Abstract
Objective
To compare the clinical efficacy of posterior percutaneous endoscopic unilateral laminotomy (PPEUL) and anterior cervical decompression and fusion (ACDF) in the treatment of single‐segment spondylotic myelopathy (CSM).
Methods
This is a retrospective research, from January 2017 to December 2019, 30 cases were included in the PPEUL group and 32 cases were included in the ACDF group. The operative duration, blood loss, length of stay, complications, Japanese Orthopaedic Association (JOA) score, visual analogue scale (VAS) score, MacNab classification and imaging data were collected preoperatively, postoperative 1‐week, final follow‐up and statistically analyzed.
Results
The surgery was completed successfully on all patients, and there were no serious complications, such as nerve or spinal cord injury or infection. In the PPEUL and ACDF groups, the operative duration were 56.63 ± 1.40 and 65.21 ± 2.45 min, the intraoperative blood loss were 51.69 ± 3.23 and 50.51 ± 5.48 mL, and the hospitalization duration was 5.75 ± 1.43 and 6.38 ± 2.16 days. The follow‐up period in the PPEUL and ACDF groups was 24.96 ± 1.12 months and 25.65 ± 1.45 months, respectively. There was no significant difference in intraoperative blood loss between the two groups, but the hospitalization and operative durations in the PPEUL group were significantly shorter than those in the ACDF group (P < 0.05). The VAS scores at postoperative 1 week and final follow‐up were significantly improved compared with those before surgery. The JOA scores at postoperative 1 week and final follow‐up were significantly improved compared with those before surgery, but there was no significant difference between the two groups at the last follow‐up. The intervertebral disc height of the adjacent segment at the last follow‐up was significantly lower in the ACDF group than in the PPEUL group (P < 0.05), but there was no significant difference between the two groups in the intervertebral disc height of the surgical segment (P > 0.05). The rate of excellent and good results was 90.0% and 87.5%, respectively. Postoperative cervical CT and MRI showed that the spinal canal was fully decompressed and spinal cord compression was relieved.
Conclusion
PPEUL has the advantages of reduced trauma, rapid recovery and remarkable curative efficacy, so it is a new choice for the treatment of CSM.
Keywords: Cervical spondylotic myelopathy, Delta system, Unilateral approach bilateral decompression
Posterior percutaneous endoscopic unilateral approach bilateral decompression for CSM
Introduction
Cervical spondylotic myelopathy (CSM) 1 is a disease based on spinal cord compression due to cervical degeneration, which leads to disturbances in the spinal cord blood supply and finally spinal cord dysfunction. Severe CSM can cause neck and shoulder pain, numbness and weakness of limbs, even paralysis, seriously affect the quality of life of patients, and bring huge economic burden to society. Its incidence is increasing year by year. The disease has unique anatomical, physiological and pathological characteristics. Early surgical treatment to relieve spinal cord compression is an important method for the treatment of CSM. The anterior cervical approach has become the main method for the treatment of single‐segment and double‐segment lesions of the cervical spine. The main anterior surgical methods are anterior cervical discectomy and fusion (ACDF) 2 , anterior cervical corpectomy and fusion (ACCF) 3 and artificial cervical disc replacement (ACDR) 4 . Previous studies have shown that the symptoms of nerve compression in patients with single segment ACDF can be effectively alleviated. However, the use of a long titanium plate will increase the exposure range of surgical field and prolong the operation time. Complications such as screw loosening and falling off, titanium plate loosening and breaking, soft tissue injury, dysphagia and hoarseness may occur after operation. It is difficult to operate under naked eye because of insufficient illumination, incomplete decompression and high risk of dural sac injury. What is more, the fusion segment 2 is likely to reduce the range of movement of cervical spine and decreased from 50.79° ± 12.88° to 30.76° ± 8.85° at postoperative 1 year after ACDF with zero‐profile anchored spacer. Although the ACDR was a feasible method for the treatment of degenerative cervical spondylosis, studies 5 showed that restrictions of the range of motion were present in eight cases (10.4%, 8/77) because of heterotopic ossifications, seven cases (9.1%, 7/77) had a spontaneous fusion of the treated segment 1 year postoperatively.
The main posterior surgical methods are laminectomy and extended laminoplasty 6 . Laminectomy includes simple laminectomy, laminectomy with fixation fusion and skip laminectomy 7 , while extended laminoplasty includes single‐open‐door and French‐door laminoplasty 8 . After decades of development, cervical laminoplasty and laminectomy have gradually become the most commonly used posterior approach for the treatment of severe multilevel cervical disc herniation and ossification of cervical posterior longitudinal ligament. However, complications such as cervical instability, adjacent segment degeneration (ASD) 9 , kyphosis 10 , and axial pain 11 , 12 have been reported in the literature.
To reduce the occurrence of cervical muscle detachment, axial pain and limited neck movement, it has been reported that bilateral decompression by hemilaminectomy can be performed under microscope or channel retraction, with the removal of paravertebral muscle on only one side intraoperatively, thereby preserving the integrity of the contralateral paravertebral muscle and bone structure and reducing the occurrence of axial pain after laminectomy 13 , 14 , 15 .
In order to further reduce trauma, endoscopic technology is widely used in CSM and other spinal diseases, and endoscopic surgery has achieved good clinical results in the treatment of cervical spondylotic radiculopathy, thoracic spinal stenosis 16 , 17 and degenerative lumbar diseases 18 , 19 . Eicker et al. performed total endoscopic multisegmental laminectomy on more than 10 cadavers, proving that resection of the upper part of the inferior lamina and ligamentum flavum can provide sufficient decompression of the cervical spinal cord. The minimal invasive technique protects most of the dorsal structures and preserves biomechanical functions, which provides a basis for the feasibility of cervical spinal canal decompression in the treatment of CSM under spinal endoscopy 16 , 17 , 20 .
Recently, Delta system has been reported for the treatment of cervical spondylotic radiculopathy 21 , which was a threaded working channel. However, there have been few reports on endoscopic unilateral laminotomy and bilateral spinal canal decompression in the treatment of CSM using the Delta system. During the operation, we used a threaded working cannula, that is, the new Delta operation system, through step‐by‐step expansion, blunt separation of the paraspinal muscle on one side to the posterior side of the lamina, unilateral laminectomy and spinal canal decompression, and then we completed contralateral decompression by changing the channel angle. At the same time, the threaded working casing can reduce stimulation of the cervical spinal cord caused by lateral sloshing and floating of the channel and improve the safety of the operation. Besides, good illumination, enlarged field of vision and three‐dimensional images under spinal endoscopy can clearly show the anatomical structures, better distinguish the boundary between posterior longitudinal ligament and dura mater, find out and electrocoagulate tiny bleeding points as soon as possible, reduce the irritation and injury to the nerve roots and spinal cord, and more accurately remove the vertebral posterior margin osteophytes.
The clinical data of patients with single‐segment CSM treated by posterior percutaneous endoscopic unilateral laminotomy (PPEUL) with bilateral decompression using the endoscopic Delta system were retrospectively analyzed, and the clinical effects were compared with those treated by ACDF. The purpose of our research was to: (i) demonstrate the techniques of endoscopic unilateral laminotomy with bilateral decompression; (ii) evaluate the primary efficacy and feasibility of PPEUL by comparing with traditional ACDF; and (iii) demonstrate the advantages of Delta system in PPEUL surgery.
Patients and Methods
Inclusion criteria: (i) obvious symptoms and signs of cervical spinal cord injury, motor or sensory dysfunction of extremities, and positive pathological signs; X‐ray, CT, MRI and other imaging findings of cervical spinal cord compression by intervertebral disc protrusion, imaging findings consistent with clinical symptoms and signs, and single‐segment lesions; more than two segments lesion; high cervical level, i.e., C1/2 and C2/3 are not included; failure of regular conservative treatment for 3 months and gradual worsening of symptoms; (ii) underwent PPEUL or ACDF; (iii) operative duration, blood loss, length of stay, complications, Japanese Orthopaedic Association (JOA) score, visual analogue scale (VAS) score, MacNab classification and imaging data were collected; (iv) followed up for more than 2 years; and (v) a clinical retrospective study.
Exclusion criteria: (i) signs of cervical instability and cervical kyphosis under cervical hyperextension and flexion; (ii) previous cervical surgery history; (iii) severe damage to the heart, liver, kidney or other important organs with inability to tolerate the operation; (iv) other immune system diseases, such as rheumatoid disease, infection, severe osteoporosis and spinal cord tumor; and (v) severe mental disorders.
Patient Data
From January 2017 to December 2019, we retrospectively analyzed 62 patients with CSM in this study. According to the surgical method, the patients were divided into two groups: the Delta system endoscopic posterior cervical unilateral laminotomy and bilateral decompression group (PPEUL group) and the ACDF group. There were 30 patients in the PPEUL group and 32 patients in the ACDF group. The average age in the PPEUL group was 57.53 ± 4.34 years, with 18 males and 12 females, while that in the ACDF group was 56.78 ± 3.96 years, including 20 males and 12 females. The period of disease course in the PPEUL group was 5.34 ± 2.04 months and that in the ACDF group was 5.18 ± 2.67 months. There was no significant difference in sex, age, period of disease course between the two groups (P > 0.05) (Table 1).
TABLE 1.
Comparison of the baseline data between the PPEUL and ACDF groups (mean ± SD, %)
| Category | PPEUL group (n = 30) | ACDF group (n = 32) | t/x 2 | P‐value |
|---|---|---|---|---|
| Sex (M/F, n) | 18/12 | 20/12 | 0.048 | 0.840 |
| Age (years) | 57.53 ± 4.34 | 56.78 ± 3.96 | 0.712 | 0.480 |
| Period (months) | 5.34 ± 2.04 | 5.18 ± 2.67 | 0.263 | 0.793 |
| Segment (n) | – | |||
| C3‐4 | 5 | 4 | – | |
| C4‐5 | 8 | 8 | – | |
| C5‐6 | 10 | 14 | – | |
| C6‐7 | 7 | 6 | – | |
| Smoking (Yes/No) | 10/20 | 9/23 | 0.198 | 0.657 |
| Diabetes (Yes/No) | 4/26 | 6/26 | 0.055 | 0.815 |
| Operative duration (min) | 56.63 ± 1.40 | 65.21 ± 2.45 | 16.779 | 0.00 |
| Estimated blood loss (mL) | 51.69 ± 3.23 | 50.51 ± 5.48 | 1.024 | 0.310 |
| Hospital stay (day) | 5.75 ± 1.43 | 6.38 ± 2.16 | 2.052 | 0.045 |
Surgical Interventions
ACDF Group
Anesthesia and Position
After successful general anesthesia, the patient was placed in supine position and the neck was fixed in slightly supine position.
Approach and Exposure
The right approach and a transverse cervical incision were used in the ACDF group, starting from the medial edge of the sternocleidomastoid muscle to the cervical midline, over a distance of approximately 5 cm, exposing the vertebral body and intervertebral space layer by layer. After the responsible segment of the vertebral body was determined by fluoroscopy, the anterior longitudinal ligament and the responsible intervertebral disc were cut open, and the lateral side of the Luschka joint was reached.
Decompression
If prevertebral osteophyte hyperplasia was present, a Kerrison punch was used to remove the osteophyte in front of the upper and lower endplates. The intervertebral space was opened, the intervertebral space was fully exposed, the nucleus pulposus was removed with nucleus pulposus forceps, and the upper and lower endplates were treated with a scraper or drill by grinding to the subchondral bone. Then, the hard osteophyte and posterior longitudinal ligament were removed from the posterior edge of the vertebral body, and the medial edge of the Luschka joint was reached on both sides.
Fusion and Fixation
A zero‐notch interbody fusion cage filled with autogenous bone and allogeneic bone was placed into the treated intervertebral space, and the distractor was loosened to approximately 2 mm from the anterior edge of the vertebral body such that the fusion cage was inserted tightly, and the screw was obliquely fixed to the upper and lower vertebral body. After C‐arm fluoroscopy was used to determine that the physiological degree of curvature, the intervertebral height of the cervical segment had returned to normal, and the internal fixation was suitably positioned, the operative field was irrigated, and bipolar electrocoagulation was used to stop bleeding.
Close
After confirming that there was no continuous bleeding, a drainage tube was placed, and the incision was closed layer by layer. The drainage tube was removed 2 days postoperatively when the drainage volume was less than 10 ml/day.
PPEUL Group
Anesthesia and Position
After general anesthesia was established with tracheal intubation, the patient lay prone with the head high and the feet low on the fluoroscopic operating table. The cervical vertebrae were fixed with a head holder and wide tape, and the shoulders were pulled backward and downward with wide tape to facilitate intraoperative fluoroscopy and positioning. Towels were routinely sterilized.
Approach and Exposure
With the aid of C‐arm fluoroscopy, the operative segment was located first. Approximately 1.5 cm along the posterior median side of the cervical vertebra, under observation of the lateral film, an 18‐gauge needle was inserted into the corresponding operation space, while the posterior film was directly facing the medial edge of the superior and inferior articular process. Taking the guide needle as the center, a sharp knife was used to cut the skin and subcutaneous fascia longitudinally along the guide needle. The incision was approximately 10 mm long. After gradually expanding the incision along the puncture needle, the Delta working channel and the endoscope were placed, and repeat fluoroscopy was performed to ensure that the position of the channel was correct (Figs. 1 and 2h).
Fig. 1.
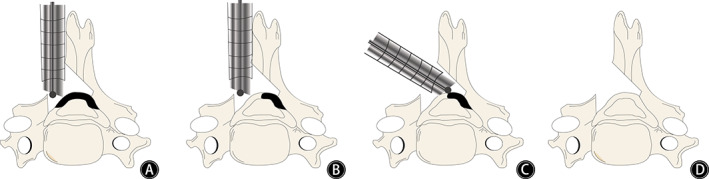
Schematic diagram of posterior percutaneous endoscopic decompression of the cervical spine. (A) Revealed unilateral laminotomy, (B) showed the removal of ligamentum flavum after laminotomy, which indicates that one side of the spinal canal has been fully decompressed. (C) Revealed contralateral removal of ligamentum flavum by changing the angle of the channel without contralateral laminectomy. (D) Revealed bilateral decompression through a unilateral approach through laminotomy in a posterior approach to the cervical spine.
Fig. 2.
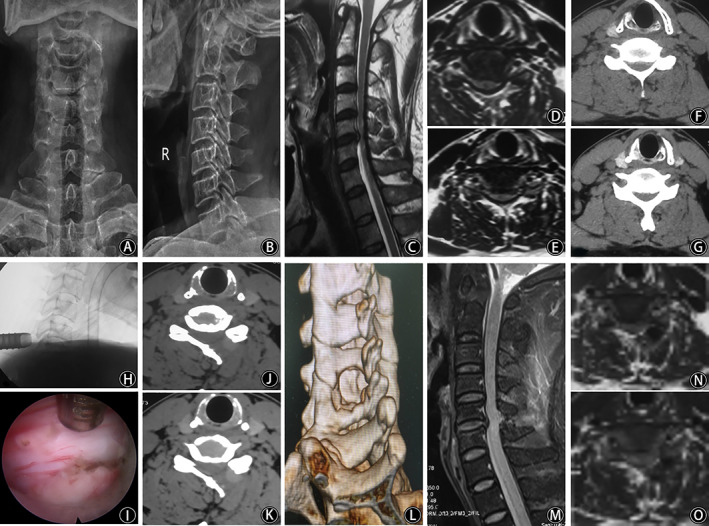
X‐ray films of cervical in the anterior (A) and lateral positions (B). Preoperative sagittal MRI (C) showed C5/6 disc herniation, and axial MRI (D and E) showed the disc herniation was on the left side, dural sac compression, and spinal canal stenosis. Preoperative axial CT (F and G) showed spinal canal stenosis. Fluoroscopy of the working channel intraoperatively (H) and spinal cord decompression under endoscopy (I). Axial CT (J and K) and three‐dimensional reconstruction (L) showing that one side of the lamina was resected and that the spinal canal was decompressed sufficiently. Postoperative sagittal MRI (M) showed that spinal canal decompression at C5/6 segment was sufficient, axial MRI (N and O) revealed that the left lamina was removed and dural sac compression was relieved.
Decompression
The soft tissue around the bony anatomical landmark “V” point was cleaned to clearly expose the landmark; if obvious hyperplasia was noted, a high‐speed burr or an Endo‐Kerrison punch was used to remove the hyperplastic bone. Then, an Endo‐Kerrison punch was used to remove part of the upper lamina along the “V” point to expose the starting point of the upper lamina of the ligamentum flavum, and then remove the lower lamina and expose the end of the inferior lamina of the ligamentum flavum. The ligamentum flavum was removed in a retrograde manner upward along the end point, paying attention to whether there were adhesions between the ligamentum flavum and dural sac to avoid cerebrospinal fluid leakage caused by tearing of the dural sac; when the ligamentum flavum is calcified, it is thinned with a spherical high‐speed burr, and then removed with the Endo‐Kerrison punch. Part of the upper and lower articular process was removed laterally. To avoid the occurrence of cervical segmental instability at a later stage, the scope of facet joint resection was less than 50%. Then, part of the bone at the root of the spinous process was ground off, the working channel was adjusted to the opposite side without compressing the dural sac. The hypertrophic ligamentum flavum and hyperplastic osteophyte on the contralateral side were removed, the spinal canal was enlarged, and compression of the contralateral nerve was relieved (Fig. 2i).
Close
Finally, bipolar electrocoagulation was used to stop bleeding of the peripheral venous plexus. After bilateral nerve root and dural sac decompression were achieved, the endoscope and working channel were removed step by step, and one or two stitches were placed at the incision.
Postoperative Management
Postoperative dehydration and anti‐inflammatory and analgesic drugs can be used for symptomatic treatment. Considering the short operative duration and the unconventional preventive application of antibiotics without implantation, patients began to move under the protection of a cervical brace 4–6 h postoperatively, and stitches were removed 1 week postoperatively. Patients wore a neck brace for 3–4 weeks.
Outcome Measures
The operative duration, blood loss volume, hospitalization duration and incidence of complications in the two groups were recorded. Routine outpatient follow‐up was performed after discharge, and clinical and imaging data were collected before the operation (Fig. 2a–g) and 1 week, 3 months, and 2 years after the operation.
Japanese Orthopaedic Association
JOA score was used to evaluate the function of patients before and after surgery. JOA scores contains motor, sensory and bladder dysfunction, with the highest score of 17. In terms of upper limb motor function, 0: unable to eat with chopsticks or spoon; 4: normal. In aspect of lower limb motor function, 0: unable to walk; 4: normal. The sensory scores of upper limbs, lower limbs and body is 2 points each with a total of 6 points. 0: obvious sensory disturbance; 2: normal. Bladder function, 0: urinary retention; 3: normal.
Visual Analogue Scale
Neck and shoulder pain was measured with a VAS ranging from 0 to 10; the VAS score is as follows: 0: no pain; less than 3: slight pain, can endure; 4 to 6: patients with moderate pain and affect sleep; 7 to 10: patients have gradually severe pain, which is unbearable, affecting appetite and sleep; the higher the score, the more severe the pain.
MacNab's Criteria
At the final follow‐up, the clinical efficacy was evaluated using the modified MacNab criteria including four grades, as follows: Excellent: complete disappearance of symptoms and restoration of original work and life; Good: mild symptoms, mild limitation of activity, and no effect on work or life; Fair: relief of symptoms, limitation of activity, effects on normal work and life; and Poor: no difference between before and after treatment, or even aggravation of symptoms.
Height of the Intervertebral Disc
The height of the intervertebral disc was defined as the average value of the anterior intervertebral space and the posterior intervertebral space, the anterior height of the intervertebral space refers to the length of the connecting line between the front end of the lower edge of the upper vertebral body and the front end of the upper edge of the lower vertebral body. The posterior height of the intervertebral space refers to the length of the connecting line between the posterior end of the lower edge of the upper vertebral body and the posterior end of the upper edge of the lower vertebral body. And the loss of its height represented degeneration of the intervertebral disc.
Evaluation of Cervical Instability
The angular displacement (AD) of the vertebral body was measured by drawing a straight line at the lower edge of the adjacent vertebral body, and the angle of intersection of the two lines was the AD of the vertebral body. The HD was measured as the horizontal distance between the posterior edge of the superior vertebral body and the posterior edge of the inferior vertebral body. Instability assessment criteria was defined as HD of the vertebral body >3.5 mm and AD of the vertebral body ≥12°. Postoperative CT and MRI of the cervical spine were performed to evaluate nerve decompression.
Statistical Analysis
The data were analyzed by SPSS (Chicago, IL) 22.0 software, and measurement data are expressed as the mean ± standard deviation. Measurement data in the two groups with a normal distribution and homogeneity of variance were compared by two independent sample t‐tests, and data recorded at different times for the same observation index were analyzed by repeated measures analysis of variance. Count data are expressed as the number of cases (%) using the χ 2 test, and the difference was considered statistically significant when P < 0.05.
Results
Follow‐Up
The follow‐up period in the PPEUL and ACDF groups was 24.96 ± 1.12 months and 25.65 ± 1.45 months, respectively. The surgery was completed successfully on all patients, and there were no serious complications, such as nerve or spinal cord injury or infection.
General Results
In the PPEUL group (n = 30), the operative duration was 56.63 ± 1.40 min, the intraoperative blood loss was 51.69 ± 3.23 ml, and the hospitalization duration was 5.75 ± 1.43 days. In the ACDF group (n = 32), the operative duration was 65.21 ± 2.45 min, the blood loss was 50.51 ± 5.48 mL, and the hospitalization duration was 6.38 ± 2.16 days. There was no significant difference in intraoperative blood loss between the two groups, but the hospitalization and operative durations in the PPEUL group were significantly shorter than those in the ACDF group (P < 0.05), as shown in Table 1.
Clinical Improvement
The VAS scores at postoperative 1 week and final follow‐up (PPEUL: 4.02 ± 1.25, 1.66 ± 0.27; ACDF: 4.89 ± 1.56, 1.57 ± 0.41) were significantly improved compared with those before surgery (PPEUL: 6.46 ± 1.33; ACDF: 7.00 ± 1.01). The JOA scores at postoperative 1 week and final follow‐up (PPEUL: 12.35 ± 0.68, 14.88 ± 1.46; ACDF: 13.44 ± 0.83, 15.01 ± 1.32) were significantly improved compared with those before surgery (PPEUL: 7.38 ± 0.21; ACDF: 7.53 ± 0.49), but there was no significant difference between the two groups at the last follow‐up (Table 2).
TABLE 2.
Change in the JOA and VAS scores at each time point postoperatively compared with preoperatively in the two groups
| Time | JOA score | VAS score | ||
|---|---|---|---|---|
| PPEUL group (n = 30) | ACDF group (n = 32) | PPEUL group (n = 30) | ACDF group (n = 32) | |
| Pre‐op | 7.38 ± 0.21 | 7.53 ± 0.49 | 6.46 ± 1.33 | 7.00 ± 1.01 |
| Postop 1 week | 12.35 ± 0.68* | 13.44 ± 0.83*, † | 4.02 ± 1.25* | 4.89 ± 1.56*, † |
| Last Follow‐up | 14.88 ± 1.46* | 15.01 ± 1.32* | 1.66 ± 0.27* | 1.57 ± 0.41* |
| F | 496.7 | 559.1 | 152.3 | 198.6 |
| P‐value | <0.01 | <0.01 | <0.01 | <0.01 |
JOA, Japanese Orthopaedic Association. VAS, visual analogue scale.
Compared with the preoperative value, P < 0.05.
Compared with the PPEHL group, P < 0.05.
Radiographic Improvement
Comparison of the Cervical Intervertebral Disc Height between the Two Groups
In the PPEUL group, the intervertebral disc height of the adjacent and surgical segments at the last follow‐up was compared with that preoperatively (preoperative adjacent segment: 5.19 ± 0.28, 5.80 ± 0.44; postoperative adjacent segment: 5.17 ± 0.21, 5.75 ± 0.16); although the intervertebral disc height decreased to some extent, there was no significant difference (P > 0.05) (Table 3).
TABLE 3.
Comparison of imaging indexes in the two groups of patients between preoperatively and at the last follow‐up
| Index | PPEUL group (n = 30) | t | P‐value | ACDF group (n = 32) | t | P‐value | ||
|---|---|---|---|---|---|---|---|---|
| Pre‐op | Last F‐up | Pre‐op | Last F‐up | |||||
| UDH (mm) | 5.19 ± 0.28 | 5.17 ± 0.21 | 0.313 | 0.755 | 5.19 ± 0.54 | 5.01 ± 0.08* | 1.865 | 0.067 |
| LDH (mm) | 5.80 ± 0.44 | 5.75 ± 0.16 | 0.585 | 0.561 | 5.84 ± 0.52 | 5.63 ± 0.20* | 2.132 | 0.037 |
| ODH (mm) | 5.64 ± 0.52 | 5.48 ± 0.65 | 1.053 | 0.297 | 5.65 ± 0.74 | 5.58 ± 0.43 | 0.463 | 0.645 |
UDH, upper disc height, DDH, lower disc height, ODH, operative disc height.
Compared with the PPEHL group, P < 0.05.
In the ACDF group, the intervertebral disc height of the adjacent segment at the last follow‐up was significantly lower than that preoperatively (preoperative adjacent: 5.19 ± 0.54, 5.84 ± 0.52; postoperative adjacent: 5.01 ± 0.08, 5.63 ± 0.20) (P < 0.05).
The intervertebral disc height of the adjacent segment at the last follow‐up was significantly lower in the ACDF group than in the PPEUL group (P < 0.05), but there was no significant difference between the two groups in the intervertebral disc height of the surgical segment (PPEUL: 5.48 ± 0.65; ACDF: 5.58 ± 0.43) (P > 0.05).
Incidence of Cervical Instability and ASD
Instability occurred in three spaces in the ACDF group, however, only one patient in the ACDF group developed clinical symptoms of ASD. This patient was treated with conservative anti‐halo therapy, anti‐inflammatories and pain relievers, and the symptoms were relieved after 2 weeks of nutritional nerve therapy.
At the last follow‐up, the MacNab criteria were used to evaluate the clinical efficacy of PPEUL and ACDF, and the rates of excellent and good results were 90.0% and 87.5%, respectively. There was no significant difference in the results of the two surgical methods as evaluated using the modified MacNab classification (Table 4).
TABLE 4.
Comparison of modified MacNab criteria between the two groups
| Group | MacNab classification (n) | E/G rate (n, %) | |||
|---|---|---|---|---|---|
| Excellent | Good | Fair | Poor | ||
| PPEUL group | 22 | 5 | 2 | 1 | 27, 90% |
| ACDF group | 24 | 4 | 3 | 1 | 28, 87.5% |
| χ 2 | 0.334 | 0.097 | |||
| P | 0.954 | 0.756 | |||
Postoperative re‐examination by cervical CT and MRI showed that the bilateral spinal canal was fully decompressed and enlarged, relieving the spinal cord compression (Fig. 2j–o).
Complications
One case of dural tearing occurred in the PPEUL group, and a drainage tube was placed. Depending on the drainage condition, the drain was pulled out the next day, and the incision healed well. Two patients with unilateral mild upper limb activity disorder were treated with methylprednisolone sodium, and the symptoms decreased gradually. The treatment was stopped on the 3rd day; with the addition of rehabilitation training, the muscle strength recovered gradually. Early postoperative pain and numbness in both upper limbs occurred in one case, which was treated with anti‐inflammatory and neurological drugs, and the symptoms disappeared after 1 month.
In the ACDF group, hoarseness occurred in one case early after the operation and was treated conservatively; there were two cases of slight dysphagia, and the symptoms disappeared 6 months after the operation. There was one case of poor positioning of the fusion cage with no clinical symptoms, so conservative observation and follow‐up were performed. Two years after the operation, the imaging data showed that all of the interbody bone grafts were fused. The incidence of complications in the two groups was 13.3% and 12.5%, respectively. There was no significant difference between the two groups (P > 0.05).
Discussion
Techniques of Endoscopic Unilateral laminotomy with Bilateral Decompression
The posterior percutaneous cervical endoscopic technique involves inserting the working channel through the posterior interlaminar approach, which can reduce destruction of the facet joint and maintain stability of the posterior structure of the spine. In this study, we used the Delta system to perform posterior cervical unilateral laminotomy and decompression, involving a minimally invasive approach to the posterior cervical vertebra. The cervical extensors were dilated step by step until passing the lamina, and then a tubular retractor was placed on the target lamina under X‐ray fluoroscopy. The spinal canal, ligamentum flavum and existing nerve root interface were visualized with endoscopy, and bilateral decompression was performed through a unilateral approach through an inclined working channel; this method is called “unilateral bilateral decompression”, or ULBD (Fig. 1). This operation has the advantages of a small skin incision, little tissue damage, good visibility, high safety, and good efficacy, among others.
Comparison with Traditional ACDF Procedure
In both groups of patients in this study, good decompression was achieved, and the postoperative JOA and VAS scores were significantly better than the preoperative scores. Additionally, there were no significant differences in the VAS or JOA scores, incidence of complications or satisfaction rate between PPEUL and ACDF. This shows that the PPEUL method can achieve the clinical efficacy of the open operation. At the same time, our results are consistent with the clinical efficacy of ACDF and unilateral bilateral decompression under microscopy in previous studies 13 , 14 , 15 , 22 , 23 . PPEUL for bilateral decompression could avoid injury to the esophagus, superior larynx, recurrent laryngeal nerve, vertebral artery and other important tissues encountered via an anterior approach 24 . Large‐channel endoscopy does not require decompression through the intervertebral disc or vertebral body 25 , which avoids endplate inflammation and reduction of the vertebral body height caused by the operation 26 . During the process of decompression, no signs of sagittal curvature straightening and kyphosis were found on X‐ray observation of cervical hyperextension or flexion during follow‐up for more than 2 years after the operation. Due to the minimal invasiveness of the surgery, functional exercise can be carried out as soon as possible after the operation, which is beneficial for the recovery of neurological function and the relief of pain.
Comparison in the Complications
Adjacent segment disease (ASD) refers to a disease with new clinical symptoms caused by new degenerative changes in adjacent segments after spinal surgery, accompanied by radiative pain, spinal cord symptoms and spinal instability. There were no imaging changes indicating cervical instability in the PPEUL group at the last follow‐up, which may be related to the patients' short history or insufficiency of the follow‐up time. In the ACDF group, three cases of instability occurred in the short term, and only one patient in the ACDF group developed clinical symptoms, such as radiative pain and spinal instability, 2 years postoperatively, so whether imaging changes will affect clinical symptoms needs further follow‐up study. Although the interbody fusion device can maintain the height of the intervertebral space, the fusion device will lead to the redistribution of biomechanical stress in the whole cervical vertebra, with the remaining intervertebral space bearing more stress, especially at the site of fusion of adjacent segments. The long‐term effect may accelerate the decrease in the intervertebral space height and the occurrence of cervical instability and ASD. Robertson and other scholars 27 , 28 reported two‐year follow‐up results and showed that the incidence of postoperative adjacent segment degeneration in the ACDF group was significantly higher than that in the artificial disc replacement group. However, the causes are complicated; for example, age may also lead to degeneration.
Advantages of Delta System in PPEUL Surgery
The external threads of the working channel increase the friction between the tube and soft tissue, which can greatly reduce injury to the spinal cord or nerve root caused by movement of the working channel; furthermore, it is not easy to enter the spinal canal, which can greatly improve the safety of the operation and reduce complications. In addition, compared with the traditional key‐hole system, the diameter of this channel is approximately 1.2 cm, and the field of vision is wider. Thus, it can be used to quickly identify tissue structures and avoid disorientation under endoscopy. Li reported 29 that 32 patients with CSM underwent bilateral or unilateral for spinal canal decompression using an large channel endoscopic system, with good results. At the same time, our procedure also causes less trauma, which are consistent with Li's research.
Comparison with Traditional Decompression under Microscopy
Compared with traditional decompression under microscopy 13 , 14 , 15 , PPEUL for bilateral decompression with the help of a high‐speed burr or an Endo‐Kerrison punch was completed by changing the channel angle. This method has the following advantages. First, under continuous saline lavage, the levels of inflammatory substances in the spinal canal are reduced, and at the same time, the water pressure helps to reduce intraoperative bleeding and keep the operative field clear, helping to reduce the incidence of dural tears. Second, under the premise of sufficient decompression at the root of the spinous process, contralateral decompression can be completed by tilting the working channel and going deep into the spinal canal, which not only preserves the integrity of the contralateral paraspinal muscle and lamina structure but also reduces the occurrence of cervical muscle detachment, axial pain and postoperative cervical instability in the later stage. In this study, the neck VAS and JOA scores after the operation were significantly improved compared with those before the operation; at the same time, according to the modified MacNab criteria, the rate of excellent and good results was 90%, and the curative effect was satisfactory. In addition, compared with ACDF, PPEUL involved no implantation and had a low cost.
Limitations
Although PPEUL for bilateral decompression is effective in the treatment of CSM, the technique still has a specific learning curve and a certain incidence of complications. In elderly patients, cervical vertebral hyperplasia is serious, structure identification under endoscopy is relatively difficult, and dural sac and ligamentum flavum adhesion and dural tears easily occur during surgery. The risk of increased intracranial pressure (IICP) increases in cases of intraoperative dural tears, especially cervical tears. A few critical complications, such as retinal hemorrhage and cerebral infarction, have been reported. It is suggested that anatomical structure should be carefully identified under endoscopy during the operation, and decompression should be carried out gently to reduce dural sac tears.
In addition, this operation has the risk of intraspinal venous plexus bleeding, which is common. In severe cases, there is a risk of nerve and dural injury and even paralysis. The follow‐up period in this study was short, and the long‐term effect needs further follow‐up; the number of cases in this group was small, and this was a retrospective study. Thus, further confirmation by multicenter randomized controlled trials is required.
Conclusions
PPEUL is a safe, minimally invasive and effective method for the treatment of CSM. Although it may not be the final treatment for CSM, it is an important step in the treatment of CSM. This method has the advantages of a small skin incision, little tissue injury, good visibility, safety, and the same efficacy as conventional ACDF.
Conflict of Interest
The authors declare that there is no conflict of competing financial and non‐financial interests.
Acknowledgments
Science and Technology joint project of Henan Provincial Health Commission (LHGJ20190859); Overseas Research and Training Project of Health Science and Technology Talents in Henan Province (HWYX 2019159); Key science and technology research and development projects of department of Science and Technology of Henan Province (212102310130).
Xiao‐bing Zhao and Ya‐jie Ma contributed equally.
References
- 1. Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007;60:S35–41. [DOI] [PubMed] [Google Scholar]
- 2. Cui W, Wu B, Liu B, Li D, Wang L, Ma S. Adjacent segment motion following multi‐level ACDF: a kinematic and clinical study in patients with zero‐profile anchored spacer or plate. Eur Spine J. 2019;28:2408–16. [DOI] [PubMed] [Google Scholar]
- 3. Traynelis VC, Arnold PM, Fourney DR, Bransford RJ, Fischer DJ, Skelly AC. Alternative procedures for the treatment of cervical spondylotic myelopathy: arthroplasty, oblique corpectomy, skip laminectomy: evaluation of comparative effectiveness and safety. Spine (Phila Pa 1976). 2013;38:S210–31. [DOI] [PubMed] [Google Scholar]
- 4. Staudt MD, Das K, Duggal N. Does design matter? Cervical disc replacements under review. Neurosurg Rev. 2018;41:399–407. [DOI] [PubMed] [Google Scholar]
- 5. Mehren C, Suchomel P, Grochulla F, Barsa P, Sourkova P, Hradil J, et al. Heterotopic ossification in total cervical artificial disc replacement. Spine (Phila Pa 1976). 2006;31:2802–6. [DOI] [PubMed] [Google Scholar]
- 6. Satomi K, Nishu Y, Kohno T, Hirabayashi K. Long‐term follow‐up studies of open‐door expansive laminoplasty for cervical stenotic myelopathy. Spine (Phila Pa 1976). 1994;19:507–10. [DOI] [PubMed] [Google Scholar]
- 7. Yuan W, Zhu Y, Liu X, Zhou X, Cui C. Laminoplasty versus skip laminectomy for the treatment of multilevel cervical spondylotic myelopathy: a systematic review. Arch Orthop Trauma Surg. 2014;134:1–7. [DOI] [PubMed] [Google Scholar]
- 8. Wang L, Wang Y, Yu B, Li Z, Liu X. Open‐door versus French‐door laminoplasty for the treatment of cervical multilevel compressive myelopathy. J Clin Neurosci. 2015;22:450–5. [DOI] [PubMed] [Google Scholar]
- 9. Hirvonen T, Siironen J, Marjamaa J, Niemelä M, Koski‐Palkén A. Anterior cervical discectomy and fusion in young adults leads to favorable outcome in long‐term follow‐up. Spine J. 2020;20:1073–84. [DOI] [PubMed] [Google Scholar]
- 10. Miyamoto H, Maeno K, Uno K, Kakutani K, Nishida K, Sumi M. Outcomes of surgical intervention for cervical spondylotic myelopathy accompanying local kyphosis (comparison between laminoplasty alone and posterior reconstruction surgery using the screw‐rod system). Eur Spine J. 2014;23:341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Epstein NE. Laminectomy for cervical myelopathy. Spinal Cord. 2003;41:317–27. [DOI] [PubMed] [Google Scholar]
- 12. Hosono N, Yonenobu K, Ono K. Neck and shoulder pain after laminoplasty. A noticeable complication. Spine (Phila Pa 1976). 1996;21:1969–73. [DOI] [PubMed] [Google Scholar]
- 13. Mielke D, Rohde V. Bilateral spinal canal decompression via hemilaminectomy in cervical spondylotic myelopathy. Acta Neurochir. 2015;157:1813–7. [DOI] [PubMed] [Google Scholar]
- 14. Hernandez RN, Wipplinger C, Navarro‐Ramirez R, Soriano‐Solis S, Kirnaz S, Hussain I, et al. Ten‐step minimally invasive cervical decompression via unilateral tubular Laminotomy: technical note and early clinical experience. Oper Neurosurg (Hagerstown). 2020;18:284–94. [DOI] [PubMed] [Google Scholar]
- 15. Dahdaleh NS, Wong AP, Smith ZA, Wong RH, Lam SK, Fessler RG. Microendoscopic decompression for cervical spondylotic myelopathy. Neurosurg Focus. 2013;35:E8. [DOI] [PubMed] [Google Scholar]
- 16. Xiaobing Z, Xingchen L, Honggang Z, Xiaoqiang C, Qidong Y, Haijun M, et al. "U" route transforaminal percutaneous endoscopic thoracic discectomy as a new treatment for thoracic spinal stenosis. Int Orthop. 2019;43:825–32. [DOI] [PubMed] [Google Scholar]
- 17. An B, Li XC, Zhou CP, Wang BS, Gao HR, Ma HJ, et al. Percutaneous full endoscopic posterior decompression of thoracic myelopathy caused by ossification of the ligamentum flavum. Eur Spine J. 2019;28:492–501. [DOI] [PubMed] [Google Scholar]
- 18. Zhao XB, Ma HJ, Geng B, Zhou HG, Xia YY. Early clinical evaluation of percutaneous full‐endoscopic Transforaminal lumbar interbody fusion with pedicle screw insertion for treating degenerative lumbar spinal stenosis. Orthop Surg. 2021;13:328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao XB, Ma HJ, Geng B, Zhou HG, Xia YY. Percutaneous endoscopic unilateral Laminotomy and bilateral decompression for lumbar spinal stenosis. Orthop Surg. 2021;13:641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eicker SO, Klingenhöfer M, Stummer W, Steiger HJ, Hänggi D. Full‐endoscopic cervical arcocristectomy for the treatment of spinal stenosis: results of a cadaver study. Eur Spine J. 2012;21:2487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haijun M, Xiaobing Z, Bin G, Jinwen H, Dacheng Z, Shenghong W, et al. Trans‐interlamina percutaneous endoscopic cervical discectomy for symptomatic cervical spondylotic radiculopathy using the new Delta system. Sci Rep. 2020;10:10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schneeberger AG, Boos N, Schwarzenbach O, Aebi M. Anterior cervical interbody fusion with plate fixation for chronic spondylotic radiculopathy: a 2‐ to 8‐year follow‐up. J Spinal Disord. 1999;12:215–20; discussion 221. [PubMed] [Google Scholar]
- 23. Hacker RJ, Cauthen JC, Gilbert TJ, Griffith SL. A prospective randomized multicenter clinical evaluation of an anterior cervical fusion cage. Spine (Phila Pa 1976). 2000;25:2646–54; discussion 2655. [DOI] [PubMed] [Google Scholar]
- 24. Quillo‐Olvera J, Lin GX, Kim JS. Percutaneous endoscopic cervical discectomy: a technical review. Ann Transl Med. 2018;6:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang JS, Chu L, Chen L, Chen F, Ke ZY, Deng ZL. Anterior or posterior approach of full‐endoscopic cervical discectomy for cervical intervertebral disc herniation? A comparative cohort study. Spine (Phila Pa 1976). 2014;39:1743–50. [DOI] [PubMed] [Google Scholar]
- 26. Nakamura S, Taguchi M. Percutaneous endoscopic cervical discectomy: surgical approaches and postoperative imaging changes. Asian Spine J. 2018;12:294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robertson JT, Papadopoulos SM, Traynelis VC. Assessment of adjacent‐segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2‐year study. J Neurosurg Spine. 2005;3:417–23. [DOI] [PubMed] [Google Scholar]
- 28. Luo J, Gong M, Huang S, Yu T, Zou X. Incidence of adjacent segment degeneration in cervical disc arthroplasty versus anterior cervical decompression and fusion meta‐analysis of prospective studies. Arch Orthop Trauma Surg. 2015;135:155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li C, Tang X, Chen S, Meng Y, Zhang W. Clinical application of large channel endoscopic decompression in posterior cervical spine disorders. BMC Musculoskelet Disord. 2019;20:548. [DOI] [PMC free article] [PubMed] [Google Scholar]


