Coronavirus disease 2019 (COVID‐19) has been proved as a multi‐organ disease with deleterious effect on the cardiovascular system. 1 COVID‐19 infection has been associated with impaired subclinical markers of cardiovascular and endothelial function even in patients with mild severity of COVID‐19. 2 Furthermore, subclinical myocardial and vascular dysfunction during COVID‐19 disease have been associated with worse outcomes and higher mortality risk. 3
Carotid‐femoral pulse wave velocity (PWVc‐f) and central systolic blood pressure (SBPcentral) are reliable markers of aortic elastic properties and have been suggested as valuable prognostic markers for cardiovascular events. 4 Glycocalyx damage, as assessed by perfused boundary region (PBR5–25) of sublingual microvessel, impaired artery flow‐mediated dilatation (FMD) and coronary flow reserve (CFR) may represent early manifestations of endothelial dysfunction with prognostic value. 5 , 6 Left and right ventricular global longitudinal strain (GLS) and myocardial work indices permit early detection of subclinical myocardial deformation. 7
In our previous study, 8 COVID‐19 patients displayed impaired endothelial function, aortic elasticity, CFR and myocardial deformation 4 months after COVID‐19 infection compared to healthy controls with similar age, sex and risk factors. Additionally, we observed a 10‐fold increase of malondialdehyde (MDA), an oxidative stress biomarker, suggesting that oxidative stress mediates cardiovascular damage. 8 There are no studies to evaluate whether these changes are reversible in a longer‐term follow‐up. The aim of the present study is to examine markers of endothelial, vascular and myocardial function during a follow‐up visit at 12 months after COVID‐19 infection in order to clarify whether the changes observed at 4 months post‐infection are reversible in the long term.
In a prospective, observational study, we consecutively recruited 70 patients (62.85% male; mean age 54.53 years) who were examined in a dedicated post‐COVID‐19 outpatient clinic during a scheduled follow‐up visit at 4 and 12 months after a confirmed COVID‐19 infection and 70 healthy individuals with similar clinical characteristics. In all participants, the medication remained unchanged during the study. A total of 24 patients (34.28%) were diagnosed with mild disease and were not subsequently hospitalized at any time of the course of the disease, whereas 23 (32.85%) patients were diagnosed to have moderate and 23 (32.85%) severe disease and thus were admitted to hospital. None of the examined patients required mechanical ventilation. Inclusion and exclusion criteria, study design and procedures have been previously described in detail. 8 At 4 and 12 months we measured (a) PBR of the sublingual arterial microvessels (online supplementary Appendix S1 ), (b) PWV and SBPcentral, (c) FMD, (d) CFR, (e) LVGLS, RVGLS and right ventricular free wall strain (RVFWS), (f) myocardial global work index (GWI) global constructive work (GCW), global wasted work (GWW) and the myocardial global work efficiency (GWE) (online supplementary Appendix S1 ), and (g) MDA as oxidative stress marker. ANOVA (factorial or for paired comparisons) was used for statistical analysis.
Online supplementary Table S 1 shows alterations in endothelial, vascular, and echocardiographic markers of myocardial function in COVID‐19 patients at 4 and 12 months after the infection compared to the control group. The results regarding the effect of COVID‐19 at 4 months after the infection have been already published. 8
At 12‐month follow‐up, COVID‐19 patients displayed an increase of PBR5–25 values compared to 4 months (p < 0.001). Likewise, FMD values were similar between 4 and 12 months (p = 0.198) and higher than those in controls (p < 0.001), suggesting persistence of endothelial damage. PWV and SBPcentral values remained similar between 4 and 12 months (p = 0.883 and p = 0.776, respectively) and were increased compared to controls (p = 0.057 and p = 0.003, respectively). Conversely, COVID‐19 patients at 12 months presented higher CFR values than at 4 months (p = 0.002). However, CFR values remained decreased compared to controls (p = 0.003). At 12 months post‐infection, LVGLS values in COVID‐19 patients showed a borderline improvement compared to values at 4 months (p = 0.069), though again these remained impaired compared to controls (p = 0.003). Conversely, RVGLS, RVFWS and tricuspid annular plane systolic excursion in COVID‐19 patients at 12 months were significantly improved compared to 4 months (p = 0.001, p < 0.001, and p = 0.002, respectively) and showed no significant difference compared to controls (p > 0.05 for all comparisons). At 4 and 12 months, 72.5% and 42.8% of the patients had abnormal (> −20%) LVGLS. The results regarding the effect of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) on myocardial work markers 4 months after the infection have not been published previously. COVID‐19 patients at 4 months displayed higher myocardial wasted work and decreased myocardial efficiency compared to controls (p = 0.01 and p = 0.006, respectively). There was a modest improvement in GWW and GWE at 12 months, compared to 4 months in COVID‐19 patients (p = 0.043 and p = 0.001, respectively); however, these markers remained impaired compared to controls (p > 0.05). At 12 months after COVID‐19 infection, MDA levels were significantly decreased compared to 4 months (p < 0.001); however, these values remained higher than in controls (p = 0.002). Figure 1 shows the most important alterations in above markers in COVID‐19 patients at 4 and 12 months after the infection compared to the control group.
Figure 1.
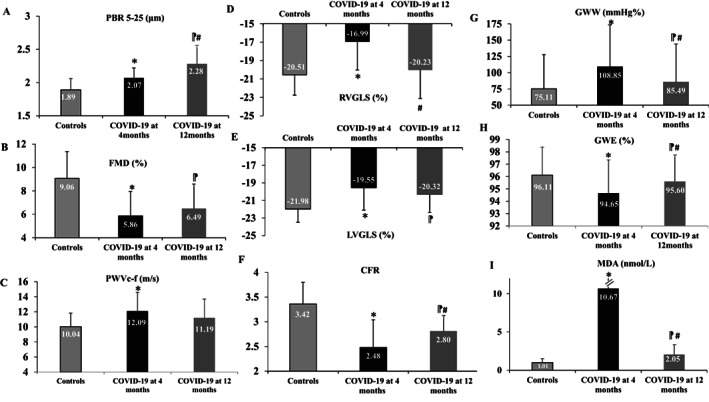
Changes in (A) perfused boundary region (PBR, µm), (B) flow‐mediated dilatation (FMD, %), (C) pulse wave velocity carotid to femoral (PWVc‐f, m/s), (D) right ventricular global longitudinal strain (RVGLS,%), (E) left ventricular global longitudinal strain (LVGLS, %), (F) coronary flow reserve (CFR), (G) global wasted work (GWW, mmHg%), (H) global work efficiency (GWE, %), (I) malondialdehyde (MDA, nmol/L) of COVID‐19 patients at 12 months, in comparison with COVID‐19 patients at 4 months and controls. *p < 0.05 for comparisons between COVID‐19 group at 4 months after the infection and control group. #p < 0.05 for comparisons between COVID‐19 group at 4 months and COVID‐19 group at 12 months after the infection.  p < 0.05 for comparisons between COVID‐19 group at 12 months after the infection and control group.
p < 0.05 for comparisons between COVID‐19 group at 12 months after the infection and control group.
At 4 months, 37.87% of the patients had symptoms (fatigue, dyspnoea, cough and chest pain) but only 4.25% at 12 months.
Our study supports that SARS‐CoV‐2 causes endothelial and cardiovascular dysfunction which are partially restored at 12 months after the infection. In our study, the values of right ventricular echocardiography markers in COVID‐19 patients improved significantly at 1 year compared to their values at 4 months. Thus, right ventricular function markers in COVID‐19 patients became in close range to those of controls with similar cardiovascular risk factors possibly due to complete resolution of lung lesions 1 year after the infection and thus resolution of myocardial stress caused by the preceding infection. In the current study, after 1 year of follow‐up, the significant reduction in MDA levels and the subsequent improvement of cardiovascular markers suggest that oxidative stress mediates the cardiovascular derangements. However, oxidative stress remained nearly two‐fold higher at 1 year compared to controls. Furthermore, COVID‐19 patients at 12 months after infection presented greater values of PBR and similar values of PWV compared to 4 months and higher than controls indicating persistence of endothelial and vascular derangement 1 year after the infection. According to research data, increased arterial stiffness and endothelial glycocalyx damage have been associated with adverse cardiovascular events. 4 , 6
In conclusion, arterial stiffening, endothelial dysfunction and a persistently high oxidative burden may lead to a compromised cardiac performance as indicated by the impaired values of myocardial work and LVGLS in COVID‐19 patients at 12 months compared to controls. To our knowledge, this is the first study to evaluate markers of endothelial and cardiovascular function as well as oxidative stress at 4 and 12 months after COVID‐19 infection and to report that the alterations observed at 4 months post‐infection are only partially reversed at 12 months.
Supporting information
Appendix S1. Supporting Information.
Acknowledgment
The present study has been supported by the Hellenic Cardiology Society.
Conflict of interest: none declared.
Contributor Information
Ignatios Ikonomidis, Email: ignoik@gmail.com.
Vaia Lambadiari, Email: vlambad@otenet.gr.
References
- 1. Akhmerov A, Marban E. COVID‐19 and the heart. Circ Res. 2020;126:1443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turan T, Özderya A, Şahin S, Konuş AH, Kul S, Akyüz AR, et al. Left ventricular global longitudinal strain in low cardiac risk outpatients who recently recovered from coronavirus disease 2019. Int J Cardiovasc Imaging. 2021;37:2979–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schnaubelt S, Oppenauer J, Tihanyi D, Mueller M, Maldonado‐Gonzalez E, Zejnilovic S, et al. Arterial stiffness in acute COVID‐19 and potential associations with clinical outcome. J Intern Med. 2021;290:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. European Network For Non‐Invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605. [DOI] [PubMed] [Google Scholar]
- 5. Lekakis J, Abraham P, Balbarini A, Blann A, Boulanger CM, Cockcroft J, et al. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehabil. 2011;18:775–89. [DOI] [PubMed] [Google Scholar]
- 6. Ikonomidis I, Thymis J, Simitsis P, Koliou GA, Katsanos S, Triantafyllou C, et al. Impaired endothelial glycocalyx predicts adverse outcome in subjects without overt cardiovascular disease: a 6‐year follow‐up study. J Cardiovasc Transl Res 2021. doi: 10.1007/s12265‐021‐10180‐2. [DOI] [PubMed] [Google Scholar]
- 7. Schroeder J, Hamada S, Gründlinger N, Rubeau T, Altiok E, Ulbrich K, et al. Myocardial deformation by strain echocardiography identifies patients with acute coronary syndrome and nondiagnostic ECG presenting in a chest pain unit: a prospective study of diagnostic accuracy. Clin Res Cardiol. 2016;105:248–56. [DOI] [PubMed] [Google Scholar]
- 8. Lambadiari V, Mitrakou A, Kountouri A, Thymis J, Katogiannis K, Korakas E, et al. Association of COVID‐19 with impaired endothelial glycocalyx, vascular function and myocardial deformation 4 months after infection. Eur J Heart Fail. 2021;23:1916–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


