Evidence of SARS‐CoV‐2 vertical transmission is scarce. At present, the influence of SARS‐CoV‐2 as a cause of late pregnancy loss is unclear. Increases in stillbirth rates have been reported during the pandemic 1 ; however, only a few cases of late pregnancy loss were deemed to be related to SARS‐CoV‐2 infection. 2 , 3 The present study sought to assess whether SARS‐CoV‐2 vertical transmission is a contributing factor to late pregnancy loss.
A cross‐sectional study of pregnant women with SARS‐CoV‐2 infection, confirmed by polymerase chain reaction (PCR) test, compared patients presenting with intrauterine fetal death (IUFD; study group) to patients bearing live infants (control group). In the study group, in addition to placental histopathology, placental and fetal samples were tested for SARS‐CoV‐2. In the control group, SARS‐CoV‐2 PCR testing was completed by testing the umbilical cord and maternal blood, placental membrane, and neonatal nasopharyngeal swabs.
Four cases of late pregnancy loss were identified, with two cases of stillbirth (25 and 35 weeks of gestation) and two cases of late missed abortion (17 and 18 weeks of gestation). There were eleven patients in the control group. No significant difference was found between the two groups in rate of COVID‐19 symptoms, and no patients had severe COVID‐19 infection. Additionally, there were no cases of diabetes or hypertensive disorders and there was no evidence for vertical transmission in the control group.
The diagnosis of pregnancy loss was made within days of COVID‐19 diagnosis. In one of these four cases, a SARS‐CoV‐2 infection of the placenta was established by PCR testing—this was a case of IUFD at 25 weeks of gestation. The patient had mild respiratory symptoms with no fever 3 days prior. While maternal nasopharyngeal samples yielded high cycle threshold (Ct) values (29.4 for E‐gene and 31.6 for N‐gene), placental samples were positive with an average Ct value of 20, which represents a higher viral load. The higher viral load measured by placental samples was suggestive of SARS‐CoV‐2 vertical transmission. SARS‐CoV‐2 genome sequencing of this case identified the B.1.1.7 (alpha) variant. Placental pathology examinations revealed significant findings of diffuse trophoblastic damage with intervillositis (Figure 1). These findings are in alignment with a recent study by Garrido‐Pontnou et al. 4 which suggested that the hallmark of SARS‐CoV‐2 placental infection is trophoblastic damage.
FIGURE 1.
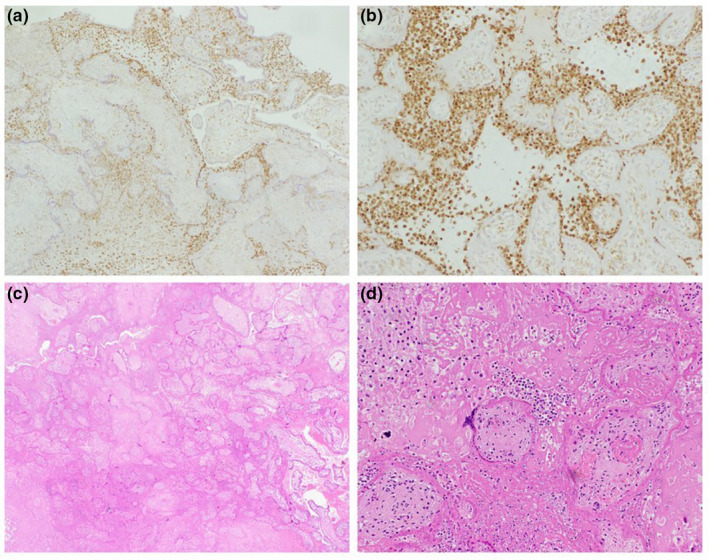
Placental pathology case of IUFD at 25 weeks of gestation depicting perivillous fibrin deposition and villous agglutination. A positive CD68 immunostain in histiocytes at ×4 magnification (a) and ×20 magnification (b). H&E at x4 (c) and x20 magnification (d).
In conclusion, the combination of placental pathology and high placental SARS‐CoV‐2 viral load, and the normal anatomic fetal scan a week prior, was highly indicative of intrauterine SARS‐CoV‐2 infection as a cause for IUFD in one of our patients. The present study's case also adds information to the current literature around Ct values indicating vertical transmission, as well as SARS‐CoV‐2 genome sequencing identifying the B.1.1.7 (alpha) variant.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
YGP, EB, TB‐N, DG, MS, and JT were responsible for the study conception and design. SS, TBV, NS, TK, AS, MM, and MP contributed to data collection. EB, YGP, TB‐N, DG, NS, TK, AS, MM, MP, MS, ON, and JT were responsible for the analysis of data and interpretation of results. YGP, TB‐N and EB were responsible for drafting the manuscript. All authors reviewed the results, and contributed to and approved of the final version of the manuscript.
Ganor Paz Y, Shiloh S, Brosh‐Nissimov T, et al. The association between SARS‐CoV‐2 infection and late pregnancy loss. Int J Gynecol Obstet. 2022;157:208–209. doi: 10.1002/ijgo.14025
REFERENCES
- 1. Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O’Brien P, Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID‐19 pandemic. JAMA. 2020;324(7):705‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richtmann R, Torloni MR, Oyamada Otani AR, et al. Fetal deaths in pregnancies with SARS‐CoV‐2 infection in Brazil: a case series. Case Rep Women’s Health. 2020;12(27):e00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baud D, Greub G, Favre G, et al. Second‐trimester miscarriage in a pregnant woman with SARS‐CoV‐2 infection. JAMA. 2020;323(21):2198‐2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garrido‐Pontnou M, Navarro A, Camacho J, et al. Diffuse trophoblast damage is the hallmark of SARS‐CoV‐2‐ associated fetal demise. Mod Pathol. 2021;34:1704‐1709. [DOI] [PMC free article] [PubMed] [Google Scholar]


