, Cagliostro M, Joshi AA, Rivas‐Lasarte M, et al. Prognostic impact of prior heart failure in patients hospitalized with COVID‐19. J Am Coll Cardiol. 2020;76:2334–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rey JR, Caro‐Codon J, Rosillo SO, Iniesta ÁM, Castrejón‐Castrejón S, Marco‐Clement I, et al.; CARD‐COVID Investigators . Heart failure in COVID‐19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail. 2020;22:2205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tomasoni D, Inciardi RM, Lombardi CM, Tedino C, Agostoni P, Ameri P, et al. Impact of heart failure on the clinical course and outcomes of patients hospitalized for COVID‐19. Results of the Cardio‐COVID‐Italy multicentre study. Eur J Heart Fail. 2020;22:2238–47. [DOI] [PubMed] [Google Scholar]
- 29. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L, Taylor DDH. Excess deaths from COVID‐19 and other causes, March‐July 2020. JAMA. 2020;324:1562–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bromage DI, Cannata A, Rind IA, Gregorio C, Piper S, Shah AM, et al. The impact of COVID‐19 on heart failure hospitalization and management: report from a heart failure unit in London during the peak of the pandemic. Eur J Heart Fail. 2020;22:978–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kirkpatrick JN, Hull SC, Fedson S, Mullen B, Goodlin SJ. Scarce‐resource allocation and patient triage during the COVID‐19 pandemic: JACC review topic of the week. J Am Coll Cardiol. 2020;76:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Auerbach A, O'Leary KJ, Greysen SR, Harrison JD, Kripalani S, Ruhnke GW, et al. Hospital ward adaptation during the COVID‐19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15:483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hauck KD, Hochman KA, Pochapin MB, Zabar SR, Wilhite JA, Glynn G, et al. The COVID‐19 Army: experiences from the deployment of non‐hospitalist physician volunteers during the COVID‐19 pandemic. Disaster Med Public Health Prep. 2021;1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mehra MR, Ruschitzka F. COVID‐19 illness and heart failure: a missing link? JACC Heart Fail. 2020;8:512–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lowenstein CJ, Solomon SD. Severe COVID‐19 is a microvascular disease. Circulation. 2020;142:1609–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Metkus TS, Sokoll LJ, Barth AS, Czarny MJ, Hays AG, Lowenstein CJ, et al. Myocardial injury in severe COVID‐19 compared with non‐COVID‐19 acute respiratory distress syndrome. Circulation. 2021;143:553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Table S1. Cohort derivation.
Table S2. Get With The Guidelines‐Heart Failure quality measures.
Table S3. Adjusted odds of mortality in each quarter during the COVID‐19 period, compared to March 2020.
Table S4. Outcomes of patients hospitalized for heart failure prior to the pandemic and during the pandemic across regions of the United States.
Table S5. Adherence to guidelines of patients hospitalized for heart failure and discharged alive, with comparison before and during the COVID‐19 pandemic, admitted to the Northeast region.
Table S6. Adherence to guidelines of patients hospitalized for heart failure and discharged alive, with comparison before and during the COVID‐19 pandemic, admitted to the Midwest region.
Table S7. Adherence to guidelines of patients hospitalized for heart failure and discharged alive, with comparison before and during the COVID‐19 pandemic, admitted to the South region.
Table S8. Adherence to guidelines of patients hospitalized for HF and discharged alive, with comparison before and during the COVID‐19 pandemic, admitted to the West region.
Table S9. Baseline patient and hospital characteristics before and during the COVID‐19 pandemic excluding centres that did not report both before and during the pandemic.
Table S10. Outcomes of patients hospitalized for heart failure prior to the pandemic and during the pandemic excluding centres that did not report both before and during the pandemic.
Table S11. Review of previously published evaluations of the association of heart failure and COVID‐19 and in‐hospital mortality.
Figure S1. Time‐to‐event analysis for in‐hospital mortality among patients hospitalized before the pandemic versus patients hospitalized during the pandemic.
Figure S2. Time‐to‐event analysis for in‐hospital mortality among patients hospitalized with COVID‐19 versus patients hospitalized without COVID‐19.
Figure S3. Adherence to guidelines of patients hospitalized for heart failure and discharged alive, with comparison before and during the COVID‐19 pandemic, excluding centres that did not report both before and during the pandemic.


