Abstract
Objective
To determine the acceptance rate of treatment alternatives for women with either preinvasive conditions or gynecologic cancers during the COVID‐19 pandemic among Latin American gynecological cancer specialists.
Methods
Twelve experts in gynecological cancer designed an electronic survey, according to recommendations from international societies, using an online platform. The survey included 22 questions on five topics: consultation care, preinvasive cervical pathology, and cervical, ovarian, and endometrial cancer. The questionnaire was distributed to 1052 specialists in 14 Latin American countries. A descriptive analysis was carried out using statistical software.
Results
A total of 610 responses were received, for an overall response rate of 58.0%. Respondents favored offering teleconsultation as triage for post‐cancer treatment follow‐up (94.6%), neoadjuvant chemotherapy in advanced stage epithelial ovarian cancer (95.6%), and total hysterectomy with bilateral salpingo‐oophorectomy and defining adjuvant treatment with histopathological features in early stage endometrial cancer (85.4%). Other questions showed agreement rates of over 64%, except for review of pathology results in person and use of upfront concurrent chemoradiation for early stage cervical cancer (disagreement 56.4% and 58.9%, respectively).
Conclusion
Latin American specialists accepted some alternative management strategies for gynecological cancer care during the COVID‐19 pandemic, which may reflect the region’s particularities.
The COVID‐19 pandemic led Latin American specialists to accept alternative management strategies for gynecological cancer care, especially regarding surgical decisions.
Keywords: Cancer management, Coronavirus, COVID‐19, Gynecologic cancer, Gynecologic neoplasms, Latin America, Survey
Short abstract
The COVID‐19 pandemic led Latin American specialists to accept alternative management strategies for gynecological cancer care, especially regarding surgical decisions.
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic has posed the greatest challenge to global health services this century. By June 2, 2020, there were 6 194 533 cases of COVID‐19 and 376 320 deaths due to associated complications. 1 Gynecologic oncology care is affected by this health crisis because new cases of cancer will be diagnosed continuously during the pandemic. Patients require the best treatment options, according to local and national resources. In addition, oncologists are aware that cancer patients are at an increased risk of fatal outcomes if they become infected with the virus causing COVID‐19; however, ceasing treatment due to the pandemic could signify disease progression and death. 2
Brazil reported the first case in Latin America and the Caribbean on February 26, 2020. Since then, COVID‐19 has spread to 50 countries and territories in the Americas. 3 During 2018, 730 239 new cases of neoplasms in women were reported in Latin America. 4 Different barriers such as restricted healthcare coverage, insufficient funding and human resources, and limited access to surgery, radiation, and chemotherapy have been identified in the region. 5 This, added to the impact of the COVID‐19 pandemic, makes it necessary for specialists to adopt alternative management strategies for patients with gynecological cancer to optimize health resources and avoid contagion for patients and doctors.
As the COVID‐19 pandemic spreads, several international scientific societies have published their recommendations for gynecologic cancer care. 6 , 7 , 8 In addition, some Latin American societies have published documents, 9 , 10 but there are several countries where no guidance has been generated. Furthermore, to date, there are no data regarding Latin American gynecologic and surgical oncologists’ acceptance of these strategies.
The aim of the present study was to determine the acceptance rate among Latin American gynecological cancer specialists of treatment strategies for women diagnosed with either preinvasive conditions or gynecological cancers during the COVID‐19 pandemic.
2. MATERIALS AND METHODS
Ten gynecologic oncologists, all members of the Asociación Colombiana de Ginecólogos Oncólogos (ACGO), a radiation oncologist, and a medical oncologist participated in a videoconference meeting on March 25, 2020. The study group defined, by majority, clinical scenarios for gynecological cancer management during the COVID‐19 pandemic, based on expertise, society recommendations, and clinical studies. 6 , 7 , 8 , 9 , 10 Differences of opinion were resolved by discussion.
A questionnaire that included demographic characteristics and specific considerations was developed in Spanish (Appendix S1). The survey was subdivided into five topics: consultation care, preinvasive cervical pathology, and cervical, ovarian, and endometrial cancer. For each item, published information on alternative management was included as a statement before each question. In total, 22 questions were considered with dichotomic answers (agree/disagree). The questions were designed with a mandatory answer, and the respondent had to answer the entire form so that the system included it in the analysis database. Google Forms (Google LLC, Mountain View, CA, USA) was used to create the instrument.
Inclusion criteria were respondents currently providing treatment for preinvasive cervical conditions and gynecological cancer as either a gynecologic oncologist or surgical oncologist in Latin America. Trainees (residents, fellows) were excluded. A convenience sampling method was adopted and a pilot test was carried out within the study group to test the platform and instrument. Initially the survey was distributed electronically to the 90 active members of ACGO on April 9, 2020. A message indicating the objectives of the study, data protection, and confidentiality was included. Reminders were sent to increase the response rate every 24 hours. The questionnaire closed on April 12, 2020.
Subsequently, through a meeting of the International Gynecologic Cancer Society (IGCS)—project ECHO (Extension for Community Healthcare Outcomes) on April 13, 2020, specialists from another 13 Latin American countries were invited via videoconference to participate in distribution of the survey. Each representative created a database with the emails of the specialists in their respective countries to avoid duplicates. This information was obtained either through oncological societies or by their local specialist groups. In Brazil, one of the researchers (RR) translated the survey into Portuguese (Appendix S2), and a pilot test with five specialists was carried out to trial the translated document. Initial email contact was made by each researcher on April 14, 2020. If the participants agreed to take part, the questionnaire link was sent. Reminders were sent every 24 hours. The questionnaire was available until April 17, 2020.
Only one questionnaire was allowed per participant. Duplicate email questionnaires identified on the platform were removed. The responses were exported to Excel (Microsoft Corp, Redmond, WA, USA). An investigator (JR) had access to the results. A descriptive analysis of the information was carried out expressing the qualitative variables in absolute and percentage frequencies. Response rates were measured by number of respondents. Statistical analysis was performed using SPSS version 21.0 (IBM, Armonk, NY, USA).
The study was approved by the academic committee of ACGO.
3. RESULTS
A total of 1052 invitations to participate were sent, with 610 questionnaires answered for an overall response rate of 58.0%. In five countries, the response rate was 100.0%, in six it was between 60.0% and 99.0%, and in three it was less than 60.0%. The country with the lowest response rate was Brazil with 132 questionnaires answered (28.7%) (Table 1). The country with the highest number of respondents was Mexico (n=155), making up 25.4% of the total respondents (Fig. 1). Most respondents were men (n=450, 73.8%) and 361 (59.2%) respondents were under 45 years of age (Fig. 2). When analyzing each question, there was a similar agreement percentage between countries; however, in seven questions a difference of over 30% in agreement was evident (Table 2).
Table 1.
Survey response rate by country.
| Country | Surveys sent | Answers | Response rate, % |
|---|---|---|---|
| Guatemala | 11 | 11 | 100.0 |
| Panamá | 12 | 12 | 100.0 |
| Perú | 31 | 31 | 100.0 |
| Uruguay | 12 | 12 | 100.0 |
| Venezuela | 24 | 24 | 100.0 |
| Costa Rica | 18 | 17 | 94.4 |
| Colombia | 90 | 81 | 90.0 |
| Mexico | 184 | 155 | 84.2 |
| Bolivia | 20 | 16 | 80.0 |
| Ecuador | 10 | 7 | 70.0 |
| Argentina | 98 | 64 | 65.3 |
| Chile | 70 | 41 | 58.6 |
| El Salvador | 12 | 7 | 58.3 |
| Brazil | 460 | 132 | 28.7 |
| Total | 1052 | 610 | 58.0 |
Figure 1.
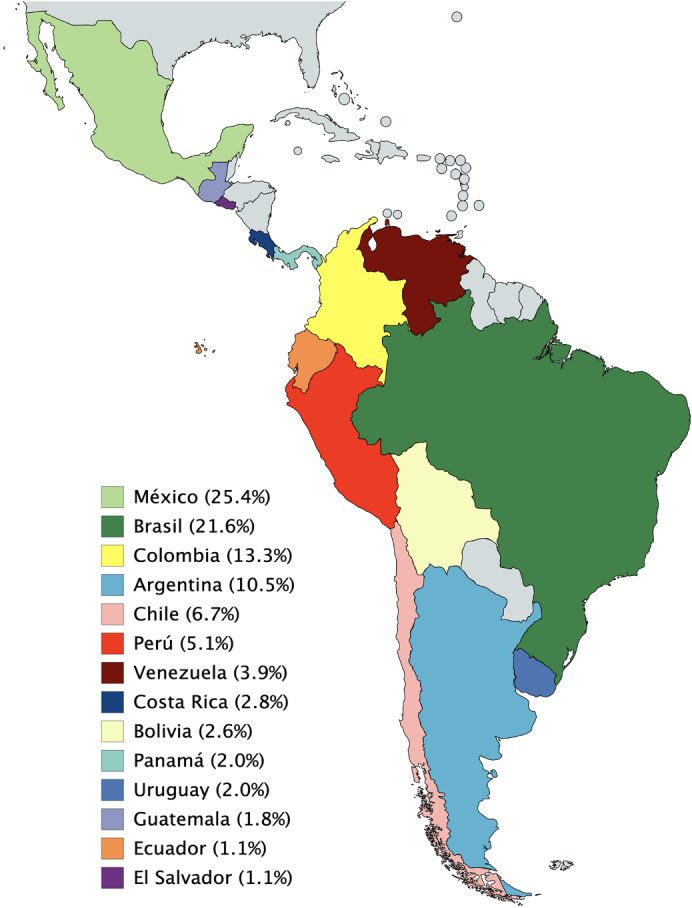
Percentage of total respondents (n=610) by country.
Figure 2.
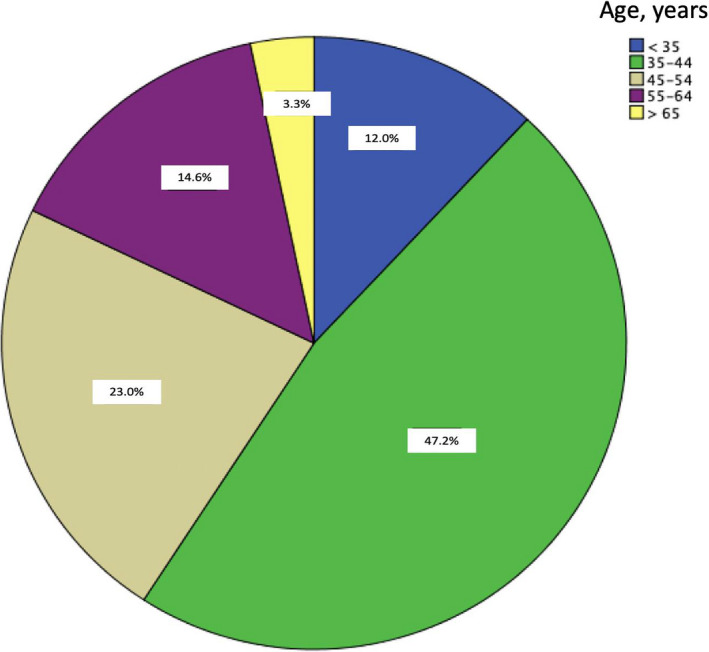
Age distribution of the respondents (n=610).
Table 2.
Agreement percentage by country according to survey question.
| Question | Agreement (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MX | BR | CO | AR | CL | PE | VE | CR | BO | PA | UY | GT | EC | SV | Overall | |
| 1. Do you consider that patients with a high suspicion of gynecological neoplasia (adnexal mass) or with confirmed histological diagnosis of gynecological cancer should be evaluated in person for the first time? 11 | 92.9 | 97.7 | 93.8 | 89.1 | 92.7 | 90.3 | 100.0 | 82.4 | 81.3 | 100.0 | 91.7 | 100.0 | 85.7 | 100.0 | 93.4 |
| 2. Do you consider that review of pathology reports after surgery for gynecological cancer should be evaluated in person? 11 | 36.1 | 81.1 | 46.9 | 28.1 | 24.4 | 22.6 | 25.0 | 17.6 | 18.8 | 50.0 | 41.7 | 45.5 | 14.3 | 14.3 | 43.6 |
| 3. Do you think that patients diagnosed with gynecological neoplasia should be contacted by teleconsultation for post‐treatment follow‐up, queried about symptoms that may lead to suspicion of recurrent disease and, if present, direct them to physical examination and paraclinical tests to rule out tumor relapse? 12 | 96.8 | 90.2 | 98.8 | 93.8 | 95.1 | 90.3 | 95.8 | 94.1 | 93.8 | 100.0 | 100.0 | 90.9 | 85.7 | 100.0 | 94.6 |
| 4. Do you think that patients diagnosed with gynecological neoplasia should be contacted by teleconsultation for post‐treatment follow‐up, queried about symptoms that may lead to suspicion of recurrent disease and, in the event of no symptoms, schedule the next visit in 3 mo, after the pandemic is controlled, to continue institutional monitoring? 12 | 96.8 | 91.7 | 97.5 | 93.8 | 100.0 | 96.8 | 95.8 | 94.1 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 95.9 |
| 5. Do you think that teleconsultation should be carried out by the gynecologist‐oncologist who treats at the institutions? 11 , 12 | 96.1 | 90.9 | 97.5 | 96.9 | 82.9 | 93.5 | 100.0 | 76.5 | 81.3 | 100.0 | 100.0 | 100.0 | 100.0 | 85.7 | 93.6 |
| 6. If you consider that the patient should be evaluated in person, do you suggest that a triage should be done by teleconsultation to screen for a suspected case of COVID‐19, according to current epidemiological criteria? 11 | 96.1 | 89.4 | 96.3 | 95.3 | 97.6 | 96.8 | 87.5 | 100.0 | 100.0 | 100.0 | 83.3 | 100.0 | 100.0 | 100.0 | 94.6 |
| 7. Do you agree that patients presenting with cervicovaginal cytology who report a low‐grade lesion with or without an HPV‐DNA (+) test could be deferred for colposcopic evaluation for at least 6 mo? 13 | 96.8 | 90.2 | 100.0 | 98.4 | 100.0 | 93.5 | 100.0 | 88.2 | 100.0 | 91.7 | 100.0 | 90.9 | 85.7 | 100.0 | 95.7 |
| 8. Do you agree that patients presenting with cervicovaginal cytology reporting a high‐grade epithelial lesion with or without an HPV‐DNA (+) test could be deferred for colposcopic evaluation for 3 mo? 13 | 71.0 | 65.9 | 86.4 | 73.4 | 75.6 | 74.2 | 70.8 | 58.8 | 75.0 | 83.3 | 66.7 | 90.9 | 85.7 | 57.1 | 73.0 |
| 9. Do you agree that for patients who present a biopsy with CIN II‐III, or adenocarcinoma in situ, without suspected infiltration, excision management could be delayed for 3 mo? 13 | 64.5 | 46.2 | 81.5 | 71.9 | 78.0 | 64.5 | 66.7 | 47.1 | 56.3 | 75.0 | 58.3 | 90.9 | 71.4 | 57.1 | 64.4 |
| 10. Do you agree that for patients who present a biopsy with a high‐grade lesion and suspected microinvasion on colposcopy, excision management could be delayed for a maximum of 1 mo? 13 | 80.0 | 80.3 | 84.0 | 84.4 | 87.8 | 74.2 | 79.2 | 82.4 | 93.8 | 100.0 | 83.3 | 90.9 | 85.7 | 100.0 | 82.6 |
| 11. Do you agree that for patients who present a lack of correlation between cytology/colposcopy/biopsy result or a positive endocervical curettage, excision management could be delayed for 3 mo? 13 | 73.5 | 46.2 | 88.9 | 71.9 | 80.5 | 61.3 | 66.7 | 76.5 | 75.0 | 83.3 | 58.3 | 90.9 | 57.1 | 71.4 | 69.2 |
| 12. Do you agree to offer primary treatment with radiotherapy (+concomitant chemotherapy) to a postmenopausal patient (>50 y) with early stage cervical cancer, with a visible lesion or with postconization positive margins, who would normally be a candidate for surgical management? 14 | 38.1 | 31.1 | 54.3 | 37.5 | 43.9 | 48.4 | 29.2 | 35.3 | 75.0 | 75.0 | 25.0 | 45.5 | 28.6 | 85.7 | 41.1 |
| 13. Do you agree to defer minimum definitive treatment by 3 mo for a patient with cervical cancer with postconization negative margins, or with a biopsy diagnosis without a visible cervical disease, with no desire to preserve fertility, with no suspicion of lymph node involvement by imaging? 15 , 16 | 85.2 | 67.4 | 85.2 | 84.4 | 63.4 | 80.6 | 62.5 | 82.4 | 100.0 | 91.7 | 75.0 | 100.0 | 100.0 | 100.0 | 79.5 |
| 14. Do you agree that it is not necessary to perform laparoscopic surgical staging in patients with locally advanced cervical cancer before treatment with chemoradiotherapy? 17 | 92.3 | 90.9 | 97.5 | 96.9 | 90.2 | 87.1 | 95.8 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 93.9 |
| 15. Do you agree not to schedule surgery for patients with central recurrence of cervical cancer with more than a 6‐mo disease‐free period (candidates for exenteration) and instead refer them to clinical oncology for chemotherapy treatment? 18 | 78.7 | 50.8 | 80.2 | 84.4 | 82.9 | 83.9 | 70.8 | 82.4 | 81.3 | 100.0 | 75.0 | 90.9 | 100.0 | 1000 | 74.9 |
| 16. Do you agree that for patients with apparent advanced ovarian epithelial cancer who have ascites and carcinomatosis, CA 125 elevation, with pathology demonstrated by cytology or by cutting needle biopsy (if possible, not laparoscopic Fagotti triage), it is preferable to use neoadjuvant chemotherapy with carboplatin–paclitaxel for 3–4 cycles, and then interval surgery, according to clinical and imaging evolution? 19 | 98.7 | 94.7 | 95.1 | 92.2 | 97.6 | 90.3 | 95.8 | 94.1 | 93.8 | 91.7 | 100.0 | 90.9 | 100.0 | 100.0 | 95.6 |
| 17. Do you agree that in platinum‐sensitive patients with current recurrence of more than one site, platinum‐based chemotherapy (with or without bevacizumab) is recommended, rather than secondary cytoreduction? 20 | 89.7 | 83.3 | 96.3 | 96.9 | 97.6 | 80.6 | 83.3 | 100.0 | 87.5 | 100.0 | 83.3 | 100.0 | 100.0 | 85.7 | 90.3 |
| 18. Do you agree that patients with pelvic masses and suspected ovarian cancer should be selected by gynecologic oncologists? Those with a significant risk of malignancy, based on clinical criteria, images and available markers, will undergo surgery with frozen section biopsy (according to the availability of this service). Others will be managed by gynecologists and obstetricians 21 , 22 | 91.0 | 75.8 | 93.8 | 79.7 | 87.8 | 93.5 | 91.7 | 100.0 | 75.0 | 100.0 | 83.3 | 100.0 | 85.7 | 85.7 | 86.7 |
| 19. Do you agree that all patients with presumed early stage endometrial cancer receive exclusive initial management with total hysterectomy and bilateral salpingo‐oophorectomy with or without sentinel lymph node (according to availability) and define adjuvant treatment with uterine histopathological features? 23 , 24 | 82.6 | 87.9 | 90.1 | 84.4 | 82.9 | 77.4 | 83.3 | 94.1 | 81.3 | 91.7 | 91.7 | 81.8 | 85.7 | 85.7 | 85.4 |
| 20. Do you agree to defer hysterectomy + adnexectomy in patients with endometrial cancer FIGO Stage I without myometrial invasion on MRI, offering them temporary hormonal management with levonorgestrel IUDs (when the resource is available) or oral hormone therapy? 25 , 26 | 76.8 | 58.3 | 65.4 | 81.3 | 78.0 | 71.0 | 50.0 | 70.6 | 75.0 | 91.7 | 41.7 | 81.8 | 71.4 | 71.4 | 69.8 |
| 21. Do you agree that patients with advanced stage endometrial cancer (Stage III) undergoing surgical management are provided sequential adjuvant therapy initially with radiotherapy and later with chemotherapy, but chemotherapy differs for grade 1 and 2 during the pandemic? 23 , 27 , 28 | 76.8 | 53.0 | 74.1 | 67.2 | 68.3 | 74.2 | 83.3 | 58.8 | 87.5 | 58.3 | 41.7 | 90.9 | 71.4 | 71.4 | 68.7 |
| 22. Would you consider it appropriate to offer hormonal management in palliative patients with endometrioid FIGO grade 1 and 2 relapse without acute symptoms, while medical treatment with chemotherapy or radiotherapy (as appropriate) could be started after pandemic control? 29 | 94.8 | 73.5 | 97.5 | 92.2 | 95.1 | 90.3 | 95.8 | 82.4 | 93.8 | 100.0 | 83.3 | 100.0 | 100.0 | 100.0 | 89.8 |
Abbreviations: AR, Argentina, CL, Chile; BO, Bolivia; BR, Brazil; CO, Colombia; CR, Costa Rica; EC, Ecuador; GT, Guatemala; MX, Mexico; PA, Panamá; PE, Perú; SV, El Salvador; UY, Uruguay; VE, Venezuela.
3.1. Outpatient care
In total, 93.4% of respondents agreed with a first time in‐person evaluation for women with a histologically confirmed neoplasia or those with an adnexal mass suggestive of cancer. Over half of respondents (56.4%) considered that review of surgical pathology reports and further treatment decisions could be done by teleconference.
Almost all (94.6%) respondents agreed with teleconsultation (including email or phone calls) for follow‐up after cancer treatment. Symptoms of suspected recurrent disease can be queried and, if present, an in‐person evaluation would be necessary for physical examination and tests requested to help confirm recurrence. If no symptoms are present, 95.9% considered scheduling the next visit in 3 months.
Concerning teleconsultation, 93.6% considered that it should be done by the gynecologist oncologist. Twenty‐nine respondents (4.8%) suggested that other professionals including nurses, general practitioners, family doctors, gynecology residents, gynecologists, or oncologic gynecologist fellows could conduct teleconsultation. For in‐person consults, 94.6% of the respondents suggested performing a telemedicine “triage” to rule out COVID‐19 infection prior to the visit, according to current epidemiological criteria in their countries.
3.2. Preinvasive cervical pathology
For patients with cytology reporting a low‐grade lesion, 95.7% of respondents considered that colposcopic evaluation could be deferred for at least 6 months. For a cytologically suspected high‐grade epithelial lesion, 73.0% agreed to defer for 3 months. In the case of a biopsy with high‐grade cervical intraepithelial neoplasia (CIN II‐III), or adenocarcinoma in situ, without suspected infiltration, 64.4% of respondents agreed with delaying the excision for 3 months. If microinvasion is suspected, 82.6% considered it appropriate to delay excision for a maximum of 1 month. For discordance between the cytology/colposcopy/biopsy result or a positive endocervical curettage for CIN II–III, 69.2% of respondents agreed to delay conization for 3 months.
3.3. Cervical cancer
When respondents were asked if they would offer primary treatment with concurrent chemoradiation to postmenopausal patients (>50 years) with Stage IB1 cervical cancer (any macroscopic lesion or postconization positive margins) rather than offering surgical management, 58.9% disagreed. A total of 79.5% supported to defer definitive treatment by a minimum of 3 months for patients with cervical cancer with postconization negative margins or microscopic disease on biopsy (Stages IA1 and IA2), without lymph node involvement. Furthermore, the majority (93.9%) of respondents considered that it was not necessary to perform laparoscopic surgical staging in patients with locally advanced cancer before treatment with chemoradiotherapy. Finally, for central recurrences, most (74.9%) agreed with referring patients to chemotherapy treatment instead of exenterative procedures.
3.4. Ovarian cancer
The majority (95.6%) of respondents favored neoadjuvant chemotherapy (NACT) with 3–4 cycles of carboplatin–paclitaxel, followed by interval surgery according to response, in advanced stage epithelial ovarian cancer confirmed by pathology, obtained from cytology or percutaneous biopsy (rather than laparoscopic triage). In patients with platinum‐sensitive recurrence of more than one site, platinum‐based chemotherapy was preferred over secondary cytoreduction (90.3%). Regarding pelvic masses, 86.7% agreed that subspecialists should select patients and offer surgery with frozen section biopsy (when available) to those with a significant risk of malignancy according to images and tumor markers.
3.5. Endometrial cancer
Concerning early stage endometrial cancer, most respondents (85.4%) agreed on exclusive initial management with total hysterectomy and bilateral salpingo‐oophorectomy, with or without sentinel lymph node, and to define adjuvant treatment with uterine histopathological features. Moreover, for low‐risk early endometrial cancer (FIGO Stage I without myometrial invasion on magnetic resonance imaging), 69.8% believed it suitable to offer temporary hormonal management with the levonorgestrel intrauterine device (LIUD) or oral hormone therapy. In postsurgical grade 1 and 2 advanced Stage III endometrial cancer, 68.7% agreed that adjuvant radiotherapy could be administered before adjuvant chemotherapy. Finally, 89.8% of respondents considered it appropriate to offer hormonal management for endometrioid FIGO grade 1 and 2 relapse without acute symptoms, while medical treatment with chemotherapy or radiotherapy could be started after pandemic control.
4. DISCUSSION
The COVID‐19 pandemic has spread rapidly, with a consequent change in oncological care. To our knowledge, this is the first survey among specialists in Latin America to report acceptance rates for practice change in different clinical scenarios for gynecological cancers during the COVID‐19 pandemic.
Several scientific societies have given recommendations concerning treatment for patients diagnosed with or being followed‐up for gynecologic oncologic malignancies during the COVID‐19 pandemic. 6 , 10 , 30 In these publications, authors addressed similar topics that we also considered when designing our survey.
There was a noticeable agreement among clinicians to conduct follow‐up visits by teleconsultation methods, according to symptoms reported by patients, and if there is a suspicion of relapse, an in‐person appointment should be scheduled in order to perform a proper physical exam. Routine diagnostic imaging studies have a poor performance in absence of symptoms. 12 Postoperative pathology review appointments can be made either by teleconsultation or in person at the specialists’ preference.
Although the American Society for Colposcopy and Cervical Pathology (ASCCP) recently suggested to delay colposcopy or conization, 13 we observed distinct opinions for some questions. This could reflect not only the differences in the availability of cervical pathology units between countries, but also the idea that much of the population in Latin America has difficulties in accessing medical care in time. This means further delaying intervention, thus missing an opportunity for cervical cancer diagnosis and treatment.
Of all 22 questions, only one showed major disagreement (58.9%) (question 12, Table 2). Although radiotherapy is an alternative treatment for inoperable patients with early stage cervical cancer, 14 disagreement in changing primary surgery was perhaps due to greater knowledge of radiation’s adverse effects and also because the number of visits needed for completion of treatment implies greater exposure to COVID‐19 infection. In contrast, other societies suggested NACT as an option, 3 , 9 , 10 but as it was not a standard nor widely disseminated strategy, it was not considered in this survey.
Despite a greater agreement on every other question for cervical cancer, a non‐negligible difference between countries was noted for one question (question 15, Table 2). Exenterations require prolonged intensive care unit and hospital stay, 31 which may explain why for recurrent cervical cancer most respondents considered opting for chemotherapy, as some societies do. 9 However, half of the respondents in one country and nearly 25% of the whole cohort considered that surgery should be done, likely reflecting the urge to offer a salvage treatment that has shown 5‐year survival rates of over 40%. 31 This should be balanced by the high risk of severe or fatal COVID‐19 infection in this context and the potential resource expenditure.
For advanced ovarian cancer, administering NACT to patients with confirmed histologically advanced epithelial ovarian cancer was widely accepted. This has been recommended by others, 6 , 7 , 8 , 9 , 10 , 30 based on at least three prospective clinical trials that have shown similar oncological outcomes compared with upfront cytoreductive surgery in noninferiority analysis. 19 , 32
In endometrial cancer, temporary hormonal management for patients with grade 1 and 2 endometrioid neoplasms without suspected myometrial invasion is an option. 25 , 26 In our survey, it is worth mentioning that 30.2% disagreed with this recommendation. Reasons might include that straightforward surgical management is still preferred by some clinicians, that LIUD availability is still limited in some locations, and that there could be some concern regarding the use of a potential thrombogenic therapy that could worsen outcomes in patients who develop COVID‐19.
Adjuvant chemotherapy–radiotherapy for Stage III endometrial cancer is favored. 27 , 28 Nevertheless, postponing chemotherapy for grade 1 and 2 endometrioid cancers during the pandemic was proposed; not so in grade 3 or other high‐risk histologies where chemotherapy has shown a greater impact on disease‐specific survival. 33 However, respondents from some countries disagreed, probably due to concerns over occult disseminated micrometastatic disease and that many Latin American regions still have limited access to radiotherapy. Depending on the country, a case‐by‐case decision including clinical oncologists and radiotherapists is recommended.
Various aspects should be viewed with caution. Although Latin American gynecological cancer specialists have accepted alternative management strategies for their patients during the COVID‐19 pandemic, these can be changed according to the time frame of the pandemic in each hospital, region, or country. Respondents from 14 countries with different disease burdens participated in this survey, 1 each with their own health infrastructure, and hence with different adaptation strategies according to individual needs and resources. These results cannot be considered clinical practice guidance because they are based on opinions and the methodology is not robust enough to provide high‐level scientific evidence. There is no consensus on care for COVID‐positive patients. Finally, the oncological impact of delaying treatments or providing alternative management in the context of the pandemic is unknown.
The main strength of the present study is that it gathers the opinions of 610 surgical and gynecologic oncologists from 14 countries currently involved in gynecological cancer care in Latin America. The dissemination of the survey was coordinated by regional experts linked to scientific societies and local groups, with access to complete databases that allowed invitation of a high number of specialists.
We identified several limitations of this study. Although the 58.0% response rate is acceptable, a significant number of specialists did not participate. The survey included limited clinical scenarios selected by the authors in a nonsystematic manner, therefore other alternative options concerning gynecologic cancers may not have been considered, just as the use of minimally invasive surgery was not contemplated. The questionnaire was not validated, which could generate misinterpretation of the questions by the respondents. Finally, the type of questions (closed‐ended) can bias the answers and limit the opinions of the participants.
In conclusion, this survey from Latin America brings together the views of a large cohort of gynecologic and surgical oncologists on how to manage gynecologic cancer during the unique and challenging global health situation caused by the COVID‐19 pandemic. Despite some differences, there were similar acceptance rates for change in practice. It is recommended that, as in common practice, all treatment decisions must be thoroughly discussed with the patient and in multidisciplinary boards.
AUTHOR CONTRIBUTIONS
JR and RP contributed to the conception and design of the study. JR, RP, AF, RF, HS, DS, JB, EG, MP, GR, IR, and LT designed the survey, after reviewing the literature. RR and GB contributed to the translation of the survey into Portuguese. FH, RR, GB, AL, MM, JH, AB, JL, SC, JL, EE, AG, GH, DC, GM, LP, JS, FN, and DA contributed to the acquisition of data, coordinating the dissemination of the survey in each country. AF and JR performed the data analysis. JR, AF, RP, FH, RR, and GB contributed to writing the manuscript. All authors approved the final version of the manuscript for publication.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
Supporting information
Appendix S1. Questionnaire in Spanish. Available at following link: https://docs.google.com/forms/d/e/1FAIpQLSey2RTCxJMykOysVjG0J7zHwCkfKRXcNdZMyKq28POTErp1YA/viewform?vc=0&c=0&w=1.
Appendix S2. Questionnaire in Portuguese. Available at following link: https://docs.google.com/forms/d/e/1FAIpQLSetzKxwpa-AZNRyeDC2Y7BXY8pYN0tRI-VgElh9pCrOfK1cKw/viewform?vc=0&c=0&w=1.
Acknowledgments
We thank the following academic societies for their support in the local coordination and dissemination of the survey: Asociación Colombiana de Ginecólogos Oncólogos (ACGO); Sociedad Chilena de Ginecología Oncológica (SCHIGO); Brazilian Society of Surgical Oncology (BSSO); Sociedad Peruana de Ginecología Oncológica; Sociedad de Obstetricia y Ginecología de Venezuela (Sección Ginecología Oncológica); Sociedad Uruguaya de Ginecología Oncológica; Asociación de Profesionales Especialistas en Ginecooncológica ASPROESGI C R; Colegio Mexicano de Ginecólogos Oncólogos; Sociedad Boliviana de Cancerología; Sociedad Ecuatoriana de Oncología; Asociación Argentina de Ginecología Oncológica (AAGO); and Asociación Salvadoreña de Oncología.
REFERENCES
- 1. World Health Organization . Coronavirus disease (COVID‐2019) situation reports. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. Accessed May 9, 2020.
- 2. Burki TK. Cancer guidelines during the COVID‐19 pandemic. Lancet Oncol. 2020;2020:629–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pan American Health Organization . COVID‐19 Situation Reports‐PAHO. 2020. https://www.paho.org/en/tag/covid-19-situation-reports. Accessed May 9, 2020.
- 4. IARC-WHO. Cancer today . Population fact Sheets. 2020. https://gco.iarc.fr/today/data/factsheets/populations/904-latin-america-and-the-caribbean-fact-sheets.pdf. Accessed May 9, 2020.
- 5. Strasser‐Weippl K, Chavarri‐Guerra Y, Villarreal‐Garza C, et al. Progress and remaining challenges for cancer control in Latin America and the Caribbean. Lancet Oncol. 2015;16:1405–1438. [DOI] [PubMed] [Google Scholar]
- 6. Ramirez PT, Chiva L, Eriksson AGZ, et al. COVID‐19 global pandemic: Options for management of gynecologic cancers. Int J Gynecol Cancer. 2020;30:561–563. [DOI] [PubMed] [Google Scholar]
- 7. Society of Gynecologic Oncology . Gynecologic Oncology Considerations during the COVID‐19 Pandemic. Society of Gynecologic Oncology. 2020. https://www.sgo.org/clinical-practice/management/covid-19-resources-for-health-care-practitioners/gyn-onc-considerations-during-covid-19/. Accessed April 18, 2020.
- 8. European Society for Medical Oncology (ESMO) . Guidelines: Cancer patient management during the covid‐19 pandemic. 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic. Accessed April 24, 2020.
- 9. Brazilian Society of Surgical Oncology . Considerations on gynecologic cancer management during COVID‐19 pandemic. 2020. http://sbco.org.br/. Accessed April 25, 2020.
- 10. Sociedad Chilena de Ginecología Oncológica (SCHIGO) . Considerations to take into account in the management of the Chilean population that presents with cancers of gynecologic origin. https://www.facebook.com/schigo.cl/photos/pcb.2538641476402929/2538641179736292/?type=3&theater. Accessed April 30, 2020.
- 11. Xiao Y, Tan C, Duan J, Wu A,Li C. An effective model for the outpatient management of COVID‐19. Infect Control Hosp Epidemiol. 2020. 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salani R, Khanna N, Frimer M, et al. An update on post‐treatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncology (SGO) recommendations. Gynecol Oncol. 2017;146:3–10. [DOI] [PubMed] [Google Scholar]
- 13. ASCCP Interim Guidance for Timing of Diagnostic and Treatment Procedures for Patients with Abnormal Cervical Screening Tests. https://www.asccp.org/covid-19. Accessed April 1, 2020.
- 14. Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib‐IIa cervical cancer. Lancet. 1997;350:535–540. [DOI] [PubMed] [Google Scholar]
- 15. Ramirez PT, Pareja R, Rendón GJ, et al. Management of low‐risk early‐stage cervical cancer: Should conization, simple trachelectomy, or simple hysterectomy replace radical surgery as the new standard of care? Gynecol Oncol. 2014;132:254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Halaska MJ, Uzan C, Han SN, et al. Characteristics of patients with cervical cancer during pregnancy: a multicenter matched cohort study. An initiative from the International Network on Cancer, Infertility and Pregnancy. Int J Gynecol Cancer. 2019; 676–82. [DOI] [PubMed] [Google Scholar]
- 17. Ramirez PT, Pareja R, Eriksson AGZ, Frumovitz M. International Gynecologic Cancer Society 2019 meeting summary. International Journal of Gynecologic Cancer. 2020;30:167–173. [DOI] [PubMed] [Google Scholar]
- 18. Tewari KS, Sill MW, Long HJ 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vergote I, Coens C, Nankivell M, et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo‐ovarian cancers: Pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 2018;19:1680–1687. [DOI] [PubMed] [Google Scholar]
- 20. Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Timmerman D, Van Calster B, Testa A, et al. Predicting the risk of malignancy in adnexal masses based on the simple rules from the International Ovarian Tumor Analysis Group. Am J Obstet Gynecol. 2016;214:424–437. [DOI] [PubMed] [Google Scholar]
- 22. Andreotti RF, Timmerman D, Strachowski LM, et al. O‐RADS US risk stratification and management system: A consensus guideline from the ACR Ovarian‐Adnexal Reporting and Data System Committee. Radiology. 2020;294:168–185. [DOI] [PubMed] [Google Scholar]
- 23. NCCN . Clinical Practice Guidelines in Oncology (NCCN Guidelines). Uterine Neoplasms Version 1. 2020 – March 06, 2020. https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. Accessed April 1, 2020.
- 24. Ballester M, Bendifallah S, Daraï E. European guidelines (ESMO‐ESGO‐ESTRO consensus conference) for the management of endometrial cancer [in French]. Bull Cancer. 2017;104:1032–1038. [DOI] [PubMed] [Google Scholar]
- 25. Fan Z, Li H, Hu R, et al. Fertility‐preserving treatment in young women with grade 1 presumed stage IA endometrial adenocarcinoma: A meta‐analysis. Int J Gynecol Cancer. 2018;28:385–393. [DOI] [PubMed] [Google Scholar]
- 26. Baker WD, Pierce SR, Mills AM, et al. Nonoperative management of atypical endometrial hyperplasia and grade 1 endometrial cancer with the levonorgestrel intrauterine device in medically ill post‐menopausal women. Gynecol Oncol. 2017;146:34–38. [DOI] [PubMed] [Google Scholar]
- 27. Latham AH, Chen L, Hou JY, et al. Sequencing of therapy in women with stage III endometrial carcinoma receiving adjuvant combination chemotherapy and radiation. Gynecol Oncol. 2019;155:13–20. [DOI] [PubMed] [Google Scholar]
- 28. Goodman CR, Hatoum S, Seagle BL, et al. Association of chemotherapy and radiotherapy sequence with overall survival in locoregionally advanced endometrial cancer. Gynecol Oncol. 2019;153:41–48. [DOI] [PubMed] [Google Scholar]
- 29. Jerzak KJ, Duska L, MacKay HJ. Endocrine therapy in endometrial cancer: An old dog with new tricks. Gynecol Oncol. 2019;153:175–183. [DOI] [PubMed] [Google Scholar]
- 30. Pothuri B, Secord AA, Armstrong DK, et al. Anti‐cancer therapy and clinical trial considerations for gynecologic oncology patients during the COVID‐19 pandemic crisis. Gynecol Oncol. 2020;158:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fleisch MC, Pantke P, Beckmann MW, et al. Predictors for long‐term survival after interdisciplinary salvage surgery for advanced or recurrent gynecologic cancers. J Surg Oncol. 2007;95:476–484. [DOI] [PubMed] [Google Scholar]
- 32. Onda T, Satoh T, Ogawa G, et al. Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomised trial. Eur J Cancer. 2020;130:114–125. [DOI] [PubMed] [Google Scholar]
- 33. Chapman BV, Swanick CW, Ning MS, et al. Adjuvant combined‐modality therapy for Stage IIIC endometrioid and non‐endometrioid endometrial cancer. Gynecol Oncol. 2019;154:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Questionnaire in Spanish. Available at following link: https://docs.google.com/forms/d/e/1FAIpQLSey2RTCxJMykOysVjG0J7zHwCkfKRXcNdZMyKq28POTErp1YA/viewform?vc=0&c=0&w=1.
Appendix S2. Questionnaire in Portuguese. Available at following link: https://docs.google.com/forms/d/e/1FAIpQLSetzKxwpa-AZNRyeDC2Y7BXY8pYN0tRI-VgElh9pCrOfK1cKw/viewform?vc=0&c=0&w=1.


