To the editor,
Several case reports have described development of liver injury following severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) vaccination in hepatology.[ 1 , 2 ] These cases showed autoimmune hepatitis (AIH) features, and all cases responded well to corticosteroid therapy. Herein, we present a patient who underwent liver transplantation due to fulminant liver failure following vaccination with the BioNTech vaccine.
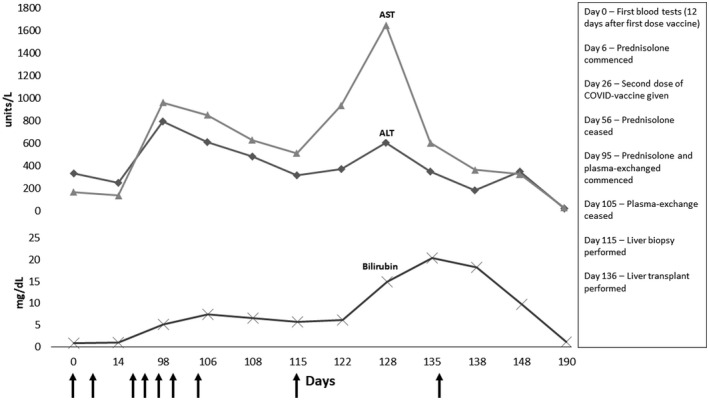
A 53‐year‐old previously healthy man received a first dose of BioNTech vaccine on the June 5, 2021. Ten days after vaccination, he developed mild abdominal pain, erythematous skin eruption, and pruritus. Initial laboratory findings were alanine aminotransferase (ALT) 333 (upper limit of normal [ULN] 55 U/L), aspartate aminotransferase (AST) 168 (ULN 34 U/L), alkaline phosphatase 102 (ULN 150 U/L), and total bilirubin 0.8 (ULN 1.2 mg/dl). The patient’s symptoms were considered to be a hypersensitivity reaction. Oral antihistaminic therapy and topical steroids did not lead to clinical improvement. On day 18 after vaccination, prednisolone 32 mg/day for 10 days followed by 16 mg/day for 20 days was commenced. One week into the steroid course, symptoms significantly improved, and aminotransferases decreased (Figure 1).
FIGURE 1.
Evolution of clinical and laboratory findings
The patient was reluctant to receive a second vaccine dose for fear of additional side effects but had travel plans and needed a full vaccination chart. He, therefore, scheduled a second Pfizer‐BioNTech vaccine dose 6 weeks after the first dose. Following the second vaccination, similar symptoms reoccurred after a few days. The dose of prednisolone was increased to 32 mg/day, gradually tapered, and discontinued in mid‐August 2021. One month after, he developed abdominal pain, myalgia, fatigue, and jaundice. The symptoms and laboratory findings did not improve over 10 days, and he was referred to a tertiary liver transplant center. At admission, ALT was 485, AST was 629, total bilirubin was 6.6 mg/dl, and the international normalized ratio was 1.36. Serum IgG levels were 28.3 (7.0–16.4 g/L). Viral serology was negative for hepatitis A–E, Epstein‐Barr virus, cytomegalovirus, and herpes simplex virus. Antinuclear antibodies, smooth muscle actin, antimitochondrial antibodies, anti–liver–kidney microsome 1, liver cytosolic antigen 1, and anti–soluble liver antigen were all negative; and ceruloplasmin was normal.
The liver biopsy showed portal inflammation with interface activity and significant lobular necroinflammatory activity, hepatocellular rosette formation and emperipolesis that are typical components of AIH. Treatment with prednisolone (40 mg/day, i.v.) and plasma exchange did not improve the liver function. The patient developed HE and underwent living donor liver transplantation. One month after liver transplantation, he was alive with significantly improved laboratory findings.
We presented a report of severe outcome of liver injury that developed after SARS‐CoV‐2 vaccination. We are aware that it is difficult to establish a definitive causality between SARS‐CoV‐2 vaccine and hepatitis. However, our case demonstrates strong evidence of vaccine‐induced immune‐mediated liver injury. The patient developed liver injury after a first dose of Pfizer‐BioNTech vaccine which on reexposure led to severe liver injury. Our case, along with other reports, suggests that AIH‐like hepatitis may develop after SARS‐CoV‐2 vaccination. Early diagnosis and effective management seem to be very important in this emerging condition.
CONFLICT OF INTEREST
Nothing to report.
AUTHOR CONTRIBUTIONS
Cumali Efe, Murat Harputluoğlu, and Sezai Yilmaz conceptualized the study. Cumali Efe, Murat Harputluoğlu, and Neşe Karadağ Soylu collected and analyzed data. All authors approved the final version of the manuscript.
DATA AVAILABILITY STATEMENT
All data that support the findings of this study are presented in the text.
REFERENCES
- 1. Palla P, Vergadis C, Sakellariou S, Androutsakos T. Letter to the editor. Autoimmune hepatitis after COVID‐19 vaccination: a rare adverse effect? Hepatology. 2022;75:489–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cao Z, Gui H, Sheng Z, Xin H, Xie Q. Letter to the editor. Exacerbation of autoimmune hepatitis after COVID‐19 vaccination. Hepatology. 2022;75(3):757–9. 10.1002/hep.32269 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this study are presented in the text.


