Abstract
Background
There is significant risk of complications and vulnerability to severe COVID‐19 disease in pregnancy, yet hesitancy exists around COVID‐19 vaccination during pregnancy and lactation.
Objective
To summarize the safety, immunogenicity, and effectiveness of COVID‐19 vaccines in pregnancy and lactation.
Search strategy
A systematic search of MEDLINE, Embase, PubMed, medRxiv, and bioRxiv.
Selection criteria
Identified original studies published on pregnant and/or lactating individuals who received one or more doses of a COVID‐19 vaccine.
Data collection and analysis
A descriptive summary organized by safety, immunogenicity, and effectiveness outcomes of COVID‐19 vaccination in pregnancy and lactation.
Main results
In total, 23 studies were identified. Humoral response and functional immunity were interrogated and found. Increasing placental transfer ratios in cord blood were associated with increasing time from the first vaccine dose to delivery. Safety data indicated that pregnant and lactating populations experienced vaccine‐related reactions at similar rates to the general population. No increased risk of adverse obstetrical or neonatal outcomes were reported. One study demonstrated that pregnant individuals were less likely to experience COVID‐19 when vaccinated.
Conclusion
COVID‐19 vaccination in pregnant and lactating individuals is immunogenic, does not cause significant vaccine‐related adverse events or obstetrical and neonatal outcomes, and is effective in preventing COVID‐19 disease.
Keywords: breastfeeding, COVID‐19, COVID‐19 vaccines, lactation, pregnancy, SARS‐CoV‐2
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is particularly impactful for pregnant women who experience increased risk of severe disease upon infection. 1 , 2 This may be due to physiologic changes in pregnancy including reduced lung capacity, increased metabolic and cardiovascular demands, and immune‐mediated changes 3 , 4 that can predispose patients to more severe respiratory disease than their non‐pregnant counterparts. 5
Given this significant risk of complications and vulnerability to severe COVID‐19 disease, it is important that the safety, immunogenicity, and effectiveness of COVID‐19 vaccines be understood to guide clinical maternal care during this pandemic. The National Advisory Committee on Immunizations (NACI), 6 Society of Obstetricians and Gynaecologists of Canada (SOGC), 7 Centers for Disease Control and Prevention (CDC), 8 American College of Obstetricians and Gynecologists (ACOG), 9 and Society for Maternal‐Fetal Medicine (SMFM) 10 have recognized pregnancy as a risk factor for severe illness and recommend vaccines to pregnant and lactating individuals. Unfortunately, this population was excluded from participation in initial COVID‐19 vaccine trials, 11 necessitating the use of real‐world data to understand how the immunologic response in pregnant and lactating states differs from that of non‐pregnant states as well as antibody transfer to neonates. These data are critical to guiding ongoing policy recommendations in this key population. Of note, many vaccines are routinely and safely used in pregnancy, including influenza, hepatitis B, tetanus, diphtheria, pertussis, polio, meningococcal, and pneumococcal vaccines.
Based on COVID‐19 immunization registries, maternal and neonatal safety signals have not been reported. 6 , 8 Furthermore, WHO, NACI, SOGC, CDC, ACOG, and SMFM all recommend that lactating individuals obtain a vaccine if they fall within priority groups for vaccination and continue breastfeeding their infants uninterrupted. 12 , 13 However, vaccine hesitancy remains high among some pregnant and lactating individuals, particularly due to the perceived lack of data from randomized clinical trials. 14 The aim of the present review was to combine safety, immunogenicity, and effectiveness data on COVID‐19 vaccines in pregnancy to provide an integrated review of a rapidly developing situation.
2. MATERIALS AND METHODS
A systematic search was conducted of MEDLINE, Embase, PubMed, medRxiv, and bioRxiv to identify original studies published on pregnant and lactating individuals who received one or more doses of a COVID‐19 vaccine. For PubMed, MEDLINE, and Embase, a combination of pregnancy and lactation‐related terms, vaccine‐related terms, and the COVID‐19 search strings developed by the Canadian Agency for Drugs and Technologies in Health were used (Appendix 1). 15 The search was conducted from September 1, 2020, to June 29, 2021, and results were restricted to the English language. For medRxiv and bioRxiv, the “medrxivr” R package was used to search preprint repository data (Appendix 1). The present study followed PRISMA guidelines and was registered on PROSPERO (CRD42021258912).
Records were screened by title, abstract, and full text for inclusion. Covidence and Excel were used to record decisions. Original studies that evaluated the immunogenicity, safety, and effectiveness of COVID‐19 vaccines in pregnant and lactating individuals were included. Cohort studies, case‐control studies, and cross‐sectional studies were included. Studies with less than 10 participants were excluded from analysis. Data extracted from studies included the following: first author; country; study design; population characteristics; vaccine type and characteristics; and study outcomes (as related to safety and immunogenicity). Data were recorded using Covidence and Excel. We assessed the quality of each study using the National Institute of Health (NIH) Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies and the NIH Quality Assessment of Case‐Control Studies.
Two reviewers independently screened records, extracted data, and assessed study quality. Disagreements between reviewers were resolved by consensus. Immunogenicity, safety, and effectiveness outcomes were described qualitatively.
3. RESULTS
The search returned 650 unique records. After screening the titles and abstracts, 42 full texts were reviewed. Of these articles, 13 were included in the review. Ten additional pre‐print and in‐press articles were included, resulting in a total of 23 articles included in the review (Figure 1).
FIGURE 1.
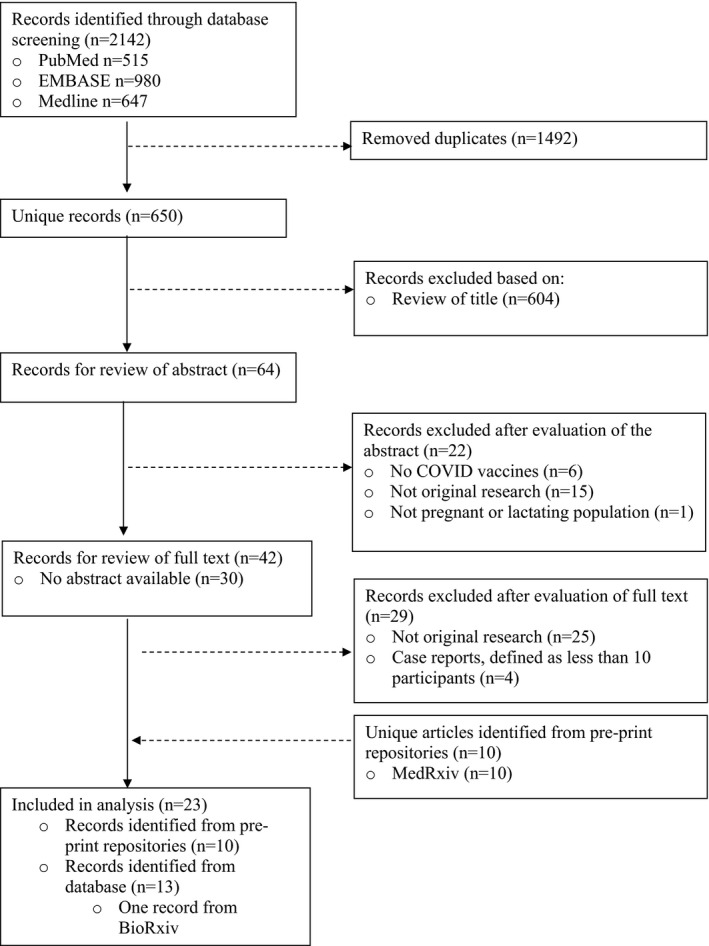
Study selection flow diagram
In total, 23 studies were included in the systematic review (Table 1). All studies were conducted in high‐income countries. The majority were observational cohort studies (n = 18), followed by case‐control (n = 3) and cross‐sectional (n = 2) studies. Eight studies looked exclusively at participants who had received BNT162b2 (Pfizer) vaccines while 14 studies included participants who had received Pfizer or mRNA‐1273 (Moderna) vaccines. Of the studies that looked at both mRNA‐based vaccines, one study also included one participant who had received the Janssen (Johnson & Johnson) vaccine, while one study included participants who had received the ChAdOx1 nCoV‐19 (AstraZeneca/Oxford) vaccine. Nine studies included reference cohorts (non‐pregnant, unvaccinated, and/or natural SARS‐CoV‐2 infection) for comparison.
TABLE 1.
Study characteristics
| Author | Country | Study design | Vaccine type | No. of doses administered before analysis | N | Population type |
|---|---|---|---|---|---|---|
| Esteve‐Paula et al. 23 | Spain | Observational cohort | Pfizer | Two | 18 | Lactating, vaccinated |
| Bertrand et al. 32 | USA | Observational cohort |
Moderna (n = 52) Pfizer (n = 128) |
Moderna took both doses (n = 52) Not clear for Pfizer |
180 | Lactating, vaccinated |
| Low et al. 28 | Singapore | Observational cohort | Pfizer | Two | 25 |
Lactating, vaccinated (n = 10) Lactating, infected, unvaccinated (n = 6) Lactating, unvaccinated (n = 9) |
| Theiler et al. 37 | USA | Case‐control |
Johnson (n = 1) Modern (n = 12) Pfizer (n = 127) |
One (n = 37) Two (n = 103) |
2002 |
Pregnant, unvaccinated (n = 1862) Pregnant, vaccinated (n = 140) |
| Selma‐Royo et al. 29 | Spain | Observational cohort |
Pfizer (n = 30) Moderna (n = 21) AZ (n = 24) |
Two for Pfizer and Moderna (n = 51), one for AZ (n = 24) | 75 |
Lactating, vaccinated (n=75) Lactating, infected, unvaccinated (n=19) Lactating, pre‐pandemic (n=13) |
| Collier et al. 16 | USA | Observational cohort |
Pfizer and Moderna (pregnant: Pfizer = 11, Moderna = 19; lactating: Pfizer = 11, Moderna = 5; non‐pregnant: Pfizer = 34, Moderna = 23) |
Not reported | 131 |
Non‐pregnant, nonlactating, vaccinated (n = 57) Pregnant, vaccinated (n = 30) Lactating, vaccinated (n = 16) Non‐pregnant, unvaccinated, infected (n = 6) Pregnant, unvaccinated, infected (n = 22) |
| Beharier et al. 17 | Israel | Case‐control | Pfizer | One and two, numbers not specified | 213 |
Pregnant, vaccinated (n = 86) Pregnant, unvaccinated, infected (n = 65) Pregnant, unvaccinated, non‐infected (n = 62) |
| Gray et al. 18 | USA | Observational cohort | Pfizer and Moderna (pregnant: Pfizer = 41, Moderna = 43; lactating: Pfizer = 16, Moderna = 15; non‐pregnant: Pfizer = 8, Moderna = 8) |
One (n = 6) Two (n = 125) |
131 |
Non‐pregnant, vaccinated (n = 16) Pregnant, vaccinated (n = 84) Lactating, vaccinated (n = 31) Infected, unvaccinated (n = 37) |
| Mithal et al. 19 | USA | Observational cohort |
Pfizer (n = 18) Moderna (n = 6) Unknown (n = 4) |
One (n = 5) Two (n = 22) |
27 | Pregnant, vaccinated |
| Perl et al. 30 | Israel | Observational cohort | Pfizer | Two | 84 | Lactating, vaccinated |
| Friedman et al. 24 | Israel | Observational cohort | Pfizer | Two | 10 | Lactating, vaccinated |
| Golan et al. 25 | USA | Observational cohort |
Moderna (n = 9) Pfizer (n = 14) |
Two | 23 |
Lactating, vaccinated (n = 23) Lactating, infected (n = 3) |
| Prabhu et al. 20 | USA | Observational cohort |
Pfizer (n = 85) Moderna (n = 37) |
One (n = 55) Two (n = 67) |
122 | Pregnant, vaccinated |
| Rottenstreich et al. 21 | Israel | Observational cohort | Pfizer | Two | 20 | Pregnant, vaccinated |
| Shanes et al. 39 | USA | Case‐control | Not reported | Not reported | 200 |
Pregnant, vaccinated (n = 84) Pregnant, unvaccinated (n = 116) |
| Atyeo et al. 22 | USA | Observational cohort |
Pfizer (n = 65) Moderna (n = 66) |
Two | 131 |
Pregnant, vaccinated (n = 84) Lactating, vaccinated (n = 31) Non‐pregnant, vaccinated (n = 16) |
| Fox et al. 31 | USA | Observational cohort |
Pfizer (n = 6) Moderna (n = 4) |
Two | 10 | Lactating, vaccinated |
| Valcarce et al. 26 | USA | Observational cohort |
Pfizer (n = 14) Moderna (n = 7) |
Two | 21 | Lactating, vaccinated |
| Shimabukuro et al. 33 | USA | Observational cohort |
Pfizer (n = 19 252) Moderna (n = 16 439) |
One (n = 16 982) Two (n = 12 273) |
35 691 | Pregnant, vaccinated |
| Juncker et al. 27 | Netherlands | Observational cohort | Pfizer |
One (n = 6) Two (n = 20) |
26 | Lactating, vaccinated |
| Kadali et al. 34 | USA | Cross‐sectional |
Pfizer (n = 20) Moderna (n = 18) |
One (n = 7) Two (n = 31) |
38 | Pregnant, vaccinated |
| Low et al. 35 | Singapore | Observational cohort | Pfizer | Two | 88 | Lactating, vaccinated |
| McLaurin‐Jiang 36 | USA | Cross‐sectional |
Pfizer (n = 2702) Moderna (n = 1714) |
One (n = 2627) Two (n = 1828) |
4455 | Lactating, vaccinated |
The quality of each study, independently assessed by two reviewers using the National Institute of Health (NIH) Quality Assessment Tool, is presented in Tables S1 and S2.
3.1. Immunogenicity overview
Seventeen studies evaluated and quantified the humoral response in breast milk, cord blood, and/or maternal blood after vaccination (Table 2).
TABLE 2.
Maternal and cord blood seroconversion and breast milk antibodies
| Author | Antibodies in breast milk | Seropositivity in maternal blood | Seropositivity in cord blood |
|---|---|---|---|
| N positive/N tested (antibody–antigen) | |||
| Esteve‐Paula et al. 23 | 18/18 (IgG–S1) | 18/18 (IgG–S1) | N/A |
| Low et al. 28 | 10/10 (IgG–spike) | N/A | N/A |
| 10/10 (IgG–RBD) | |||
| 9/10 (IgA–spike) | |||
| 9/10 (IgA–RBD) | |||
| Selma‐Royo et al. 29 | Moderna and Pfizer | N/A | N/A |
| 40/40 (IgG–RBD) | |||
| 24/40 (IgA–RBD) | |||
| AZ | |||
| 17/20 (IgG–RBD) | |||
| 6/20 (IgA–RBD) | |||
| Collier et al. 16 | N/S | 44/44 (IgG–RBD) | 9/9 (IgG–RBD) |
| Beharier et al. 17 | N/A | N/S | N/S |
| Gray et al. 18 | N/S | N/S | 10/10 (IgG–RBD) |
| 10/10 (IgG–spike) | |||
| Mithal et al. 19 | N/A | 26/27 (IgG–RBD) | 25/28 (IgG–RBD) |
| 15/27 (IgM–RBD) | 0/28 (IgM–RBD) | ||
| Perl et al. 30 | 68/70 | N/A | N/A |
| Friedman et al. 24 | N/S | N/S | N/A |
| Golan et al. 25 | 13/15 (IgA–RBD) | 14/14 (IgG–RBD) | N/A |
| Prabhu et al. 20 | N/A | 87/122 (IgG–S‐RBD) | N/S |
| Rottenstreich et al. 21 | N/A | 20/20 (IgG–RBD) | 20/20 (IgG–RBD) |
| 20/20 (IgG–spike) | 20/20 (IgG–spike) | ||
| 6/20 (IgM–S1 RBD) | 0/20 (IgM–S1‐RBD) | ||
| Shanes et al. 39 | N/A | 30/52 (IgM–spike) | N/A |
| 50/52 (IgG–spike) | |||
| Atyeo et al. 22 | N/S | N/S | N/S |
| Fox et al. 31 | 10/10 (IgG–spike) | N/A | N/A |
| 6/10 (IgA–spike) | |||
| Valcarce et al. 26 | 18/21 (IgA–?) | 18/21 (IgA–?) | N/A |
| 10/10 (IgG–?) | 21/21 (IgG–?) | ||
| Juncker et al. 27 | 26/26 (IgA–spike) | 26/26 (IgG–spike) | N/A |
Abbreviations: N/A, study did not assay for humoral response in this sample type; N/S, study did not specify antibody conversion rates.
3.2. Placental transfer
Seven studies assessed antibody titers in vaccinated maternal and cord blood. 16 , 17 , 18 , 19 , 20 , 21 , 22 Transfer of IgG to cord blood was noted in all studies. All studies assessed IgG to RBD and/or spike protein. Placental transfer ratios (cord blood antibody concentration/maternal serum antibody concentration) differed between and within studies. Five studies observed that increasing transfer ratios of IgG in cord blood were associated with having received the second vaccine dose before delivery, and with increasing time from the first vaccine dose to delivery. 18 , 19 , 20 , 21 , 22 In their study of 13 individuals, Gray et al. 18 reported a median of 36.5 days (interquartile range [IQR] 30–42 days) and 14 days (IQR 11–16 days) from the first and second vaccine to delivery, respectively. Similarly, Rottenstreich et al., 21 in their study of 20 individuals, reported a median of 33 days (IQR 30–37 days) and 11 days (IQR 9–15 days), respectively. Mithal et al. 17 and Beharier et al. 19 studied 27 and 86 individuals, respectively, and reported time from the first vaccine dose to delivery up to 12 weeks, while Prabhu et al. 20 had one participant out of 67 who received their second dose more than 10 weeks before delivery. Generally, all studies noted a positive linear relationship between placental transfer ratio 19 , 20 or IgG titers in cord blood 17 , 18 , 21 and time since the first and/or second vaccine dose to delivery. Additionally, Gray et al. 18 noted that the time‐dependent nature of transfer efficiency may only affect specific IgG subpopulations. A significant increase in transfer of IgG1 spike was correlated with increased time from dose 2 to delivery, but this correlation was not observed for IgG1 RBD.
Functional antibody subpopulations were also studied. Atyeo et al., 22 in their study of seven individuals, noted that equivalent phagocytic antibodies and lower FcR binding and NK‐cell activating antibodies against SARS‐CoV‐2 spike antigen were found in cord blood compared to maternal serum. Conversely, in the same study, higher FcR binding and NK‐cell activating antibodies were observed for influenza hemagglutinin (HA)‐specific antibodies in the same maternal‐cord dyads. The decreased functional antibody transfer observed for SARS‐CoV‐2 spike antigen was attributed to the reduced time from vaccination to delivery (participants received the vaccine in the third trimester), resulting in less efficient transfer of antibodies to the neonate. Despite the low rates of antibody transfer, cord blood was enriched for FcgR3a antibodies against SARS‐CoV‐2 RBD.
Collier et al. 16 evaluated binding and neutralizing antibodies in nine maternal and cord blood samples and found that compared to infected, non‐vaccinated maternal‐infant dyads, vaccinated dyads produced a more robust binding (IgG RBD) and neutralizing antibody response. Gray et al. 18 also assayed for neutralizing antibodies in 10 maternal‐infant dyads. All but two maternal‐infant pairs had detectable antibodies. For the two cord blood samples without neutralizing antibody present, the pregnant individual had either not received dose 2 or received it only 7 days prior.
3.3. Temporal profile of humoral response
Eight studies noted an increase in IgG, IgM, and/or IgA in the sera of pregnant and lactating individuals after vaccination. 16 , 18 , 22 , 23 , 24 , 25 , 26 , 27 Gray et al. 18 observed that IgM and IgA titers were strongly induced after the first dose in 115 pregnant and lactating individuals. IgG was robustly induced after both doses, and by 2 weeks after the second vaccine, the dominant serum antibody response was IgG. Collier et al. 16 noted that levels of IgG were higher for pregnant and lactating individuals (n = 44) after vaccination than the infected, non‐vaccinated, pregnant comparator group (n = 26). While most studies sampled 2–3 timepoints, Juncker et al. 24 and Friedman et al. 27 sampled sera in lactating individuals at multiple timepoints (>3), noting general trends of IgG induction. Juncker et al. 24 noted a gradual increase in IgG over four timepoints (pre‐dose 1, 14 days after dose 1, 20 days after dose 1, 15 days after dose 2) in 26 lactating individuals without any declines (median breastfeeding time of 7 months). In their study of 10 individuals, Friedman et al. 27 sampled over four timepoints (7 days after dose 1, 14 days after dose 1, 7 days after dose 2, and 14 days after dose 2) and noted an increase in IgG between all timepoints except for the last two, which showed a plateau (mean postpartum time of 5 months). Esteve‐Palau et al. 23 also noted a similar plateau between later median time points at 14 and 28 days after dose 2, with median levels of IgG serum at 11 505 (IQR 8933–21 184) and 8311 (IQR 5578–17 419), respectively, in 18 lactating individuals (mean postpartum time of 19 months). Taken together, the studies from Friedman et al. 27 and Esteve‐Palau et al. 23 suggest that peak antibody titers may occur as early as 7 days after the second dose and last as long as 28 days after the second dose.
Similar to antibodies in sera, IgG, IgA, and IgM titers in breast milk also were detected after vaccination. 16 , 18 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 Gray et al. 18 found that levels of IgA and IgM were detected after the first dose and IgG increased significantly after the first and second dose in 31 individuals. Several studies that collected samples of breast milk at enough timepoints observed a biphasic IgA response in breast milk, with a rise in IgA immediately after the first dose, a decline before administration of the second dose, a rise after the second dose, and another decline thereafter. 24 , 25 , 27 , 29 , 30 However, Low et al. 28 only observed an increase in IgA titers after the second dose and not after the first dose in 10 individuals.
A few studies compared antibody titers in breast milk between vaccinated, lactating individuals and unvaccinated, infected individuals. In vaccinated, lactating individuals compared to lactating individuals infected with the virus, Low et al. 28 found higher levels of IgA (n = 10 vs n = 6), Golan et al. 25 did not observe differences in IgA titers (n = 23 vs n = 3), and Collier et al. 16 and Selma‐Royo et al. 29 found lower levels of IgA (n = 16 vs n = 6 and n = 51 vs n = 19, respectively). Meanwhile, Selma‐Royo et al. 29 found higher levels of IgG29 and Collier et al. 16 found lower levels of IgG in vaccinated, lactating individuals compared to their COVID‐19–infected, lactating counterparts. Collier et al. 16 also noted lower neutralizing antibody titers in vaccinated individuals compared to infected controls (n = 16 vs n = 6). There was a lack of consensus in antibody trends between these studies. Of note, these studies did not match the unvaccinated, infected individuals as controls; they were simply included as a reference, comparator cohort. Confounders such as variable sample collection time and waning immunity with natural infection may preclude clinically relevant conclusions from this comparison. As such, larger sample sizes with matched controls are needed to draw definitive comparisons on antibody titers in breast milk between vaccinated and unvaccinated individuals.
3.4. Antibody features and functions
Multiple studies profiled the functional response of the antibodies produced. Neutralizing antibodies were assayed and found in maternal sera in two studies, 16 , 18 cord blood in two studies, 16 , 18 and breast milk in two studies. 16 , 24
Collier et al. 16 (n = 17) found a higher phagocytic score for antibody‐dependent complement deposition (ADCD) and antibody‐dependent monocyte cellular phagocytosis (ADCP) in pregnant, vaccinated individuals (n = 8) compared to their non‐pregnant, vaccinated counterparts (n = 49). Conversely, the phagocytic score for antibody‐dependent neutrophil phagocytosis (ADNP), ADCD, and ADCP in lactating individuals (n = 9) was lower than their non‐pregnant counterparts (n = 49). Unlike Collier et al., 16 Atyeo et al. 22 found that ADCP was comparable between pregnant (n = 84), lactating (n = 31), and non‐pregnant individuals (n = 16). This was not augmented by the second dose. However, while ADNP was similar between groups after the first dose, this response was enhanced after the second dose, with the most significant boost occurring in the lactating cohort. Atyeo et al. 22 also evaluated transfer of ADCP and ADNP into breast milk. While ADCP and ADNP were transferred at comparable ratios after the first dose, ADCP was transferred at a higher ratio than ADNP after the second dose.
3.5. T‐cell response
Collier et al. 16 examined spike‐specific T‐cell responses in the peripheral blood mononuclear cells of 18 pregnant, 7 lactating, and 12 non‐pregnant patients. It was noted that the percentage of spike‐specific IFN‐gamma production by CD4 T‐cells, CD4 central memory T‐cells, CD8 T‐cells, and CD8 central memory T‐cells was similar across all three groups.
3.6. Maternal safety events: Overall reactogenicity profile
Nine different studies looked at symptom profiles in pregnant and/or lactating patients after the first and/or second dose (Tables 3 and 4). 16 , 18 , 29 , 30 , 32 , 33 , 34 , 35 , 36 Five studies profiled symptoms in lactating individuals, 29 , 30 , 32 , 35 , 36 two studies looked at pregnant individuals, 33 , 34 and two studies looked at both pregnant and lactating individuals. 16 , 18 Of note, these studies were based on self‐reported symptoms and adverse events and, as such, are subject to accurate reporting from participants. Studies with smaller participant sizes may not accurately reflect reactogenicity profiles and thus may not be generalizable, while larger participant sizes may more accurately capture symptoms with less bias.
TABLE 3.
Local and systemic maternal adverse effects after dose 1 of mRNA‐based vaccines a
| Gray et al. 18 | Bertrand et al. 32 | Perl et al. 30 | Selma‐Royo et al. 29 | Shimabukuro et al. 33 | Collier et al. 16 | McLaurin‐Jiang et al. 36 | |
|---|---|---|---|---|---|---|---|
| N (pregnant and/or lactating individuals) | 109 | 178 | 84 | 51 | 16 982 | 46 | 2627 |
| Injection site soreness/Pain | 93 (85.3) | 155 (87.1) | 40 (47.6) | N/A | 14 962 (88.1) | N/A | 1374 (52.3) |
| Injection site reaction/Rash | 1 (0.9) | N/A | N/A | N/A | N/A | N/A | N/A |
| Headache | 16 (14.7) | 41 (23.0) | N/A | 9 (17.6) | 3078 (18.1) | N/A | 646 (24.6) |
| Muscle aches/Pain | 6 (5.5) | 22 (12.4) | N/A | N/A | 1962 (11.6) | N/A | 431 (16.4) |
| Fatigue | 16 (14.7) | 43 (24.2) | 8 (9.5) | N/A | 5022 (29.6) | N/A | 749 (28.5) |
| Fever/Chills | 2 (1.8) | N/A | N/A | N/A | N/A | N/A | N/A |
| Allergic reaction | 0 | N/A | N/A | N/A | N/A | N/A | 13 (0.5) |
| Anaphylaxis | N/A | N/A | N/A | N/A | N/A | N/A | 0 (0.0) |
| Fever | N/A | 3 (1.7) | 0 (0.0) | 2 (3.9) | 709 (4.2) | 0 (0.0) | 60 (2.3) |
| Chills | N/A | 13 (7.3) | N/A | N/A | 696 (4.1) | N/A | 208 (7.9) |
| Injection site redness | N/A | 12 (6.7) | N/A | N/A | 508 (3.0) | N/A | N/A |
| Rash (body) | N/A | 2 (1.1) | N/A | N/A | 42 (0.2) | N/A | N/A |
| Injection site swelling | N/A | 13 (7.3) | N/A | N/A | 1057 (6.2) | N/A | N/A |
| Injection site itching | N/A | 7 (3.9) | N/A | N/A | 260 (1.5) | N/A | N/A |
| Nausea | N/A | 6 (3.4) | N/A | N/A | 1130 (6.7) | N/A | N/A |
| Joint pain | N/A | 8 (4.5) | N/A | N/A | 551 (3.2) | N/A | N/A |
| Abdominal pain | N/A | 1 (0.6) | N/A | N/A | 277 (1.6) | N/A | N/A |
| Diarrhea | N/A | 2 (1.1) | N/A | N/A | 367 (2.2) | N/A | N/A |
| Vomiting | N/A | 0 (0.0) | N/A | N/A | 159 (0.9) | N/A | N/A |
| Change in milk supply (more milk) | N/A | 4 (2.2) | N/A | N/A | N/A | N/A | N/A |
| Change in milk supply (less milk) | N/A | 15 (8.4) | N/A | N/A | N/A | N/A | N/A |
| Change in milk color | N/A | 3 (1.7) | N/A | N/A | N/A | N/A | N/A |
Abbreviations: N/A, not applicable.
Values are given as number (percentage).
TABLE 4.
Local and systemic maternal adverse effects after dose 2 of mRNA‐based vaccines a
| Gray et al. 18 | Bertrand et al. 32 | Perl et al. 30 | Selma‐Royo et al. 29 | Shimabukuro et al. 33 | Collier et al. 16 | Low et al. 35 | McLaurin‐Jiang et al. 36 | Kadali et al. b , 34 | |
|---|---|---|---|---|---|---|---|---|---|
| N (pregnant and/or lactating individuals) | 105 | 175 | 84 | 51 | 12 273 | 45 | 88 | 1828 | 38 |
| Injection site soreness/Pain | 61 (58.1) | 150 (85.7) | 34 (40.5) | N/A | 11 274 (91.9) | N/A | 57 (includes swelling) (64.8) | 1312 (71.8) | 37 (97.4) |
| Injection site reaction/Rash | 1 (1.0) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Headache | 36 (34.3) | 109 (62.3) | N/A | 20 (39.2) | 6800 (55.4) | N/A | N/A | 1002 (54.8) | 19 (50.0) |
| Muscle aches/Pain | 53 (50.5) | 112 (64.0) | N/A | N/A | 6638 (54.1) | N/A | N/A | 941 (51.5) | 13 (34.2) |
| Fatigue | 55 (52.4) | 124 (70.9) | 28 (33.3) | N/A | 8772 (71.5) | N/A | 54 (61.4) | 1177 (64.4) | 22 (57.9) |
| Fever/Chills | 37 (35.2) | N/A | N/A | N/A | N/A | N/A | 38 (43.2) | N/A | N/A |
| Allergic reaction | 1 (1.0) | N/A | N/A | N/A | N/A | N/A | 5 (0.3) | N/A | |
| Anaphylaxis | N/A | N/A | N/A | N/A | N/A | N/A | 0 (0.0) | 4 (0.2) | N/A |
| Fever | N/A | 51 (29.1) | 10 (11.9) | 16 (11.9) | 4242 (34.6) | 11 (24.4) | 386 (21.1) | 6 (15.8) | |
| Chills | N/A | 91 (52.0) | N/A | N/A | 4502 (36.7) | N/A | N/A | 808 (44.2) | 18 (47.4) |
| Injection site redness | N/A | 18 (10.3) | N/A | N/A | 660 (5.4) | N/A | N/A | N/A | N/A |
| Rash (body) | N/A | 2 (1.1) | N/A | N/A | 36 (0.3) | N/A | N/A | N/A | 4 (10.5) |
| Injection site swelling | N/A | 21 (12.0) | N/A | N/A | 1462 (11.9) | N/A | N/A | N/A | N/A |
| Injection site itching | N/A | 13 (7.4) | N/A | N/A | 302 (2.5) | N/A | N/A | N/A | N/A |
| Nausea | N/A | 31 (17.7) | N/A | N/A | 3265 (26.6) | N/A | 1 (1.1) | N/A | 11 (28.9) |
| Joint pain | N/A | 47 (26.9) | N/A | N/A | 3138 (25.6) | N/A | N/A | N/A | 3 (7.9) |
| Abdominal pain | N/A | 7 (4.0) | N/A | N/A | 717 (5.8) | N/A | N/A | N/A | N/A |
| Diarrhea | N/A | 6 (3.4) | N/A | N/A | 609 (5.0) | N/A | N/A | N/A | 0 (0.0) |
| Vomiting | N/A | 5 (2.9) | N/A | N/A | 558 (4.5) | N/A | N/A | N/A | 1 (2.6) |
| Change in milk supply (more milk) | N/A | 7 (4.0) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Change in milk supply (less milk) | N/A | 20 (11.4) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Change in milk color | N/A | 2 (1.1) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
Abbreviations: N/A, not applicable.
Values are given as number (percentage).
Only 31/38 received two doses and adverse effect was not specified after each dose.
3.7. Reactogenicity in pregnant populations
Shimabukuro et al. 33 conducted the study with the largest sample size (first dose: n = 16 982, second dose: n = 12 273) and demonstrated that pain at injection site, fatigue, headache, and myalgia were the most frequently reported vaccine‐related symptoms after either dose for mRNA‐based vaccines in pregnant individuals. These symptoms were more frequently reported after dose 2 for both vaccines than dose 1. The vaccine‐related symptom profile was similar for pregnant versus non‐pregnant patients in most cases aside from pain at injection site more frequently reported in pregnant individuals and “other systemic reactions” reported more frequently among non‐pregnant individuals. Kadali et al. 34 conducted a cross‐sectional study on 1029 female healthcare workers, 38 of whom were pregnant, and found that there were no differences between pregnant and non‐pregnant women for any of the symptoms and adverse events assessed, including pain at injection site, fever, and nausea.
3.8. Reactogenicity in lactating populations
Bertrand et al. 32 (n = 180) found that more than 85% of lactating participants reported local or systemic symptoms for both mRNA vaccines after the first or second dose. Higher frequencies of symptoms were reported after the second dose of either mRNA vaccine. Meanwhile, Perl et al. 30 (n = 84) found that 56% of lactating persons reported a vaccine‐related adverse event after the first dose versus 62% after the second dose: pain at injection site was the most commonly reported event. Similarly, McLaurin‐Jiang et al. 36 (n = 4455) found that most post‐vaccination symptoms were more commonly reported after the second dose of either mRNA vaccine than the first dose for lactating individuals. Interestingly, participants more frequently reported fatigue, headache, pain at injection site, muscle pain, chills, fever, and allergic reactions with the Moderna vaccine as opposed to Pfizer (Pfizer = 1714, Moderna = 2702).
Selma‐Royo et al. 29 was one of the few studies that included patients who had received one dose of the Oxford/AstraZeneca vaccine. Patients who had received Oxford/AstraZeneca reported more symptoms after vaccination compared to those receiving mRNA‐based vaccines (AZ = 24, Moderna = 21, Pfizer = 30). This was particularly notable for fever (P = 0.0005) and headache (P < 0.0001).
With regard to lactation‐specific symptoms, McLaurin‐Jiang et al. 36 (n = 4455) found that 2% of lactating patients reported an adverse impact on breastfeeding after vaccination. Of them, 90% reported no change in production of milk while 4% reported an increase in production and 6% reported a decrease. These impacts on breastfeeding were not associated with vaccine type. The adverse impacts on breastfeeding were more commonly associated with the second dose, reported symptoms in the infant, and intensity of intake of breast milk after adjusting for confounders. Low et al. 28 noted that 6% of their lactating study population (n = 88) experienced axillary or neck lymph node swelling after vaccination while 3% reported mastitis. None of the participants reported changes to milk supply. With regard to changes in supply of breast milk, Bertrand et al. 32 found very low frequencies of a reported reduction in milk supply but noted that all cases (n = 180) returned to normal within 72 h.
Gray et al. 18 found that there were no significant differences in post‐vaccine reactions between pregnant patients (n = 84), lactating patients (n = 31), and their non‐pregnant counterparts (n = 16).
3.9. Pregnancy loss and neonatal outcomes
Five studies reported on pregnancy loss and/or neonatal outcomes after maternal vaccination (Table 5). 17 , 18 , 33 , 37 The two studies with the largest participant sizes did not find an increased risk of pregnancy loss or adverse neonatal outcomes. Shimabukuro et al. 33 compared all reported outcomes to published incidences among the general population and found that rates of spontaneous abortion (12.6%), stillbirth (0.1%), preterm birth (9.4%), small size for gestational age (3.2%), congenital anomalies (2.2%), and neonatal death (0%) all fell within the range or lower than published incidences. Theiler et al. 37 compared a pregnant, vaccinated cohort to a pregnant, unvaccinated cohort (n = 140 vs n = 1862) and found that the adverse outcome index (a composite of adverse maternal events) did not differ by vaccination status.
TABLE 5.
Pregnancy loss and neonatal outcomes after vaccination a
| Shimabukuro et al. 33 | Beharier et al. 17 | Gray et al. 18 | Theiler et al. 37 | Kadali et al. 34 | |
|---|---|---|---|---|---|
| Spontaneous abortion/miscarriage | 104/827 (12.6) | N/A | N/A | N/A | 1/38 |
| Stillbirth | 1/725 (0.1) | N/A | N/A | 0 (0.0) | N/A |
| Preterm birth <37 week | 60/636 (9.4) | 4/92 (4.3) | 1/13 (8) | N/A | 1/38 |
| SGA/fetal growth restriction | 23/724 (3.2) | N/A | 0/13 (0.0) | N/A | N/A |
| Congenital anomalies | 16/724 (2.2) | N/A | N/A | N/A | N/A |
| Neonatal death | 0/724 | N/A | N/A | N/A | N/A |
| Admission to NICU | N/A | 4/92 (4.3) | 2/13 (15) | 1/140 (0.7) | N/A |
| Supplemental oxygen/CPAP | N/A | N/A | 1/13 (8) | N/A | N/A |
| TTN | N/A | N/A | 1/13 (8) | N/A | N/A |
| 5‐min APGAR <7 | N/A | N/A | N/A | 3/140 (2.1) | N/A |
| Low birth weight <2500 g | N/A | N/A | N/A | 11/140 (7.9) | N/A |
| Very low birth weight <1500 g | N/A | N/A | N/A | 3/140 (2.1) | N/A |
Abbreviations: N/A, not applicable; NICU, neonatal intensive care unit; SGA, small for gestational age; TTN, transient tachypnea of the newborn.
Values are given as number (percentage).
3.10. Effectiveness
Only one study examined the effectiveness of the vaccine in pregnant patients. Theiler et al. 37 found that vaccinated pregnant patients were less likely than unvaccinated patients to experience SARS‐CoV‐2 infection before delivery. Out of 140 vaccinated pregnant patients, approximately 2 (1.4%) experienced infection while 210 out of 1862 (11.3%) unvaccinated patients experienced COVID‐19 illness (P < 0.001).
4. DISCUSSION
The present systematic review describes the current literature on the immunogenicity, safety, and effectiveness of COVID‐19 vaccines in pregnant and lactating individuals. Immunogenicity data indicated that robust humoral responses were mounted in maternal sera, and transferred to cord blood and breast milk. Seroconversion was close to 100% for all studies that assessed humoral response. Functional immunity, antibody‐dependent activity, and T‐cell response were also interrogated and found in several studies. It is worthwhile to note that there appears to be increasing transfer of antibodies to the neonate with increasing time from vaccination to delivery. This suggests that vaccinating earlier in pregnancy would not only confer immunity to the pregnant individual as early as possible but may also maximize transfer of antibodies to the fetus. Most importantly, one study demonstrated that pregnant individuals were less likely to experience COVID‐19 when vaccinated, indicating that vaccines appear to be effective in preventing COVID‐19.
Safety data indicated that pregnant and lactating populations experienced non‐severe vaccine‐related local and systemic reactions after the first and second dose. The overall reactogenicity profile was similar between pregnant and non‐pregnant populations. Importantly, higher incidences of adverse obstetrical and neonatal outcomes were not seen in vaccinated cohorts compared to prior published incidences/unvaccinated cohorts.
Due to rapid research outputs during the COVID‐19 pandemic, many publications were submitted to the pre‐print repositories medRxiv and bioRxiv. About half of the studies from the present review were from these repositories (n = 11) and, as such, these papers have not undergone a rigorous peer‐review process. Furthermore, because many of the studies used different assays when assessing for humoral response, values reported from these studies were not comparable and could not be meta‐analyzed (Table S3). As additional immunogenicity data are published, studies with similar assays may be compared for pooled analysis.
Future research should incorporate larger sample sizes that are more diverse in nature and not limited to specific populations (i.e. healthcare workers). Of note, while many studies from the present review included reference cohorts (non‐pregnant, unvaccinated, and/or natural SARS‐CoV‐2 infection) for comparison, these were not matched controls, and may have contributed to conflicting data. Furthermore, while this review identified several studies that looked at antibody titers in breast milk, none of these studies assessed corresponding levels of infant serum to evaluate the transfer of antibodies to the infant. Future studies should determine if efficient passive immunity may be conferred from the lactating individual to the infant. Finally, the lack of effectiveness data to date underscores the need for further research in this area, particularly as there is no known immune correlate of protection.
A systematic review on COVID‐19 vaccination in pregnancy was recently published on MedRxiv. 38 That review limited their analysis to pregnant populations and included 12 observational studies, two of which were not included here because they had less than 10 participants. Overall, this systematic review reached similar conclusions to those of the present study and reported a robust immunogenic response without any adverse obstetrical or neonatal outcomes.
It is believed that this is the first systematic review on the safety, immunogenicity, and effectiveness of COVID‐19 vaccines in both pregnant and lactating individuals. Overall, the review demonstrated that COVID‐19 vaccination in pregnant and lactating individuals can induce a robust immunogenic response, does not raise significant vaccine‐related adverse events or obstetrical and neonatal outcomes, and is effective in preventing the transmission of COVID‐19. Taken together, these results support current recommendations from WHO and various obstetrical and gynecologic societies that COVID‐19 vaccines can and should be used during pregnancy and lactation.
CONFLICTS OF INTEREST
CE has received consulting fees from Gilead for work related to HIV, outside the submitted work. GO is a co‐investigator on an investigator‐led trial funded by Hologic Inc and Roche. DM has received funding in kind from Merck for unrelated vaccine projects. She has also received funding for unrelated clinical trials by GSK, Merck, Novartis, and Sanofi‐Pasteur. The other authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
WF and EM contributed to the protocol/project development. WF and BS contributed to the systematic review and manuscript writing. WF, EM, CE, GO, and DM contributed to manuscript editing and review. DM oversaw the project. All authors approved the final version of the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We thank Julie Hanna for assisting with project management. Specific funding was not obtained for this review. WF was supported by UBC’s Faculty of Medicine Summer Student Research Program. EM was supported by CANFAR/CTN & MSFHR postdoctoral fellowships.
Fu W, Sivajohan B, McClymont E, et al. Systematic review of the safety, immunogenicity, and effectiveness of COVID‐19 vaccines in pregnant and lactating individuals and their infants. Int J Gynecol Obstet. 2022;156:406–417. doi: 10.1002/ijgo.14008
REFERENCES
- 1. Lokken EM, Huebner EM, Taylor GG, et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obs Gynec. 2021;225(1):77.e1‐77.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory‐confirmed SARS‐CoV‐2 infection by pregnancy status — United States, January 22–October 3, 2020. Morb Mortal Wkly Rep. 2020;69(44):1641‐1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mertz D, Kim TH, Johnstone J, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta‐analysis. BMJ. 2013;347:f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obs Gynec. 2011;205(1):10‐18. [DOI] [PubMed] [Google Scholar]
- 5. Galang RR, Chang K, Strid P, et al. Severe coronavirus infections in pregnancy: a systematic review. Obs Gynec. 2020;136(2):262‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. NACI. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI) Accessed July 10, 2021. https://www.canada.ca/en/public‐health/services/immunization/national‐advisory‐committee‐on‐immunization‐naci/recommendations‐use‐covid‐19‐vaccines.html
- 7. Society of Obstetricians and Gynaecologists of Canada SOGC statement on COVID‐19 vaccination in pregnancy. Accessed July 10, 2021. https://sogc.org/common/Uploadedfiles/LatestNews/SOGC_Statement_COVID‐19_Vaccination_in_Pregnancy.pdf
- 8. CDC . COVID‐19 vaccines while pregnant or breastfeeding. Published 2021. Accessed August 14, 2021. https://www.cdc.gov/coronavirus/2019‐ncov/vaccines/recommendations/pregnancy.html
- 9. American College of Obstetricians and Gynecologists . Vaccinating pregnant and lactating patients against COVID‐19: practice advisory—December 2020. Accessed July 10, 2021. https://www.acog.org/clinical/clinical‐guidance/practice‐advisory/articles/2020/12/vaccinating‐pregnant‐and‐lactating‐patients‐against‐covid‐19
- 10. Society for Maternal‐Fetal Medicine . Society for Maternal‐Fetal Medicine (SMFM) statement: SARS‐CoV‐2 vaccination in pregnancy. Accessed July 10, 2021. https://s3.amazonaws.com/cdn.smfm.org/media/2591/SMFM_Vaccine_Statement_12‐1‐20_(final).pdf
- 11. Rasmussen SA, Kelley CF, Horton JP, Jamieson DJ. Coronavirus disease 2019 (COVID‐19) vaccines and pregnancy: what obstetricians need to know. Obstet Gynecol. 2021;137(3):408‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The Moderna COVID‐19 (mRNA‐1273) vaccine: what you need to know. Accessed July 9, 2021. https://www.who.int/news‐room/feature‐stories/detail/the‐moderna‐covid‐19‐mrna‐1273‐vaccine‐what‐you‐need‐to‐know
- 13. The Pfizer BioNTech (BNT162b2) COVID‐19 vaccine: what you need to know. Accessed July 13, 2021. https://www.who.int/news‐room/feature‐stories/detail/who‐can‐take‐the‐pfizer‐biontech‐covid‐19‐‐vaccine
- 14. Skjefte M, Ngirbabul M, Akeju O, et al. COVID‐19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36(2):197‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Canadian Agency for Drugs and Technologies in Health . CADTH COVID‐19 Search Strings. Accessed June 30, 2021. https://covid.cadth.ca/literature‐searching‐tools/cadth‐covid‐19‐search‐strings/
- 16. Collier A‐R, McMahan K, Yu J, et al. Immunogenicity of COVID‐19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325(23):2370‐2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beharier O, Plitman Mayo R, Raz T, et al. Efficient maternal to neonatal transfer of antibodies against SARS‐CoV‐2 and BNT162b2 mRNA COVID‐19 vaccine. J Clin Invest. 2021;131(13):e150319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obs Gynec. 2021;225(3):303.e1‐303.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mithal LB, Otero S, Shanes ED, Goldstein JA, Miller ES. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am J Obs Gynec. 2021;225(2):192‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prabhu M, Murphy EA, Sukhu AC, et al. Antibody response to coronavirus disease 2019 (COVID‐19) messenger RNA vaccination in pregnant women and transplacental passage into cord blood. Obs Gynec. 2021;138(2):278‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rottenstreich A, Zarbiv G, Oiknine‐Djian E, Zigron R, Wolf DG, Porat S. Efficient maternofetal transplacental transfer of anti‐ SARS‐CoV‐2 spike antibodies after antenatal SARS‐CoV‐2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Atyeo C, DeRiso EA, Davis C, et al. COVID‐19 mRNA vaccines drive differential Fc‐functional profiles in pregnant, lactating, and non‐pregnant women. bioRxiv. 2021;1‐35. https://www.biorxiv.org/content/10.1101/2021.04.04.438404v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Esteve‐Palau E, Gonzalez‐Cuevas A, Guerrero ME, et al. Quantification of specific antibodies against SARS‐CoV‐2 in breast milk of lactating women vaccinated with an mRNA vaccine. medRxiv. 2021;1‐8. https://www.medrxiv.org/content/10.1101/2021.04.05.21254819v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Friedman MR, Kigel A, Bahar Y, et al. BNT162b2 COVID‐19 mRNA vaccine elicits a rapid and synchronized antibody response in blood and milk of breastfeeding women. medRxiv. 2021;1‐8. https://www.medrxiv.org/content/10.1101/2021.03.06.21252603v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Golan Y, Prahl M, Cassidy A, et al. Immune response during lactation after anti‐SARS‐CoV2 mRNA vaccine. medRxiv. 2021;1‐15. https://www.medrxiv.org/content/10.1101/2021.03.09.21253241v2 [Google Scholar]
- 26. Valcarce V, Stafford LS, Neu J, et al. Detection of SARS‐CoV‐2 specific IgA in the human milk of COVID‐19 vaccinated, lactating health care workers. medRxiv. 2021;1‐16. https://www.medrxiv.org/content/10.1101/2021.04.02.21254642v1 [DOI] [PubMed] [Google Scholar]
- 27. Juncker HG, Mulleners SJ, van Gils MJ, et al. The levels of SARS‐CoV‐2 specific antibodies in human milk following vaccination. J Hum Lact. 2021;1‐8. doi: 10.1177/08903344211027112 [DOI] [PubMed] [Google Scholar]
- 28. Low J, Gu Y, Shu Feng Ng M, et al. BNT162b2 vaccination induces SARS‐CoV‐2 specific antibody secretion into human milk with minimal transfer of vaccine mRNA. medRxiv. 2021:1‐38. https://www.medrxiv.org/content/10.1101/2021.04.27.21256151v1 [Google Scholar]
- 29. Selma‐Royo M, Bauerl C, Mena‐Tudela D, et al. Anti‐SARS‐Cov‐2 IgA and IgG in human milk after vaccination is dependent on vaccine type and previous Sars‐Cov‐2 exposure a longitudinal study. medRxiv. 2021;1‐22. https://www.medrxiv.org/content/10.1101/2021.05.20.21257512v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perl SH, Uzan‐Yulzari A, Klainer H, et al. SARS‐CoV‐2–specific antibodies in breast milk after COVID‐19 vaccination of breastfeeding women. JAMA. 2021;325(19):2013‐2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fox A, Norris C, Amanat F, Zolla‐Pazner S, Powell R. The vaccine‐elicited immunoglobulin profile in milk after COVID‐19 mRNA‐ based vaccination is IgG‐dominant and lacks secretory antibodies. medRxiv. 2021;1‐14. https://www.medrxiv.org/content/10.1101/2021.03.22.21253831v1 [Google Scholar]
- 32. Bertrand K, Honerkamp‐Smith G, Chambers C. Maternal and child outcomes reported by breastfeeding women following mRNA COVID‐19 vaccination. medRxiv. 2021;1‐8. https://www.medrxiv.org/content/10.1101/2021.04.21.21255841v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid‐19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273‐2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kadali RAK, Janagama R, Peruru SR, et al. Adverse effects of COVID‐19 messenger RNA vaccines among pregnant women: a cross‐sectional study on healthcare workers with detailed self‐reported symptoms. Am J Obs Gynec. 2021;1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Low JM, Lee LY, Ng YPM, Zhong Y, Amin Z. Breastfeeding mother‐child clinical outcomes after COVID‐19 vaccination. medRxiv. 2021;1‐17. https://www.medrxiv.org/content/10.1101/2021.06.19.21258892v1 [DOI] [PubMed] [Google Scholar]
- 36. McLaurin‐Jiang S, Garner CD, Krutsch K, Hale TW. Maternal and child symptoms following COVID‐19 vaccination among breastfeeding mothers. Breastfeed Med. 2021;16(9):702–709. [DOI] [PubMed] [Google Scholar]
- 37. Theiler RN, Wick M, Mehta R, Weaver A, Virk A, Swift M. Pregnancy and birth outcomes after SARS‐CoV‐2 vaccination in pregnancy. medRxiv. 2021;1‐15. https://www.medrxiv.org/content/10.1101/2021.05.17.21257337v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pratama NR, Wafa IA, Budi DS, Putra M, Wardhana MP, Wungu CDK. Covid‐19 vaccination in pregnancy. A systematic review. Medrxiv. 2021;1‐32. https://www.medrxiv.org/content/10.1101/2021.07.04.21259985v1.full [Google Scholar]
- 39. Shanes ED, Otero S, Mithal LB, Mupanomunda CA, Miller ES, Goldstein JA. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccination in pregnancy: measures of immunity and placental histopathology. Obs Gynecol. 2021;138(2):281‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


