Abstract
The emergence of SARS‐CoV‐2 Omicron variant (B.1.1.529) with major spike protein mutations has raised concern over potential neutralization escape and breakthrough infections among vaccinated and previously SARS‐CoV‐2‐infected subjects. We measured cross‐protective antibodies against variants in health care workers (HCW, n = 20) and nursing home residents (n = 9) from samples collected at 1–2 months, following the booster (3rd) dose. We also assessed the antibody responses in subjects infected before the Omicron era (n = 38) with subsequent administration of a single mRNA vaccine dose. Following booster vaccination, HCWs had high IgG antibody concentrations to the spike protein and neutralizing antibodies (NAb) were detectable against all variants. IgG concentrations among the elderly remained lower, and some lacked NAbs against the Beta and Omicron variants. NAb titers were significantly reduced against Delta, Beta, and Omicron compared to WT virus regardless of age. Vaccination induced high IgG concentrations and variable titers of cross‐reactive NAbs in previously infected subjects, whereas NAb titers against Omicron were barely detectable 1 month postinfection. High IgG concentrations with cross‐protective neutralizing activity were detected after three Coronavirus Disease 2019 (COVID‐19) vaccine doses in HCWs. However, lower NAb titers seen in the frail elderly suggest inadequate protection against Omicron breakthrough infections, yet protection against severe COVID‐19 is expected.
Keywords: COVID‐19, neutralizing antibodies, SARS‐COV‐2, variants of concern, vaccination
Neutralizing antibodies against variants of concern were detected up to 2.5 months after the third COVID‐19 mRNA vaccination in adults, whereas some elderly subjects did not have neutralization capacity against Beta and Omicron variants at 1 month after third dose. Single mRNA vaccine after SARS‐CoV‐2 infection induced comparable antibody levels.
Introduction
As of 26 November 2021, the World Health Organization (WHO) classified the Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) B.1.1.529 Omicron variant as a Variant of Concern (VOC) [1]. The Omicron variant is antigenically more distant from the original SARS‐CoV‐2 vaccine strain than the previously most distant VOC strains Beta and Delta [2].
Omicron shares several mutations in the receptor‐binding domain (RBD) with previously identified VOC including Beta (K417N, N501Y) and Delta (T478K). These mutations influence the ability of the variants to resist the neutralizing activity of antibodies [3, 4, 5, 6]. Omicron has several additional mutations in the RBD, including E484A [7], that are likely to further enhance its ability to escape neutralizing antibodies (NAbs) and suggest a significant potential for vaccine escape compared with the Delta variant [8].
Omicron has an increased growth rate, household transmission risk, and secondary attack rate compared to the Delta variant [9] and it is expected to become the dominant strain worldwide. This has resulted in urgent administration of booster vaccinations and the development of variant‐specific vaccine compositions.
Both Coronavirus disease 2019 (COVID‐19) mRNA vaccines were initially shown to be highly immunogenic, also among elderly subjects [10, 11], and were found to be 94 to 95% effective in preventing WT SARS‐CoV‐2 infection in short‐term after the second dose [12, 13]. Effectiveness against Delta variant infection has been shown to be slightly lower but remains relatively good at over 80% during the first 3 months, yet lower in the elderly [14]. However, longer follow‐up data from vaccine effectiveness (VE) studies have indicated that protection wanes more steeply against Delta variant infection. In the elderly, substantial waning to low VE levels of 20% after 5 months has been reported following the second vaccine dose [14]. For Omicron variant infection, first VE estimates show considerable and rapid waning after two vaccine doses, but restoration of high effectiveness after an mRNA booster [15, 16].
NAb levels are highly predictive of protection against infection and clinical disease [17]. However, no absolute antibody titer threshold has been established as a correlate of protection for SARS‐CoV‐2 [18]. Omicron has been shown to escape vaccine‐induced humoral immunity; substantially reduced NAb titers have been seen in recently vaccinated subjects [2, 19, 20]. Also, substantial ability to evade immunity from prior infection has been suggested [21]. However, previously infected subjects with subsequent vaccination were found to retain relatively high neutralization titers [20].
In this study, we measured the NAb titers against a WT virus, and three variants isolated in Finland during 2021: Beta (B.1.351), Delta (B.1.617.2), and Omicron (B.1.1.529) in health care workers (HCW) and elderly subjects, who received a booster vaccination 6 to 9 months after the second dose. We also measured NAb responses against Beta, Delta, and Omicron as well as Alpha (B.1.1.7) to a single vaccination in subjects infected before the Omicron era and compared the NAb titers to the peaking antibody response 1 month after infection.
Results
Higher IgG antibody concentrations after booster vaccination in HCWs compared to the elderly
To estimate the extent of cross‐protective immunity following COVID‐19 booster vaccination, we measured the concentration of IgG antibodies to spike protein (anti‐S IgG) and NAb titers from HCWs and elderly subjects sampled ≥21 days after the third vaccine dose (Fig. 1, Table 1). We found that the anti‐S IgG geometric mean concentrations (GMCs) 21 to 42 days following a booster dose (Comirnaty) were 1.4‐ (RBD) to 1.8‐fold (full‐length spike glycoprotein, SFL) higher in HCWs compared to the elderly. The anti‐S IgG GMCs were equally high in HCWs sampled at 43 to 77 days after the booster, compared to those sampled earlier. Subjects who received a Spikevax booster had 2.2‐ (RBD) to 2.5‐fold (SFL) higher anti‐S IgG GMCs compared to those who were vaccinated with Comirnaty. None of the differences was statistically significant (Kruskall–Wallis) due to the very small number of subjects.
Figure 1.
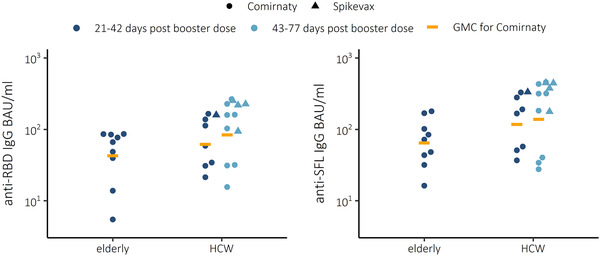
IgG concentrations and geometric mean concentrations (GMC) expressed as BAU/mL for WT spike proteins (SFL and RBD) in sera of elderly (n = 9) and health care workers (HCW) 21–42 (n = 8) or 43–77 (n = 12) days after booster mRNA vaccination (third dose) using Comirnaty or 21–42 (n = 1) and 43–77 (n = 4) days after using Spikevax. Each sample was tested as a technical duplicate in each experiment and the average of the two duplicates is shown. The experimental precision was confirmed by two positive control samples in each independent experiment.
Table 1.
Geometric mean IgG concentrations, GMC [95% CI] expressed as BAU/mL for wild‐type (WT) spike proteins (SFL and RBD) and geometric mean titers, GMT [95% CI] of neutralizing antibodies (NAb) against WT virus and three variants of concern Delta (B.1.617.2), Beta (B.1.351), and Omicron (B.1.1.529) in elderly (n = 7–9) and health care workers (HCW) 21–42 (n = 7) or 43–77 (n = 8) days after booster mRNA vaccination (third dose of Comirnaty)
| Anti‐S IgG BAU/mL | NAb to SARS‐CoV‐2 | ||||||
|---|---|---|---|---|---|---|---|
| Days post booster | SFL [95% CI] | RBD [95% CI] | WT [95% CI] | Delta [95% CI] | Beta [95% CI] | Omicron [95% CI] | |
| Elderly | 21–42 |
65 [35–120] n = 9 |
43 [20–90] n = 9 |
180 [55–560] n = 7 |
24 [5.8–100] n = 7 |
12 [3.3–44] n = 7 |
6.7 [2.7–17] n = 7 |
| HCW | 21–42 |
120 [52–270] n = 7 |
62 [29–130] n = 7 |
290 [130–660] n = 7 |
140 [77–250] n = 7 |
64 [37–110] n = 7 |
24 [12–50] n = 7 |
| 43–77 |
140 [50–380] n = 8 |
84 [34–200] n = 8 |
420 [180–1000] n = 8 |
150 [48–450] n = 8 |
78 [22–280] n = 8 |
41 [14–115] n = 8 |
|
Each sample was tested a technical duplicate in each experiment and the experimental precision was confirmed by two positive control samples in each independent experiment.
Correction added on 8th April 2022, after first online publication: display of Table 1 has been updated.
Booster vaccination induced variable concentrations of cross‐NAbs
Following booster vaccination, we observed measurable NAb titers and likely strong and moderate cross‐protection against Delta and Beta variants tested in HCWs, respectively (Fig. 2, Table 1). NAb titers to Omicron were decreased by 91 and 96% (p < 0.001 and 0.0021, Wilcoxon rank‐sum test) compared to WT SARS‐CoV‐2 in HCW and elderly, respectively. NAb titers to Beta variant were decreased by 80 and 93% (p < 0.001 and 0.0053) and NAb titers to Delta variant were decreased by 61 and 87% (p = 0.023 and 0.029) compared to NAb to WT SARS‐CoV‐2, in HCWs and elderly, respectively (Fig. 3). The NAb titers against WT virus were 1.6‐fold and 3.6‐fold higher against Omicron in HCWs compared to the elderly, sampled 21 to 42 days following the Comirnaty booster vaccination (Table 1). The NAb titers against Delta were 4.4‐ and 3.6‐fold higher compared to Omicron in HCW and elderly, respectively. Furthermore, two of seven elderly did not have NAb to Omicron despite receiving a booster dose only 21–42 days prior.
Figure 2.
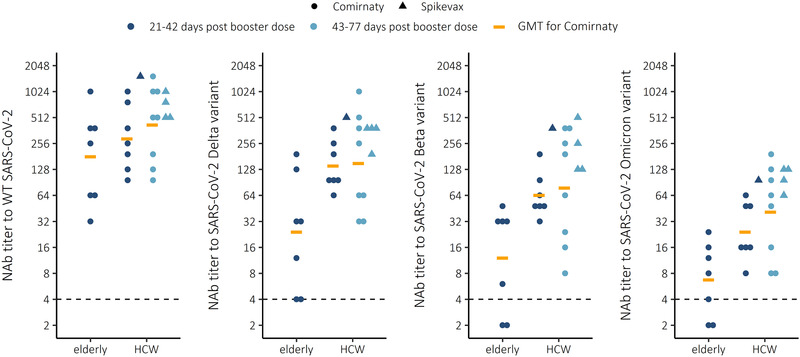
Neutralizing antibody (NAb) titers to WT virus and three variants of concern Delta (B.1.617.2), Beta (B.1.351), and Omicron (B.1.1.529) in elderly (n = 7) and health care workers (HCW) 21–42 (n = 8) or 43–77 (n = 12) daysafter third booster mRNA vaccination with Comirnaty or Spikevax COVID‐19 vaccine. Each sample was tested as a technical duplicate in each experiment and 50% inhibition of SARS‐CoV‐2 infection observed by the cytopathic effect of inoculated cells is shown. The experimental precision was confirmed by two positive control samples in each independent experiment.
Figure 3.
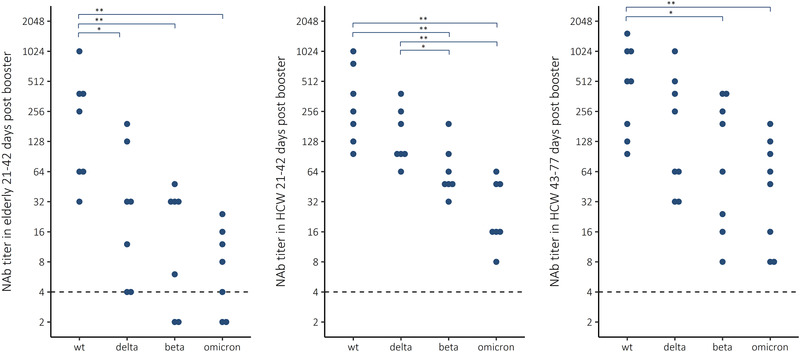
Neutralizing antibody (NAb) titers to WT virus and three variants of concern Delta (B.1.617.2), Beta (B.1.351), and Omicron (B.1.1.529) in elderly (n = 7) and health care workers (HCW) 21–42 (n = 7) or 43–77 (n = 8) days after third booster mRNA vaccination with Comirnaty COVID‐19 vaccine. Wilcoxon rank‐sum test * p < 0.05, **p < 0.01. Each sample was tested as a technical duplicate in each experiment and 50% inhibition of SARS‐CoV‐2 infection observed by the cytopathic effect of inoculated cells is shown. The experimental precision was confirmed by two positive control samples in each independent experiment.
Previous infection and a single vaccine dose induced high IgG concentrations and variable NAb titers
We measured anti‐S IgG concentrations and NAb titers from samples collected from previously infected subjects at 1–2 months following a laboratory‐confirmed SARS‐CoV‐2 infection by WT (n = 13), Alpha (n = 20), or Beta variant (n = 5). The anti‐S IgG concentrations measured at 26–70 days after infection were on average 6.3 and 6.4 binding antibody unit [BAU]/mL for RBD and SFL, respectively. The anti‐S IgG levels after infection and a single dose of the Comirnaty COVID‐19 vaccine are presented in Supporting information Fig. S1.
Mild infection induced the strongest NAb titers against the homologous variant strain, but only moderate or low cross‐protection against other variants (Fig. 4). For Omicron, infection by heterologous variant induced no NAb titers in subjects who had faced WT or Beta variant infection, and only one subject with prior Alpha variant infection had a borderline positive NAb titer against Omicron.
Figure 4.
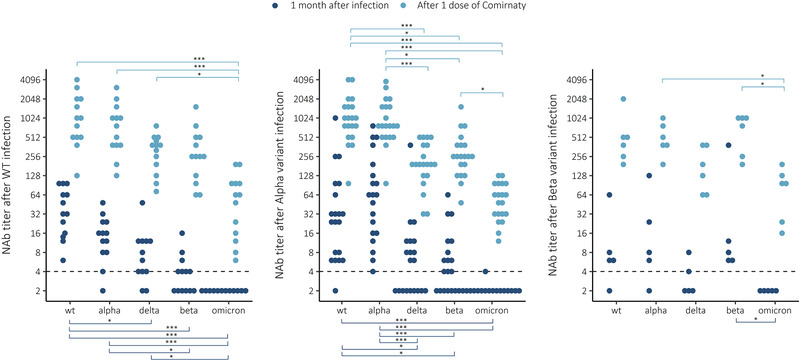
Neutralizing antibody (NAb) titers to WT virus and four variants Alpha (B.1.1.7), Delta (B.1.617.2), Beta (B.1.351), and Omicron (B.1.1.529) 1 month after infection with WT, Alpha, or Beta variant and after one dose of Comirnaty COVID‐19 vaccine. Wilcoxon rank‐sum test *p < 0.05, **p < 0.01, ***p < 0.001. Each sample was tested as a technical duplicate in each experiment and 50% inhibition of SARS‐CoV‐2 infection observed by the cytopathic effect of inoculated cells is shown. The experimental precision was confirmed by two positive control samples in each independent experiment.
We then measured NAb from samples collected from the previously infected subjects who had received a single COVID‐19 vaccine dose (Comirnaty) 3 to 6 months following infection. A single dose induced very strong anti‐S IgG responses (16‐ to 27‐fold, for RBD and SFL, respectively), and the anti‐S IgG concentrations were comparable to the levels following a Comirnaty booster dose in HCWs (75 vs. 130 BAU/mL for RBD and 140 vs. 170 BAU/mL for SFL) 21–77 days after booster dose. Most importantly, we measured a marked increase in cross‐protective antibody responses in previously infected subjects against all variants including Omicron (Fig. 4). The NAb titers remained elevated for at least 2 months following vaccination.
Discussion
Following a booster vaccination, given at 6 to 9 months following the second COVID‐19 mRNA vaccine dose, we found high anti‐S IgG and detectable NAb concentrations, suggesting cross‐protection against all variants among HCWs. The anti‐S IgG concentrations and the ability to neutralize variants, including Omicron, remained equally high in samples collected from HCW from 1 to up to 2.5 months following the booster. However, among the frail elderly living in a residential care home the antibody concentrations measured a month after the booster dose were lower compared to those among HCW and not all subjects had NAbs against the Beta and Omicron variants.
The age‐dependency of the antibody responses suggests that protection against breakthrough infections following a booster dose may be partial and transient in the frail elderly. Antibody responses to COVID‐19 vaccination have been shown to correlate negatively with age; vaccination induces highest antibody responses in younger subjects, and lower among subjects aged 65 and older [11, 22, 23]. The concentration of NAbs has also been shown to decline faster with increasing age [24, 25].
Furthermore, the response to a booster vaccination is likely dose‐dependent. The mRNA content in the Comirnaty vaccine is 30 μg compared to 100 μg in the Spikevax; the currently licensed booster dose for Spikevax is 50 μg. A 50 μg Spikevax booster dose was shown to markedly increase the NAb titers against Omicron in subjects previously immunized with two doses of the Spikevax vaccine [26]. Preliminary data indicate that a full dose would result in a twofold concentration of NAbs against the Omicron variant [27]. In this study, we found that the HCWs who received a full dose of Spikevax as a booster tended to have higher concentrations of cross‐NAbs compared to those vaccinated with Comirnaty (2.4‐fold against Omicron). However, the difference was not statistically significant due to the small number of subjects.
Effectiveness against infection and severe disease has been reported to be higher for Spikevax compared to Comirnaty [28]. In subjects vaccinated with two doses of an mRNA vaccine, Spikevax has been shown to induce twofold antibody concentrations compared to Comirnaty [23]. A high concentration of NAbs has previously been shown to correlate with protection against breakthrough infections [29, 30]. These data suggest that the stronger the initial immune response to the vaccine, the longer the immunity will persist. Also, our finding that the booster vaccination induced weaker immune responses among the elderly subjects suggests that the added benefit of a booster vaccination against breakthrough infections is likely shorter with older age.
The ability of sera from subjects vaccinated with two doses of a mRNA vaccine (Comirnaty), collected 56 days following the first vaccine dose, to neutralize Omicron variants has been shown to be markedly reduced compared to the ancestral, vaccine‐type virus [19]. Only 20 and 24% of the vaccinated subjects had NAb against the two Omicron variants tested, compared to 100% against the vaccine‐type, Beta and Delta variants [19]. Similar findings of very low NAb activity against Omicron were reported in the South‐African study of subjects vaccinated with two doses of the Comirnaty vaccine [20]. Another study using live virus neutralization assay found that subjects recently vaccinated with two doses of an adenovirus vector vaccine (Vaxzevria) or mRNA vaccine (Comirnaty) with an extended interval of 8 to 11 weeks between the two vaccine doses had substantially reduced NAb titers against Omicron, but only some subjects had no NAb [2]. A longer interval between the two doses of the primary series has been shown to result in significantly higher antibody responses, indicative of at least partial booster response [31, 32]. A booster vaccination, when given as a third dose several months after the second dose, has been shown to significantly increase the IgG and NAb concentrations [32, 33] and markedly improve neutralization of the Omicron variant [26]. This is in line with our finding of positive NAb titers against Omicron in all working‐age subjects, who had received the booster vaccination.
We also found that the anti‐S IgG concentrations among previously infected, once‐vaccinated subjects were high and NAb titers variable, but comparable to the antibody concentrations following a third vaccine dose among the HCWs. The South‐African study also reported that all the previously infected and subsequently vaccinated subjects (5/5) had NAbs against Omicron [20]. The kinetics of antibody concentrations following infection may be different compared to vaccine‐induced immunity, and the breadth of cross‐protective immunity may be qualitatively different. Data suggest that although vaccination may induce higher initial antibody concentrations compared to infection, these antibodies appear to decline much faster [34]. A follow‐up study of 2653 subjects vaccinated with two doses of Comirnaty, and 4361 convalescent COVID‐19 patients found that IgG antibody titers decreased by up to 40% each subsequent month in vaccinated subjects, while in the convalescents they decreased by less than 5% per month [34]. We have previously shown that anti‐S IgG antibodies can be detected up to 13 months following SARS‐CoV‐2 infection in 97% of the subjects, and that cross‐protective NAb against Alpha, Beta, and Delta variants persisted in the majority of subjects with severe COVID‐19 disease [35].
NAb levels induced by infection or vaccination are predictive of protection from symptomatic SARS‐CoV‐2 infection [17, 29, 30]. Although no exact serological correlates have been established, protection from an infection likely requires higher NAb levels, whereas the estimated NAb level for protection from severe infection is sixfold lower [17]. SARS‐CoV‐2 infection induces long‐lived BM plasma cells that continue to produce antibodies which likely explains why following a rapid decline of antibody concentrations during the first few months, low but stable concentrations can be detected a year after infection [36]. Circulating, resting memory B cells have also been detected in convalescent individuals [36]; a memory B‐cell response to SARS‐CoV‐2 evolves during the first 6 months after infection, characterized with greater somatic hypermutation, resistance to mutations in the RBD of the spike protein, and increased potency [37]. Persistent antigen stimulation in previously infected subjects likely drives this type of maturation of the B‐cell response. In vaccinated subjects, a booster dose is expected to induce a memory B‐cell response. In previously infected subjects, vaccination with a single dose of a mRNA vaccine induced not only higher NAb concentrations compared to naïve subjects, but also higher affinity of antibodies, suggesting that in convalescent individuals, the first vaccination recalls pre‐existing memory B cells against SARS‐CoV‐2 that undergo rapid affinity maturation [38]. Higher antibody affinity was found to correlate with improved NAb titers against multiple SARS‐CoV‐2 variants [38]. Susceptibility to infection increases over time, as the level of NAbs declines, especially against antigenically different strains such as Omicron [16]. However, boosting immunity with third doses not only increases the concentration of antibodies, but also improves the quality of the B‐cell response, and potential long‐term immunity against severe COVID‐19 disease.
We acknowledge several limitations in our study due to the small number of subjects and HCW gender distribution. Also, in HCW and elderly subjects, we analyzed sera after the third dose, but we did not have prebooster samples available for comparison. However, prebooster samples likely had very low NAb titers against variants as low NAb activity against Omicron has been reported following the second dose [19, 20, 26]; and especially as 6 to 9 months had passed before the third dose was administered. In this study, we only assessed humoral immune responses that are critical immune mechanisms for reducing infection. SARS‐CoV‐2 infection and vaccination also induce durable T‐cell immunity, which targets multiple epitopes in the SARS‐CoV‐2 spike protein and is likely to enhance protection against severe COVID‐19 disease against SARS‐CoV‐2 variants [39]. Analysis of the mutations in the spike protein sequence of the Omicron variant with computational modeling suggested that the T‐cell response against Omicron would remain broadly cross‐protective, as only a small number of the CD4+ and CD8+ T‐cell epitopes, and none of the immunodominant epitopes, are affected by the Omicron‐specific mutations [40].
In summary, the results of our study support previous findings indicating that COVID‐19 booster vaccinations raise IgG concentrations, although NAb titers remain low against Omicron compared to WT and Delta variants. In this study, we were able to demonstrate measurable NAbs against Delta, Beta, and Omicron VOCs up to 2.5 months after the third mRNA vaccine dose in adults, whereas some elderly subjects did not have neutralization capacity against Beta and Omicron variants at 1 month after third dose. We observed that the NAb titers against Omicron were barely detectable already 1 month after mild SARS‐CoV‐2 infection caused by WT virus, Alpha or Beta variant, suggesting an increased risk of reinfection caused by Omicron. However, single mRNA COVID‐19 vaccine dose after SARS‐CoV‐2 infection induced NAb titers comparable to third mRNA COVID‐19 vaccination. Booster vaccinations likely improve protection against infections at least temporarily, which may help reduce transmission of the virus in the pandemic situation. Immunity against severe diseases is also based on memory B cells and T‐cell‐mediated immunity, which is expected to improve and last longer after booster vaccination.
Materials and methods
Study design and participants
This was an observational clinical vaccine study in which the vaccinations were administered according to the national COVID‐19 vaccination campaign. Clinical trial registration: EudraCT 2021‐004788‐29.
We invited HCWs (n = 20, median age 50.2 [27.2–63.1], 100% female) and elderly in a residential care home (n = 9, 84.2 [71.5–89.6], 44% female) to participate and donate a blood sample for measurement of SARS‐CoV‐2‐specific serum antibodies (Supporting information Table S1). The HCWs and care home residents had received the first and second vaccination (COVID‐19 mRNA vaccine, Comirnaty) at a median of 21 [21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35] days apart, and the booster dose (Comirnaty, n = 15; Spikevax, n = 5) had been given at a median of 245 [206–291] days following the second dose. The blood samples were collected at a median of 40 [21–77] days following the booster dose. None of the participants had documentation of previous SARS‐CoV‐2 infection in the National Infectious Diseases Register.
Second, we invited 480 subjects with laboratory‐confirmed SARS‐CoV‐2 infection identified in the National Infectious Disease Register (between Oct 2020 and March 2021). A total of 82 (17%) subjects participated, from which a total of 38 participants with a blood sample taken both before and after their first COVID‐19 mRNA vaccine dose (Comirnaty) were selected for this study. The cause of infection of the selected participants had been identified as WT (n = 13, median age 54.5 [44.7–80.8], 62% female), Alpha (n = 20, median age 51.2 [27.4–71.4], 55% female), or Beta variant of SARS‐CoV‐2 (n = 5, median age 44.0, [32.7–50.5], 40% female). Only one of the 38 selected subjects had a history of severe COVID‐19 disease (hospitalization), caused by WT. Blood samples were collected at a median of 50 [26–70] days after the COVID‐19 infection and 10 to 20 (n = 11), 21 to 42 (n = 17), and ≥43 days (n = 11) after subsequent administration of a single dose of a COVID‐19 mRNA vaccine given at a median of 181, 182, and 158 days after infection, subsequently. From one participant, with Beta variant infection, two postvaccination samples were included (14 and 76 days after vaccination). The COVID‐19 vaccination history of all the participants was collected from the National Vaccination Registry.
Serum specimens were separated by centrifugation, aliquoted, and stored at −20°C or below. For assessment of NAbs, sera were heat‐inactivated at 56°C for 30 min.
SARS‐CoV‐2 viruses selected for microneutralization test (MNT)
We utilized five SARS‐CoV‐2 viruses for determining NAb titers. WT virus (B lineage) indicates hCoV‐19/Finland/1/2020 (GISAID accession ID EPI_ISL_407079; GenBank accession ID MZ934691). WT virus isolation and propagation were performed in African green monkey kidney epithelial (Vero E6) cells [41]. All variant viruses were isolated and propagated in VeroE6‐TMPRSS2‐H10 cells [42] and further propagated in Vero E6 cells for MNT. Alpha variant (B.1.1.7) indicates the isolate hCoV‐19/Finland/THL‐202102301/2021 (EPI_ISL_2590786; MZ944886), Beta variant (B.1.351) the isolate hCoV‐19/Finland/THL‐202101018/2021 (EPI_ISL_3471851; MZ944846), Delta variant (B.1.617.2) the isolate hCoV‐19/Finland/THL‐202117309/2021 (EPI_ISL_2557176; MZ945494), and Omicron variant (B.1.1.529/BA.1) the isolate hCoV‐19/Finland/THL‐202126660/2021 (EPI_ISL_8768822; OM393712). A tissue culture infectious dose 50% assay was performed for all viruses to achieve the comparable virus concentration (of 100 × tissue culture infectious dose 50% per well) among the different variants.
SARS‐CoV‐2 MNT
We performed a cytopathic effect‐based MNT as previously described [35, 41]. Briefly, serum samples were twofold serially diluted starting at 1:4 in Eagle's minimum essential medium supplemented with penicillin, streptomycin, and 2% of heat‐inactivated fetal bovine serum. At the biosafety level 3 laboratory, pretitrated virus was added to obtain 100× tissue culture infectious dose of 50% per well following incubation for 1 h at +37°C, 5% CO2. African green monkey kidney epithelial (VeroE6) cells were added, and the 96‐well tissue culture plates were incubated at +37°C, 5% CO2 for 4 days. Wells were fixed with 30% formaldehyde and stained with crystal violet. Results were expressed as MNT titers corresponding to the reciprocal of the serum dilution that inhibited 50% of SARS‐CoV‐2 infection observed by the cytopathic effect of inoculated cells. MNT titer ≥6 was considered positive, borderline when 4, and negative when <4. Borderline values were further confirmed with biological repeats. For titer comparison, a titer of 192, 96, 32, 8, and <4 was measured for the WHO International Standard (NIBSC 20/136 [43]) using the WT virus, Alpha, Delta, Beta, and Omicron variant, respectively.
SARS‐CoV‐2 fluorescent multiplex immunoassay
We measured the concentration of IgG antibodies to spike glycoprotein of WT SARS‐CoV‐2 (S‐IgG Ab) with in‐house fluorescent multiplex immunoassay, as previously described [44]. Briefly, sera from donors were incubated with microspheres covered with SARS‐CoV‐2 RBD and SFL glycoprotein, and bound antibodies were detected with R‐phycoerythrin‐conjugated secondary antibody. Antibody concentrations were measured with MAGPIX system using xPONENT software version 4.2 (Luminex Corporation, Austin, TX) and converted into BAU/mL, which is calibrated with WHO international standard for SARS‐CoV‐2 antibody assays.
Statistical methods
The study analysis is descriptive. We calculated the GMC and titers Geometric mean titer with 95% confidence intervals for IgG and NAb levels, respectively. MNT titers <4 were assigned a titer value of 2. We assessed the statistical differences in antibody levels between groups using the Kruskal–Wallis test with Bonferroni correction. Differences in NAb titers between viruses were assessed with the Wilcoxon rank‐sum test. The statistical significance level of difference was set to p < 0.05. Statistical analyses were performed using SPSS version 27 and R (v4.0.4) with Rstudio (v1.4.1106).
Conflict of interest
Finnish Institute for Health and Welfare has received research funding for unrelated studies from GlaxoSmithKline Vaccines (N.E., A.A.P., and M.M. as investigators), Pfizer (A.A.P.), and Sanofi Pasteur (A.A.P.). The other authors report no potential conflicts of interest.
Ethics approval and patient consent statement
The study protocol of the COVID‐19 vaccine immunological studies in Finland was approved by the National Committee on Medical Research Ethics (TUKIJA/347/2021) and by the Finnish Medicines Agency Fimea as the regulatory authority (European Union clinical trials database code of EudraCT 2021‐004788‐29). For the follow‐up of COVID‐19, the study protocol of the serological population study of the coronavirus epidemic was approved by the ethical committee of the Hospital District of Helsinki and Uusimaa (HUS/1137/2020). Written informed consent was obtained from all study subjects before sample collection.
Author contributions
M.M. and A.H. designed the experiments. M.M., A.A.P., and H.N. contributed to the study design. A.S. performed the FMIA tests. A.H. developed and performed the microneutralization tests. P.Ö. coordinated the virus isolations. A.S., A.H., and T.N. analyzed the data. N.E coordinated the participant recruitment, sample collection, and sample processing. A.H., A.S., N.E., and M.M. wrote the manuscript and all coauthors contributed to the critical revision of the text.
Peer review
The peer review history for this article is available at https://publons.com/publon/10.1002/eji.202149785.
Abbreviations
- anti‐S IgG
IgG antibodies to spike protein
- BAU
binding antibody unit concentration
- COVID‐19
Coronavirus Disease 2019
- GMC
geometric mean concentration
- HCW
health care worker
- MNT
microneutralization test
- NAb
neutralizing antibody
- RBD
receptor‐binding domain
- SARS‐CoV‐2
Severe acute respiratory syndrome coronavirus 2
- SFL
full‐length spike glycoprotein
- VE
vaccine effectiveness
- VOC
variants of concern
- WHO
World Health Organization
Supporting information
Supplementary material
Acknowledgments
We thank all the study participants. We also thank Juha Oksanen, Esa Ruokokoski, Elina Isosaari, Tommi Korhonen, Joni Niemi, Oona Liedes, Saimi Vara, Päivi Siren, Maila Kyrölä, Ritva Syrjänen, Heta Nieminen, Camilla Virta, Lotta Hagberg, Marja Leinonen, Arja Rytkönen, Mervi Lasander, Marja‐Liisa Ollonen, Larissa Laine, Marika Skön, Tiina Sihvonen, Elina Virtanen, Johanna Rintamäki, Leena Saarinen, Marja Suorsa, Minna Haanpää, Johanna Mustajoki, Mervi Eskelinen, Niina Ikonen, Kirsi Liitsola, Soile Blomqvist, Erika Lindh, and Esa Rönkkö.
We gratefully acknowledge the authors and their respective laboratories, who analyzed and submitted the sequences to GISAID's EpiCoV and GenBank Database.
This study was funded by the Finnish Institute for Health and Welfare and the Academy of Finland (Decision number 336431).
Data availability statement
Data subject to third party restrictions. The data that support the findings of this study are available from Finnish Social and Health Data Permit Authority Findata. Restrictions apply to the availability of these data, which were used under license for this study under informed consent form. Anonymized data are available after permission by Findata.
References
- 1. World Health Organization , Classification of omicron (B.1.1.529): SARS‐CoV‐2 variant of concern. 2021. https://www.who.int/news/item/26‐11‐2021‐classification‐of‐omicron‐(b.1.1.529)‐sars‐cov‐2‐variant‐of‐concern.
- 2. Dejnirattisai, W. , Shaw, R. H. , Supasa, P. , Liu, C. , Stuart, A. S. , Pollard, A. J. , Liu, X. , et al., Reduced neutralisation of SARS‐CoV‐2 omicron B.1.1.529 variant by post‐immunisation serum. Lancet 2022. 399: 234–236. S0140‐6736(21)02844‐0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou, D. , Dejnirattisai, W. , Supasa, P. , Liu, C. , Mentzer, A. J. , Ginn, H. M. , Zhao, Y. , et al., Evidence of escape of SARS‐CoV‐2 variant B.1.351 from natural and vaccine‐induced sera. Cell 2021. 184: 2348–2361.e6. S0092‐8674(21)00226‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casadevall, A. , Henderson, J. P. , Joyner, M. J. , Pirofski, L. A. , SARS‐CoV‐2 variants and convalescent plasma: reality, fallacies, and opportunities. J. Clin. Invest. 2021. 131: e148832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang, P. , Nair, M. S. , Liu, L. , Iketani, S. , Luo, Y. , Guo, Y. , Wang, M. , et al., Antibody resistance of SARS‐CoV‐2 variants B.1.351 and B.1.1.7. Nature 2021. 593: 130–135. [DOI] [PubMed] [Google Scholar]
- 6. Wu, K. , Werner, A. P. , Koch, M. , Choi, A. , Narayanan, E. , Stewart‐Jones, G. B. E. , Colpitts, T. , et al., Serum neutralizing activity elicited by mRNA‐1273 vaccine. N. Engl. J. Med. 2021. 384: 1468–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu, Z. , VanBlargan, L. A. , Bloyet, L. M. , Rothlauf, P. W. , Chen, R. E. , Stumpf, S. , Zhao, H. , et al., Identification of SARS‐CoV‐2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell. Host Microbe 2021. 29: 477–488.e4. S1931‐3128(21)00044‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen, J. , Wang, R. , Gilby, N. B. and Wei, G. W. , Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J. Chem. Inf. Model. 62:412‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. UK Health Security Agency , SARS‐CoV‐2 variants of concern and variants under investigation in England. Technical Briefing 31 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1042367/technical_briefing‐31‐10‐december‐2021.pdf. [Google Scholar]
- 10. Anderson, E. J. , Rouphael, N. G. , Widge, A. T. , Jackson, L. A. , Roberts, P. C. , Makhene, M. , Chappell, J. D. , et al., Safety and immunogenicity of SARS‐CoV‐2 mRNA‐1273 vaccine in older adults. N. Engl. J. Med. 2020. 383: 2427–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walsh, E. E. , Frenck, R. W. ,Jr, Falsey, A. R. , Kitchin, N. , Absalon, J. , Gurtman, A. , Lockhart, S. , et al., Safety and immunogenicity of two RNA‐based covid‐19 vaccine candidates. N. Engl. J. Med. 2020. 383: 2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polack, F. P. , Thomas, S. J. , Kitchin, N. , Absalon, J. , Gurtman, A. , Lockhart, S. , Perez, J. L. , et al., Safety and efficacy of the BNT162b2 mRNA covid‐19 vaccine. N. Engl. J. Med. 2020. 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baden, L. R. , El Sahly, H. M. , Essink, B. , Kotloff, K. , Frey, S. , Novak, R. , Diemert, D. , et al., Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N. Engl. J. Med. 2021. 384: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Young‐Xu, Y. , Zwain, G. M. , Powell, E. I. and Smith, J. , Estimated effectiveness of COVID‐19 messenger RNA vaccination against SARS‐CoV‐2 infection among older male veterans health administration enrollees, January to September 2021. JAMA Netw. Open 2021. 4: e2138975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andrews, N. , Stowe, J. , Kirsebom, F. , Toffa, S. , Rickeard, T. , Gallagher, E. , Gower, C. , et al., Effectiveness of COVID‐19 vaccines against the omicron (B.1.1.529) variant of concern. MedRxiv 2021. medRxiv [Google Scholar]
- 16. Gardner, B. J. and Kilpatrick, A. M. , Estimates of reduced vaccine effectiveness against hospitalization, infection, transmission and symptomatic disease of a new SARS‐CoV‐2 variant, omicron (B.1.1.529), using neutralizing antibody titers. MedRxiv 2021. medRxiv [Google Scholar]
- 17. Khoury, D. S. , Cromer, D. , Reynaldi, A. , Schlub, T. E. , Wheatley, A. K. , Juno, J. A. , Subbarao, K. , et al., Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat. Med. 2021. 27:1205‐1211. [DOI] [PubMed] [Google Scholar]
- 18. Krammer, F. , A correlate of protection for SARS‐CoV‐2 vaccines is urgently needed. Nat. Med. 2021. 27: 1147–1148. [DOI] [PubMed] [Google Scholar]
- 19. Lu, L. , Mok, B. W. , Chen, L. L. , Chan, J. M. , Tsang, O. T. , Lam, B. H. , Chuang, V. W. , et al., Neutralization of SARS‐CoV‐2 omicron variant by sera from BNT162b2 or coronavac vaccine recipients. Clin. Infect. Dis. 2021. ciab1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cele, S. , Jackson, L. , Khoury, D. S. , Khan, K. , Moyo‐Gwete, T. , Tegally, H. , San, J. E. , et al., Omicron extensively but incompletely escapes pfizer BNT162b2 neutralization. Nature 2021. 602, 654–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pulliam, J. R. C. , van Schalkwyk, C. , Govender, N. , von Gottberg, A. , Cohen, C. , Groome, M. J. , Dushoff, J. , et al., Increased risk of SARS‐CoV‐2 reinfection associated with emergence of the omicron variant in South Africa. MedRxiv 2021. medRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amodio, E. , Capra, G. , Casuccio, A. , Grazia, S. , Genovese, D. , Pizzo, S. , Calamusa, G. , et al., Antibodies responses to SARS‐CoV‐2 in a large cohort of vaccinated subjects and seropositive patients. Vaccines (Basel) 2021. 9: 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steensels, D. , Pierlet, N. , Penders, J. , Mesotten, D. and Heylen, L. , Comparison of SARS‐CoV‐2 antibody response following vaccination with BNT162b2 and mRNA‐1273. Jama 2021. 326: 1533–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levin, E. G. , Lustig, Y. , Cohen, C. , Fluss, R. , Indenbaum, V. , Amit, S. , Doolman, R. , et al., Waning immune humoral response to BNT162b2 Covid‐19 vaccine over 6 months. N. Engl. J. Med. 2021. 385: e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doria‐Rose, N. , Suthar, M. S. , Makowski, M. , O'Connell, S. , McDermott, A. B. , Flach, B. , Ledgerwood, J. E. , et al., Antibody persistence through 6 months after the second dose of mRNA‐1273 vaccine for covid‐19. N. Engl. J. Med. 2021. 384: 2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doria‐Rose, N. , Shen, X. , Schmidt, S. D. , O'Dell, S. , McDanal, C. , Feng, W. , Tong, J. , et al., Booster of mRNA‐1273 strengthens SARS‐CoV‐2 omicron neutralization. MedRxiv 2021. medRxiv [Google Scholar]
- 27. Moderna Press Release , Authorized booster (50 μg of mRNA‐1273) increases omicron neutralizing antibody levels approximately 37‐fold; a 100 μg booster dose of mRNA‐1273 increases omicron neutralizing antibody levels approximately 83‐fold. 2021.
- 28. Dickerman, B. A. , Gerlovin, H. , Madenci, A. L. , Kurgansky, K. E. , Ferolito, B. R. , Figueroa Muñiz, M. J. , Gagnon, D. R. and Gaziano, J. M. , Comparative effectiveness of BNT162b2 and mRNA‐1273 vaccines in U.S. veterans. NEJM 2022. 386:105‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bergwerk, M. , Gonen, T. , Lustig, Y. , Amit, S. , Lipsitch, M. , Cohen, C. , Mandelboim, M. , et al., Covid‐19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021. 385: 1474‐1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feng, S. , Phillips, D. J. , White, T. , Sayal, H. , Aley, P. K. , Bibi, S. , Dold, C. , et al., Correlates of protection against symptomatic and asymptomatic SARS‐CoV‐2 infection. Nat. Med. 2021. 27: 2032–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Payne, R. P. , Longet, S. , Austin, J. A. , Skelly, D. T. , Dejnirattisai, W. , Adele, S. , Meardon, N. , et al., Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021. 184: 5699–5714.e11. S0092‐8674(21)01221‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Flaxman, A. , Marchevsky, N. G. , Jenkin, D. , Aboagye, J. , Aley, P. K. , Angus, B. , Belij‐Rammerstorfer, S. , et al., Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV‐19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002). Lancet 2021. 398: 981–990. S0140‐6736(21)01699‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Falsey, A. R. , Frenck, R. W. ,Jr, Walsh, E. E. , Kitchin, N. , Absalon, J. , Gurtman, A. , Lockhart, S. , et al., SARS‐CoV‐2 neutralization with BNT162b2 vaccine dose 3. N. Engl. J. Med. 2021. 385: 1627–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Israel, A. , Shenhar, Y. , Green, I. , Merzon, E. , Golan‐Cohen, A. , Schäffer, A. A. , Ruppin, E. , et al., Large‐scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS‐CoV‐2 infection. MedRxiv 2021. medRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haveri, A. , Ekström, N. , Solastie, A. , Virta, C. , Österlund, P. , Isosaari, E. , Nohynek, H. , et al., Persistence of neutralizing antibodies a year after SARS‐CoV‐2 infection in humans. Eur. J. Immunol. 2021. 51: 3202‐3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Turner, J. S. , Kim, W. , Kalaidina, E. , Goss, C. W. , Rauseo, A. M. , Schmitz, A. J. , Hansen, L. , et al., SARS‐CoV‐2 infection induces long‐lived bone marrow plasma cells in humans. Nature 2021. 595: 421‐425. [DOI] [PubMed] [Google Scholar]
- 37. Gaebler, C. , Wang, Z. , Lorenzi, J. C. C. , Muecksch, F. , Finkin, S. , Tokuyama, M. , Cho, A. , et al., Evolution of antibody immunity to SARS‐CoV‐2. Nature 2021. 591: 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tang, J. , Grubbs, G. , Lee, Y. , Huang, C. , Ravichandran, S. , Forgacs, D. , Golding, H. , et al., Antibody affinity maturation and cross‐variant activity following SARS‐CoV‐2 mRNA vaccination: Impact of prior exposure and sex. EBioMedicine 2021. 74: 103748. S2352‐3964(21)00542‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Redd, A. D. , Nardin, A. , Kared, H. , Bloch, E. M. , Pekosz, A. , Laeyendecker, O. , Abel, B. , et al., CD8+ T‐cell responses in COVID‐19 convalescent individuals target conserved epitopes from multiple prominent SARS‐CoV‐2 circulating variants. Open Forum. Infect. Dis. 2021. 8: ofab143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ahmed, S. F. , Quadeer, A. A. and McKay, M. R. , SARS‐CoV‐2 T cell responses elicited by COVID‐19 vaccines or infection are expected to remain robust against omicron. Viruses 2022. 14: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haveri, A. , Smura, T. , Kuivanen, S. , Osterlund, P. , Hepojoki, J. , Ikonen, N. , Pitkapaasi, M. , et al., Serological and molecular findings during SARS‐CoV‐2 infection: The first case study in Finland, January to February 2020. Euro Surveill. 2020. 25: 2000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rusanen, J. , Kareinen, L. , Szirovicza, L. , Uğurlu, H. , Levanov, L. , Jääskeläinen, A. , Ahava, M. , et al., A generic, scalable, and rapid time‐resolved förster resonance energy transfer‐based assay for antigen detection‐SARS‐CoV‐2 as a proof of concept. mBio 2021. 12: e00902–e00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mattiuzzo, G. , Bentley, E. M. , Hassall, M. , Routley, S. , Richardson, S. , Bernasconi, V. , Kristiansen, P. , et al., Establishment of the WHO international standard and reference panel for anti‐SARS‐CoV‐2 antibody, 2020.
- 44. Solastie, A. , Virta, C. , Haveri, A. , Ekström, N. , Kantele, A. , Miettinen, S. , Lempainen, J. , et al., A highly sensitive and specific SARS‐CoV‐2 spike‐ and nucleoprotein‐based fluorescent multiplex immunoassay (FMIA) to measure IgG, IgA, and IgM class antibodies. Microbiol. Spectr. 2021. 9: 113121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data subject to third party restrictions. The data that support the findings of this study are available from Finnish Social and Health Data Permit Authority Findata. Restrictions apply to the availability of these data, which were used under license for this study under informed consent form. Anonymized data are available after permission by Findata.


