Abstract
Objective
Data on the immune response to SARS‐CoV‐2 during pregnancy are lacking and the potential role and effect of SARS‐CoV‐2 vaccination in pregnancy is yet to be completely investigated.
Method
This is a cross‐sectional observational study wherein pregnant women were tested for SARS‐CoV‐2 immunoglobulin M and immunoglobulin G levels, irrespective of their infective status or presence or symptomatology.
Result
Of the 220 pregnant women tested, 160 (72.7%) were SARS‐CoV‐2 IgG positive, 37 (16.8%) were SARS‐CoV‐2 IgM positive and 27 (16.9%) were both IgG and IgM positive. The average antibody titer found was 10.49 BAU/ml (±14.0) and 0.6 (±0.55) for anti‐SARS‐CoV‐2 IgG and IgM non neutralizing antibodies respectively. ROC analysis for SARS‐CoV‐2 IgG positivity showed a cut‐off value of 1.19 with a sensitivity of 99.3% (0.99 AUC, 95% CI) and specificity of 98.3% (0.99 AUC, 95% CI), respectively. Similarly, ROC analysis for SARS‐CoV‐2 IgM positivity showed a cut‐off value of 1 with a sensitivity of 97.3% (0.99 AUC, 95% CI) and specificity of 98.9% (0.99 AUC, 95% CI), respectively.
Conclusion
First trimester sero‐molecular screening suggests a high prevalence of COVID antibodies in the study population of pregnant women in the first trimester, without the patients being symptomatic.
Keywords: COVID, first trimester, pandemic, pregnancy, SARS‐CoV‐2, seroprevalence, wave
1. INTRODUCTION
The World Health Organization was informed of a cluster of pneumonia cases of unknown origin in Wuhan City, China in December 2019. Since then, and as of 26th September 2021, about 33.6 million cases of COVID‐19 with 450 000 deaths have been reported in India, and Delhi recorded 1.4 million cases and about 26 000 deaths. 1 All age groups are susceptible to COVID‐19 infection; however, impact in pregnant women has drawn much attention because of the unique immunological state of pregnancy and the increased risk of respiratory infections. 2 , 3 Recent data from the United Kingdom has confirmed that pregnant women are at more risk of severe illness from SARS‐CoV‐2 infection, compared with non‐pregnant women. Furthermore, infection is associated with increased risk of stillbirth, growth restriction and preterm birth. 4
Data on the immune response to SARS‐CoV‐2 during pregnancy are lacking and the potential role and effect of SARS‐CoV‐2 vaccination in pregnancy is yet to be completely investigated. 5 The Indian Council of Medical Research has validated and approved IgG kits for SARS‐CoV‐2 to be used to conduct serosurveys in India. 6 Reports of cases of SARS‐CoV‐2 infection in pregnancy have been documented but are concentrated mainly in the second and third trimester of pregnancy. 7 , 8 , 9 , 10 However, viral infections can be harmful to the fetus during the first trimester of pregnancy as well; SARS‐CoV‐2 is one of these serious infections is creating concerns for obstetricians 11 , 12 , 13 and pregnant women. Screening pregnant women has gained importance because of the high proportion of asymptomatic cases and because of the increasing evidence of adverse maternal and fetal outcomes related to COVID‐19. 14 Data on the immune response to SARS‐CoV‐2 during pregnancy are lacking and the potential role and effect of SARS‐CoV‐2 vaccination in pregnancy is yet to be completely investigated. The aim of this study was to evaluate the seropositivity among pregnant women in their first trimester during the pandemic. This data will be further help, when the pregnancy outcomes are evaluated.
2. METHODS
We report epidemiologic data from a study investigating a cohort of women who became pregnant just before or during the COVID‐19 pandemic during the second peak, from April 2021 to August 2021. Ethical approval was given by the institutional ethical committee. 298 pregnant women in the first trimester (11–13 weeks of gestation) were recruited at the rural center of the All India Institute of Medical Sciences, New Delhi. Data on demographic characteristics and COVID‐19‐related symptoms were collected using a structured questionnaire. Patients were tested for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) immunoglobulin M and immunoglobulin G levels. Only asymptomatic women, who have not been diagnosed with COVID‐19 in the past 3 months, were recruited. Written, informed consent was obtained from all participants.
VIDAS® (Biomerieux) SARS‐CoV‐2 IgM (qualitative) and VIDAS® SARS‐CoV‐2 IgG II (semi‐quantitative) assay was used with automated VIDAS® system for detection of IgM and IgG respectively. Both are specific for the SARS‐CoV‐2 receptor binding domain of the spike protein in human serum which is based on the Enzyme‐linked fluorescent immunoassay (ELFA) technique.
Data analysis was carried out using STATA version 16.0. Quantitative variables were expressed as the mean ± standard deviation and qualitative categorical variables were expressed as frequency and percentages. Mean values of normally distributed data were compared using the Student's t‐ test Qualitative variables were compared using the χ 2 test or Fisher's exact test, as appropriate. To decide the cut‐off values of IgG and IgM markers for an optimum level of sensitivity and specificity, ROC analysis was carried out. Area under curve (AUC) with 95% was presented. A two‐sided probability of P < 0.05 was considered to be statistically significant.
3. RESULTS
A total of 298 women in the first trimester of pregnancy (11–13 weeks of pregnancy), were included in the study. Participants had an average age of 24.0 ± 4.1 years and a body‐mass index of 22.51 ± 4.3 kg/m2. Of the 298 women, 94 (31.5%) were primigravidae, 61 (20.5%) had given birth once, 143 (47.9%) had been pregnant more than once. All women were homemakers, and none were smokers. One woman (0.3%) had essential hypertension. No women had associated medical disorders like type 1 or type 2 diabetes mellitus, chronic kidney disease or any other autoimmune disease. Other demographic details are presented in Table 1.
TABLE 1.
Baseline characteristics in the study population
| Characteristics | IgG positive (n = 160) | IgG negative (n = 60) | P value | IgM positive (n = 38) | IgM negative (n = 182) | P value |
|---|---|---|---|---|---|---|
| Mean age (in years) | 24.27 | 23.28 | 0.10 | 23.97 | 24.14 | 0.82 |
| Mean gestation (in weeks) | 13.3 | 13.6 | 0.61 | 13.3 | 14.04 | 0.37 |
| BMI (kg/m2) | 22.44 | 22.54 | 0.95 | 22.8 | 20.8 | 0.30 |
| Multiparity | 147 | 53 | 0.56 | 33 | 176 | 0.28 |
Pregnant women were asked questions regarding symptoms related to Covid‐19 infection during their first trimester. Symptom profile showed that 31 (10.4%) had fever, 12 (4%) had coughs, eight (2.7%) had shortness of breath, three (1%) experienced headache, two (0.9%) had lethargy and one (0.3%) experienced vomiting during their first trimester. None had joint pains, loss of smell/taste, rhinorrhea or diarrhea. Nasopharyngeal and throat swabs for COVID‐19 RT PCR for five symptomatic women (who presented with current symptoms and not just history of symptoms in the first trimester) included in study were negative. None had exposure to a case of Covid‐19 infection at home, in community settings or in hospital, nor did anyone have a history of traveling to an abroad destination. Of the 298 women eligible women who were recruited, 78 were unwilling to participate in serological prevalence study. Around 20% of these women had symptoms suggestive of COVID. As shown in Tables 2 and 3, the presence or absence of symptomatology in their first trimester is not related to IgG or IgM positivity.
TABLE 2.
Correlation of symptomatology with IgG positivity
| IgG positive (%) | IgG negative | Exact significance (two‐sided) | |
|---|---|---|---|
| Symptoms present | 33 (20.6%) | 13 (21.7%) | 0.854 |
| Symptoms absent | 127 (79.3%) | 47 (78.3%) | |
| Total | 160 | 60 |
TABLE 3.
Correlation of Symptomatology with IgM positivity
| IgM positive (%) | IgM negative | Exact significance (two‐sided) | |
|---|---|---|---|
| Symptoms present | 5 (13.5%) | 41 (22.4%) | 0.273 |
| Symptoms absent | 32 (86.5%) | 142 (77.6%) | |
| Total | 37 | 183 |
Of the 220 patients tested for IgG and IgM, 160 (72.7%; 95% CI: 66.8–78.6%) were SARS‐CoV‐2 IgG positive, 37 (16.8%; 95% CI: 11.8–21.8%) were SARS‐CoV‐2 IgM positive and 27 (16.9%; 95% CI: 7.9–1.6%) were both IgG and IgM positive. The temporal association of the antibodies prevalence in shown in Figure 1. The average (Sd) antibody titer found was 10.49 BAU/ml (±14.0) and 0.6 (±0.55) for anti‐SARS‐CoV‐2 IgG and IgM non neutralizing antibodies, respectively. ROC analysis for SARS‐CoV‐2 IgG positivity showed a cut‐off value of 1.19 with a sensitivity of 99.3% (0.9949 AUC, 95% CI) and specificity of 98.3% (0.9949 AUC, 95% CI) respectively (Figure 2). Similarly, ROC analysis for SARS‐CoV‐2 IgM positivity showed a cut‐off value of 1 with a sensitivity of 97.3% (0.9935 AUC, 95% CI) and specificity of 98.9% (0.9935 AUC, 95% CI) (Figure 3). ROC analysis for SARS‐CoV‐2 IgG positivity showed a cut‐off value of 1.19 with a sensitivity of 99.3% and specificity of 98.3% contributing AUC with 0.995. Similarly, ROC analysis for SARS‐CoV‐2 IgM positivity showed a cut‐off value of 1 with a sensitivity of 97.3% and specificity of 98.9% yielding AUC with 0.993. Even though the IgG and IgM positivity was determined based on manufacturer cut‐off value, the cut‐off value derived from the data may have implications for the Indian population to correctly classify the true positivity and the true negativity.
FIGURE 1.
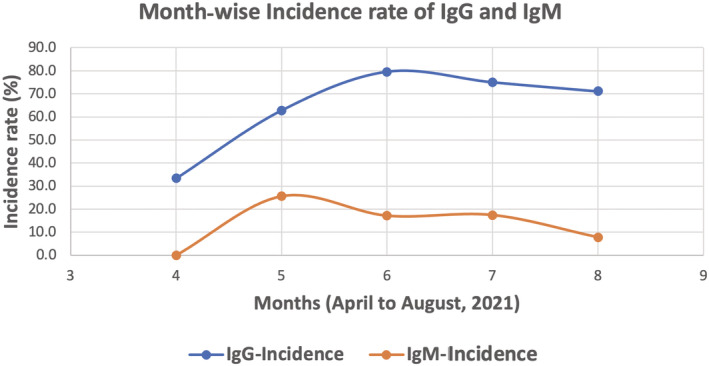
Distribution of IgG and IgM levels in pregnant women in their first trimester, during the second wave of the COVID‐19 pandemic in Delhi, India
FIGURE 2.
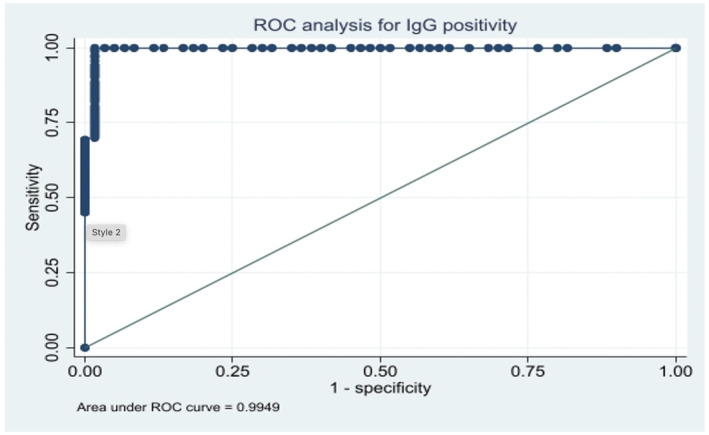
ROC analysis for serum IgG levels among pregnant women in their first trimester
FIGURE 3.
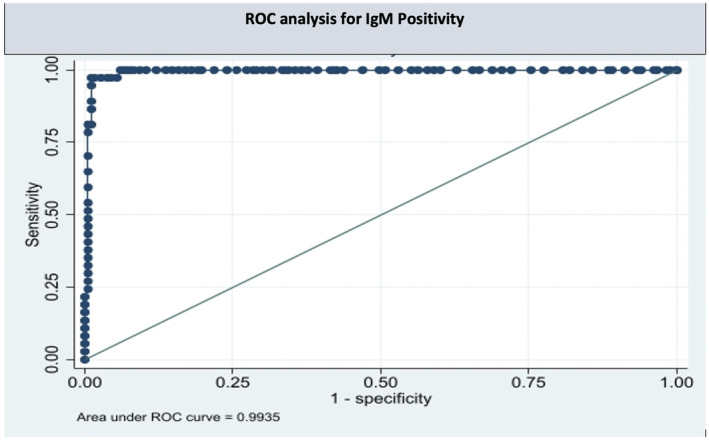
ROC analysis for serum IgM levels among pregnant women in their first trimester
4. DISCUSSION
4.1. Principal findings
In this study of 220 patients, 160 (72.7%; 95% CI: 66.8–78.6%) were SARS‐CoV‐2 IgG positive, 37 (16.8%; 95% CI: 11.8–21.8%) were SARS‐CoV‐2 IgM positive and 27 (16.9%; 95% CI: 7.9–1.6%) were both IgG and IgM positive.
4.2. Results
A study evaluated the progression of seroprevalance of COVID antibodies in pregnant population of the south of Madrid, Spain, during the first wave of the COVID‐19 pandemic. They reported that seropositivity increased from 0% to 21.4% (95% CI 11.8–31.0) during the study period, of which 27.9% had an asymptomatic course. They tested 769 serum samples during the first and third trimesters of pregnancy for specific IgG anti SARS‐CoV‐2 RBD and S proteins. 17 In another study from New York City, 19 out of 47 (40.4%) women tested positive for antibodies. 18 Of the 19 women with antibodies detected, three noted symptoms of COVID‐19 prior to enrollment and four developed symptoms after study enrollment. Our study showed a high prevalence of 72.7% of IgG antibodies in the study population, as the data was collected during the second peak of pandemic. The ICMR data during this time period also showed similar seropositivity in general population. 6
4.3. Clinical implications
The present work highlights the crucial role of serum antibodies for early diagnosis of SARS‐CoV‐2 among asymptomatic pregnant patients. The specificity of real‐time reverse transcription polymerase chain reaction (RT‐PCR) for the detection of COVID‐19 is remarkable, but its accuracy depends on the sampling quality. 15 Advantages of testing pregnant women for antibody response to COVID‐19 are bring able to identify possibly “healed” women (e.g., IgG positive) who were never tested with RT‐PCR assay using nasopharyngeal (NP) swab specimens and to also detect women who are still at risk for COVID‐19 infection (e.g. IgM and IgG negative). Women who do not know their infective status represent a potential threat to others, including healthcare workers (HCWs) and other patients. Antibodies to SARS‐CoV‐2 could serve as the basis for an “immunity passport” or “risk‐free certificate” (digital or physical documents that certify an individual has been infected and is purportedly immune to SARS‐CoV‐2). 16 This statement is yet not verified. Also, while evaluating the effect of COVID on pregnancy outcomes, the antibody evaluation might be useful. However, as seen from the data analysis, there was a high prevalence of COVID‐like symptoms in seronegative women and vice‐versa; that is say, no symptoms in women with positive IgG or IgM antibodies (Tables 2, 3).
4.4. Research implications
According to the Indian Council of Medical Research, IgG antibody test for COVID‐19 may be useful in serosurveys among asymptomatic individuals and the high‐risk or vulnerable population to understand the proportion of population exposed to infection with SARS‐CoV2 and thus, appropriate public health interventions for prevention and control of disease can be planned and implemented accordingly. 6 As our study clearly shows a high percentage of seropositivity in asymptomatic women, any research on maternal and neonatal outcomes, only on the basis of nasopharyngeal or oral testing in symptomatic women, may be flawed.
4.5. Strengths and limitations
This study may serve as a basic framework to detect vertical transmission of SARS‐CoV‐2 from mothers to fetuses and later to detect neonatal outcomes. A further follow‐up of these pregnant woman may enlighten with the impact of COVID seropositivity on materno‐fetal outcomes, which our study is currently lacking.
5. CONCLUSION
We report epidemiologic data from this study investigating a cohort of women who became pregnant just before or during the COVID‐19 pandemic during the second peak. First trimester seromolecular screening suggests a high prevalence of COVID antibodies in the study population of pregnant women in the first trimester during the COVID‐19 wave. Thus, this fact needs to be taken into account when evaluating the effect of COVID‐19 on pregnancy.
AUTHOR CONTRIBUTIONS
KAS: Planning of study, conception of idea, data compilation, manuscript drafting. NS: Data compilation, manuscript preparation, data Collection. SH: Planning of study, conception of idea, final manuscript drafting. PM: Data collection, data analysis, final manuscript drafting. KY: Data collection, final manuscript drafting. AG: Data collection, manuscript preparation. VD: Planning of study, conception of idea, final manuscript drafting. NB: Planning of study, final manuscript drafting.
CONFLICTS OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Sharma KA, Singh N, Hillman S, et al. Seroprevalence of SARS‐CoV‐2 antibodies among first‐trimester pregnant women during the second wave of the pandemic in India. Int J Gynecol Obstet. 2022;00:1–5. doi: 10.1002/ijgo.14189
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Johns Hopkins Coronavirus Resource Center . Jhu.edu. [cited 2021 Oct 11]. Available from: https://coronavirus.jhu.edu/map.html.
- 2. Cosma S, Borella F, Carosso A, et al. The “scar” of a pandemic: cumulative incidence of COVID‐19 during the first trimester of pregnancy. J Med Virol. 2021;93(1):537‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cosma S, Carosso AR, Cusato J, et al. Coronavirus disease 2019 and first‐trimester spontaneous abortion: a case‐control study of 225 pregnant patients. Am J Obstet Gynecol. 2021;224(4):391.e1‐391.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. RCOG Guidelines on coronavirus (COVID) infection in pregnancy. https://www.rcog.org.uk/globalassets/documents/guidelines/2021‐08‐25‐coronavirus‐covid‐19‐infection‐in‐pregnancy‐v14.pdf.
- 5. Benedetto C, Carosso A, Corezzi M, Zotti CM. EBCOG EBCOG position statement: vaccination in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2019;240:375‐376. doi: 10.1016/j.ejogrb.2019.04.022 [DOI] [PubMed] [Google Scholar]
- 6. Gov.in . [cited 2021 Oct 11]. Available from: https://www.icmr.gov.in/pdf/covid/strategy/New_additional_Advisory_23062020_3
- 7. Jiao J. Under the epidemic situation of COVID‐19, should special attention to pregnant women be given? J Med Virol. 2020;92(9):1371‐1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carosso A, Cosma S, Borella F, et al. Pre‐labor anorectal swab for SARS‐CoV‐2 in COVID‐19 patients: is it time to think about it? Eur J Obstet Gynecol Reprod Biol. 2020. Apr 11;249:98‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen S, Liao E, Cao D, Gao Y, Sun G, Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol. 2020;92(9):1556‐1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223(1):111‐e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrazzi E, Frigerio L, Savasi V, et al. Vaginal delivery in SARS‐CoV‐2‐infected pregnant women in northern Italy: a retrospective analysis. BJOG. 2020;127(9):1116‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liao J, He X, Gong Q, Yang L, Zhou C, Li J. Analysis of vaginal delivery outcomes among pregnant women in Wuhan, China during the COVID‐19 pandemic. Int J Gynaecol Obstet. 2020;150(1):53‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breslin N, Baptiste C, Gyamfi‐Bannerman C, et al. COVID‐19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020;2(2):100118. doi: 10.1016/j.ajogmf.2020.100118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ali A, Rashid Z, Zhou J, et al. Evaluating antibody response pattern in asymptomatic virus infected pregnant females: human well‐being study. J King Saud Univ Sci. 2021;33(1):101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zullo F, Di Mascio D, Saccone G. Coronavirus disease 2019 antibody testing in pregnancy. Am J Obstet Gynecol MFM. 2020;2(3):100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Villalaín C, Herraiz I, Luczkowiak J, et al. Seroprevalence analysis of SARS‐CoV‐2 in pregnant women along the first pandemic outbreak and perinatal outcome. PLoS ONE. 2020;15(11):e0243029. doi: 10.1371/journal.pone.0243029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baptiste C, Breslin N, Chong A, et al. 919 rates of seroprevalance of COVID‐19 among pregnant patients in New York City. Am J Obstet Gynecol. 2021;224(2):S571. doi: 10.1016/j.ajog.2020.12.944 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


