Abstract
The severity of the COVID‐19 pandemic and subsequent mitigation strategies have varied across the Nordic countries. In a joint Nordic population‐based effort, we compared patterns of new cancer cases and notifications between the Nordic countries during 2020. We used pathology notifications to cancer registries in Denmark, the Faroe Islands, Finland, Iceland, Norway and Sweden to determine monthly numbers of pathology notifications of malignant and in situ tumours from January to December 2020 compared to 2019 (2017‐2019 for Iceland and the Faroe Islands). We compared new cancer cases per month based on unique individuals with pathology notifications. In April and May 2020, the numbers of new malignant cases declined in all Nordic countries, except the Faroe Islands, compared to previous year(s). The largest reduction was observed in Sweden (May: −31.2%, 95% CI −33.9, −28.3), followed by significant declines in Finland, Denmark and Norway, and a nonsignificant decline in Iceland. In Denmark, Norway, Sweden and Finland the reporting rates during the second half of 2020 rose to almost the same level as in 2019. However, in Sweden and Finland, the increase did not compensate for the spring decline (annual reduction −6.2% and −3.6%, respectively). Overall, similar patterns were observed for in situ tumours. The COVID‐19 pandemic led to a decline in rates of new cancer cases in Sweden, Finland, Denmark and Norway, with the most pronounced reduction in Sweden. Possible explanations include the severity of the pandemic, temporary halting of screening activities and changes in healthcare seeking behaviour.
Keywords: cancer, COVID‐19, incidence, Nordic, SARS‐CoV‐2
What's new?
The severity of the COVID‐19 pandemic and subsequent mitigation strategies have varied across the Nordic countries. This is the first international comparison of cancer notification rates during the first year of the COVID‐19 pandemic, including six countries with similar tax‐funded healthcare systems and population‐based cancer reporting. The findings suggest that, despite differences in pandemic mitigation efforts, the severity of the pandemic may have had a larger effect on cancer detection than strict societal restrictions. In all countries, it will be of importance to monitor future trends in late‐stage cancer incidence and survival.
Abbreviations
- CI
confidence Interval
- COVID‐19
coronavirus disease 2019
- ICD‐O
International Classification of Diseases for Oncology
- NORPAT
Norwegian Pathology codes
- PC
percentage change
- RR
relative rate
- SNOMED
Systematized Nomenclature of Medicine
1. INTRODUCTION
The COVID‐19 pandemic has had profound global impacts on public health and delivery of healthcare, including reports of fewer patients seeking care for life‐threatening conditions. 1 For cancer, there have been reports of declines in number of newly diagnosed cases during the pandemic, patterns that are likely to reflect postponement of seeking healthcare, lower diagnostic activity and lower attendance in outreach screening programmes. 2 , 3 , 4 Several countries temporarily halted screening activities in the first half of 2020, with effects on rates of newly diagnosed cancer cases. 2 , 5 , 6 , 7 , 8 , 9
Pandemic mitigation efforts, including bans on large gatherings and closing of schools, workplaces and restaurants, were implemented to varying degrees in all Nordic countries (Denmark, Norway, Sweden, Finland, Iceland and the Faroe Islands) in March 2020 following the first reported COVID‐19 cases (Table S1). In Sweden, restrictions were mainly based on recommendations and individual responsibility without full societal lock‐down, with schools remaining open for children under 17, elderly over 70 strongly recommended to shield and with employers and employees adhering to work‐from‐home policies. Contrary, in the other Nordic countries there were stricter assembly bans and societal lock‐downs, including closing of schools and workplaces from March. All countries implemented strict domestic and international travel restrictions, including between the Nordic countries. From June to August 2020, restrictions were lifted gradually as the community transmission of COVID‐19 declined in all countries, with Iceland and the Faroe Islands reporting no confirmed COVID‐19 cases during some periods. However, in August, the COVID‐19 rates increased again in the Faroe Islands, and also in the other countries in September and October. During this second surge, restrictions were resumed across all Nordic countries, albeit with larger regional variations, and remained in effect at the end of 2020. In Sweden, these restrictions were again based on recommendations, and in Denmark the restrictions were gradually implemented with schools, shops and restaurants not closing until late December 2020. By the end of December 2020, the COVID‐19 incidence soared in Sweden and Denmark, while it was lower in the other countries.
Across the Nordic countries, there were marked differences in COVID‐19 incidence, hospitalisations and deaths during 2020 (Table S2). Although testing strategies differed between the countries, Sweden had markedly higher rates of infection, hospitalisation and mortality than the other Nordic countries. By the end of 2020, more than 9800 COVID‐19 related deaths were reported in Sweden corresponding to a mortality rate of 97.2 per 100 000 compared to 22.4 (Denmark), 11.4 (Finland), 8.5 (Norway), 8.5 (Iceland) and 1.7 (the Faroe Islands) (Table S2).
The Nordic region has a combined population of 27.2 million with an annual number of new cancer cases of 179 166 in 2019 and the five most common cancers being prostate, breast, colorectal, hematologic and lung cancer (Table 1). All Nordic countries have similar tax‐funded healthcare systems, with universal access to care for all residents, including national screening programmes for breast and cervical cancer (and colorectal cancer in Denmark, the Faroe Islands and regionally in Sweden). During March‐April 2020, screening activities were fully or partly halted due to impact of the pandemic in Norway, Sweden and Iceland, while they remained operational although with lower attendance rates in Denmark and Finland. The Faroe Islands reported no reductions in either screening activities or attendance during 2020.
TABLE 1.
Population size, most common and annual number of cancer cases and screening programmes in the Nordic countries
| Denmark | Norway | Sweden | Finland | Iceland | Faroe Islands | |
|---|---|---|---|---|---|---|
| Population size a | 5 792 203 | 5 421 242 | 10 099 270 | 5 540 718 | 341 250 | 52 305 |
| Annual new cancer cases b | 44 349 | 35 228 | 62 541 | 35 038 | 1774 | 236 |
| Top 5 common cancer types b | Breast, prostate, lung, colorectal, hematologic malignancies | Prostate, breast, colorectal, lung, hematologic malignancies | Prostate, breast, colorectal, hematologic malignancies, skin melanoma | Prostate, breast, colorectal, hematologic malignancies, lung | Breast, prostate, hematologic malignancies, colorectal, lung |
Colorectal, prostate, breast, lung, hematologic malignancies |
| National Screening programmes, age range c |
Breast 50‐69 Cervical 23‐64 Colorectal 50‐74 |
Breast 50‐69 Cervical 25‐69 |
Breast 40‐74 Cervical 23‐64 Colorectal 60‐69 d |
Breast 50‐69 Cervical 30‐60 |
Breast 40‐69 Cervical 23‐64 |
Breast 50‐69 Cervical 23‐64 Colorectal 50‐74 |
| COVID‐19 impact on screening in 2020 | Screening was never halted or reduced, although attendance was lower | Screening was halted in March, resumed in June, fully operational in Aug | Screening was halted in some regions in March, resumed in May with regional variations, delays throughout 2020 mainly in metropolitan regions | No national restrictions on cancer screening, but some municipalities temporarily suspended screening programmes | Screening was halted in some regions in March, resumed in May | Screening was never halted or reduced |
Total population numbers 2020 from GLOBOCAN (https://gco.iarc.fr). Accessed June 15, 2021.
New cancer cases 2019 from NORDCAN, 2019 (https://nordcan.iarc.fr). Accessed January 23, 2022.
Lundberg et al, Trends in cancer survival in the Nordic countries 1990‐2016: the NORDCAN survival studies. Acta Oncol 2020;59 (11):1266‐1274.
Only a few Swedish regions have implemented colorectal cancer screening, national implementation is ongoing.
Since the pandemic has had profound affects across all levels of healthcare, we aimed to assess both the impact on diagnostic activity and new cancer cases using data from pathology notifications. The aim of our study was therefore to compare patterns of cancer notifications and new cases during 2020, the first year of the COVID‐19 pandemic, across the Nordic countries that experienced marked differences in COVID‐19 infection rates and related deaths. For this purpose, we took advantage of similar systems for collection of high quality population‐based cancer registry data in the Nordic region. 10
2. MATERIALS AND METHODS
In this population‐based study, we used pathology notifications to assess patterns of diagnostic activity and new cancer cases during 2020 in the Nordic countries. For this purpose, we used two separate measures: the “number of notifications” as an indicator of diagnostic activity and the “number of new cases” as an indicator of incidence. It should be noted that a case definition based on pathology notifications is not the same as the definition used in official cancer incidence statistics that is based on additional notifications other than pathology and includes additional quality assurance. 10 Hence, in the present study the ‘number of new cases’ represents a proxy for cancer incidence.
In all Nordic countries, the law mandates notification of cancer to the national cancer registries. Cancer notifications originate from several clinical sources of which pathology notifications represent the most rapid data source reaching the cancer registries. 10 The pathology notifications are based on cytology (including fine needle biopsy and other malignant cytology), biopsies (including core biopsy, true cut and excisions) and surgery.
We included pathology notifications to the Nordic national cancer registries covering 100% of the population in Denmark, the Faroe Islands, Finland, Iceland and Norway from January 1, 2020 until December 31, 2020. For Sweden, where national cancer registration is decentralised to six Regional Cancer Centers, we included pathology notifications from 15 out of 31 laboratories with electronic reporting (covering approximately 55% of population), and pathological and/or clinical notifications (to cover 100% of population) to estimate new cancer cases during 2020 (Table S3). The comparison period was from January until December 2019 for all countries except for Iceland and the Faroe Islands where average numbers across 2017 to 2019 were used to reduce random variation due to low numbers.
During diagnostic work‐up, one cancer case may generate several pathology notifications. We used two separate counts of pathology notifications to describe the diagnostic activity and the number of new cases (Table S3). First, the “number of notifications” per month was counted as all pathology notifications in a given month (regardless of previous history of cancer or previous notifications in these individuals). This number included more notifications than new cases and can be viewed as a measure of the diagnostic activity and work‐up intensity. Second, the “number of new cases” per month was counted as all unique cases based on one or more pathology notifications per individual in a given month, that is, if there were multiple notifications related to the same case, only the first was counted. For Sweden, the “number of new cases” was based on pathology and/or clinical reports. For the “number of new cases,” we excluded cases with any malignant cancer notification within 5 years prior to the notification in 2020 or 2019 (2017‐2019 for Iceland and Faroe Islands), except for Finland where cases with any previous pathology notification within 2 years prior were excluded due to technical changes made in the pathology notification database in 2017. For in situ cases, we excluded cases with a previous notification of in situ or malignant diagnosis within 5 years.
We used International Classification of Diseases for Oncology third edition (ICD‐O‐3) or Systematized Nomenclature of Medicine (SNOMED)/Norwegian Pathology codes (NORPAT) to define the diagnosis in the pathology notifications (Table S3). Malignant diagnoses were defined according to ICD‐O‐3 as any topography (ICD‐O‐3: C00.0‐C80.9) and morphology (ICD‐O‐3: fifth digit code 3, 6 or 9), excluding basal cell carcinoma (morphology codes: 80901‐80983), and according to SNOMED/NORPAT as any T code and M codes M80000‐M99999 with last digit 3‐9, excluding basal cell carcinoma (M80901‐M80999). In situ diagnoses were defined according to ICD‐O‐3 as any topography (ICD‐O‐3: C00.0‐C80.9) and morphology (ICD‐O‐3: fifth digit code 2), and according to SNOMED/NORPAT as any T code and M codes M80000‐M99999 with last digit 2. In case of multiple diagnoses on the same notification, an invasive diagnosis had priority over an in situ diagnosis. In situ diagnoses for Finland were not used due to a major increase in coverage of in situ reporting during the study period.
2.1. Statistical methods
Monthly numbers of new cases and notifications were compared between 2020 and 2019 (for Iceland and the Faroe Islands the comparison period was 2017‐2019) for malignant and in situ diagnoses. For the Faroe Islands, bimonthly intervals were used to reduce random variation. Percentage change within each country was calculated as the difference in monthly numbers (new cases, notifications) between 2020 and 2019 divided by the corresponding monthly numbers in 2019 (for Iceland and the Faroe Islands compared to the average monthly numbers 2017‐2019). Ninety‐five percent confidence intervals (CI) for the percentage change were estimated from a Poisson regression model for the number of new cases (notifications) for each month as outcome and including year and country as covariates (and their interaction), where the resulting rate ratio (RR) is a direct transformation of the percentage change (percentage change = [RR − 1] * 100) assuming same population size at risk for 2020 and 2019 (for Iceland and the Faroe Islands the population at risk was assumed the same 2017‐2020). This is a reasonable assumption given that we only studied two (four) consecutive years. The monthly percentage changes across countries were compared to a likelihood ratio test of the models with and without the interaction of year and country. The Faroe Islands were excluded from the tests due to bimonthly intervals. Cumulative percentage change in 2020 within each country was calculated as the difference in monthly cumulative numbers (new cases, notifications) between 2020 and 2019 divided by the corresponding total number at the end of 2019 (for Iceland and the Faroe Islands compared to average monthly cumulative numbers 2017‐2019 and average total numbers at end of 2017‐2019). Thus, the estimated cumulative percentage change represents the change in 2020 standardised to the expected number in a reference year (2019 or 2017‐2019) and is a measure of the cumulative deviation at each month from a year unaffected by the pandemic. Total annual numbers (new cases, notifications) during 2020 and the comparison year(s) were calculated by sex and age groups (18‐49, 50‐69, 70+ years), and the differences in absolute numbers are presented. Age groups were selected to broadly reflect screening ages and high risk groups for COVID‐19.
Data were compiled and aggregated in each country, and anonymous tabulated data were analysed in Sweden. The statistical analysis was performed in Stata 17.0/BE (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC).
3. RESULTS
In all Nordic countries, except for Iceland and the Faroe Islands, there was a significant reduction in both the number of new malignant cases and notifications to the cancer registries in April and May 2020 (Table 2, Figure 1). The largest decline was observed in Sweden (malignant cases April: −25.0% and May: −31.2%) followed by Finland (April: −11.3% and May: −24.2%), while the reductions in Denmark and Norway were around 15% in both months (Denmark April: −13.6%, May: −15.7%, Norway April: −15.3%, May: −14.3%). In Iceland, there were nonsignificant reductions of −13.4% (Apr) and −17.2% (May), while there was no reduction in the Faroe Islands. In Iceland, there was an indication of a rebound in numbers during the second half of 2020 that largely compensated for the deficit earlier in the year, while the recovery in Denmark, Norway, Sweden and Finland was less pronounced. Numbers in the Faroe Islands were stable between June and August followed by a nonsignificant decline in number of cases and notifications between September and December coinciding with a rise of COVID‐19 cases. For in situ diagnoses, reported new cases and notifications from March and onwards largely followed the pattern for malignant diagnoses. In Sweden, the reduction in in situ reporting was more pronounced than that for malignant reporting during the second half of 2020.
TABLE 2.
Monthly percentage change of new cases and pathology notifications to the Nordic cancer registries, comparing 2020 vs 2019
| Denmark | Norway | Sweden a | Finland | Iceland b | Faroes c | ||
|---|---|---|---|---|---|---|---|
| Perc. change (95% CI) | Perc. change (95% CI) | Perc. change (95% CI) | Perc. change (95% CI) | Perc. change (95% CI) | Perc. change (95% CI) | P‐value d | |
| Cases malignant | |||||||
| Jan | −1.8 (−6.1, 2.8) | 3.5 (−1.6, 8.8) | −1.7 (−5.3, 1.9) | −4.2 (−9.1, 0.9) | 6.3 (−14.7, 32.5) | −6.3 (−43.0, 54.3) | .275 |
| Feb | 4.4 (−0.5, 9.6) | 6.2 (0.7, 12.0) | 0.5 (−3.3, 4.4) | 2.9 (−2.6, 8.6) | −4.7 (−24.6, 20.6) | .467 | |
| Mar | −1.7 (−6.2, 3.1) | 0.9 (−4.4, 6.4) | −4.3 (−7.9,−0.6) | 6.3 (0.8, 12.1) | −13.4 (−31.7, 9.8) | 29.2 (−24.2, 120.1) | .019 |
| Apr | −15.7 (−20.0,−11.3) | −15.3 (−20.0,−10.4) | −25.0 (−28.1,−21.9) | −11.3 (−16.1,−6.3) | −17.2 (−35.6, 6.5) | <.001 | |
| May | −13.6 (−17.6,−9.4) | −14.3 (−18.8,−9.5) | −31.2 (−33.9,−28.3) | −24.2 (−28.2,−20.0) | −10.1 (−27.9, 12.2) | 17.7 (−30.0, 97.9) | <.001 |
| Jun | 12.6 (7.4, 18.0) | 9.1 (3.5, 15.0) | −1.6 (−5.5, 2.4) | −1.9 (−7.0, 3.5) | 19.3 (−5.1, 49.9) | <.001 | |
| Jul | 3.0 (−1.9, 8.2) | −2.0 (−7.5, 3.8) | −9.3 (−13.0,−5.3) | −1.1 (−6.4, 4.5) | 10.7 (−13.7, 41.9) | 29.4 (−31.3, 143.7) | .002 |
| Aug | 5.4 (0.5, 10.6) | 0.3 (−5.1, 6.1) | −4.5 (−8.4,−0.5) | −9.6 (−14.3,−4.6) | 8.5 (−14.6, 37.9) | <.001 | |
| Sep | 9.1 (4.2, 14.1) | −2.5 (−7.5, 2.7) | 5.3 (1.4, 9.3) | 1.5 (−3.5, 6.8) | 12.2 (−10.1, 40.2) | −42.6 (−67.8, 2.6) | .018 |
| Oct | −3.8 (−8.1, 0.7) | −0.8 (−5.8, 4.3) | −6.5 (−9.8,−3.0) | −3.8 (−8.6, 1.1) | 5.2 (−14.3, 29.1) | .363 | |
| Nov | 5.5 (0.8, 10.4) | 3.2 (−2.0, 8.6) | 4.9 (1.1, 9.0) | −0.8 (−5.7, 4.5) | 6.7 (−13.8, 32.0) | −27.7 (−59.3, 28.5) | .429 |
| Dec | 11.1 (5.8, 16.6) | 4.0 (−1.5, 9.8) | 1.5 (−2.5, 5.6) | 6.6 (0.9, 12.7) | 34.3 (6.2, 69.9) | .014 | |
| TOTAL Jan‐Dec | 1.1 (−0.3, 2.4) | −0.7 (−2.2, 0.9) | −6.2 (−7.2,−5.1) | −3.6 (−5.1,−2.1) | 4.2 (−2.4, 11.3) | −4.0 (−23.2, 19.9) | <.001 |
| TOTAL Mar‐Dec | 1.0 (−0.5, 2.6) | −1.8 (−3.5,−0.1) | −7.3 (−8.5,−6.2) | −4.2 (−5.7,−2.5) | 4.9 (−2.4, 12.7) | −3.4 (−24.7, 23.8) | <.001 |
| Cases in situ | |||||||
| Jan | 3.3 (−5.7, 13.1) | 4.0 (−3.6, 12.2) | −1.1 (−6.2, 4.4) | N/A | 31.3 (−20.7, 117.2) | N/A e | .494 |
| Feb | 24.7 (12.9, 37.8) | 3.6 (−4.4, 12.4) | 7.8 (1.9, 14.0) | N/A | 67.8 (−4.0, 193.3) | .012 | |
| Mar | −2.0 (−10.8, 7.8) | −11.8 (−18.6,−4.4) | 0.6 (−4.9, 6.3) | N/A | −38.6 (−65.9, 10.4) | N/A e | .025 |
| Apr | −28.9 (−36.1,−20.9) | −25.1 (−31.4,−18.3) | −23.7 (−28.1,−19.0) | N/A | −51.9 (−75.2,−6.7) | .378 | |
| May | −16.6 (−24.4,−8.1) | −16.1 (−22.6,−9.1) | −25.9 (−30.1,−21.5) | N/A | −20.5 (−54.6, 39.2) | N/A e | .048 |
| Jun | 2.6 (−6.5, 12.6) | 12.5 (4.0, 21.7) | −5.7 (−11.1,−0.0) | N/A | 79.0 (4.5, 206.7) | <.001 | |
| Jul | 0.0 (−10.6, 11.8) | −2.2 (−11.6, 8.1) | −19.2 (−24.8,−13.1) | N/A | 11.1 (−41.2, 110.0) | N/A e | .002 |
| Aug | −3.7 (−12.5, 6.1) | −12.6 (−19.5,−5.1) | −17.9 (−23.0,−12.5) | N/A | −11.5 (−49.5, 55.0) | .060 | |
| Sep | 14.0 (4.0, 25.0) | 7.9 (0.2, 16.1) | −6.5 (−11.4,−1.2) | N/A | 9.1 (−30.3, 70.8) | N/A e | <.001 |
| Oct | −7.9 (−16.1, 1.0) | −3.4 (−10.0, 3.7) | −19.2 (−23.4,−14.7) | N/A | 18.8 (−25.8, 90.0) | <.001 | |
| Nov | 2.8 (−5.9, 12.3) | 3.3 (−3.9, 11.1) | −12.6 (−17.3,−7.8) | N/A | −5.3 (−39.9, 49.4) | N/A e | <.001 |
| Dec | 7.7 (−2.8, 19.4) | 11.7 (2.6, 21.6) | −6.8 (−12.3,−1.1) | N/A | −12.9 (−53.7, 63.8) | .003 | |
| TOTAL Jan‐Dec | −0.8 (−3.5, 2.0) | −2.5 (−4.7,−0.2) | −10.9 (−12.3,−9.4) | N/A | 3.3 (−11.4, 20.4) | 45.7 (−30.8, 207.0) | <.001 |
| TOTAL Mar‐Dec | −3.4 (−6.3,−0.4) | −3.7 (−6.1,−1.3) | −13.8 (−15.3,−12.1) | N/A | −4.0 (−18.9, 13.6) | 87.5 (−20.5, 342.2) | <.001 |
| Notifications malignant | Malignant and in situ | ||||||
| Jan | −1.2 (−4.3, 2.1) | 4.4 (1.1, 7.9) | −0.3 (−3.0, 2.4) | −5.8 (−9.2,−2.3) | 15.9 (−2.1, 37.1) | −7.0 (−39.5, 43.1) | <.001 |
| Feb | 3.9 (0.3, 7.5) | 6.6 (2.9, 10.3) | 1.0 (−1.8, 3.9) | 2.7 (−1.2, 6.7) | 7.4 (−10.4, 28.7) | .201 | |
| Mar | 2.1 (−1.2, 5.6) | 7.3 (3.7, 11.0) | 0.6 (−2.2, 3.4) | 0.2 (−3.6, 4.0) | −5.8 (−21.3, 12.9) | 46.7 (−7.3, 132.2) | .026 |
| Apr | −10.8 (−14.0,−7.5) | −9.7 (−12.8,−6.4) | −16.3 (−18.7,−13.8) | −8.7 (−12.2,−5.1) | −3.5 (−20.4, 17.0) | .001 | |
| May | −15.2 (−18.1,−12.2) | −8.6 (−11.7,−5.5) | −25.9 (−28.0,−23.6) | −25.8 (−28.7,−22.9) | −11.5 (−25.5, 5.2) | 22.2 (−21.3, 89.9) | <.001 |
| Jun | 10.4 (6.7, 14.1) | 5.0 (1.5, 8.6) | −4.5 (−7.3,−1.7) | −7.1 (−10.5,−3.5) | 27.7 (7.0, 52.5) | <.001 | |
| Jul | 2.1 (−1.4, 5.7) | 1.0 (−2.6, 4.7) | −11.6 (−14.3,−8.8) | −6.8 (−10.3,−3.1) | 21.8 (0.8, 47.2) | 14.1 (−34.3, 98.1) | <.001 |
| Aug | 3.2 (−0.2, 6.8) | 1.6 (−2.0, 5.3) | −8.0 (−10.7,−5.1) | −13.5 (−16.8,−10.0) | 10.0 (−8.7, 32.7) | <.001 | |
| Sep | 9.1 (5.6, 12.7) | −2.6 (−5.9, 0.7) | 5.7 (2.8, 8.7) | 7.2 (3.4, 11.2) | 19.5 (0.8, 41.6) | −31.8 (−57.1, 8.4) | <.001 |
| Oct | −5.6 (−8.6,−2.5) | −7.9 (−10.8,−4.8) | −11.2 (−13.6,−8.8) | −4.1 (−7.5,−0.6) | 12.0 (−4.5, 31.3) | .001 | |
| Nov | 4.3 (0.9, 7.7) | 6.2 (2.7, 9.8) | −3.0 (−5.6,−0.2) | −6.6 (−10.0,−3.0) | 4.9 (−11.0, 23.7) | −33.6 (−59.2, 8.0) | <.001 |
| Dec | 12.8 (8.9, 16.7) | 7.0 (3.3, 10.9) | 5.3 (2.2, 8.4) | 0.1 (−3.8, 4.1) | 32.9 (11.0, 59.1) | <.001 | |
| TOTAL Jan‐Dec | 1.1 (0.1, 2.1) | 0.7 (−0.3, 1.7) | −5.8 (−6.6,−5.1) | −5.9 (−6.9,−4.8) | 10.3 (4.8, 16.0) | −2.3 (−19.1, 18.0) | <.001 |
| TOTAL Mar‐Dec | 1.1 (−0.0, 2.1) | −0.3 (−1.4, 0.8) | −7.1 (−7.9,−6.3) | −6.7 (−7.8,−5.6) | 9.9 (4.0, 16.2) | −1.1 (−19.9, 22.0) | <.001 |
| Notifications in situ | |||||||
| Jan | 0.8 (−5.6, 7.7) | 5.8 (0.5, 11.5) | N/A | N/A | 50.0 (0.2, 124.5) | N/A e | .106 |
| Feb | 19.6 (11.2, 28.5) | 4.2 (−1.4, 10.2) | N/A | N/A | 74.2 (12.0, 170.9) | .002 | |
| Mar | 0.3 (−6.3, 7.4) | −6.2 (−11.2,−0.9) | N/A | N/A | −25.6 (−53.6, 19.3) | N/A e | .183 |
| Apr | −22.3 (−28.0,−16.1) | −16.7 (−21.4,−11.6) | N/A | N/A | −40.5 (−64.5,−0.3) | .176 | |
| May | −15.0 (−20.9,−8.7) | −12.5 (−17.2,−7.5) | N/A | N/A | −26.8 (−54.3, 17.2) | N/A e | .641 |
| Jun | 8.7 (1.6, 16.4) | 4.6 (−1.0, 10.5) | N/A | N/A | 60.8 (3.7, 149.5) | .120 | |
| Jul | 2.6 (−5.1, 11.0) | −9.8 (−15.8,−3.3) | N/A | N/A | 50.0 (−12.5, 157.3) | N/A e | .014 |
| Aug | 3.3 (−3.6, 10.7) | −17.9 (−22.5,−13.0) | N/A | N/A | −15.9 (−48.2, 36.7) | <.001 | |
| Sep | 9.6 (2.5, 17.1) | −2.8 (−7.8, 2.4) | N/A | N/A | 41.6 (−0.2, 100.9) | N/A e | .004 |
| Oct | −5.7 (−11.8, 0.8) | −12.1 (−16.5,−7.5) | N/A | N/A | 23.3 (−15.5, 79.9) | .069 | |
| Nov | 4.3 (−2.2, 11.2) | −3.0 (−7.9, 2.1) | N/A | N/A | 18.9 (−17.5, 71.3) | N/A e | .141 |
| Dec | 14.9 (6.9, 23.5) | 5.7 (−0.3, 12.1) | N/A | N/A | 7.8 (−32.3, 71.5) | .216 | |
| TOTAL Jan‐Dec | 1.4 (−0.6, 3.5) | −5.1 (−6.6,−3.6) | N/A | N/A | 16.4 (2.9, 31.7) | 57.9 (−21.9, 219.2) | <.001 |
| TOTAL Mar‐Dec | −0.1 (−2.2, 2.2) | −7.2 (−8.8,−5.5) | N/A | N/A | 8.6 (−5.2, 24.4) | 88.9 (−15.8, 323.7) | <.001 |
Note: Both men and women, ages 18+.
Abbreviations: N/A, not available; Perc. change, percentage change.
Sweden: Cases from malignant and in situ tumours separately. Notifications from combined malignant and in situ tumours.
Iceland: Comparison to average numbers 2017‐2019.
Faroes: Comparison to average numbers 2017‐2019 in bimonthly intervals (Jan/Feb, Mar/Apr, May/Jun, Jul/Aug, Sep/Oct, Nov/Dec).
Tests excluding the Faroe Islands, due to bimonthly intervals.
Faroes: Bimonthly numbers of cases and notifications of in situ diagnoses not presented due to small numbers.
FIGURE 1.
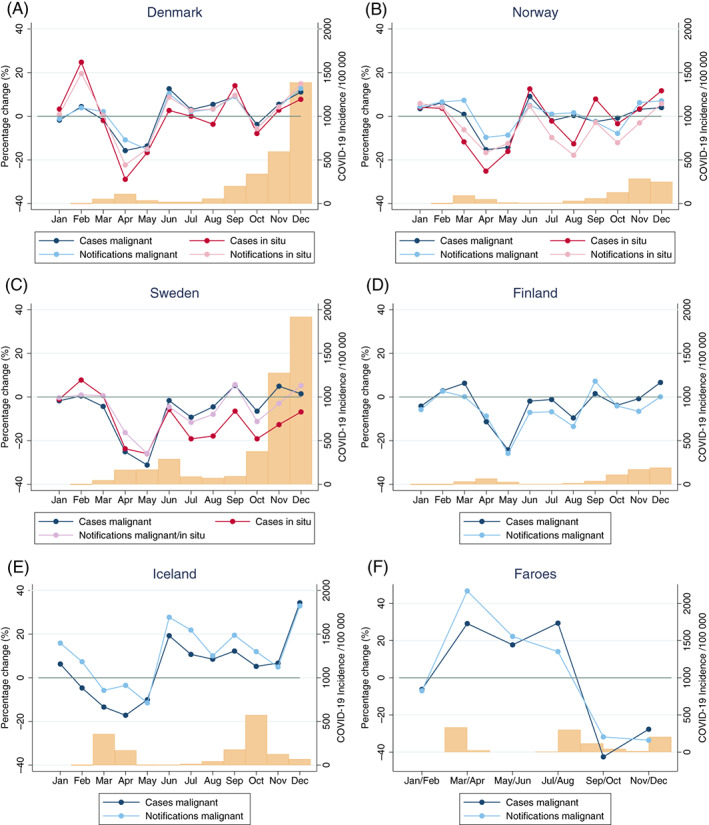
Percentage change (monthly) of new malignant and in situ cases and notifications to the Nordic cancer registries comparing 2020 to 2019. Both men and women, ages 18+. The bars at the bottom of each panel represents the incidence of COVID‐19 (per 100 000) per month. Panels (A‐F) Population sizes are 5 792 203 (Denmark), 5 421 242 (Norway), 10 099 270 (Sweden), 5 540 718 (Finland), 341 250 (Iceland), 52 305 (Faroe Islands). Panel (C) (Sweden): Notifications from both malignant and in situ tumours. Cases of COVID‐19 from Table S2. Panel (E) (Iceland): Comparison to average numbers 2017‐2019. Panel (F) (Faroe Islands): Comparison to average numbers 2017‐2019 in bimonthly intervals (Jan/Feb, Mar/Apr, May/Jun, Jul/Aug, Sep/Oct, Nov/Dec) [Color figure can be viewed at wileyonlinelibrary.com]
The cumulative deficit of new malignant cases and notifications to the Nordic cancer registries across months in 2020 compared to a reference year was largest in Sweden, followed by Finland (Figure 2). At the end of 2020, the total decline in new malignant cases in Sweden was −6.2% (95% CI −7.2, −5.1) and −3.6% (−5.1, −2.1) in Finland, while there was no deficit in Denmark, Norway and the Faroe Islands, and a nonsignificant surplus in Iceland (+4.2%; −2.4, 11.3) (Table 2). The cumulative deficit of new in situ cases was also largest in Sweden, with a total decline at the end of 2020 of −10.9% (−12.3, −9.4). The underlying reported monthly numbers are presented in Figures S1 and S2.
FIGURE 2.
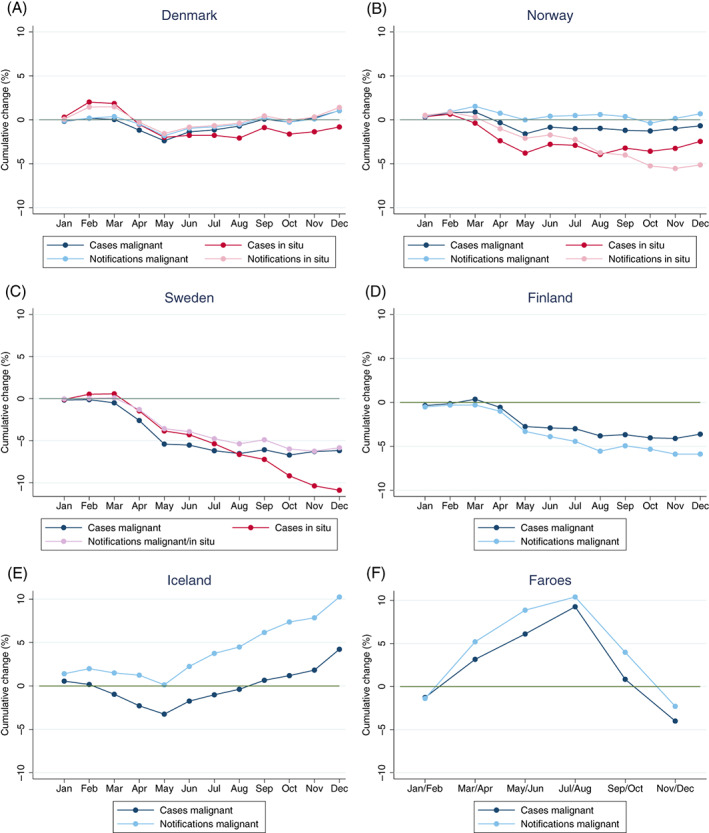
Cumulative percentage change of malignant and in situ cases and notifications to the Nordic cancer registries at each month 2020 compared to total number 2019. Both men and women, ages 18+. Panels (A‐F): Population sizes are 5 792 203 (Denmark), 5 421 242 (Norway), 10 099 270 (Sweden), 5 540 718 (Finland), 341 250 (Iceland), 52 305 (Faroe Islands). Panel (C) (Sweden): Notifications from both malignant and in situ tumours. Panel (E) (Iceland): Comparison to average numbers 2017‐2019. Panel (F) (Faroe Islands): Comparison to average numbers 2017‐2019 in bimonthly intervals (Jan/Feb, Mar/Apr, May/Jun, Jul/Aug, Sep/Oct, Nov/Dec) [Color figure can be viewed at wileyonlinelibrary.com]
To assess how the percentage change influenced absolute numbers of new cases and notifications to the Nordic cancer registries, the total annual numbers and percentage change by sex and age groups are presented in Table 3 and Figure 3. For malignant diagnoses, there were declines in numbers of new cases among women in Norway, Sweden and Finland, and in the age group 50 to 69 years in both sexes combined across all countries except Iceland and the Faroe Islands. For in situ diagnoses, there were reduced numbers of new cases in both sexes and across age groups in Denmark and Sweden. Overall, the patterns of number of notifications mimicked the patterns in number of new cases.
TABLE 3.
Total numbers and difference in numbers of new malignant and in situ cases and notifications to the Nordic cancer registries, Jan‐Dec 2020 vs 2019
| Denmark | Norway | Sweden | ||||
|---|---|---|---|---|---|---|
| 2020/2019 | Diff | 2020/2019 | Diff | 2020/2019 | Diff | |
| N/N | N (PC) | N/N | N (PC) | N/N | N (PC) | |
| Cases, malignant | ||||||
| Total | 41 387/40 956 | 431 (1.1) | 32 192/32 411 | −219 (−0.7) | 58 786/62 649 | −3863 (−6.2) a |
| Men | 20 678/20 524 | 154 (0.8) | 17 079/16 831 | 248 (1.5) | 30 358/32 856 | −2498 (−7.6) a |
| Women | 20 709/20 432 | 277 (1.4) | 15 113/15 580 | −467 (−3.0) a | 28 428/29 793 | −1365 (−4.6) a |
| 18‐49 | 3661/3618 | 43 (1.2) | 3536/3606 | −70 (−1.9) | 5509/5741 | −232 (−4.0) a |
| 50‐69 | 16 626/17 078 | −452 (−2.6) a | 12 514/13 324 | −810 (−6.1) a | 20 910/22 548 | −1638 (−7.3) a |
| 70+ | 21 100/20 260 | 840 (4.1) a | 16 142/15 481 | 661 (4.3) a | 32 367/34 360 | −1993 (−5.8) a |
| Cases, in situ | ||||||
| Total | 9892/9973 | −81 (−0.8) | 14 372/14 734 | −362 (−2.5) a | 26 218/29 417 | −3199 (−10.9) a |
| Men | 3666/3492 | 174 (5.0) a | 3585/3731 | −146 (−3.9) | 6919/7556 | −637 (−8.4) a |
| Women | 6226/6481 | −255 (−3.9) a | 10 787/11 003 | −216 (−2.0) | 19 299/21 861 | −2562 (−11.7) a |
| 18‐49 | 2957/3161 | −204 (−6.5) a | 5871/6033 | −162 (−2.7) | 10 942/12 713 | −1771 (−13.9) a |
| 50‐69 | 3396/3587 | −191 (−5.3) a | 3644/3852 | −208 (−5.4) a | 6391/6884 | −493 (−7.2) a |
| 70+ | 3539/3225 | 314 (9.7) a | 4857/4849 | 8 (0.2) | 8885/9820 | −935 (−9.5) a |
| Notifications, malignant | Malignant and in situ | |||||
| Total | 81 251/80 389 | 862 (1.1) a | 78 638/78 098 | 540 (0.7) | 110 071/116 889 | −6818 (−5.8) a |
| Men | 40 118/39 570 | 548 (1.4) | 40 662/37 776 | 2886 (7.6) a | 45 278/47 318 | −2040 (−4.3) a |
| Women | 41 133/40 819 | 314 (0.8) | 37 976/40 322 | −2346 (−5.8) a | 64 793/69 571 | −4778 (−6.9) a |
| 18‐49 | 6633/6665 | −32 (−0.5) | 8367/8734 | −367 (−4.2) a | 21 515/24 023 | −2508 (−10.4) a |
| 50‐69 | 33 179/34 246 | −1067 (−3.1) a | 31 060/32 861 | −1801 (−5.5) a | 35 720/37 698 | −1978 (−5.2) a |
| 70+ | 41 439/39 478 | 1961 (5.0) a | 39 211/36 503 | 2708 (7.4) a | 52 836/55 168 | −2332 (−4.2) a |
| Notifications, in situ | ||||||
| Total | 19 255/18 988 | 267 (1.4) | 29 315/30 900 | −1585 (−5.1) a | N/A | N/A |
| Men | 7215/6758 | 457 (6.8) a | 7509/7896 | −387 (−4.9) a | N/A | N/A |
| Women | 12 040/12 230 | −190 (−1.6) | 21 806/23 004 | −1198 (−5.2) a | N/A | N/A |
| 18‐49 | 5417/5783 | −366 (−6.3) a | 12 055/12 527 | −472 (−3.8) a | N/A | N/A |
| 50‐69 | 6201/6337 | −136 (−2.1) | 6870/7877 | −1007 (−12.8) a | N/A | N/A |
| 70+ | 7637/6868 | 769 (11.2) a | 10 390/10 496 | −106 (−1.0) | N/A | N/A |
| Finland | Iceland | Faroes | ||||
|---|---|---|---|---|---|---|
| 2020/2019 | Diff | 2020/2017‐2019 b | Diff b | 2020/2017‐2019 b | Diff b | |
| N/N | N (PC) | N/N | N (PC) | N/N | N (PC) | |
| Cases, malignant | ||||||
| Total | 31 984//33 186 | −1202 (−3.6) a | 1809/1735.7 | 73.3 (4.2) | 152/158.3 | −6.3 (−4.0) |
| Men | 16 388/16 995 | −607 (−3.6) a | 876/847.0 | 29.0 (3.4) | 83/82.3 | 0.7 (0.8) |
| Women | 15 596/16 191 | −595 (−3.7) a | 933/888.7 | 44.3 (5.0) | 69/76.0 | −7.0 (−9.2) |
| 18‐49 | 2532/2631 | −99 (−3.8) | 198/233.7 | −35.7 (−15.3) | 11/15.0 | −4.0 (−26.7) |
| 50‐69 | 12 205/13 030 | −825 (−6.3) a | 776/754.3 | 21.7 (2.9) | 53/65.3 | −12.3 (−18.9) |
| 70+ | 17 247/17 525 | −278 (−1.6) | 859/754.3 | 104.7 (13.9) a | 88/78.0 | 10.0 (12.8) |
| Cases, in situ | ||||||
| Total | N/A | N/A | 333/322.3 | 10.7 (3.3) | 17/11.7 | 5.3 (45.7) |
| Men | N/A | N/A | 107/105.7 | 1.3 (1.3) | 14/4.0 | 10.0 (250.0) a |
| Women | N/A | N/A | 225/218.3 | 6.7 (3.1) | 3/7.7 | −4.7 (−60.9) |
| 18‐49 | N/A | N/A | 44/55.3 | −11.3 (−20.5) | 9/4.0 | 5.0 (125.0) |
| 50‐69 | N/A | N/A | 106/97.7 | 8.3 (8.5) | 2/4.0 | −2.0 (−50.0) |
| 70+ | N/A | N/A | 185/167.7 | 17.3 (10.3) | 6/3.7 | 2.3 (63.6) |
| Notifications, malignant | ||||||
| Total | 61 902/65 773 | −3871 (−5.9) a | 3137/2845.3 | 291.7 (10.3) a | 213/218.0 | −5.0 (−2.3) |
| Men | 28 731/29 984 | −1253 (−4.2) a | 1483/1334.7 | 148.3 (11.1) a | 110/113.7 | −3.7 (−3.2) |
| Women | 33 171/35 789 | −2618 (−7.3) a | 1654/1510.7 | 143.3 (9.5) a | 103/104.3 | −1.3 (−1.3) |
| 18‐49 | 5327/5675 | −348 (−6.1) a | 369/375.7 | −6.7 (−1.8) | 19/17.0 | 2.0 (11.8) |
| 50‐69 | 24 444/27 168 | −2724 (−10.0) a | 1377/1277.7 | 99.3 (7.8) | 68/85.7 | −17.7 (−20.6) |
| 70+ | 32 131/32 930 | −799 (−2.4) a | 1391/1192.0 | 199.0 (16.7) a | 126/115.3 | 10.7 (9.2) |
| Notifications, in situ | ||||||
| Total | N/A | N/A | 547/470.0 | 77.0 (16.4) a | 20/12.7 | 7.3 (57.9) |
| Men | N/A | N/A | 156/149.3 | 6.7 (4.5) | 15/4.3 | 10.7 (246.2) a |
| Women | N/A | N/A | 391/320.7 | 70.3 (21.9) a | 5/8.3 | −3.3 (−40.0) |
| 18‐49 | N/A | N/A | 68/66.0 | 2.0 (3.0) | 10/4.3 | 5.7 (130.8) |
| 50‐69 | N/A | N/A | 180/156.7 | 23.3 (14.9) | 2/4.0 | −2.0 (−50.0) |
| 70+ | N/A | N/A | 299/247.3 | 51.7 (20.9) a | 8/4.3 | 3.7 (84.6) |
Note: Total and by sex and age.
Abbreviation: PC, percentage change.
Significant difference (P‐value <.05).
Iceland and Faroes: Comparison to average numbers 2017‐2019.
FIGURE 3.
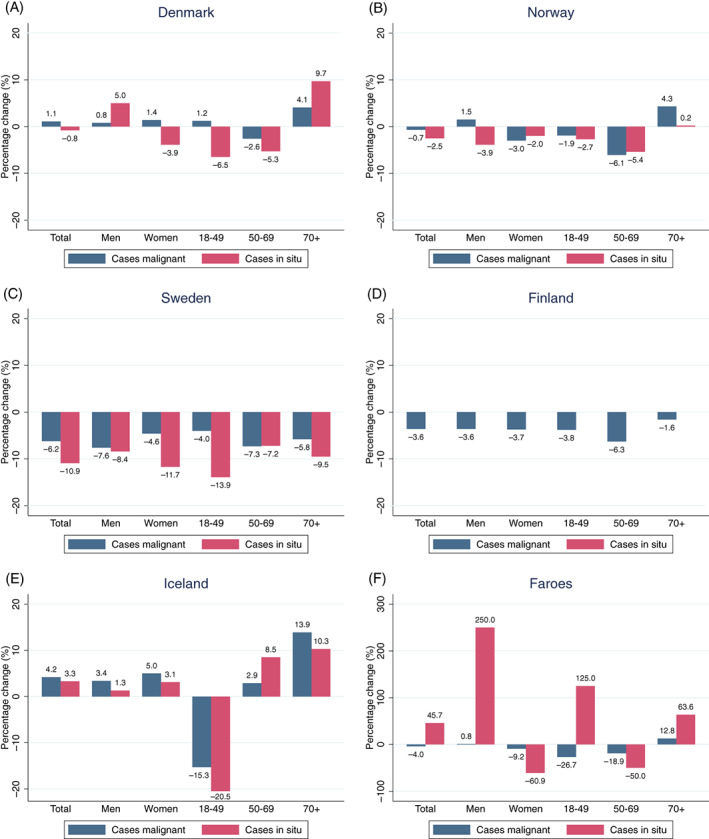
Percentage change (Jan‐Dec) of new malignant and in situ cases reported to the Nordic cancer registries in 2020 compared to 2019. Total and by sex and age. Panels (A‐F): Population sizes are 5 792 203 (Denmark), 5 421 242 (Norway), 10 099 270 (Sweden), 5 540 718 (Finland), 341 250 (Iceland), 52 305 (Faroe Islands). Panel (E) (Iceland): Comparison to average numbers 2017‐2019. Panel (F) (Faroe Islands): Comparison to average numbers 2017‐2019 [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
4.1. Main findings
Despite pronounced differences in rates of COVID‐19 cases and deaths between Sweden and the other Nordic countries, we found similar marked declines in new cases of histologically verified tumours during April and May 2020 across the Nordic region, that was most pronounced in Sweden. These initial reductions were followed by rebounds closer to expected levels during the rest of 2020 in Denmark, Norway, Sweden and Finland. In Iceland, rates of new cases remained at higher levels throughout 2020 compared to previous years. Although based on small numbers, the pattern in the Faroe Islands was different, with no apparent decline in cancer reporting during the first wave of the pandemic followed by a decline in from September to December 2020. The similar declines for number of notifications and new cases, as well as for malignant and in situ diagnosis, are likely to reflect an effect of temporary halting or reductions of cancer screening and changes of healthcare seeking behaviour, rather than lower cancer diagnostic work‐up intensity.
Due to demographic shifts, the number of cancer cases is expected to rise between 1.2% and 2.2% in the Nordic countries each year. 11 At the end of 2020, reporting in Sweden had not compensated for the spring deficit leading to an overall −6.2% decline in malignant cases for the entire year despite an expected annual increase of around 1.4%. In Denmark and Norway, with an expected yearly increase of around 1.6% to 2.2%, the reporting is unlikely to have been fully recovered since the yearly accumulated reporting ended around 0% to 1%. In Iceland, we observed a complete recovery with an increase in cancer reporting of 4.2% that was higher than expected. In Finland, with an expected annual increase of around 1.4%, the annual deficit was −3.6%. The overall patterns of reported notifications followed the patterns for cases, indicating that the reductions are likely to reflect fewer patients seeking care, reduced screening and lower attendance. Fewer patients seeking care will likely mostly affect the detection of malignant tumours, while lower screening activity will have the highest impact on in situ diagnoses and early stage invasive tumours. However, in all countries, the overall patterns of reporting during 2020 was similar for malignant and in situ tumours. Of special note was that the in situ reporting in Sweden during the autumn of 2020 was markedly lower than the reporting of malignant tumours.
4.2. Effect of COVID‐19 epidemic on cancer healthcare in the Nordic countries
While the available data did not allow for a direct assessment of COVID‐19 related factors (severity of pandemic, mitigation efforts) that might have affected similarities and dissimilarities in cancer incidence and diagnostic work‐up, a descriptive comparison between the Nordic countries may yet provide some insights. In Norway, Sweden and Iceland, screening programmes were temporarily halted and attendance lower when resumed during the first surge of the pandemic, whereas screening remained open in Denmark and Finland, yet with lower attendance rates, in contrast to the Faroe Islands where attendance did not fall. Of special note is that despite reallocation of healthcare resources, the capacity to provide care for individuals seeking healthcare for cancer related symptoms appears to have been largely upheld in all Nordic countries during the pandemic. In Sweden, there were few or no delays in start of treatment in newly diagnosed cancer patients. 12 The rebound in cancer reporting during the second surge in COVID‐19 infections in the autumn of 2020 may reflect changes in patient perceptions of the safety of seeking care. Furthermore, our results indicate that the restrictions implemented in Norway and later in Denmark during the fourth quarter of 2020 appear to have had little or no impact on cancer detection. In all Nordic countries, healthcare services remained opened and available to non‐COVID‐19 related conditions, although fewer patients sought healthcare in particular in the early phase of the pandemic. In Sweden, the number of visits to hospital emergency services declined by 16% from March to September 2020 compared to 2017 to 2019. 13 Declines were also observed in other Nordic countries. 14 , 15
4.3. Other settings outside the Nordic region
Our findings corroborate previous reports of cancer reporting patterns in the Nordic countries and are also broadly in line with results from other countries. 16 , 17 , 18 , 19 , 20 , 21 , 22 In both Europe and the United States, there have been reports of marked reductions in the number of newly diagnosed cancer cases, particularly during the first phase of the pandemic between March and May 2, 2020, 4 , 6 , 8 , 9 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 Reductions in number of reported cases of breast and cervical cancer have been suggested to reflect not only temporary halting of screening programmes, but also lower attendance rates that have remained also after screening programmes were resumed. 2 , 5 , 6 , 7 , 8 , 9 , 31 Furthermore, there are reports that rates of PSA testing for prostate cancer in asymptomatic men have declined, 3 , 32 which are likely to reflect hesitancy to contact healthcare during surges in COVID‐19 transmission. Previous Nordic national reports have shown particular sharp declines in newly reported cases of cervical cancer in situ, prostate cancer and breast. 16 , 19 , 22 In Iceland, the temporary reductions have mainly been observed for cancers of the breast, colorectum and skin. 17
Only a few studies have compared the stage distribution of newly diagnosed cancer before, during and following the first wave of the pandemic. In the Netherlands, marked reductions were observed for in situ (cTis, DCIS) and early stage (cT1, stage I) breast tumours early in the pandemic with no evidence of a shift to advanced stage breast cancer in early autumn 2020 following restart of screening. 33 Similarly, Dutch data on colorectal cancer found decreases in incidence, particularly for stage I tumours. 34 From the UK, a shift towards more advanced breast cancer stages was reported between May and October 2020 after a substantial drop from 43.8% to 9.2% screen‐detected cases compared to 2019. 35 Another study from the UK reported reductions in number of low‐stage cases of cervical cancer in the early phase of 2020, 36 while no difference in pathological stage of skin cancer was found in the autumn of 2020 (Oct‐Dec) when referral rates where back to normal levels. 37 In Denmark, a recent study did not find any significant changes in stage distribution across seven major cancer sites between 2019 and 2020. 22
In Denmark, Norway and Sweden, we found particularly large cumulative annual declines of malignant cases in screening ages 50 to 69 years, indicating that the large reductions in the spring were not fully compensated during the summer and autumn with lower COVID‐19 infection rates. By the end of 2020, there was a deficit in the number of new malignant cases in both sexes in Sweden, while the reduction of in situ cases was confined to women, representing a substantial deficit of undetected cases in line with a previous national report. 16 Breast cancer and cervical cancer screening was temporarily halted or reduced, albeit with large regional variations within Sweden. Following resumptions of services, several breast cancer screening units in Sweden reported remaining back‐logs in the autumn 2020. 38
In December 2020, 10 out of 21 Swedish healthcare regions reported cervical cancer screening deficits (personal communication). Similarly, six regions reported a decline of more than 30% of screen‐detected breast cancer in 2020. 16 In the Nordic countries, approximately a third of breast cancers are detected by screening, with a higher proportion in Sweden where the screening age range is 40 to 74 years. 39 , 40 , 41 Data based on clinical registers indicate no or minor changes in the detection rates of colorectal cancer in Sweden in 2020 compared to earlier years. 42 For colorectal cancer, incidence rates during the past 10 years have been relatively stable in all Nordic countries, with the exception of declining rates in Denmark and Norway in recent years. 43 The decline in Denmark is likely to reflect the introduction of a national screening programme between 2014 and 2017.
The strengths of our study included the use of data from essentially complete pathology notification registration across a region with more than 27 million inhabitants. The Nordic cancer registries are based on robust national systems for cancer reporting with a high degree of completeness and timeliness. In addition, the Nordic countries have similar tax‐funded national healthcare systems aiming to ensure equal access to care, including national cancer screening programmes at low out of pocket cost for the individual.
A limitation of our study was the ecological study design. The available data cannot explain the observed intra‐ and intercountry differences, but merely provide a perspective in relation to the development of the pandemic and mitigation efforts undertaken in each country. A major limitation was that the numbers by tumour site was unavailable in the dataset at hand, since tumour location was not consistently included in pathology notifications in all countries. Also, no data was available on tumour stage. We used the difference in reporting between 2020 and 2019 (for Iceland and the Faroe Islands: 2017‐2019) as a measure of pandemic impact, an approach that assumes stable rates (of notifications) over years if there was no pandemic, that is, everything else held equal across calendar years. Since the cancer burden continuously increases with 1.2% to 2.2% new cases per year because of demographic changes (increasing population size, ageing population), the observed deficits are likely to be underestimated, and in particularly so for Iceland and the Faroe Islands where the comparison period was an average over 3 years. Additionally, the percentage change across the compared years assumes stable underlying incidence rates; however, since the comparison covers a short period the impact of long‐term trends should be minor. Number of cancer notifications are not comparable to official incidence, as one case can give rise to multiple notifications. For number of notifications, we counted all notifications, regardless of previous cancer diagnosis. This represents an indirect measure of diagnostic work‐up activity, and will thus only be comparable over calendar time if the rates of second cancer or recurrence (secondary cancer) are similar across years. For number of new cases, the lack of nationwide timely data on pathology notifications in Sweden was a limitation. For that reason, the assessment of number of new cases in Sweden was based on available data on pathological and/or clinical notifications. Since clinical reporting is less rapid this is likely to have led to an underestimation of monthly numbers at the end of 2020. For Finland, the number of new cases was restricted to patients with no previous cancer within 2 years, compared to 5 years for the other countries, meaning that numbers for Finland are likely overestimated compared to the other countries. For Finland, we also did not extract pathology notifications of in situ diagnoses due to a major increase in coverage between 2019 and 2020. In addition, new cancer cases based on pathology notifications alone are not directly comparable to official incidence, since it does not include confirmatory clinical notifications or have undergone quality checks by cancer registry staff. Some comparisons, in particular those based on data from Iceland and the Faroe Islands, were hampered by low numbers and random variation. However, internal comparisons within countries should be valid since coding and reporting practices were similar within each country during the study period.
5. CONCLUSION
In conclusion, despite marked differences between the Nordic countries regarding the severity of the COVID‐19 pandemic and mitigation efforts, similar patterns with the largest declines in cancer notification were observed during April and May of 2020, with the most pronounced reduction observed in Sweden. Of note was the minimal impact of the COVID‐19 pandemic in the two island nations Iceland and the Faroe Islands, where containment strategies were easier to implement, yet due to smaller numbers in these two countries the reported findings are subject to more uncertainty, and it cannot be excluded that findings are due to chance. In all Nordic countries, the monthly variations in the number of cancer notifications are likely to reflect combined effects of societal restrictions, temporary halting on screening activities, and public perceptions of risk of infection with fewer individuals seeking care when COVID‐19 infection rates were high. A remaining whole‐year deficit in Sweden, the country with the highest rates of COVID‐19 cases and deaths, may be explained by continued high community transmission of COVID‐19 throughout 2020 with a remaining hesitancy to seek healthcare. Our findings may suggest that the severity of the pandemic has had larger effect on cancer detection than strict societal restrictions. In all countries, the consequences of the pandemic include risks of diagnostic delays that may lead to future increased rates of late‐stage cancer with poorer prognosis in cancer patient subgroups. 44 , 45
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
AUTHOR CONTRIBUTIONS
Anna L. V. Johansson, Siri Larønningen, Charlotte Wessel Skovlund, Marnar Fríðheim Kristiansen, Laufey Tryggvadóttir, Janne Pitkäniemi, Tomas Tanskanen, Giske Ursin, and Mats Lambe conceived and designed the study. Siri Larønningen, Charlotte Wessel Skovlund, Marnar Fríðheim Kristiansen, Tomas Tanskanen, Laufey Tryggvadóttir, and Mats Lambe compiled the national data from their respective countries. Anna L. V. Johansson analysed the aggregated Nordic data. Anna L. V. Johansson, Siri Larønningen, Charlotte Wessel Skovlund, Marnar Fríðheim Kristiansen, Janne Pitkäniemi, Tomas Tanskanen, Laufey Tryggvadóttir, Giske Ursin, and Mats Lambe interpreted the data and results. Anna L. V. Johansson and Mats Lambe wrote the first draft of the study. Siri Larønningen, Charlotte Wessel Skovlund, Marnar Fríðheim Kristiansen, Janne Pitkäniemi, Tomas Tanskanen, Laufey Tryggvadóttir, Giske Ursin, Lina Steinrud Mørch, Søren Friis, Tom Børge Johannesen, Tor Åge Myklebust, Anna Skog, David Pettersson, Helgi Birgisson, Anni Virtanen, and Nea Malila critically reviewed and edited the article. All authors approved the final article. The work reported in the article has been performed by the authors, unless clearly specified in the text.
ETHICS STATEMENT
Denmark: Legal approval to use data was obtained from The Danish Health Data Authority. Ethical review is not required for register‐based studies in Denmark. The Faroe Islands: The data collection for this project has been approved by the Faroese Data Protection Authority. Finland: The Finnish Cancer Registry (FCR) provided statistical data for our study. The FCR has permission to collect, process and report statistical data without the need to seek consent. Iceland: The Icelandic Cancer Registry (ICR) provided statistical data for our study. The ICR has permission to collect, process and report statistical data without the need to seek consent. Norway: The Norwegian Cancer Registry provided statistical data for our study. The CRN has permission to collect, process and report statistical data without the need to seek consent. This project also has an ethical permission in Norway (REK 136767). Sweden: The project was conducted under ethical permission from the Swedish Ethical Review Authority (Dnr. 2020‐0427, 2020‐07218, 2021‐03041), which granted the use of the data for research purposes, including combining Nordic data. The Swedish data was compiled and aggregated by the Regional Cancer Center West, Göteborg, Sweden. All countries: Fully anonymized aggregated data were shared with and analysed by Anna L. V. Johansson.
Supporting information
Table S1Overview of COVID‐19 strategies in the Nordic countries Jan‐Dec 2020
Table S2Number of new COVID‐19 cases by month and the mortality rate from COVID‐19 in the year 2020 in the Nordic countries
Table S3Data sources of newly diagnosed cancer cases and inclusion and exclusion criteria used by each Nordic country
Figure S1Number of cases reported to the Nordic cancer registries, 2020 vs 2019 (vs average 2017‐2019 for Iceland/Faroes). Both men and women, ages 18+
Figure S2Number of notifications to the Nordic cancer registries, 2020 vs 2019 (vs average 2017‐2019 for Iceland/Faroes). Both men and women, ages 18+
ACKNOWLEDGEMENTS
We would like to thank Anna Genell and Chenyang Zhang at the Regional Cancer Center West, Göteborg, Sweden and Hólmfríður Hilmarsdóttir at the Icelandic Cancer Registry, for data extraction and statistical support.
Johansson ALV, Larønningen S, Skovlund CW, et al. The impact of the COVID‐19 pandemic on cancer diagnosis based on pathology notifications: A comparison across the Nordic countries during 2020. Int J Cancer. 2022;151(3):381‐395. doi: 10.1002/ijc.34029
Funding informationOur study was funded by the Nordic Cancer Union (NCU: R276‐A15785; PI: Ursin). The funder had no role in the data collection or interpretation of the findings.
DATA AVAILABILITY STATEMENT
Our study was based on national and regional cancer registry data. The data are available from each national and regional cancer registry under the use of appropriate ethical and legal permissions, including the GDPR. Further details and other data that support the findings of our study are available from the corresponding author upon request.
REFERENCES
- 1. Solomon MD, Nguyen‐Huynh M, Leong TK, et al. Changes in patterns of hospital visits for acute myocardial infarction or ischemic stroke during COVID‐19 surges. JAMA. 2021;326:82‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh QD. Cancer screening tests and cancer diagnoses during the COVID‐19 pandemic. JAMA Oncol. 2021;7:458‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID‐19 pandemic. JAMA Oncol. 2021;7:878‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patt D, Gordan L, Diaz M, et al. Impact of COVID‐19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform. 2020;4:1059‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dinmohamed AG, Cellamare M, Visser O, et al. The impact of the temporary suspension of national cancer screening programmes due to the COVID‐19 epidemic on the diagnosis of breast and colorectal cancer in the Netherlands. J Hematol Oncol. 2020;13:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferrara G, De Vincentiis L, Ambrosini‐Spaltro A, et al. Cancer diagnostic delay in northern and central Italy during the 2020 lockdown due to the coronavirus disease 2019 pandemic. Am J Clin Pathol. 2021;155:64‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gathani T, Clayton G, MacInnes E, Horgan K. The COVID‐19 pandemic and impact on breast cancer diagnoses: what happened in England in the first half of 2020. Br J Cancer. 2021;124:710‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maluchnik M, Podwojcic K, Wieckowska B. Decreasing access to cancer diagnosis and treatment during the COVID‐19 pandemic in Poland. Acta Oncol. 2021;60:28‐31. [DOI] [PubMed] [Google Scholar]
- 9. Peacock HM, Tambuyzer T, Verdoodt F, et al. Decline and incomplete recovery in cancer diagnoses during the COVID‐19 pandemic in Belgium: a year‐long, population‐level analysis. ESMO Open. 2021;6:100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pukkala E, Engholm G, Hojsgaard Schmidt LK, et al. Nordic Cancer Registries—an overview of their procedures and data comparability. Acta Oncol. 2018;57:440‐455. [DOI] [PubMed] [Google Scholar]
- 11. Larønningen S, Ferlay J, Bray F, et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 9.0 (01.03.2021). Association of the Nordic Cancer Registries. Cancer Registry of Norway. https://nordcan.iarc.fr/en/dataviz/predictions_tables?sexes=0&year_prediction=2020&cancers=990. Accessed September 29, 2021.
- 12. Regional Cancer Centres in Cooperation . [Regionala Cancercentrum i Samverkan], Sweden. Shorter Waiting Times in Cancer Care—Status of Inclusions and Waiting Times in Standardized Care. March 2021 [Kortare väntetider i cancervården – statust för inklusions‐och ledtidsmål i SVF] [in Swedish]. https://cancercentrum.se/samverkan/vara-uppdrag/statistik/svf-statistik/vantetider-i-svf/. Accessed August 23, 2021.
- 13. National Board of Health and Welfare, Sweden . Analysis of Patient Visits to Hospital Emergency Services During and After the First Wave of COVID‐19 [Analys av hur patienter besöker somatiska akutmottagningar under och efter första covid‐19‐vågen]. Publication 2020‐12‐7089 [in Swedish]. https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/ovrigt/2020-12-7089.pdf. Accessed January 18, 2022.
- 14. Report from the Corona Commission, Norway . [Myndighetenes håndtering av koronapandemien. Rapport fra Koronakommisjonen. Norges offentlige utredninger 2021: 6] [in Norwegian]. https://files.nettsteder.regjeringen.no/wpuploads01/blogs.dir/421/files/2021/04/Koronakommisjonens_rapport_NOU.pdf. Accessed February 11, 2022.
- 15. Report from the Directorate of Health, Iceland . [Talnabrunnur Desember_2020] [in Icelandic]. https://www.landlaeknir.is/servlet/file/store93/item44014/Talnabrunnur_Desember_2020.pdf. Accessed February 11, 2022.
- 16. Regional Cancer Centres in Co‐operation [Regionala Cancercentrum i Samverkan], Sweden . Postponed Cancer Care, Report 5—Reporting Statistics: Cancer Notifications to the Cancer Registry, Comparison of 2020 to 2019. [Uppskjuten cancervård, Delrapport 5 – Inrapporteringsstatistik: canceranmälan till cancerregistret, jämförelse diagnosår 2020 och 2019]; June 2021 [in Swedish]. https://cancercentrum.se/samverkan/covid-19/uppskjuten-cancervard/. Accessed September 7, 2021.
- 17. COVID‐aim1 , Birgisson H, Hilmarsdottir H, Palsson RLT. COVID‐19 and cancer incidence in Iceland during year 2020. Letter to Laeknabladid [in Icelandic]. Laeknabladid. 2021;107:206‐207. [Google Scholar]
- 18. Pitkäniemi J, Virtanen A, Tanskanen T. Cancer Samples Declined in Spring 2020: Is the Coronavirus Pandemic Reflected in the Cancer Burden? [Syöpänäytteet vähenivät keväällä 2020: Heijastuuko koronaviruspandemia syöpätaakkaan?] Duodecim; 2021:137 [in Finnish].
- 19. Larønningen S, Skog A, Gulbrandsen J, et al. Cancer Diagnostics During COVID‐19 [Kreftdiagnostikk under Covid‐19.]. Oslo: Kreftregisteret; 2021. ISBN 978–82–93804‐02‐4 [in Norwegian]. [Google Scholar]
- 20. Kristiansen MF, Petersen MS, Strom M. Cancer diagnosed during the COVID‐19 pandemic in The Faroe Islands. Acta Oncol. 2021;60:856‐858. [DOI] [PubMed] [Google Scholar]
- 21. Laronningen S, Skog A, Gulbrandsen J, et al. Considerable Decline in Cancer Diagnoses During the COVID‐19 Pandemic. Tidsskr Nor Laegeforen; 2021:141. [DOI] [PubMed]
- 22. Skovlund CW, Friis S, Christensen J, Nilbert MC, Morch LS. Drop in cancer diagnosis during the COVID‐19 pandemic in Denmark: assessment of impact during 2020. Acta Oncol. 2022. doi: 10.1080/0284186X.2021.2024879 [DOI] [PubMed] [Google Scholar]
- 23. Andrew TW, Alrawi M, Lovat P. Reduction in skin cancer diagnoses in the UK during the COVID‐19 pandemic. Clin Exp Dermatol. 2021;46:145‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Vincentiis L, Carr RA, Mariani MP, Ferrara G. Cancer diagnostic rates during the 2020 ‘lockdown’, due to COVID‐19 pandemic, compared with the 2018‐2019: an audit study from cellular pathology. J Clin Pathol. 2021;74:187‐189. [DOI] [PubMed] [Google Scholar]
- 25. Dinmohamed AG, Visser O, Verhoeven RHA, et al. Fewer cancer diagnoses during the COVID‐19 epidemic in the Netherlands. Lancet Oncol. 2020;21:750‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID‐19) pandemic. JAMA Netw Open. 2020;3:e2017267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaufman HW, Chen Z, Niles JK, Fesko YA. Changes in newly identified cancer among US patients from before COVID‐19 through the first full year of the pandemic. JAMA Netw Open. 2021;4:e2125681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morris EJA, Goldacre R, Spata E, et al. Impact of the COVID‐19 pandemic on the detection and management of colorectal cancer in England: a population‐based study. Lancet Gastroenterol Hepatol. 2021;6:199‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turkington RC, Lavery A, Donnelly D, Cairnduff V, McManus DT, Coleman HG. The impact of the COVID‐19 pandemic on Barrett's esophagus and esophagogastric cancer. Gastroenterology. 2021;160:2169‐2171.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coma E, Guiriguet C, Mora N, et al. Impact of the COVID‐19 pandemic and related control measures on cancer diagnosis in Catalonia: a time‐series analysis of primary care electronic health records covering about five million people. BMJ Open. 2021;11:e047567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zadnik V, Mihor A, Tomsic S, et al. Impact of COVID‐19 on cancer diagnosis and management in Slovenia—preliminary results. Radiol Oncol. 2020;54:329‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fallara G, Sandin F, Styrke J, et al. Prostate cancer diagnosis, staging, and treatment in Sweden during the first phase of the COVID‐19 pandemic. Scand J Urol. 2021;55:184‐191. [DOI] [PubMed] [Google Scholar]
- 33. Eijkelboom AH, de Munck L, Lobbes MBI, et al. COVID‐19 Consortium and the COVID and Cancer‐NL Consortium. Impact of the suspension and restart of the Dutch breast cancer screening program on breast cancer incidence and stage during the COVID‐19 pandemic. Prev Med. 2021;151:106602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Toes‐Zoutendijk E, Vink G, Nagtegaal ID, et al. COVID and cancer‐NL consortium. Impact of COVID‐19 and suspension of colorectal cancer screening on incidence and stage distribution of colorectal cancers in the Netherlands. Eur J Cancer. 2022;161:38‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borsky K, Shah K, Cunnick G, Tsang‐Wright F. Pattern of breast cancer presentation during the coronavirus disease pandemic: results from a cohort study in the UK. Future Oncol. 2022;18:437‐443. doi: 10.2217/fon-2021-0970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Davies J, Spencer A, Macdonald S, et al. Cervical cancer and COVID: an assessment of the initial effect of the pandemic and subsequent projection of impact for women in England: a cohort study. BJOG. 2022. doi: 10.1111/1471-0528.17098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gaunt N, Green RL, Motta LF, Jamieson LA. Skin cancers in lockdown: no impact on pathological tumour staging. Br J Dermatol. 2021;185:844‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Regional Cancer Centres in Co‐operation [Regionala Cancercentrum i Samverkan], Sweden . Breast Cancer and Prostate Cancer: Impact of COVID‐19 Pandemic on Diagnostics and Treatments [Bröstcancer och prostatacancer: Covid‐19‐pandemins påverkan på diagnostik och behandling] June 2021 [in Swedish] https://cancercentrum.se/contentassets/7a5980d5d3e1402cac354ebf4fd5d574/kval‐reg‐rapport‐_covid19_‐bca‐och‐pca‐210609.pdf. Accessed January 27, 2022.
- 39. Regional Cancer Centres in Co‐operation [Regionala Cancercentrum i Samverkan], Sweden . Annual report 2020 of the National Quality Register for Breast Cancer (NKBC) [Årsrapport 2020 från Nationellt Kvalitetsregister för Bröstcancer (NKBC)] [in Swedish]. https://cancercentrum.se/globalassets/cancerdiagnoser/brost/kvalitetsregister/ett‐urval‐av‐data‐fran‐nkbc‐rapporten‐2020.pdf. Accessed January 26, 2022.
- 40. Hofvind S, Holen Å, Román M, Sebuødegård S, Puig‐Vives M, Akslen L. Mode of detection: an independent prognostic factor for women with breast cancer. J Med Screen. 2016;23:89‐97. [DOI] [PubMed] [Google Scholar]
- 41. Finnish Cancer Registry . Breast Cancer Screening. https://cancerregistry.fi/screening/breast‐cancer‐screening/. Accessed February 11, 2022.
- 42. Regional Cancer Centres in Co‐operation [Regionala Cancercentrum i Samverkan], Sweden . Lung Cancer and Colorectal Cancer: Impact of COVID‐19 Pandemic on Diagnostics and Treatments [Lungcancer och kolorektalcancer: Covid‐19‐pandemins påverkan på diagnostik och behandling] [in Swedish]. https://cancercentrum.se/globalassets/covid-19/20211223_covid19_lungcancer_kolorektalcancer.pdf. Accessed February 18, 2022.
- 43. Larønningen S, Ferlay J, Bray F, et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 9.0 (01.03.2021). Association of the Nordic Cancer Registries. Cancer Registry of Norway. https://nordcan.iarc.fr/en/dataviz/trends?cancers=520&sexes=1_2&populations=246_208_752_578&age_start=4&years=2000_2019. Accessed March 23, 2022.
- 44. Maringe C, Spicer J, Morris M, et al. The impact of the COVID‐19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population‐based, modelling study. Lancet Oncol. 2020;21:1023‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ward ZJ, Walbaum M, Walbaum B, et al. Estimating the impact of the COVID‐19 pandemic on diagnosis and survival of five cancers in Chile from 2020 to 2030: a simulation‐based analysis. Lancet Oncol. 2021;22:1427‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1Overview of COVID‐19 strategies in the Nordic countries Jan‐Dec 2020
Table S2Number of new COVID‐19 cases by month and the mortality rate from COVID‐19 in the year 2020 in the Nordic countries
Table S3Data sources of newly diagnosed cancer cases and inclusion and exclusion criteria used by each Nordic country
Figure S1Number of cases reported to the Nordic cancer registries, 2020 vs 2019 (vs average 2017‐2019 for Iceland/Faroes). Both men and women, ages 18+
Figure S2Number of notifications to the Nordic cancer registries, 2020 vs 2019 (vs average 2017‐2019 for Iceland/Faroes). Both men and women, ages 18+
Data Availability Statement
Our study was based on national and regional cancer registry data. The data are available from each national and regional cancer registry under the use of appropriate ethical and legal permissions, including the GDPR. Further details and other data that support the findings of our study are available from the corresponding author upon request.


