Abstract
Objective
To assess clinical impact, psychological effects, and knowledge of pregnant women during the COVID‐19 outbreak in seven cities in Colombia. Currently, there are uncertainty and concerns about the maternal and fetal consequences of SARS‐CoV‐2 infection during pregnancy.
Methods
A cross‐sectional web survey was carried out including pregnant women in seven cities in Colombia. Women were evaluated during the mitigation phase of the SARS‐CoV‐2 pandemic between April 13 and May 18, 2020. The questions evaluated demographic, knowledge, psychological symptoms, and attitudes data regarding the COVID‐19 pandemic.
Results
A total of 1021 patients were invited to participate, obtaining 946 valid surveys for analysis. The rate of psychological consequences of the pandemic was much larger than the number of patients clinically affected by the virus, with 50.4% of the entire cohort reporting symptoms of anxiety, 49.1% insomnia, and 25% reporting depressive symptoms. Poorly informed women were more likely to be younger, affiliated to the subsidized regime, and with lower levels of education.
Conclusion
The knowledge of pregnant women about SARS‐CoV‐2 infection is far from reality and this seems to be associated with an indirect effect on the concern and psychological stress of pregnant women in Colombia.
Keywords: Anxiety, Coronavirus, COVID‐19, Depression, Depressive symptoms, Mental health, Pregnancy, Sleep
Short abstract
A high degree of psychological stress in pregnant women in Colombia might be associated with a gap in knowledge about the consequences of SARS‐CoV‐2 infection during pregnancy.
1. INTRODUCTION
There is currently uncertainty regarding the impact of the infection caused by the severe acute respiratory distress syndrome coronavirus 2 (SARS‐CoV‐2) in pregnant women. 1 , 2 , 3 History has shown that emerging infections have a significant impact on the health of pregnant women and their fetuses, 4 with the highest risk of complications in pregnant women as in 2009 during the influenza A(H1N1) virus pandemic 5 and the serious fetal effects of the Zika virus as a more recent example. 6 , 7 However, descriptive series from high‐resource countries published so far have reported a variable clinical course of coronavirus disease of 2019 (COVID‐19) in pregnant women. 1 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15
On the other hand, according to recent publications, the indirect effects in public health of the COVID‐19 outbreak will probably be more severe than the outbreak itself. 16 Although mortality rates for COVID‐19 appear to be low in children aged under 5 years and in women of reproductive age, these groups might be disproportionately affected by the disruption of routine health services, particularly in low‐ and middle‐income countries. 16 Finally, the indirect psychological effects of COVID‐19 in the entire population are also high according to recently published data. 9 , 11
There is a paucity of data from low‐resource countries describing the direct and collateral psychological effects of COVID‐19 on pregnant women, neither the relationship of the potential psychological effects with other factors such as her degree of information, education, or healthcare coverage. Thus, the aim of the present study was to survey pregnant women in order to evaluate the direct and indirect psychological impact during the COVID‐19 outbreak.
2. MATERIALS AND METHODS
A web‐based cross‐sectional survey of pregnant women was carried out. Pregnant women aged over 18 years were eligible to participate. Pregnant women, regardless of their gestational age, were evaluated during the mitigation phase of the SARS‐CoV‐2 pandemic between April 13 and May 18, 2020, in seven cities in Colombia. A standardized, self‐administered questionnaire based on the knowledge, attitudes, and psychological effects, produced by the research group and adapted following WHO recommendations, 13 was sent to those patients whose providers were affiliated to the Atlantic Maternal Fetal Medicine Association (AMMFA) and the National Federation of Perinatology (FECOPEN) and who agreed to collaborate with the study. An electronic invitation to participate was sent to the patients’ smartphones using a messenger app (WhatsApp), in which the objective of the survey was explained. Next, the link of the website of the survey was sent to mothers who voluntarily agreed to participate using the same messenger app.
Before the beginning of the study, 65 questionnaires were administered to pregnant women in the cities in which the principal investigators were allocated—Barranquilla, Cartagena, Cucuta, Sincelejo, Bucaramanga, and Valledupar—as a pilot test evaluating the expected time for completion of the survey, interpretation, and clarity of questions and instructions. Although those answer were not used in the final analysis, questions reported as unclear and/or difficult to answer were then modified or excluded before the initiation of the actual study.
The present study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Hospital ESE Maternidad Rafael Calvo (Cartagena, Colombia) and the Institutional Review Board (IRB) at La Misericordia – Simon Bolivar University Medical Center (Barranquilla, Colombia). Electronic informed consent was obtained from each participant before starting the investigation. Participants were informed that the study was voluntary and would not have any impact on their clinical care. They were well‐informed that they could withdraw from the study at any moment without providing any justification.
The questions included in the survey covered different aspects about the sociodemographic characteristics of the women and their knowledge and attitudes regarding the SARS‐CoV‐2 pandemic. Questions included in the survey were completed using a multiple‐choice format, in which patients were encouraged to complete all questions by choosing their answer from three options (Yes, No, or Unknown). The survey employed is included in Supplementary File S1. The survey was structured as a total of 46 questions: 10 questions about the patients’ general knowledge about the pandemic; 10 questions about their attitudes; 10 questions on the potential psychological effects; eight questions on the participants’ demographic characteristics; and eight questions on their medical history. A combination of measures in the questionnaires were used to assess the psychological impact, depression, and anxiety. It also included questions about the development, or not, of symptoms associated with this respiratory infection, or if laboratory tests for SARS‐CoV‐2 were obtained and/or if different clinical complications or admission to hospital was indicated.
The mothers’ knowledge about the impact of COVID‐19 on their pregnancy was evaluated based on six judgment questions (Supplementary File S1). Each correct answer was given 2 points, an incorrect answer was given 1 point, and an uncertain answer was given no points (for a maximum score of 12 points and a minimum of 0). Participants with scores of 7 points or higher and less than 7 points were considered well and poorly informed, respectively. Questions within the survey were organized into five domains based on the content and information that were attempting to assess attitudes towards the virus, its psychological effects, and women’s knowledge about complications from SARS‐CoV‐2. Finally, the fourth and fifth domains enquired about symptoms and tests performed.
For the statistical analysis, descriptive analyses were conducted to describe the demographic characteristics and the participants’ knowledge about COVID‐19. Given that questions in the first two domains were subjective, responses were analyzed using descriptive statistics to determine the percentage of participants selecting each survey response of the total number of participants who answered the question. Second, the frequency of psychological symptoms stratified by patient knowledge was reported, and χ2 was used to compare the differences between groups. Linear regression was utilized to assess the association between knowledge of SARS‐CoV‐2 and participant characteristics. Data were analyzed using R software. P values of less than 0.05 were considered statistically significant (two‐sided tests).
3. RESULTS
A total of 1021 participants received the questionnaires; however, 65 patients collected during the pilot phase were excluded from the analysis. In addition, surveys from 10 patients were removed from the analysis due to incomplete data. Thus, a total of 946 surveys were included in the analysis (response rate of 92.6%). Table 1 describes the sociodemographic characteristic of the participants. Almost half of the participants were aged 25–35 years, 44% were nulliparous, and the majority of patients were in the second trimester of their pregnancy (median 24 weeks [interquartile range 17.2–31 weeks]). Regarding socioeconomic information, 58% of patients were covered by the Colombian contributory healthcare regime and 66% of the women reported having a level of education that was beyond secondary school (a mean of 8 years of schooling).
Table 1.
| Clinical characteristics | n=946 |
|---|---|
| Maternal age (years) | |
| 15–25 | 288 (30.9) |
| 25–35 | 503 (54) |
| 35–45 | 138 (14.8) |
| >45 | 2 (0.21) |
| Nulliparity | 408 (44.2) |
| Multiple gestation | 29 (3.1) |
| Gestational age (weeks) | 24 (17.2–31) |
| Health insurance | |
| None | 3 (0.3) |
| Subsidized regime | 200 (21.4) |
| Contributory regime | 549 (58.7) |
| Commercial health insurance | 183 (19.6) |
| Maternal education | |
| Primary | 62 (6.6) |
| Secondary | 257 (27.3) |
| Technician | 457 (48.5) |
| University | 167 (17.7) |
Values are given as number (percentage) or median (interquartile range).
Missing values: age (n=15), nulliparity (n=23), multiple gestation (n=12), health insurance (n=11), education (n=3).
Clinical characteristics and psychological symptoms of the participants are presented in Table 2. Only 65 (6.9%) women reported having developed symptoms compatible with SARS‐CoV‐2 infection at the time of the completion of the survey. Regarding respiratory symptoms related to SARS‐CoV‐2 infection, the most frequently reported was cough (5.24%), followed by shortness of breath (3.6%) and fever (1.4%). Ten patients required hospitalization, nine were tested for SARS‐CoV‐2 using polymerase chain reaction (PCR), and five of them had the infection confirmed with a positive PCR result. Six patients (9.2%) who were symptomatic and were not tested required admission to the hospital in the following days. Of the five patients with positive PCR results, only one developed pneumonia for COVID‐19, required admission to the intensive care unit, and had good evolution and posterior negativization of the PCR test.
Table 2.
| Clinical symptoms | n=946 |
|---|---|
| Fever (temperature >38°C) | 13 (1.39) |
| Cough | 49 (5.24) |
| Shortness of breath | 34 (3.64) |
| Sore throat | 10 (1.1) |
| Fatigue | 178 (19) |
| Consulted due to symptoms | 65 (7.08) |
| Tested with PCR for SARS‐CoV‐2 | 9 (0.95) |
| Positive PCR result for SARS‐CoV‐2 | 5 (0.52) |
| Required hospitalization due to COVID‐19 | 10 (1.05) |
Abbreviation: PCR, polymerase chain reaction.
Values are given as number (percentage).
Missing values: fever (n=12), cough (n=11), shortness of breath (n=11), sore throat (n=8), fatigue (n=10), consulted due to symptoms (n=28).
The distribution of psychological symptoms is presented in Figure 1. The presence of symptoms associated with anxiety was reported by 50.1% of the women. Similarly, 49% of the participants reported insomnia and 25.4% reported symptoms of depression. Using the score for questions related to knowledge, participants were classified according to their level of knowledge about the disease and potential effects on their pregnancies. Participants with scores of 7 points or higher and less than 7 points were considered well and poorly informed, respectively. Based on the answers reflecting their knowledge about SARS‐CoV‐2, patients were classified as poorly or well informed (78.5% and 21.5%, respectively).
Figure 1.
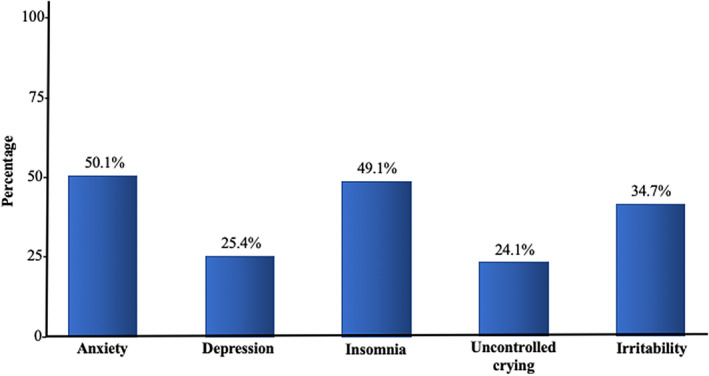
Distribution of symptoms of distress among pregnant women during the SARS‐CoV‐2 pandemic in Colombia. The presence of symptoms associated with anxiety was reported by 50.1% of the women. Similarly, 49% of them reported insomnia and 25.4% reported symptoms of depression.
Table 3 describes patients’ attitudes and fears according to their level of COVID‐19–related knowledge. Patients who were classified as better informed about COVID‐19 (score ≥7 points) were more likely to be covered by private insurance (43% had prepaid medicine), had a higher level of education (86.6% had a technical/college degree), and were in the older age range (83.3% were aged >25 years and 23% were aged >35 years). Those women with less knowledge about COVID‐19 were significantly more likely to fear the effects that SARS‐CoV‐2 can cause to their pregnancies and they were more likely to consider a preterm delivery before the peak of the pandemic (both P<0.001) (Table 3). However, there were no significant differences in the rate of psychological symptoms or levels of fear among women, irrespective of their knowledge about SARS‐CoV‐2, with a minority of patients (n=15, 1.6%) considering terminating the pregnancy due to the fear of COVID‐19.
Table 3.
Attitudes and psychological symptoms in patients according to their knowledge about the effects of SARS‐CoV‐2 in pregnancy. a
| Poorly informed (n=722) | Well informed (n=219) | P value | |
|---|---|---|---|
| Concern for SARS‐CoV‐2 | 0.25 | ||
| Yes | 659 (91.5) | 194 (88.6) | |
| No | 55 (7.64) | 24 (11.0) | |
| Unknown | 6 (0.83) | 1 (0.46) | |
| Considered terminating the pregnancy due to fear of SARS‐CoV‐2 infection | 10 (1.39) | 5 (2.28) | 0.21 |
| Affected psychologically by the SARS‐CoV‐2 pandemic | 369 (51.4) | 114 (52.1) | 0.41 |
| Feels she should deliver prematurely before the peak of the pandemic | <0.001 | ||
| Yes | 80 (11.1) | 18 (8.22) | |
| No | 456 (63.2) | 193 (88.1) | |
| Unknown | 185 (25.7) | 8 (3.65) | |
| Feels fear for the effects that SARS CoV‐2 can have on her baby | <0.001 | ||
| Yes | 652 (90.6) | 177 (80.8) | |
| No | 38 (5.28) | 39 (17.8) | |
| Unknown | 30 (4.17) | 3 (1.37) | |
| Scared when attending a medical appointment | 0.3 | ||
| Yes | 504 (70.1) | 142 (64.8) | |
| No | 204 (28.4) | 74 (33.8) | |
| Unknown | 11 (1.53) | 3 (1.37) | |
| Anxiety | 356 (49.5) | 113 (51.8) | 0.57 |
| Depression | 185 (26.1) | 50 (23.0) | 0.09 |
| Anger | 273 (38.1) | 76 (34.7) | 0.36 |
| Uncontrolled crying | 179 (24.9) | 47 (21.5) | 0.43 |
| Insomnia | 351 (49.1) | 107 (49.1) | 0.72 |
| Irritability | 14 (1.97) | 1 (0.46) | 0.33 |
Values are given as number (percentage).
4. DISCUSSION
The principal findings of the present study are: (1) the collateral psychological impact of COVID‐19 in the overall pregnant population is high, with as much as 50% of the participants describing symptoms of anxiety and 25% reporting symptoms of depression; (2) the level of knowledge of pregnant women in Colombia regarding the potential deleterious effects of COVID‐19 in pregnancy is low, with misconceptions about fetal death, vertical transmission, and desire to terminate the pregnancy; and (3) poorly informed women were more likely to be younger, with a lower level of education and subsidized healthcare coverage.
The pandemic was first reported in Latin America in late February 2020. The first case in Colombia was confirmed by real‐time reverse‐transcription PCR (rRT‐PCR) on March 6, 2020. On July 27, 2020, there have been 257 101 reported cases with 8777 deaths and 131 161 recovered patients. 16 However, pregnancy status has not been included in the national statistics so far. Nevertheless, in agreement with a recent publication by Saccone et al., 9 two‐thirds of the pregnant women in the present study surveyed in Colombia showed symptoms associated with anxiety as well as concern about the vertical transmission of the virus and the effects on the fetus. It has been proposed that isolation, social distancing, and extreme changes in daily life may increase the risk of depression among vulnerable populations such as pregnant women. Therefore, it is of paramount importance to start assessing the psychological impact of the COVID‐19 outbreak in daily practice. 9
Currently, the clinical course of COVID‐19 in pregnant women is not completely understood, but so far, publications from mainly high‐income countries suggest minimal or no deleterious effects over the fetus and the pregnancy outcome. 1 , 2 , 8 , 9 , 10 , 11 , 12 , 13 , 17 , 18 , 19 , 20 , 21 Understanding the community’s knowledge of SARS‐CoV‐2 can be an important tool when developing and implementing future COVID‐19 educational strategies and interventions. The results from the present survey reflect the general knowledge from Colombian society on this pandemic and its effects on pregnancy. The observed association between the patients’ knowledge, their attitudes, level of education, type of assurance, and maternal age are thought‐provoking. Older women with a higher level of education and higher socioeconomic status (based on their health insurance) showed a significantly higher knowledge on the effects of SARS‐CoV‐2 during pregnancy. This association is relevant and may reflect a group that is at higher risk for developing mental health complications during pregnancy or later. Specific educational strategies on COVID‐19 and its effects on pregnancy are required for young pregnant women with less education and state insurance to help maintain their psychological well‐being during the current public health contingency. Finally, these findings can be used to formulate targeted psychological interventions directed at vulnerable groups identified in the present study. Among the limitations of the study is the non‐probability sampling; thus, convenience sampling was used, which may have created bias. However, the number of patients surveyed (n=1021) in different regions of the country suggests that this is a sample representative of the reality of pregnant women in Colombia at this time of the outbreak.
In conclusion, the results from the present study suggest that although the number of cases of SARS‐CoV‐2 infections during pregnancy is still moderate in our environment, the COVID‐19 pandemic may cause a major mental health burden in pregnant women. There are gaps in knowledge and information regarding multiple aspects of COVID‐19 among pregnant women, including misconceptions about the risks of vertical transmission, risk of congenital malformations, and impact at the time and route of delivery as the most relevant points.
AUTHOR CONTRIBUTIONS
MPS: conceptualization, methodology, data curation, writing—original draft preparation. IVV, JPO, LGP, PGC, ACR, DSV, ERM, SMG, MCS, HGB, EN, KFL, and ABO: data collection, writing—reviewing and editing the final manuscript. MSC: supervision, methodology, writing—reviewing and editing the final version of the manuscript. JM: conceptualization, methodology, data curation, formal analysis, writing—preparation of the original draft.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
Supporting information
File S1. Coronavirus SARS‐CoV‐2 (COVID 19) and pregnancy survey.
Acknowledgments
The authors express their gratitude to the following doctors for their collaboration in carrying out this study: Rafael Campanella, Oscar Roncallo Navas, Carlos Becerra, Arturo Cardona, Dahiana Gallo, Esperanza Burgos, Leila Bolívar, Rodrigo Otero, Pola Royo, Francisco Camargo, Alexei Bermudez, Omar Lopez, and Fabian Nassir.
REFERENCES
- 1. Elshafeey F, Magdi R, Hindi N, et al. A systematic scoping review of COVID‐19 during pregnancy and childbirth. Int J Gynecol Obstet. 2020;150:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dashraath P, Wong JLJ, Lim MXK, et al. Coronavirus disease 2019 (COVID‐19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222:521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. Coronavirus Disease 2019 (COVID‐19) and pregnancy: What obstetricians need to know. Am J Obstet Gynecol. 2020;222:415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rasmussen SA, Hayes EB. Public health approach to emerging infections among pregnant women. Am J Public Health. 2005;95:1942–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sanz Cortes M, Rivera AM, Yepez M, et al. Clinical assessment and brain findings in a cohort of mothers, fetuses and infants infected with ZIKA virus. Am J Obstet Gynecol. 2018;218:440.e1–440.e36. [DOI] [PubMed] [Google Scholar]
- 7. Parra‐Saavedra M, Reefhuis J, Piraquive JP, et al. Serial head and brain imaging of 17 fetuses with confirmed Zika virus infection in Colombia, South America. Obstet Gynecol. 2017;130:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lokken EM, Walker CL, Delaney S, et al. Clinical characteristics of 46 pregnant women with a SARS‐CoV‐2 infection in Washington State. Am J Obstet Gynecol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saccone G, Florio A, Aiello F, et al. Psychological Impact of COVID‐19 in pregnant women. Am J Obstet Gynecol. 2020;232:293–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crovetto F, Crispi F, Llurba E, Figueras F, Gomez‐Roig MD, Gratacos E. Seroprevalence and presentation of SARS‐CoV‐2 in pregnancy. Lancet. 2020;396(10250):530–531. 10.1101/2020.06.17.20134098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zanardo V, Manghina V, Giliberti L, Vettore M, Severino L, Straface G. Psychological impact of COVID‐19 quarantine measures in northeastern Italy on mothers in the immediate postpartum period. Int J Gynecol Obstet. 2020;150:184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Z, Wang Z, Xiong G. Clinical characteristics and laboratory results of pregnant women with COVID‐19 in Wuhan, China. Int J Gynecol Obstet. 2020;150:312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen D, Yang H, Cao Y, et al. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID‐19) infection. Int J Gynecol Obstet. 2020;149:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y, Chen H, Tang K, Guo Y. Clinical manifestations and outcome of SARS‐CoV‐2 infection during pregnancy. J Infect. 2020. 10.1016/j.jinf.2020.02.028 [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID‐19: A systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020;99:823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ministerio de protección social de Colombia . Source: https://www.ins.gov.co/Noticias/paginas/coronavirus.aspx. Accessed July 27, 2020.
- 17. Roberton T, Carter ED, Chou VB, et al. Early estimates of the indirect effects of the coronavirus pandemic on maternal and child mortality in low‐ and middle‐income countries. Lancet Glob Health. 2020;8:e901–e908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 (COVID‐19) in pregnant women: A report based on 116 cases. Am J Obstet Gynecol. 2020;223:111.e1–111.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karimi‐Zarchi M, Neamatzadeh H, Dastgheib SA, et al. Vertical transmission of Coronavirus disease 19 (COVID‐19) from infected pregnant mothers to neonates: A review. Fetal Pediatr Pathol. 2020;39:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. López M, Gonce A, Meler E, et al. Coronavirus disease 2019 in pregnancy: A clinical management protocol and considerations for practice. Fetal Diagn Ther. 2020;47:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID‐19) during pregnancy: a systematic review and meta‐analysis. Am J Obstet Gynecol MFM. 2020;2:100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. Coronavirus SARS‐CoV‐2 (COVID 19) and pregnancy survey.


