Abstract
Objective
To compare risk of death due to COVID‐19 among pregnant, postpartum, and non‐pregnant women of reproductive age in Brazil, using the severe acute respiratory syndrome surveillance system (SARS‐SS).
Methods
A secondary analysis was performed of the Brazilian official SARS‐SS, with data retrieved up to August 17, 2020. Cases were stratified by pregnancy status, risk factors or co‐morbidities, and outcome (death or recovery). Multiple logistic regression was employed to examine associations between independent variables and risk of death.
Results
A total of 24 805 cases were included, with 3129 deaths (12.6%), including 271 maternal deaths. Postpartum was associated with increased risk of death, admission to the intensive care unit (ICU), and mechanical ventilation. Co‐morbidities with higher impact on case fatality rate among non‐obstetric cases were cancer and neurological and kidney diseases. Among pregnant women, cancer, diabetes mellitus, obesity, and rheumatology diseases were associated with risk of death. In the postpartum subgroup, age over 35 years and diabetes mellitus were independently associated with higher chance of death.
Conclusion
Postpartum was associated with worse outcomes among the obstetric population, despite lower risk of dying without accessing ICU care. Non‐pregnant women with cancer, neurological diseases, and kidney diseases have a higher risk of death due to COVID‐19.
Keywords: cardiovascular disease, COVID‐19, maternal death, obesity, postpartum, pregnancy, women of reproductive age
Synopsis
Postpartum increased the risk of death, admission to the ICU, and mechanical ventilation among reproductive‐age women admitted to hospital due to COVID‐19 severe acute respiratory syndrome.
1. INTRODUCTION
Since the beginning of the global COVID‐19 pandemic, several studies have shown co‐morbidities and risk factors associated with adverse outcomes of SARS‐CoV‐2 infection. Thus, obesity, diabetes, cardiovascular diseases, as well as increasing age, have been associated with case fatality among patients with COVID‐19. 1 , 2 However, whether the same conditions or further risk factors influence the prognosis of obstetric patients is still under debate.
Recently gathered evidence suggested that obesity, diabetes, and cardiovascular diseases might increase the risk of death among pregnant and postpartum women, as previously described for the general population. 3 , 4 However, further chronic conditions that might pose additional risk for the obstetric population remain to be studied. The same can be said about women of reproductive age, who are usually younger and having fewer co‐morbidities when compared to most samples of patients with COVID‐19 studied so far. Studies focusing on repercussions of COVID‐19 upon women of reproductive age comparing disease evolution throughout different phases of women's lives have shown that pregnancy and/or postpartum status are associated with worse outcomes of COVID‐19. 5 , 6
Brazil faces the second worst pandemic scenario globally, with more than 190 000 deaths as of late December 2020. 7 Reasons for worse outcomes of coronavirus infection in the country include erratic containment measures, lack of timely guidelines for COVID‐19 care, barriers to health access, inequalities among vulnerable populations, and the pandemic burden upon the already overloaded and underfinanced Brazilian public health system. 8 , 9
The aims of the present study were to analyze whether the gestational period alone encloses higher risk of death due to COVID‐19 on women of reproductive age, and to compare the findings among pregnant, postpartum, and non‐pregnant women in Brazil, using data from the severe acute respiratory syndrome (SARS) surveillance system (SARS‐SS).
2. MATERIALS AND METHODS
The present study was based on publicly available data from the Brazilian official SARS‐SS (SIVEP‐Gripe in Portuguese). SARS is a nationally notifiable disease mandatorily reported to the Ministry of Health in Brazil by public and private hospitals. 10 Cases of SARS‐SS are defined by flu‐like symptoms and at least one of the following severity criteria: dyspnea; respiratory distress; or oxygen saturation below 95%. Information on the methods were also described elsewhere. 3 Over 95% of cases of SARS‐SS COVID‐19 were diagnosed based on laboratory tests, predominantly the SARS‐CoV‐2 reverse transcription polymerase chain reaction (RT‐PCR) test.
All female cases within the age group of 10–45 years were included. The final sample was classified by pregnancy status as non‐obstetric women, pregnant women, and postpartum women (up to 42 days after childbirth). Unfortunately, the exact time from childbirth until SARS diagnosis was not available. Cases of pregnant and postpartum women were identified using a two‐step process. First, the two close‐ended fields available in the database were selected: “Pregnancy Trimester” (answer options “first,” “second,” “third,” “unknown gestational age,” or “not applicable”); and “Postpartum” (answer options “yes” or “no”). Missing information for those variables were coded as non‐pregnant women of reproductive age in the first step. Second, additional hand‐searches in the “other co‐morbidities” open‐ended field were performed, looking for mentions of pregnancy, postpartum, abortion, and childbirth/delivery.
Risk factors for death associated with COVID‐19 during pregnancy and postpartum were previously reported by the study group. 11 In the present study, non‐pregnant women of reproductive age were also included, as well as a thorough analysis of all close‐ended co‐morbidities and risk factors variables available in the database, including the open‐ended “other co‐morbidities” variable. A semi‐automatic algorithm to classify descriptive data about co‐morbidities (reported by the provider at SARS notification) was adopted, using a specifically programmed software, applying the Python language. The software singled‐out all unique “other co‐morbidities” open‐ended field entries and made it possible to classify them into a set of created close‐ended categories, subsequently assigning the close‐ended category linked to the “other co‐morbidities” of all individuals in the dataset.
The co‐morbidity categories were created by the authors. Obvious predefined reclassifications were solved by the semi‐automatic algorithm, and the remaining cases were manually and independently reviewed by two authors. The open‐ended “Other co‐morbidities” field comprised over 5300 different terms due to diverse description of the same condition (“asthma,” “severe asthma,” “asthmatic bronchitis”), as well as typos. The initial 40 categories were then regrouped, using clinical criteria defined by the authors, resulting in 14 main co‐morbidity categories: cancer; cardiovascular diseases; diabetes mellitus; gastrointestinal diseases; hematological diseases; immunosuppression or HIV/AIDS; kidney diseases; liver diseases; neurological diseases or stroke; obesity; respiratory diseases (including asthma); rheumatological diseases; thrombotic events or vascular diseases; and thyroid diseases. Co‐morbidity data derived from these procedures were grouped with data from the original SARS‐SS close‐ended co‐morbidity fields to provide a single set of co‐morbidities or risk factor data.
Independent variables were included in the risk of death analysis: age; ethnicity; and pregnancy status (non‐obstetric, pregnant, or postpartum women). Age was dichotomized as under 20 years, 20–34 years, and 35 years and above. A descriptive secondary analysis of adverse outcomes by pregnancy status was performed, with individual outcomes as follows: admission to the intensive care unit (ICU); mechanical ventilation; and death without intensive care (defined as a death event without a record of being admitted to the ICU or having received mechanical ventilation). Missing data on co‐morbidities and use of intensive care resources (admission to the ICU and mechanical ventilation) were treated as absence of the condition. Missingness on SARS‐SS was previously described. 11 , 12
All eligible records documented in the SARS‐SS until August 17, 2020, with an outcome (death or recovery) were included in the analysis. Univariate association between independent variables and death was assessed using hypothesis tests, and P values were calculated. Stepwise multiple logistic regression was employed to examine the association between independent variables and risk of death, providing adjusted odds ratio (OR) and corresponding 95% confidence interval. Statistical significance was defined at the 0.05 level, and P values were two‐tailed. Analyses were conducted using STATA 12. STROBE reporting guidelines for observational studies were followed. In accordance with Brazilian regulatory requirements, a secondary analysis of publicly available anonymized data does not require approval from an institutional ethics review board.
3. RESULTS
Table 1 shows sample characteristics by pregnancy status (n = 24 805 cases). There were 3129 fatality cases (case fatality rate of 12.6%), including 271 maternal deaths. The three subgroups were significantly different in most characteristics, with the exception of diabetes mellitus and three co‐morbidity groups with markedly reduced sample sizes (gastrointestinal diseases, hematological diseases, and thrombotic events or vascular diseases). Absence of any co‐morbidity or risk factor was found in 74.3% of all pregnant women, in 69.8% of all postpartum cases, and in 63.4% of non‐obstetric cases. Cardiovascular disease was the most prevalent co‐morbidity among all groups (range 9.5%–15.1%), followed by obesity (range 7.9%–8.9%).
TABLE 1.
Characteristics of the sample by pregnancy statusª
| Pregnant (n=2265) | Postpartum (n=630) | Non‐obstetric (n=21 910) | P value | |
|---|---|---|---|---|
| Age (years)‐median (range) | 29 (11–44) | 30 (12–44) | 36 (10–44) | <0.0001 |
| Age (years) | ||||
| <20 | 211 (9.3) | 62 (9.8) | 997 (4.6) | |
| 20–34 | 1541 (68.0) | 404 (64.1) | 8551 (39.0) | <0.0001 |
| >34 | 513 (22.6) | 164 (26.0) | 12 262 (56.4) | |
| Ethnicity | ||||
| White | 578 (25.5) | 130 (20.6) | 6612 (30.2) | <0.0001 |
| Black | 110 (4.9) | 37 (5.9) | 915 (4.2) | |
| Yellow | 20 (0.9) | 6 (1.0) | 224 (1.0) | |
| Brown/Pardo | 1040 (45.9) | 324 (51.4) | 7243 (33.1) | |
| Indigenous | 25 (1.1) | 6 (1.0) | 66 (0.3) | |
| Unknown | 492 (21.7) | 127 (20 | 6850 (31.3) | |
| SpO2 <95% at notification | 490 (21.6) | 219 (34.8) | 7762 (35.4) | <0.0001 |
| No risk factor or co‐morbidity | 1683 (74.3) | 440 (69.8) | 13 897 (63.4) | <0.0001 |
| Cancer | 5 (0.2) | 4 (0.6) | 415 (1.9) | <0.0001 |
| Cardiovascular diseases | 216 (9.5) | 95 (15.1) | 2963 (13.5) | <0.0001 |
| Diabetes mellitus | 178 (7.9) | 51 (8.1) | 1952 (8.9) | 0.20 |
| Gastrointestinal diseases | 4 (0.2) | 3 (0.5) | 55 (0.3) | 0.41 |
| Hematological diseases | 30 (1.3) | 3 (0.5) | 282 (1.3) | 0.19 |
| Immunosuppression or HIV/AIDS | 28 (1.2) | 13 (2.1) | 676 (3.1) | <0.0001 |
| Kidney diseases | 10 (0.4) | 10 (1.6) | 511 (2.3) | <0.0001 |
| Liver diseases | 5 (0.2) | 4 (0.6) | 95 (0.4) | 0.23 |
| Neurological diseases or stroke | 20 (0.9) | 4 (0.6) | 387 (1.8) | 0.0009 |
| Obesity | 92 (4.1) | 41 (6.5) | 1912 (8.7) | <0.0001 |
| Respiratory diseases (including asthma) | 104 (4.6) | 21 (3.3) | 1385 (6.3) | 0.0001 |
| Rheumatological diseases | 6 (0.3) | 3 (0.5) | 200 (0.9) | 0.003 |
| Thrombotic events or vascular diseases | 3 (0.1) | 2 (0.3) | 44 (0.2) | 0.62 |
| Thyroid diseases | 38 (1.7) | 3 (0.5) | 273 (1.2) | 0.043 |
Values are given as number (percentage) if no otherwise specified.
All independent variables were significantly associated with risk of death, except for two (gastrointestinal diseases and thrombotic events or vascular diseases) (Table 2). Figure 1 illustrates adverse outcomes of COVID‐19 among the three subgroups. Postpartum was associated with increased risk of death, admission to the ICU, and mechanical ventilation, but not with dying without ICU care. The risk of dying without access to the ICU or mechanical ventilation was significantly higher among non‐obstetric cases. Figure 2 presents descriptive data comparing the case fatality rate among cases of COVID‐19 SARS according to pregnancy status and presence of risk factor or co‐morbidity. Pregnant women without any risk factors had a lower risk of death, followed by non‐obstetric cases without risk factors. The higher case fatality rate was observed for postpartum with any risk factor, followed by non‐obstetric cases with any risk factor (Figure 2).
TABLE 2.
Case fatality rate by characteristics of the total sample a
| Cure (n=21 676) | Death (n=3129) | P value | |
|---|---|---|---|
| Age (years) | |||
| <20 | 1135 (89.4) | 135 (10.6) | <0.0001 |
| 20–34 | 9486 (90.4) | 1010 (9.6) | |
| >34 | 11 055 (84.8) | 1984 (15.2) | |
| Ethnicity | |||
| White | 6581 (89.9) | 739 (10.1) | <0.0001 |
| Black | 885 (83.3) | 177 (16.7) | |
| Asiatic | 212 (84.8) | 38 (15.2) | |
| Brown/Pardo | 7472 (84.1) | 1365 (15.9) | |
| Indigenous | 81 (83.5) | 1365 (15.9) | |
| Unknown | 6675 (89.4) | 794 (10.6) | |
| Pregnancy status | |||
| Pregnant | 2114 (93.3) | 151 (16.7) | <0.0001 |
| Postpartum | 510 (81.0) | 120 (19.0) | |
| Non‐obstetric | 19 052 (87.0) | 2858 (13.0) | |
| SpO2 <95% at notification | |||
| Yes | 12 338 (83.9) | 2373 (16.1) | <0.0001 |
| No | 9338 (92.5) | 756 (7.5) | |
| Any risk factor or co‐morbidity | |||
| Yes | 6760 (76.9) | 2025 (23.1) | <0.0001 |
| No | 14916 (93.1) | 1104 (6.9) | |
| Cancer | |||
| Yes | 198 (46.7) | 226 (53.3) | <0.0001 |
| No | 21478 (88.1) | 2903 (11.9) | |
| Cardiovascular diseases | |||
| Yes | 2537 (77.5) | 737 (22.5) | <0.0001 |
| No | 19139 (88.9) | 2392 (11.1) | |
| Diabetes mellitus | |||
| Yes | 1558 (71.4) | 623 (28.6) | <0.0001 |
| No | 20118 (88.9) | 2506 (11.1) | |
| Gastrointestinal diseases | |||
| Yes | 51 (82.3) | 11 (17.7) | 0.2234 |
| No | 21625 (87.4) | 3118 (12.6) | |
| Hematological diseases | |||
| Yes | 244 (77.5) | 72 (22.5) | <0.0001 |
| No | 21 432 (87.5) | 3058 (12.5) | |
| Immunosuppression or HIV/AIDS | |||
| Yes | 462 (64.4.) | 255 (35.6) | <0.0001 |
| No | 21214 (88.1) | 2874 (11.9) | |
| Kidney diseases | |||
| Yes | 325 (61.2) | 206 (38.8) | <0.0001 |
| No | 21 351 (88.0) | 2923 (12.0) | |
| Liver diseases | |||
| Yes | 74 (71.2) | 30 (28.8) | <0.0001 |
| No | 21 602 (87.5) | 3099 (12.5) | |
| Neurological diseases or stroke | |||
| Yes | 276 (67.2) | 135 (32.8) | <0.0001 |
| No | 21400 (87.7) | 2994 (12.3) | |
| Obesity | |||
| Yes | 1487 (72.7) | 558 (27.3) | <0.0001 |
| No | 20 189 (88.7) | 2571 (11.3) | |
| Respiratory diseases (including asthma) | |||
| Yes | 1256 (83.2) | 254 (16.8) | <0.0001 |
| No | 20 420 (87.7) | 2875 (12.3) | |
| Rheumatological diseases | |||
| Yes | 137 (65.6) | 72 (34.4) | <0.0001 |
| No | 21 539 (87.6) | 3057 (12.4) | |
| Thrombotic events or vascular diseases | |||
| Yes | 39 (79.6) | 10 (20.4) | 0.1000 |
| No | 21 637 (87.4) | 3119 (12.6) | |
| Thyroid diseases | |||
| Yes | 284 (90.4) | 30 (9.6) | 0.1002 |
| No | 21 392 (87.3) | 3099 (12.7) | |
Values are given as number (percentage) or median (range).
FIGURE 1.
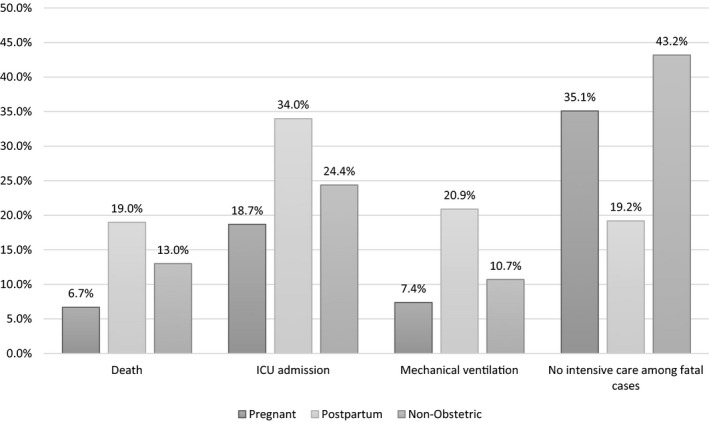
Outcomes by pregnancy status (p<0.0001 for all outcomes). Abbreviation: ICU, intensive care unit
FIGURE 2.
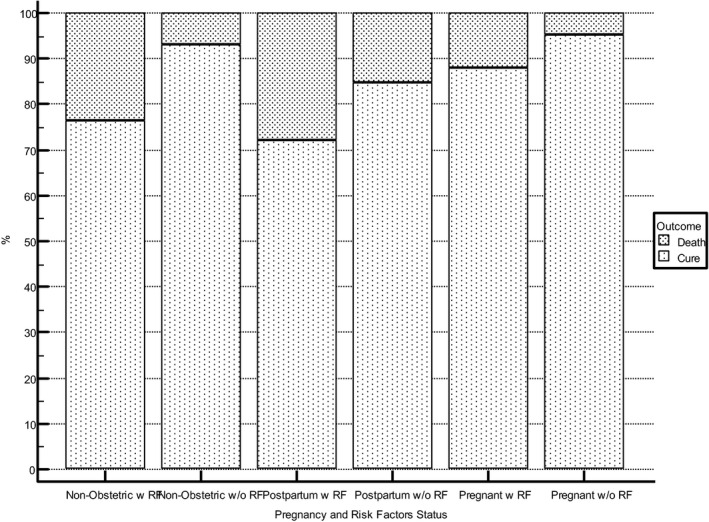
Case fatality rate by pregnancy and risk factor status (p<0.0001). Abbreviation: RF, risk factor
Multiple logistic regression models (Table 3) indicate that postpartum significantly increases the chance of death from COVID‐19 SARS (OR 1.9), while being pregnant reduces this chance (OR 0.6). Co‐morbidities with a higher impact on case fatality rate were cancer, neurological diseases, and kidney diseases. Being white or age under 35 years are protective factors. Among pregnant women, cancer, diabetes mellitus, obesity, and rheumatology diseases were associated with risk of death in the multivariate analysis. In the postpartum subgroup, age over 35 years and diabetes mellitus were independently associated with a higher chance of death.
TABLE 3.
Multivariate logistic regression model for risk of death (total sample and by pregnancy status)
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Total sample a | |||
| Age <20 years | 0.7219 | 0.5925–0.8795 | 0.001 |
| Age 20–34 years | 0.6952 | 0.6371–0.7587 | <0.0001 |
| White ethnicity | 0.5473 | 0.4958–0.6042 | <0.0001 |
| Missing data on ethnicity | 0.6291 | 0.5714–0.6927 | <0.0001 |
| Postpartum | 1.8976 | 1.5309–2.3520 | <0.0001 |
| Pregnant b | 0.6228 | 0.5219–0.7432 | <0.0001 |
| Cancer | 8.0501 | 6.5145–9.9477 | <0.0001 |
| Cardiovascular disease | 1.4049 | 1.2633–1.5623 | <0.0001 |
| Diabetes mellitus | 2.4003 | 2.1414–2.6905 | <0.0001 |
| Hematological diseases | 1.5051 | 1.1121–2.0371 | 0.008 |
| Immunosuppression or HIV/AIDS | 2.3134 | 1.9229–2.7832 | <0.0001 |
| Kidney disease | 3.1040 | 2.5485–3.7805 | <0.0001 |
| Neurological diseases or previous history of stroke | 3.3343 | 2.6542–4.1886 | <0.0001 |
| Obesity | 2.6691 | 2.3802–2.9932 | <0.0001 |
| Respiratory diseases (including asthma) | 1.3286 | 1.1423–1.5452 | 0.0002 |
| Rheumatological disease | 2.8978 | 2.1084–3.9828 | <0.0001 |
| Thyroid diseases | 0.6422 | 0.4315–0.9559 | 0.029 |
| Pregnant | |||
| Cancer | 9.9449 | 1.6234–60.9239 | 0.0130 |
| Diabetes mellitus | 1.8535 | 1.1314–3.0363 | 0.0143 |
| Obesity | 3.9556 | 2.3067–6.7834 | <0.0001 |
| Rheumatology diseases | 7.5902 | 1.3614–42.3179 | 0.0208 |
| Postpartum c | |||
| Age ≥35 years | 2.0015 | 1.2884–3.1095 | 0.0020 |
| Ethnicity unknown | 0.3861 | 0.2066–0.7215 | 0.0029 |
| Diabetes mellitus | 2.5800 | 1.3727–4.8492 | 0.0032 |
| Non‐obstetric d | |||
| Age <20 years | 0.7588 | 0.6137–0.9382 | 0.0108 |
| Age 20–34 years | 0.6842 | 0.6240–0.7501 | <0.0001 |
| White ethnicity | 0.5234 | 0.4708–0.5819 | <0.0001 |
| Black ethnicity | 0.8057 | 0.6619–0.9809 | 0.0314 |
| Unknown ethnicity | 0.6141 | 0.5543–0.6803 | <0.0001 |
| Cancer | 8.2414 | 6.6489–10.2153 | <0.0001 |
| Cardiovascular diseases | 1.4018 | 1.2539–1.5670 | <0.0001 |
| Diabetes mellitus | 2.4518 | 2.1754–2.7634 | <0.0001 |
| Hematological diseases | 1.4387 | 1.0476–1.9758 | 0.0246 |
| Immunosuppression or HIV | 2.4009 | 1.9859–2.9026 | <0.0001 |
| Kidney diseases | 3.1538 | 2.5782–3.8578 | <0.0001 |
| Liver diseases | 1.6594 | 1.0003–2.7526 | 0.0498 |
| Neurological diseases or stroke | 3.5902 | 2.8453–4.5301 | <0.0001 |
| Obesity | 2.7185 | 2.4140–3.0615 | <0.0001 |
| Respiratory diseases (including asthma) | 1.3546 | 1.1590–1.5832 | 0.0001 |
| Rheumatology diseases | 2.8050 | 2.0228–3.8896 | <0.0001 |
| Thyroid diseases | 0.5376 | 0.3452–0.8373 | 0.006 |
Abbreviations: AUC, area under the receiver operating characteristic curve; CI, confidence interval; OR, odds ratio; ROC, receiver operating characteristic.
Total sample: 87.5% correctly classified using stepwise multiple logistic regression method. AUC 0.732 (95% CI 0.727–0.738).
Pregnant: 93.4% correctly classified using stepwise multiple logistic regression method. AUC 0.578 (95% CI 0.558–0.599).
Postpartum: 81.3% correctly classified using stepwise multiple logistic regression method. AUC 0.636 (95% CI 0.597–0.674).
Non‐obstetric: 87.2% correctly classified using stepwise multiple logistic regression method. AUC 0.736 (95% CI 0.730–0.742).
4. DISCUSSION
In the present sample, the postpartum period was associated with worse outcomes in the obstetric population. When stratified by pregnancy status, postpartum women had increased rates of admission to the ICU (34%), invasive ventilation (20.9%), and death (19%) when compared to pregnant and non‐pregnant women. However, the risk of dying without accessing ICU care was lower in this subgroup (19.2% vs 35.1% among pregnant women, and 43.2% among non‐obstetric cases). The findings of the present study indicate a case fatality rate of 12.6% in the total sample (range 6.7%–19.0%). However, a sample was analyzed of girls and women aged 10–45 years already diagnosed with COVID‐19 SARS; therefore, they were younger and at reduced risk of death due to COVID‐19 compared to men or older populations. 13
Worse outcomes among postpartum women could be partially explained by the overlap of coagulation disorders in patients with COVID‐19 with the hypercoagulability of the postpartum period. 14 , 15 , 16 Unfortunately, the SARS‐SS does not provide data regarding date of birth or week of pregnancy at diagnosis, so details of onset of symptoms were not available. Thus, the postpartum subgroup in the present analysis may also comprise women infected with COVID‐19 while pregnant who evolved to SARS after delivery. In addition, clinical worsening during pregnancy may prompt delivery, leading to a SARS notification after birth. Pregnant patients with a compromised respiratory system may undergo a termination of pregnancy as a therapeutic measure. Accordingly, this may also contribute to the more frequent occurrence of death in the postpartum period.
Obstetric patients with SARS often need to be transferred to tertiary health units located away from their place of residence, contributing to worse outcomes. 9 Usually, Brazilian facilities dedicated to COVID‐19 are not equipped with midwifery or obstetrics infrastructure; therefore, delivery may be anticipated before the patient's transference. Emergency procedures such as tracheal intubation and admission to the ICU might have been postponed until after childbirth, partly explaining the paradoxical finding of fewer deaths without ICU care during postpartum in comparison to pregnancy. According to the three‐delays model, 17 performing induction of labor or cesarean delivery before providing adequate ventilatory support is not recommended. 18 , 19 , 20 Surgical trauma may contribute to the deterioration of the women's condition, as already observed in cases of non‐obstetric COVID‐19. 21 , 22
Pregnant women were less frequently admitted with low oxygen saturation when compared with postpartum or non‐pregnant women. Barriers to access health care seem to be in the origins of COVID‐19 outcomes in Brazil. 9 , 11 In this context, routine antenatal care appointments might facilitate the early identification of respiratory symptoms, inducing timely referral to higher levels of care.
Chronic conditions are risk factors for women dying with COVID‐19, confirming findings in the general population. 2 , 23 In the present sample, cancer enhanced the chance of death due to COVID‐19 by 8–9 times for women of reproductive age. Oncologic patients may have lymphopenia and neutropenia due to treatment and disease evolution, which may impair the therapeutic organic response to viral infections. Previous authors observed worse outcomes of COVID‐19 among patients with cancer, with the prognosis influenced by staging and site of cancer. 24 Unfortunately, the database in the present study did not provide information on cancer‐specific variables.
In the present sample, diabetes was a risk factor for death. Diabetes can increase respiratory infections due to altered innate immunity and elevate levels of pro‐inflammatory cytokine. A meta‐analysis of 6452 patients found that SARS‐CoV‐2 infection in patients with diabetes increased mortality and SARS by two and four times, respectively. 25 However, body mass index was the only risk factor for death or tracheal intubation in an observational study with diabetic patients infected with COVID‐19. 26
Obesity is an already known risk factor for death due to COVID‐19 for both pregnant and non‐obstetric patients, though not for postpartum women. 27 , 28 , 29 A meta‐analysis exploring the relationship between COVID‐19 and obesity showed that obese individuals had a 74% higher risk of admission to the ICU, and a 48% increased risk of death. 27 Obesity seems to be an independent risk factor, despite its association with several co‐morbidities (diabetes, hypertension, cardiovascular disease). 29 Obesity is a metabolic disease marked by insulin resistance, hyperglycemia, altered adipokines, and chronic inflammation, 27 and the production of abnormal cytokines is also observed in SARS. The disease increases the risk of thrombosis, and severe COVID‐19 is associated with prothrombotic disseminated intravascular coagulation and high rates of venous thromboembolism. 30 Two meta‐analyses showed that the prevalence of thromboembolic events in hospitalized patients with SARS‐CoV‐2 surpasses 30%. 31 , 32
In the present sample, rheumatological diseases increased the risk of death by seven times in pregnancy, and by three times in the general female population with COVID‐19. This group of conditions includes a diverse spectrum of diseases, and SARS‐SS does not provide detailed information. However, connective tissue disorders are associated with worse prognoses for SARS‐CoV‐2 infection. Typical impairment of organs and systems and treatment with high doses of immunosuppressants may conjointly contribute to clinical deterioration from COVID‐19 in rheumatological patients. 33 , 34 Rheumatological diseases are systemic inflammatory disorders in which virus infections may trigger severe features. 35 , 36 , 37
Cardiovascular diseases increased the fatality rate by 1.4 times in the overall sample. Pre‐eclampsia has been described as a potential risk factor for complications from COVID‐19, 38 , 39 although the association was not present in the present sample. SARS‐SS variables do not differentiate pre‐pregnancy from gestational hypertension, as well as heart diseases from further cardiovascular conditions. Additional affections such as nephropathies, hematological disorders, and neuropathies among others, showed an association with death due to COVID‐19 among Brazilian women of reproductive age, regardless of pregnancy status.
A systematic review on predictors of death in patients hospitalized due to COVID‐19 found that the summary relative risk (sRR) of death was higher for those with the following: age 60 years and over; men; smoking history; chronic obstructive pulmonary disease; hypertension; diabetes; heart disease; and chronic kidney disease. Excluding male patients, the main risk factors for death are consistent with our findings, 40 although there is a different age range in the present study.
The main limitations of the present study rely on its retrospective nature based on secondary database analysis, likely increasing the risk of bias due to missingness and a lack of detailed information about clinical prognostic factors. In addition, co‐morbidity information was derived using two different sources (the original coded field about risk factors and the classification of answers to open‐ended questions), meaning that not all cases were classified as having risk factors using the same standardized procedure. Another limitation is that the SARS‐SS does not provide data about other obstetric variables such as date of birth or week of pregnancy at diagnosis. It is worth mentioning that universal screening is not included among Brazilian policies during the pandemic; therefore, data on asymptomatic or mildly symptomatic women are not available through SARS‐SS.
Despite these limitations, the present study was able to provide useful information about the interaction between pregnancy status and co‐morbidities as risk factors for death due to COVID‐19, using a large dataset with nationwide coverage. The present data adds to the still preliminary knowledge about risk factors for COVID‐19‐related maternal deaths, given the fact that Brazil has overwhelming figures of cases of COVID‐19 and deaths, both in the general population and in pregnant and postpartum women.
The findings of the present study highlight the relevance of the postpartum period as a risk factor for COVID‐19 adverse outcomes, particularly when in association with co‐morbidities. Further prospective studies might clarify the identified risk factors associated with a worsening prognosis of COVID‐19 among women. Postpartum women must be considered a special population within these studies.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors contributed equally for study conception and study design. MLST, MOM, RK, and VK conducted the data collection. MLST, MOM, CBA, RK, VK, LK, MMRA, and MNP conducted the data analysis and interpretation. MLST, MOM, MNP, CBA, and RK wrote the first draft of the paper. All authors reviewed and provided comments on the first draft and approved the final manuscript. MOM and MLST are the study guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
ACKNOWLEDGMENTS
The authors thank all members of the Brazilian Group for Studies of COVID‐19 and Pregnancy for all their efforts in supporting this work and in improving maternal health.
Knobel R, Takemoto MLS, Nakamura‐Pereira M, et al. COVID‐19‐related deaths among women of reproductive age in Brazil: The burden of postpartum. Int J Gynecol Obstet. 2021;155:101–109. 10.1002/ijgo.13811
REFERENCES
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS‐CoV‐2: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takemoto M, Menezes M, Andreucci C, et al. Clinical characteristics and risk factors for mortality in obstetric patients with severe COVID‐19 in Brazil: a surveillance database analysis. BJOG An Int J Obstet Gynaecol. 2020;127(13):1618‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lumbreras‐Marquez MI, Campos‐Zamora M, Seifert SM, et al. Excess maternal deaths associated with Coronavirus Disease 2019 (COVID‐19) in Mexico. Obstet Gynecol. 2020;136(6):1114‐1116. [DOI] [PubMed] [Google Scholar]
- 5. DeBolt CA, Bianco A, Limaye MA, et al. Pregnant women with severe or critical coronavirus disease 2019 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2020;224(5):510‐e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory‐confirmed SARS‐CoV‐2 infection by pregnancy status — United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Worldometer . Coronavirus Cases [Internet]. Available from: https://www.worldometers.info/coronavirus/Acessed on: December 24, 2020.
- 8. Horton R. Editorial COVID‐19 in Brazil: “So what?”. Lancet. 2020;6736(20):31095. [Google Scholar]
- 9. Menezes MO, Takemoto MLS, Nakamura‐Pereira M, et al. Risk factors for adverse outcomes among pregnant and postpartum women with acute respiratory distress syndrome due to COVID‐19 in Brazil. Int J Gynecol Obstet. 2020;151(3):415‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde . Influenza. In: [Health Surveillance Guide]. 3rd edn. Brasília‐DF: Ministry of Health; 2019:1‐741. [Google Scholar]
- 11. Baqui P, Bica I, Marra V, Ercole A, van der Schaar M. Ethnic and regional variations in hospital mortality from COVID‐19 in Brazil: a cross‐sectional observational study. Lancet Glob Heal. 2020;8(8):e1018‐e1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takemoto ML, Menezes MD, Andreucci CB, et al. The tragedy of COVID‐19 in Brazil: 124 maternal deaths and counting. Int J Gynecol Obstet. 2020;151(1):154‐156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA. 2020;323(20):2052‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takayama W, Endo A, Yoshii J, et al. Severe COVID‐19 pneumonia in a 30‐year‐old woman in the 36th week of pregnancy treated with postpartum extracorporeal membrane oxygenation. Am J Case Rep. 2020;21:e927521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katz D, Beilin Y. Disorders of coagulation in pregnancy. Br J Anaesth. 2015;115:ii75‐ii88. [DOI] [PubMed] [Google Scholar]
- 17. Pacagnella RC, Cecatti JG, Parpinelli MA, et al. Delays in receiving obstetric care and poor maternal outcomes: results from a national multicentre cross‐sectional study. BMC Pregnancy Childbirth. 2014;14(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. RCOG . Coronavirus (COVID‐19) infection in pregnancy. Guidelines. 2020;12: 1‐7. [Google Scholar]
- 19. Narang K, Ibirogba ER, Elrefaei A, et al. SARS‐CoV‐2 in Pregnancy: A Comprehensive Summary of Current Guidelines. J Clin Med. 2020;9(5):1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brasil. Ministério da Saúde. Secretaria de Atenção Especializada . Protocolo de Manejo Clínico da Covid‐19 na Atenção Especializada. Brasília ‐ DF; 2020. Apr.
- 21. Nahshon C, Bitterman A, Haddad R, Hazzan D, Lavie O. Hazardous postoperative outcomes of unexpected COVID‐19 infected patients: a call for global consideration of sampling all asymptomatic patients before surgical treatment. World J Surg. 2020;44(8):2477‐2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aminian A, Safari S, Razeghian‐Jahromi A, Ghorbani M, Delaney CP. COVID‐19 outbreak and surgical practice: unexpected fatality in perioperative period. Ann Surg. 2020;272(1):e27‐e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hernández‐Vásquez A, Azañedo D, Vargas‐Fernández R, Bendezu‐Quispe G. Association of comorbidities with pneumonia and death among COVID‐19 patients in Mexico: a Nationwide Cross‐sectional Study. J Prev Med Public Health. 2020;53(4):211‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jee J, Foote MB, Lumish M, et al. Chemotherapy and COVID‐19 Outcomes in Patients with Cancer. J Clin Oncol. 2020;38(30):3538‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID‐19 pneumonia ‐ a systematic review, meta‐analysis, and meta‐regression. Diabetes Metab Syndr. 2020;14(4):395‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID‐19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID‐19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21(11):e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584(7821):430‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Halpern B, Louzada MLC, Aschner P, et al. Obesity and COVID‐19 in Latin America: a tragedy of two pandemics—Official document of the Latin American Federation of Obesity Societies. Obes Rev. 2021;22(3):e13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sattar N, McInnes IB, McMurray JJV. Obesity is a risk factor for severe COVID‐19 infection: multiple potential mechanisms. Circulation. 2020;142(1):4‐6. [DOI] [PubMed] [Google Scholar]
- 31. Kefale B, Tegegne GT, Degu A, Tadege M, Tesfa D. Prevalence and risk factors of thromboembolism among patients with Coronavirus Disease‐19: a systematic review and meta‐analysis. Clin Appl Thromb. 2020;26:107602962096708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Di Minno A, Ambrosino P, Calcaterra I, Di Minno MND. COVID‐19 and venous thromboembolism: a meta‐analysis of literature studies. Semin Thromb Hemost. 2020;46(07):763‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gianfrancesco M, Hyrich KL, Hyrich KL, et al. Characteristics associated with hospitalisation for COVID‐19 in people with rheumatic disease: data from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis. 2020;79(7):859‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pablos JL, Galindo M, Carmona L, et al. Clinical outcomes of hospitalised patients with COVID‐19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis [Internet]. 2020;79(12):1544‐9. [DOI] [PubMed] [Google Scholar]
- 35. Pamukçu B. Inflammation and thrombosis in patients with COVID‐19: a prothrombotic and inflammatory disease caused by SARS coronavirus‐2. Anatol J Cardiol. 2020;24(4):224‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Montero F, Martínez‐Barrio J, Serrano‐Benavente B, et al. Coronavirus disease 2019 (COVID‐19) in autoimmune and inflammatory conditions: clinical characteristics of poor outcomes. Rheumatol Int. 2020;40(10):1593‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis for the third international consensus definitions for sepsis and septic shock (sepsis‐3). JAMA. 2016;315(8):762‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Narang K, Enninga EAL, Gunaratne MDSK, et al. SARS‐CoV‐2 infection and COVID‐19 during pregnancy: a multidisciplinary review. Mayo Clin Proc. 2020;95(8):1750‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID‐19) during pregnancy: a systematic review and meta‐analysis. Am J Obstet Gynecol MFM. 2020;2(2):100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID‐19: a comprehensive systematic review and meta‐analysis of 77 studies and 38,000 patients. PLoS One. 2020;15(12):e0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]


