Abstract
A chromosomally located β-lactamase gene, cloned and expressed in Escherichia coli from a reference strain of the enterobacterial species Kluyvera cryocrescens, encoded a clavulanic acid-inhibited Ambler class A enzyme, KLUC-1, with a pI value of 7.4. KLUC-1 shared 86% amino acid identity with a subgroup of plasmid-mediated CTX-M-type extended-spectrum β-lactamases (CTX-M-1, -3, -10, -11, and -12), the most closely related enzymes, and 77% amino acid identity with KLUA-1 from Kluyvera ascorbata. The substrate profile of KLUC-1 corresponded to that of CTX-M-type enzymes.
At present, the genus Kluyvera is composed of four enterobacterial species: Kluyvera ascorbata, Kluyvera cryocrescens, Kluyvera georgiana, and Kluyvera cochleae (9). K. ascorbata is more frequently isolated from clinical specimens, while K. cryocrescens is mostly isolated from the environment (water, soil, sewage, and hospital environment) (32). Eighteen detailed cases of human K. cryocrescens infections have been reported, with some (but not all) of them occurring in immunocompromised patients (32). The detailed susceptibility of K. cryocrescens to β-lactams is not known except for its resistance to ampicillin (1, 32).
We report here on the characterization of a class A β-lactamase from K. cryocrescens with a substrate profile extended to expanded-spectrum cephalosporins. Sequence analysis revealed its similarity to several plasmid-mediated CTX-M-type extended-spectrum β-lactamases (ESBLs).
Bacterial strains and plasmid analysis
K. cryocrescens reference strain 79.54 was from the strain collection of the Institut Pasteur (Paris, France). Plasmid DNA extractions, performed as described previously (26), failed to identify plasmids.
Cloning and sequence analysis of β-lactamase gene from K. cryocrescens.
Whole-cell DNA of K. cryocrescens 79.54 was extracted as described previously (26), digested with Sau3AI, and ligated into the BamHI site of phagemid pBK-CMV (26). Thirty Escherichia coli DH10B recombinant clones were obtained after selection on kanamycin- and amoxicillin-containing plates, as described previously (26). One of the recombinant plasmids that had the shortest insert (pKC7954) was retained for further analysis. Its DNA insert (6.1 kb) was sequenced and analyzed as described previously (26).
An open reading frame (ORF) of 932 bp was identified (data not shown). The G+C content of this ORF was 54.9%, which lies within the G+C ratios for enterobacterial genes. Within the deduced protein of this ORF (311 amino acids), named KLUC-1, characteristic elements of Ambler class A β-lactamases were identified (Fig. 1) (17). Isoelectric focusing analysis, performed as reported previously (26), showed that cultures of K. cryocrescens 79.54 and E. coli DH10B(pKC7954) gave single and identical β-lactamases, each with a pI value of 7.4.
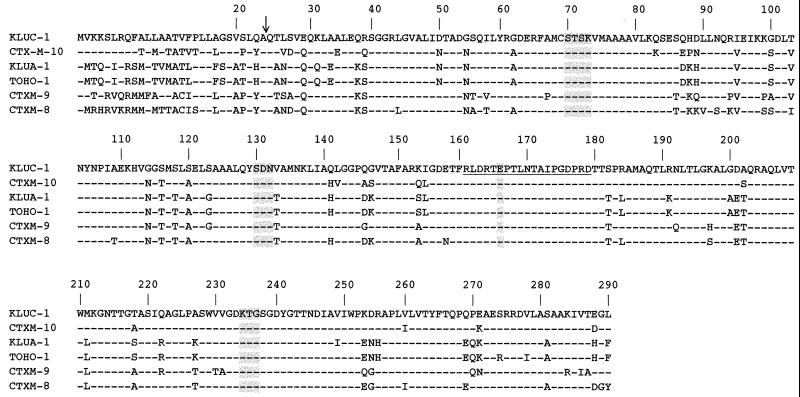
FIG. 1.
Alignment of the KLUC-1 amino acid sequence with those of CTX-M-10 from E. coli (31), KLUA-1 from K. ascorbata (GenBank accession no. CAB 59824), Toho-1 from E. coli (14), CTX-M-9 from E. coli (27), and CTX-M-8 from Enterobacter cloacae (7). The numbering is according to Ambler et al. (2). Dashes indicate identical amino acid residues. The vertical arrow indicates the putative cleavage site of the leader peptide of the mature KLUC-1 β-lactamase. Four structural elements characteristic of class A β-lactamases are shaded (17). The amino acids of the omega loop are underlined.
The KLUC-1 β-lactamase was closely related to the CTX-M-1 (MEN-1) subgroup of plasmid-mediated enzymes (CTX-M-1, -3, -10, -11, and -12), sharing 85 to 86% amino acid identity (5, 18, 21). It shared 77% identity with the CTX-M-2 subgroup (CTX-M-2, -5, -6, and -7 and Toho-1) and 76 and 78% identities with CTX-M-8 and -9, respectively (7, 15, 27, 31).
KLUC-1 from K. cryocrescens shared only 77% amino acid identity with the chromosomally encoded KLUA-1 β-lactamase from K. ascorbata (GenBank accession no. CAB59824), while the two enterobacterial species from which these β-lactamases were obtained are phylogenetically related (10). The amino acid identities of KLUC-1 with the naturally occurring class A β-lactamases from Klebsiella oxytoca (3), Serratia fonticola (22), Citrobacter koseri (formerly Citrobacter diversus) (24), Proteus vulgaris (23), Yersinia enterocolitica (29), and Klebsiella pneumoniae (SHV-1) (4) were 72, 72, 71, 63, 57, and 37%, respectively.
A 1,241-bp ORF was identified 498 bp upstream of blaKLUC-1 in the same transcription orientation; this ORF codes for a putative 401-amino-acid protein. This protein shared 96% identity with an aspartate aminotransferase from E. coli K-12 (6) and with a putative protein whose ORF was found upstream of blaKLUA-1 from K. ascorbata (GenBank accession no. 272538). Additionally, 90% nucleotide identity was found in these intergenic regions in K. ascorbata and K. cryocrescens. No upstream-located LysR-type regulator gene was identified, whereas AmpR genes are upstream-located compared to the chromosomally encoded β-lactamase genes of S. fonticola, P. vulgaris, and C. koseri (14, 16, 20).
Another ORF was identified 928 bp downstream of blaKLUC-1 in the same transcription orientation; this ORF encoded a putative protein that shared 69% amino acid identity with that identified downstream of blaKLUA-1 (GenBank accession no. 272538). No consistent nucleotide identity was found in the immediate downstream region of the β-lactamase genes identified in K. cryocrescens and K. ascorbata.
Since the ORFs located upstream and downstream of the β-lactamase genes from K. cryocrescens and K. ascorbata shared consistent identities, it is possible that they constituted similar loci. Similarly, AmpC genes in several enterobacterial species are bracketed by nucleotide sequences that encode functionally related proteins (25).
A Southern transfer of a gel containing whole-cell DNA of K. cryocrescens 79.54 was performed (28), and the DNA hybridized by PCR with an 846-bp internal fragment of blaKLUC-1 as a labeled probe (28). A positive signal was detected at the chromosomal migration position, further indicating the chromosomal origin of blaKLUC-1 (data not shown).
Susceptibility testing.
The MICs of selected β-lactams were determined as described previously (26). K. cryocrescens 79.54 was resistant to amoxicillin and ticarcillin and had reduced susceptibility to cephalothin and cefuroxime (Table 1). It was susceptible to the other β-lactam antibiotics tested. Resistance to aminopenicillins has been reported previously for K. cryocrescens (1). Once cloned in pBK-CMV (pKC7954) and expressed in E. coli DH10B (Table 1), KLUC-1 also conferred resistance or reduced susceptibility to cefotaxime, ceftriaxone, cefpirome, and aztreonam. These activities paralleled the activity of a β-lactamase from crude extracts (25) of a culture of E. coli DH10B(pKC7954), which were 40-fold higher than that of a culture of K. cryocrescens 79.54 (data not shown). The resistance profile observed for E. coli DH10B(pKC7954) corresponded to that conferred by plasmid-mediated CTX-M-type β-lactamases, which do not compromise susceptibility to ceftazidime significantly (31). The addition of clavulanic acid and tazobactam strongly lowered the β-lactam MICs (Table 1). These results indicate that KLUC-1 is a clavulanic acid-inhibited ESBL that is likely weakly expressed in K. cryocrescens.
TABLE 1.
MICs of β-lactams for reference strain K. cryocrescens 79.54, E. coli DH10B(pKC7954), and reference strain E. coli DH10B
| β-Lactam(s)b | MIC
(μg/ml)a
|
||
|---|---|---|---|
| K. cryocrescens 79.54 | E. coli DH10B (pKC7954) | E. coli DH10B | |
| Amoxicillin | 64 | >512 | 2 |
| Amoxicillin + CLA | <0.5 | 8 | 2 |
| Ticarcillin | 128 | >512 | 1 |
| Ticarcillin + CLA | 1 | 8 | 1 |
| Piperacillin | <1 | 128 | 1 |
| Piperacillin + TZB | <1 | <1 | 1 |
| Cephalothin | 8 | >512 | 2 |
| Cefuroxime | 16 | 512 | 2 |
| Cefotaxime | 0.06 | 8 | <0.06 |
| Cefotaxime + CLA | <0.06 | 0.06 | <0.06 |
| Cefotaxime + TZB | <0.06 | 0.12 | <0.06 |
| Ceftazidime | <0.06 | 0.5 | <0.06 |
| Ceftazidime + CLA | <0.06 | 0.12 | <0.06 |
| Ceftazidime + TZB | <0.06 | 0.12 | <0.06 |
| Ceftriaxone | <0.06 | 128 | <0.06 |
| Cefepime | <0.06 | 1 | <0.06 |
| Cefepime + CLA | <0.06 | 0.25 | <0.06 |
| Cefepime + TZB | <0.06 | 0.12 | <0.06 |
| Cefpirome | <0.06 | 4 | <0.06 |
| Cefpirome + CLA | <0.06 | <0.06 | <0.06 |
| Cefpirome + TZB | <0.06 | <0.06 | <0.06 |
| Cefoxitin | 1 | 4 | 2 |
| Moxalactam | 0.03 | 0.12 | 0.12 |
| Aztreonam | 0.06 | 2 | 0.06 |
| Meropenem | <0.03 | <0.06 | <0.06 |
| Imipenem | 0.06 | 0.12 | 0.12 |
K. cryocrescens 79.54 and E. coli DH10B(pKC7954) produced the KLUC-1 β-lactamase.
CLA, clavulanic acid at a fixed concentration of 2 μg/ml; TZB, tazobactam at a fixed concentration of 4 μg/ml.
The β-lactam resistance pattern conferred by KLUC-1 resembled those conferred by chromosomally encoded β-lactamases of other enterobacterial species: S. fonticola (22), C. koseri, and P. vulgaris (19).
Biochemical analysis of β-lactamase KLUC-1.
A culture of E. coli DH10B(pKC7954) was grown overnight at 37°C in 2 liters of Trypticase soy broth with amoxicillin (50 μg/ml) and kanamycin (30 μg/ml). The β-lactamase extract was obtained after sonification as described previously (26). It was further purified by a two-step ultrafiltration procedure, as recommended by the manufacturer (Vivaspin, 100,000 MWCOPES and 10,000 MWCOPES; Sartorius, Göttingen, Germany). Partially purified β-lactamase was used for kinetic measurements, performed at 30°C in 100 mM sodium phosphate (pH 7.0) as described previously (25).
The specific activity of the purified KLUC-1 β-lactamase, measured with 100 μM cephalothin as the substrate, was 4.2 U · mg of protein−1 with a 10-fold purification factor. KLUC-1 had strong activity against benzylpenicillin, piperacillin, cephalothin, cefuroxime, and ceftriaxone (Table 2). Significant hydrolytic activity was observed against cefotaxime and ceftriaxone, while a very low level of activity was detectable against ceftazidime. For this substrate, high Km and low relative Vmax values were observed (Table 2). This substrate profile corresponded to that reported for CTX-M-type enzymes (31).
TABLE 2.
Kinetic parameters of β-lactamase KLUC-1a
| Substrate | Relative Vmax | Km (μM) | Relative Vmax/Km |
|---|---|---|---|
| Benzylpenicillin | 100 | 10 | 100 |
| Amoxicillin | 65 | 30 | 25 |
| Ticarcillin | 6 | 5 | 10 |
| Piperacillin | 145 | 20 | 70 |
| Cephalothin | 1,200 | 140 | 100 |
| Cefuroxime | 175 | 30 | 65 |
| Cefoxitin | <0.1 | NDb | ND |
| Cefotaxime | 170 | 110 | 20 |
| Ceftazidime | 0.5 | 5,700 | <0.01 |
| Ceftriaxone | 170 | 45 | 45 |
| Cefpirome | 70 | 1,950 | 0.5 |
| Cefepime | 70 | 750 | 1 |
| Aztreonam | 10 | 150 | 1 |
| Imipenem | 0.01 | 30 | <0.01 |
Vmax and Vmax/Km values were relative to that of benzylpenicillin, for which the values were set equal to 100. Data are the means of three independent experiments. Standard deviations were within 15%.
ND, not determinable.
Inhibition measured as 50% inhibitory concentrations showed that the 50% inhibitory concentrations of clavulanic acid, tazobactam, and sulbactam were low, being 0.05, 0.01, and 0.15 μM, respectively. On the basis of its kinetic parameters, the KLUC-1 enzyme is a β-lactamase that may belong to the 2be group of β-lactamases of the Bush-Jacoby-Medeiros classification (8).
Conclusion.
The KLUC-1 β-lactamase from K. cryocrescens was mostly related to the subgroup of β-lactamases that comprises CTX-M-1, -3, -10, -11, and -12. However, it was not the direct progenitor of known plasmid-mediated CTX-M enzymes, in contrast to KLUA-1 from K. ascorbata, which shares 99% amino acid identity with the CTX-M-2 β-lactamase (C. Humeniuk et al., unpublished data [GenBank accession no. CAB59824]). Like the CTX-M-type enzymes (31), KLUC-1 is a clavulanic acid-inhibited Ambler class A ESBL that possesses a substrate profile that includes extended-spectrum cephalosporins but not ceftazidime. KLUC-1 possesses amino acid residues at key positions that may explain its extended spectrum of hydrolysis. The serine residue at position 237 may contribute significantly to this extended substrate profile, as reported for the β-lactamase of P. vulgaris (30). A similar omega loop sequence (residues 161 to 179) is found for KLUC-1 and the sequences of CTX-M-type enzymes such as Toho-1 (Fig. 1). The crystal structure analysis of Toho-1 shows that residue Phe160 suppresses the hydrogen bond between residues Thr160 and Ser/Thr181 that connects the N and C termini of the omega loop in non-ESBLs; this may explain, in part, the expanded substrate profile of Toho-1 (13). Since residue Phe160 was found also in the KLUC-1 sequence, the lack of a hydrogen bond may also increase the flexibility of the omega loop, extending the KLUC-1 substrate profile. Additionally, KLUC-1, like Toho-1 (13), has glycine residues at positions 232, 236, and 238 that may increase the flexibility of the B3 strand, which would make it possible for KLUC-1 to bind to bulky extended-spectrum cephalosporins.
Interestingly, most of the chromosomally encoded class A β-lactamases in members of the family Enterobacteriaceae (S. fonticola, P. vulgaris, C. koseri, K. ascorbata, and K. cryocrescens) have the same substrate profile, which includes amino- and ureidopenicillins, cephalothin, cefuroxime, cefotaxime, and ceftriaxone but not ceftazidime (19). However, KLUC-1 did not confer resistance to expanded-spectrum cephalosporins in K. cryocrescens. As reported for the expression of the naturally encoded β-lactamase from Klebsiella oxytoca, which is also independent of a LysR-type regulator (11, 12), it is likely that extended-spectrum cephalosporin-resistant K. cryocrescens mutants that would contain mutations in the blaKLUC-1 promoter region may be selected in vivo. In that case, a strong increase in the level of expression of the KLUC-1 β-lactamase would be expected (as observed when blaKLUC-1 was cloned on multicopy vector pBK-CMV [Table 1]), and this would thus confer resistance to extended-spectrum cephalosporins.
Finally, this report further underlines the fact that enterobacterial species are natural producers of either no β-lactamase or the Ambler class A and/or class C β-lactamase.
Nucleotide sequence accession number.
The nucleotide sequence of blaKLUC-1 and the 6.1-kb insert of recombinant plasmid pKC7954 has been assigned GenBank nucleotide database accession no. AY026417.
Acknowledgments
This work was funded by a grant from the Ministères de l'Education Nationale et de la Recherche (UPRES, grant JE 2227), Université Paris XI, Faculté de Médecine, Paris-Sud, Paris, France.
We thank Chantal Bizet of the Institut Pasteur strain collection for the gift of the K. cryocrescens reference strain and Samuel Bellais for advice in determination of biochemical parameters.
REFERENCES
- 1.Altwegg M, Zollinger-Iten J, Von Graevenitz A. Differentiation of Kluyvera cryocrescens from Kluyvera ascorbataby irgasan susceptibility testing. Ann Inst Pasteur Microbiol (Paris) 1986;137A:159–168. doi: 10.1016/s0769-2609(86)80021-7. [DOI] [PubMed] [Google Scholar]
- 2.Ambler R P, Coulson A F W, Frère J-M, Ghuysen J M, Joris B, Forsman M, Lévesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;276:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arakawa Y, Ohta M, Kido N, Mori M, Ito H, Komatsu T, Fujii Y, Kato N. Chromosomal β-lactamase of Klebsiella oxytoca, a new class A enzyme that hydrolyzes broad-spectrum β-lactam antibiotics. Antimicrob Agents Chemother. 1989;33:63–70. doi: 10.1128/aac.33.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthélémy M, Péduzzi J, Labia R. Complete amino acid sequence of p453-plasmid-mediated PIT-2 β-lactamase (SHV-1) Biochem J. 1988;251:73–79. doi: 10.1042/bj2510073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthélémy M, Péduzzi J, Bernard H, Tancrède C, Labia R. Close amino acid sequence relationship between the new plasmid-mediated extended-spectrum β-lactamase MEN-1 and chromosomally encoded enzymes of Klebsiella oxytoca. Biochim Biophys Acta. 1992;1122:15–22. doi: 10.1016/0167-4838(92)90121-s. [DOI] [PubMed] [Google Scholar]
- 6.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coliK-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet R, Sampaio J L M, Labia R, De Champs C, Sirot D, Chanal C, Sirot J. A novel CTX-M β-lactamase (CTX-M-8) in cefotaxime-resistant Enterobacteriaceaeisolated in Brazil. Antimicrob Agents Chemother. 2000;44:1936–1942. doi: 10.1128/aac.44.7.1936-1942.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farmer J J., III . Enterobacteriaceae: introduction and identification, p442–458. In: Murray P R, Barron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. [Google Scholar]
- 10.Farmer J J, Fanning G R, Huntley-Carter G P, Holmes B, Hickman F W, Richard C, Brenner D J. Kluyvera, a new (redefined) genus in the family Enterobacteriaceae: identification of Kluyvera ascorbata sp. nov. and Kluyvera cryocrescenssp. nov. in clinical specimens. J Clin Microbiol. 1981;13:919–933. doi: 10.1128/jcm.13.5.919-933.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fournier B, Lagrange P H, Philippon A. In-vitro susceptibility of Klebsiella oxytocastrains to 13 β-lactams in the presence and absence of β-lactamase inhibitors. J Antimicrob Chemother. 1996;37:931–942. doi: 10.1093/jac/37.5.931. [DOI] [PubMed] [Google Scholar]
- 12.Fournier B, Lagrange P H, Philippon A. Beta-lactamase gene promoters of 71 clinical strains of Klebsiella oxytoca. Antimicrob Agents Chemother. 1996;40:460–463. doi: 10.1128/aac.40.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibuka A, Taguchi A, Ishiguro M, Fushinobu S, Ishii Y, Kamitori S, Okuyama K, Yamaguchi K, Konno M, Matsuzawa H. Crystal structure of the E166A mutant of extended-spectrum β-lactamase Toho-1 at 1.8 Å resolution. J Mol Biol. 1999;285:2079–2087. doi: 10.1006/jmbi.1998.2432. [DOI] [PubMed] [Google Scholar]
- 14.Ishiguro K, Sugimoto K. Purification and characterization of the Proteus vulgarisBlaA protein, the activator of the β-lactamase gene. J Biochem. 1996;120:98–103. doi: 10.1093/oxfordjournals.jbchem.a021399. [DOI] [PubMed] [Google Scholar]
- 15.Ishii Y, Ohno A, Taguchi H, Imajo S, Ishiguro M, Matsuzawa H. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A beta-lactamase isolated from Escherichia coli. Antimicrob Agents Chemother. 1995;39:2269–2275. doi: 10.1128/aac.39.10.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones M E, Bennett P M. Inducible expression of the chromosomal cdiA from Citrobacter diversus NF85, encoding an Ambler class A β-lactamase, is under similar genetic control to the chromosomal ampC, encoding an Ambler class C enzyme from Citrobacter freundiiOS60. Microb Drug Resist. 1995;1:285–291. doi: 10.1089/mdr.1995.1.285. [DOI] [PubMed] [Google Scholar]
- 17.Joris B, Ledent P, Dideberg O, Fonze E, Lamotte-Brasseur J, Kelly J A, Ghuysen J M, Frère J-M. Comparison of the sequences of class A β-lactamases and of the secondary structure elements of penicillin-recognizing proteins. Antimicrob Agents Chemother. 1991;35:2294–2301. doi: 10.1128/aac.35.11.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kariuki S, Corkill J E, Revathi G, Musoke R, Hart C A. Molecular characterization of a novel plasmid-encoded cefotaximase (CTX-M-12) found in clinical Klebsiella pneumoniaeisolates from Kenya. Antimicrob Agents Chemother. 2001;45:2141–2143. doi: 10.1128/AAC.45.7.2141-2143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matagne A, Lamotte-Brasseur J, Frère J-M. Catalytic properties of class A β-lactamases: efficiency and diversity. Biochem J. 1998;330:581–598. doi: 10.1042/bj3300581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto Y, Inoue M. Characterization of SFO-1, a plasmid-mediated inducible class A β-lactamase from Enterobacter cloacae. Antimicrob Agents Chemother. 1999;43:307–313. doi: 10.1128/aac.43.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliver A, Perez-Diaz J C, Coque T M, Baquero F, Canton R. Nucleotide sequence and characterization of a novel cefotaxime-hydrolyzing β-lactamase (CTX-M-10) isolated in Spain. Antimicrob Agents Chemother. 2001;45:616–620. doi: 10.1128/AAC.45.2.616-620.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Péduzzi J, Farzaneh S, Reynaud A, Barthélémy M, Labia R. Characterization and amino acid sequence analysis of a new oxyimino cephalosporin-hydrolyzing class A β-lactamase from Serratia fonticolaCUV. Biochim Biophys Acta. 1997;1341:58–70. doi: 10.1016/s0167-4838(97)00020-4. [DOI] [PubMed] [Google Scholar]
- 23.Péduzzi J, Reynaud A, Baron P, Barthélémy M, Labia R. Chromosomally encoded cephalosporin-hydrolyzing β-lactamase of Proteus vulgarisRO104 belongs to Ambler's class A. Biochim Biophys Acta. 1994;1207:31–39. doi: 10.1016/0167-4838(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 24.Perilli M, Franceschini N, Segatore B, Amicosante G, Oratore A, Duez C, Joris B, Frère J-M. Cloning and nucleotide sequencing of the gene encoding the β-lactamase from Citrobacter diversus. FEMS Microbiol Lett. 1991;67:79–84. doi: 10.1016/0378-1097(91)90448-j. [DOI] [PubMed] [Google Scholar]
- 25.Poirel L, Guibert M, Girlich D, Naas T, Nordmann P. Cloning, sequence analyses, expression, and distribution of ampC-ampR from Morganella morganiiclinical isolates. Antimicrob Agents Chemother. 1999;43:769–776. doi: 10.1128/aac.43.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poirel L, Le Thomas I, Naas T, Karim A, Nordmann P. Biochemical sequence analyses of GES-1, a novel class A extended-spectrum β-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob Agents Chemother. 2000;44:622–632. doi: 10.1128/aac.44.3.622-632.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabate M, Tarrago R, Navarro F, Miro E, Verges C, Barbe J, Prats G. Cloning and sequence of the gene encoding a novel cefotaxime-hydrolyzing β-lactamase (CTX-M-9) from Escherichia coliin Spain. Antimicrob Agents Chemother. 2000;44:1970–1973. doi: 10.1128/aac.44.7.1970-1973.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Seoane A, Garcia Lobo J M. Nucleotide sequence of a new class A beta-lactamase gene from the chromosome of Yersinia enterocolitica: implications for the evolution of class A β-lactamases. Mol Gen Genet. 1991;228:215–220. doi: 10.1007/BF00282468. [DOI] [PubMed] [Google Scholar]
- 30.Tamaki M, Nukaga M, Sawai T. Replacement of serine 237 in class A beta-lactamase of Proteus vulgarismodifies its unique substrate specificity. Biochemistry. 1994;33:10200–10206. doi: 10.1021/bi00199a049. [DOI] [PubMed] [Google Scholar]
- 31.Tzouvelekis L S, Tzelepi E, Tassios P T, Legakis N J. CTX-M-type β-lactamases: an emerging group of extended-spectrum enzymes. Int J Antimicrob Agents. 2000;14:137–142. doi: 10.1016/s0924-8579(99)00165-x. [DOI] [PubMed] [Google Scholar]
- 32.West B C, Vijayan H, Shekar R. Kluyvera cryocrescensfinger infection: case report and review of eighteen infections in human beings. Diagn Microbiol Infect Dis. 1998;32:237–241. doi: 10.1016/s0732-8893(98)00087-x. [DOI] [PubMed] [Google Scholar]