Abstract
Background
Clinical presentation and outcomes of COVID‐19 infection during pregnancy remain limited and fragmented.
Objectives
To summarize the existing literature on COVID‐19 infection during pregnancy and childbirth, particularly concerning clinical presentation and outcomes.
Search strategy
A systematic search of LitCovid, EBSCO MEDLINE, CENTRAL, CINAHL, Web of Science, and Scopus electronic databases. The references of relevant studies were also searched.
Selection criteria
Identified titles and abstracts were screened to select original reports and cross‐checked for overlap of cases.
Data collection and analysis
A descriptive summary organized by aspects of clinical presentations (symptoms, imaging, and laboratory) and outcomes (maternal and perinatal).
Main results
We identified 33 studies reporting 385 pregnant women with COVID‐19 infection: 368 (95.6%) mild; 14 (3.6%) severe; and 3 (0.8%) critical. Seventeen women were admitted to intensive care, including six who were mechanically ventilated and one maternal mortality. A total of 252 women gave birth, comprising 175 (69.4%) cesarean and 77 (30.6%) vaginal births. Outcomes for 256 newborns included four RT‐PCR positive neonates, two stillbirths, and one neonatal death.
Conclusion
COVID‐19 infection during pregnancy probably has a clinical presentation and severity resembling that in non‐pregnant adults. It is probably not associated with poor maternal or perinatal outcomes.
Keywords: Childbirth, Coronavirus, COVID‐19, Neonate, Pregnancy, Systematic scoping review
Short abstract
COVID‐19 infection during pregnancy probably has clinical presentation and severity similar to non‐pregnant adults and is probably not associated with poor maternal or perinatal outcomes.
1. INTRODUCTION
COVID‐19 is caused by a virus that belongs to the coronaviruses, which are positive‐sense, single‐stranded RNA viruses. Coronaviruses contain the largest genomes of all RNA viruses. They have helical nucleocapsids and an envelope that is derived from intracellular membranes. Electron micrographs show spikes sticking out of their surfaces (due to a large glycoprotein), leading to their name (corona=crown). Seven coronaviruses cause human disease, of which three are highly pathogenic: SARS‐CoV, MERS‐CoV, and the new SARS‐COV‐2, which causes COVID‐19. Phylogenic analysis and genome sequencing identified SARS‐COV‐2 as a member of the subgenus Sarbecovirus (beta‐CoV lineage B), similar to the severe acute respiratory syndrome (SARS) virus, but in a different clade. 1
The spectrum of symptomatic infection ranges from mild to critical. In a report that included approximately 44 672 confirmed infections, the severity was mild (no or mild pneumonia) in 81%; severe (e.g. with more than 50% lung involvement on imaging within 24–48 hours) in 14%; or critical (e.g. with respiratory failure or multiorgan dysfunction) in 5%. The overall case fatality rate was 2.3%. 2
Viral pandemics threaten the general population including pregnant women. The generalization of pregnancy as a condition of immune suppression or increased risk of infection represents a misleading concept. Pregnancy represents a unique immune condition that is modulated, but not suppressed. The correct concept would allow caregivers and policy makers to make valid recommendations for treating pregnant women during pandemics. 3
In this critical time of a COVID‐19 pandemic, caregivers need to understand the spectrum of presentations and outcomes of COVID‐19 infection during pregnancy and childbirth. Therefore, the aim of the present scoping review was to systematically map the state of knowledge of COVID‐19 infection during pregnancy, particularly as it pertains to clinical presentation and outcomes.
2. MATERIALS AND METHODS
This review followed the five stages outlined in the Arksey and O’Malley framework. 4
2.1. Stage 1: Identifying research questions
The following questions guided this scoping review of COVID‐19 infection during pregnancy: What is the clinical presentation of COVID‐19 during pregnancy? What is the spectrum of COVID‐19 disease severity during pregnancy? What are the maternal adverse outcomes in cases of COVID‐19? What are the fetal and neonatal outcomes in cases of COVID‐19?
2.2. Stage 2: Identifying relevant studies
We conducted a systematic search using the LitCovid, EBSCO MEDLINE, CENTRAL, CINAHL, Web of Science, and Scopus electronic databases. The search was last updated on April 19, 2020. We did not impose any language restriction. The detailed search strategy can be found in Data S1. Following study selection (stage 3), we searched the list of references in the selected studies. We contacted the authors of published reports for additional information.
2.3. Stage 3: Study selection
Two authors (RM and FE) independently screened citation titles and abstracts, then reviewed potentially relevant articles in full. We considered any article reporting original research of COVID‐19 during pregnancy, whether diagnosis was confirmed by reverse‐transcription polymerase chain reaction (RT‐PCR) or based on clinical, imaging, and laboratory criteria. If agreement on abstract or full article inclusion could not be reached between the two reviewers, an opinion was requested from a third reviewer (AN).
2.4. Stage 4: Data charting process
A data‐charting electronic form was jointly developed by two reviewers (NH and ME) to determine which variables to extract. Two authors (NF and MS) independently extracted data and (SM, MN, AA, MK and MME) continuously updated the data‐charting form. We extracted the following data items: general data (title, year of publication, author’s name, country); methodological data (research design, setting, sample, participant characteristics—e.g. age, gestational age at diagnosis); and clinical data (clinical presentation, illness severity, gestational age at delivery, mode of delivery, mode of analgesia or anesthesia, maternal outcomes, perinatal outcomes). We did not perform a formal critical appraisal of primary studies for this scoping review.
2.5. Stage 5: Summarizing results
The results were organized under the following categories: clinical presentation, maternal outcomes, and perinatal outcomes.
We reported the review following the Preferred Reporting Items for Systematic Review and Meta‐Analysis (PRISMA) guidelines – extension for scoping review. 5
3. RESULTS
We identified 33 original studies reporting 385 women with COVID‐19 during pregnancy and childbirth. Figure 1 shows the process of study inclusion in the scoping review.
Figure 1.
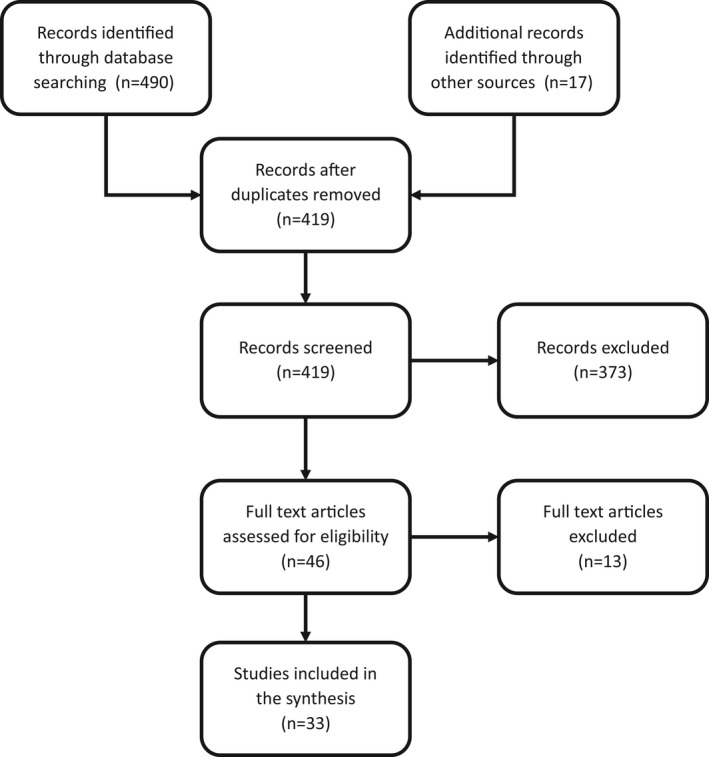
Study flowchart.
The studies included one case–control study from China, 6 16 case reports (from Australia, 7 China, 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 Honduras, 17 Iran, 18 South Korea, 19 Sweden, 20 Turkey, 21 and the USA 22 ), and 16 case series (from China, 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 Italy, 35 The Netherlands, 36 and the USA 37 , 38 ). We mapped the distribution of studies according to the study design (Table 1). The studies reported data between December 8, 2019 and April 19, 2020.
Table 1.
Distribution of study designs included in the review.
| Design | Country | Count of reports | Sum of COVID‐19 cases |
|---|---|---|---|
| Case–control | China | 1 | 16 |
| Case report | Australia | 1 | 1 |
| China | 9 | 9 | |
| Honduras | 1 | 1 | |
| Iran | 1 | 1 | |
| South Korea | 1 | 1 | |
| Sweden | 1 | 1 | |
| Turkey | 1 | 1 | |
| USA | 1 | 1 | |
| Case series | China | 12 | 154 |
| Italy | 1 | 42 | |
| Netherland | 1 | 107 | |
| USA | 2 | 50 | |
| Total | 33 | 385 |
3.1. Clinical presentation
Maternal age of the reported cases ranged from 21–42 years. Infection was asymptomatic in 29 (7.5%) women. Symptoms at the time of diagnosis were reported in most women (n=356, 92.5%). The most frequent symptoms were fever (n=259, 67.3%); cough (n=253, 65.7%); dyspnea (n=28, 7.3%); diarrhea (n=28, 7.3%); sore throat (n=27, 7.0%); fatigue (n=27, 7.0%); myalgia (n=24, 6.2%); and chills (n=21, 5.5%). Other symptoms were reported in less than 5% of women and included nasal congestion, rash, sputum production, headache, malaise, and loss of appetite. Symptoms appeared in the postpartum period in 19 (4.9%) women.
Laboratory confirmation, using RT‐PCR, was reported in 346 (89.9%) women. The specimens were collected using nasopharyngeal swabs. Additional specimens were collected in the form of nasal swab, vaginal swab, urine, stool, and sputum.
Clinical and radiological features were the basis for diagnosis in 39 (10.1%) women. Chest imaging was reported in 161 (41.8%) women, yet usable data were available for 125 (32.5%). Of these 125 women, typical features in the chest CT scan were seen bilaterally in 99 (79.2%) women and unilaterally in 22 (17.6%). No abnormality on chest CT was reported in 4 (3.2%) women. The predominant radiological pattern was ground‐glass opacity in 102 (81.6%), consolidation in 22 (17.6%), or reticular in 1 (0.8%). Additional radiological features included thickening of the adjacent pleura in 1 (0.8%), pleural effusion in 9 (7.2%), atelectasis in 1 (0.8%), and crazy paving appearance in 1 (0.8%).
Laboratory findings included elevated D‐dimer in 86 (22.3%), elevated C‐reactive protein in 72 (18.7%), lymphopenia in 54 (14.0%), modest increase in liver enzymes (AST in 22 [5.7%], ALT in 21 [5.45%]), and thrombocytopenia in 4 (1.0%) women.
3.2. Pregnancy and childbirth
Gestational age at the time of diagnosis ranged from 6–41 weeks of gestation, with 276 (71.7%) beyond 24 weeks of gestation and 109 (28.3%) in early pregnancy. The course of pregnancy included birth in 252 (65.5%), ongoing pregnancy in 124 (32.2%), induced abortion in 4 (1.0%), spontaneous abortion in 3 (0.8%), and 2 (0.5%) women with a tubal pregnancy. Among the 252 women who gave birth, 175 (69.4%) were delivered by cesarean and 77 (30.6%) had a vaginal birth. Type of anesthesia was reported in 57 women, with neuraxial anesthesia (continuous epidural, combined spinal‐epidural, or spinal) used in 53 (93.0%) women and general endotracheal anesthesia used in 4 (7.0%) women. Twelve of the continuous epidural cases experienced hypotension (<30% of basal) that was managed intraoperatively.
3.3. Maternal outcomes
Case severity was mild in 368 (95.6%) women, severe in 14 (3.6%), and critical in 3 (0.8%). The critical cases had multiple organ dysfunction syndrome (MODS) including acute respiratory distress syndrome (ARDS). Seventeen (4.4%) women required admission to an intensive care unit (ICU) and 6 (1.6%) were mechanically ventilated, including one on extracorporeal membrane oxygenation (ECMO). All ICU admissions improved and were discharged, except for one mortality and one case on ECMO. On April 6, 2020 we contacted the authors of the cases series that included the case on ECMO for an update on her outcome. We did not receive the required data.
3.4. Perinatal outcomes
Among the 252 women who gave birth, 248 had singleton and four had twin pregnancy, with a total of 256 newborns. Gestational age at birth ranged from 30–41 weeks of gestation. Preterm birth (<37 weeks of gestation) occurred in 39 (15.2%) newborns. Birthweight ranged from 1520–4050 g. Low birth weight (<2500 g) was reported in 20 (7.8%) newborns. Intrauterine fetal distress was reported in 20 (7.8%) newborns.
Among the 256 newborns, reported outcomes included admission to NICU in 8 (3.1%), neonatal mechanical ventilation in 3 (1.2%), respiratory distress syndrome in 12 (4.7%), neonatal pneumonia in 3 (1.2%), and disseminated intravascular coagulation in 3 (1.2%). Mortality occurred in 3 cases. Two stillbirths were reported for two critical women (one maternal mortality and one woman on ECMO). One early neonatal death occurred due to complications of prematurity following cesarean delivery at 34 weeks for antepartum hemorrhage.
Four (1.6%) newborns, delivered by cesarean, had a positive RT‐PCR test result and were classified as mild. The sample in one of the four newborns was collected 36 hours after birth. The four newborns recovered and were discharged. Samples from their cord blood, placenta, and amniotic fluid were negative. Three (1.2%) newborns had positive IgM and 6 (2.3%) had a positive IgG test. PCR test for COVID‐19 in samples from 30 cord blood, 23 amniotic fluid, and 12 placentas were all negative. Data on breastfeeding were reported for 29 newborns, with 18 newborns breast fed. All samples of breast milk from 26 women tested negative for COVID‐19.
4. DISCUSSION
This scoping review identified 33 primary studies addressing COVID‐19 infection during pregnancy across various settings of care. In summary, the spectrum of illness severity in 385 pregnant women was 368 (95.6%) considered mild, 14 (3.6%) considered severe, and 3 (0.8%) critical cases. Seventeen women were admitted to an ICU (five mechanically ventilated, one on ECMO). Only one woman died. Childbirth occurred in 252 women (169.4% delivered by cesarean, 30.6% vaginal birth). Outcomes of 256 newborns included four RT‐PCR positive, two stillbirths, and one neonatal death.
Accumulating evidence suggests that a subgroup of patients with severe and critical COVID‐19 might have a cytokine storm syndrome. Viral infections might trigger a syndrome known as secondary hemophagocytic lymphohistiocytosis (sHLH). Patients with sHLH have fulminant hypercytokinemia and multiorgan failure that eventually cause death. Pulmonary involvement (including ARDS) occurs in 50% of patients with sHLH. Critical cases of COVID‐19 show a cytokine profile resembling sHLH, characterized by increased proinflammatory interleukins and tumor necrosis factor‐α and associated with mortality. 39 Pregnancy modulates the immune system. Human chorionic gonadotropin and progesterone inhibit the Th1 proinflammatory pathway via decreasing tumor necrosis factor‐α. 3 We speculate that this modulated immune system might protect pregnant women from the cytokine storm syndrome and associated morbidity and mortality. This might explain the results of reported outcomes among the 385 women in 33 reports.
We extracted data regarding potential vertical transmission. In four neonates who had RT‐PCR confirmed infection, samples from cord blood and amniotic fluid were negative. Based on the available data, we are uncertain of the mode of transmission since there is no evidence that these four cases were the result of a vertical transmission. The negative tests in the breast milk of 26 cases is probably reassuring for nursing mothers. Evidently, as more data are shared, our confidence in the estimates will improve. This will allow us to make better well‐informed decisions in this critical time.
Obstetricians and those caring for mothers and newborns need more sharing of accurate, routinely collected health data of COVID‐19 cases during pregnancy. This need is currently being addressed by developing disease registries in different countries including PRIORITY in the USA, one in the Republic of Ireland, one in the Netherlands, and an international registry in Switzerland. Prospective structured data collection will help future research projects and will allow a clear understanding of the risks associated with COVID‐19 infection during pregnancy. Networks of healthcare facilities and research organizations should work to create a responsive data collection system to ensure a rapid assessment of the risks linked to future emergent pathogens.
The development of and adherence to a set of core outcomes for reporting studies of COVID‐19 during pregnancy is essential. Finally, we believe that the inclusion of pregnant women in current trials may be considered.
Our scoping review has some limitations. To make our review feasible, we were only able to include data from published or shared individual patient data. We believe that, amid a critical time of a pandemic, cases of COVID‐19 during pregnancy may not have been shared by every healthcare facility. This is a rapidly evolving healthcare issue. This scoping review was an enormous undertaking and our results are only up to date to April 20, 2020. We did not assess the quality of reports. We understand that during a pandemic, the intention to share data rapidly might have an impact on the quality of published primary reports. Some of the primary sources might overlap. We have traced the cases through careful data collection and contacting the authors to minimize the possibility of double counting.
In conclusion, the currently available data suggest that COVID‐19 infection during pregnancy has a similar clinical presentation and illness severity to non‐pregnant adults and may not be associated with poor maternal or perinatal outcomes.
AUTHOR CONTRIBUTIONS
All authors made substantive intellectual contributions to the development of this manuscript. AN contributed to the study conception and design and conceptualized the review approach. NH, FE, RM, MS, MN, NF, ME, SM, MK, AA, SG, and MME contributed to the screening, study selection, data charting, and data extraction. AN led the manuscript writing. All authors provided detailed comments on earlier drafts and approved the final manuscript.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
Supporting information
Data S1. Detailed search strategy.
Acknowledgments
We thank Mr Tianxiao Xu for his assistance with the translation of the Chinese papers. We are grateful to Professor Hassan Awwad, Chairman of the Department of Obstetrics and Gynecology at Ain Shams University for his guidance and support.
REFERENCES
- 1. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID‐19) outbreak in China. JAMA. 2020;323:1239. [DOI] [PubMed] [Google Scholar]
- 3. Mor G, Cardenas I. The immune system in pregnancy: A unique complexity. Am J Reprod Immunol. 2010;63:425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arksey H, O’Malley L. Scoping studies: Towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. [Google Scholar]
- 5. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): Checklist and explanation. Ann Intern Med. 2018;169:467–473. [DOI] [PubMed] [Google Scholar]
- 6. Li N, Han L, Peng M, et al. Maternal and neonatal outcomes of pregnant women with COVID‐19 pneumonia: A case‐control study. Clin Infect Dis. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lowe B, Bopp B. COVID‐19 vaginal delivery – a case report. Aust N Z J Obstet Gynaecol. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou R, Chen Y, Lin C, et al. Asymptomatic COVID‐19 in pregnant woman with typical chest CT manifestation: A case report. Chin J Perinat Med. 2020;23. 10.3760/cma.j.cn113903-20200220-00134. http://rs.yiigle.com/yufabiao/1187185.htm. Accessed March 4, 2020. [DOI] [Google Scholar]
- 9. Yao L, Wang J, Zhao J, Cui J, Hu Z. Asymptomatic COVID‐19 infection in pregnant woman in the third trimester: A case report. Chin J Perinat Med. 2020;23. 10.3760/cma.j.cn113903-2020221-00143. http://rs.yiigle.com/yufabiao/1183315.htm. Accessed March 2, 2020. [DOI] [Google Scholar]
- 10. Xiong X, Wei H, Zhang Z, et al. Vaginal delivery report of a healthy neonate born to a convalescent mother with COVID19. J Med Virol. 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wen R, Sun Y, Xing Q‐S. A patient with SARS‐CoV‐2 infection during pregnancy in Qingdao, China. J Microbiol Immunol Infect. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liao X, Yang H, Kong J, Yang H. Chest CT findings in a pregnant patient with 2019 Novel Coronavirus disease. Balkan Med J. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Zhao R, Zheng S, et al. Lack of vertical transmission of severe acute respiratory syndrome Coronavirus 2, China. Emerg Infect Dis. 2020;26 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kang X, Zhang R, He H, et al. Anesthesia management in cesarean section for a patient with coronavirus disease 2019 [in Chinese]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dong L, Tian J, He S, et al. Possible vertical transmission of SARS‐CoV‐2 from an infected mother to her newborn. JAMA. 2020;e204621 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X, Zhou Z, Zhang J, Zhu F, Tang Y, Shen X. A case of 2019 Novel Coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zambrano LI, Fuentes‐Barahona IC, Bejarano‐Torres DA, et al. A pregnant woman with COVID‐19 in Central America. Travel Med Infect Dis. 2020;101639 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karami P, Naghavi M, Feyzi A, et al. Mortality of a pregnant patient diagnosed with COVID‐19: A case report with clinical, radiological, and histopathological findings. Travel Med Infect Dis. 2020;101665 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee DH, Lee J, Kim E, Woo K, Park HY, An J. Emergency cesarean section on severe acute respiratory syndrome coronavirus 2 (SARS‐ CoV‐2) confirmed patient. Korean J Anesthesiol. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gidlöf S, Savchenko J, Brune T, Josefsson H. COVID‐19 in pregnancy with comorbidities: More liberal testing strategy is needed. Acta Obstet Gynecol Scand. 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21. Kalafat E, Yaprak E, Cinar G, et al. Lung ultrasound and computed tomographic findings in pregnant woman with COVID‐19. Ultrasound Obstet Gynecol. 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22. Iqbal SN, Overcash R, Mokhtari N, et al. An uncomplicated delivery in a patient with Covid‐19 in the United States. N Engl J Med. 2020;382:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020;395:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen R, Zhang Y, Huang L, Cheng B‐H, Xia Z‐Y, Meng Q‐T. Safety and efficacy of different anesthetic regimens for parturients with COVID‐19 undergoing Cesarean delivery: A case series of 17 patients. Can J Anesth. 2020;1–9 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeng L, Xia S, Yuan W, et al. Neonatal Early‐Onset Infection With SARS‐CoV‐2 in 33 Neonates Born to Mothers With COVID‐19 in Wuhan, China. JAMA Pediatr. 2020;e200878 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019‐nCoV pneumonia. Transl Pediatr. 2020;9:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen S, Liao E, Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol, 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khan S, Peng L, Siddique R, et al. Impact of COVID‐19 infection on pregnancy outcomes and the risk of maternal‐to‐neonatal intrapartum transmission of COVID‐19 during natural birth. Infect Control Hosp Epidemiol. 2020;1–3 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lei D, Wang C, Li C, et al. Clinical characteristics of COVID‐19 in pregnancy: Analysis of nine cases. Chin J Perinat Med. 2020;23. 10.3760/cma.j.cn113903-20200216-00117 [Epub ahead of print]. http://rs.yiigle.com/yufabiao/1186828.htm. Accessed March 2, 2020. [DOI] [Google Scholar]
- 30. Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with Coronavirus disease (COVID‐19) pneumonia: A preliminary analysis. Am J Roentgenol. 2020;1–6 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31. Liu Y, Chen H, Tang K, Guo Y. Clinical manifestations and outcome of SARS‐CoV‐2 infection during pregnancy. J Infect. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu X, Sun R, Chen J, Xie Y, Zhang S, Wang X. Radiological findings and clinical characteristics of pregnant women with COVID‐19 pneumonia. Int J Gynecol Obstet. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu N, Li W, Kang Q, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID‐19 in Wuhan, China: A retrospective, single‐centre, descriptive study. Lancet Infect Dis. 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng H, Xu C, Fan J, et al. Antibodies in Infants Born to Mothers With COVID‐19 Pneumonia. JAMA. 2020;e204861 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferrazzi EM, Frigerio L, Cetin I, et al. COVID‐19 Obstetrics Task Force, Lombardy, Italy: Executive management summary and short report of outcome. Int J Gynecol Obstet. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nederlandse Vereniging Voor Obstetrie en Gynaecologie . Update registration COVID‐19 positive pregnant women in NethOSS [in Dutch] [website]. 2020. https://www.nvog.nl/actueel/registratie‐van‐covid‐19‐positieve‐zwangeren‐in‐nethoss. Accessed April 18, 2020.
- 37. Breslin N, Baptiste C, Miller R, et al. COVID‐19 in pregnancy: Early lessons. Am J Obstet Gynecol MFM. 2020;100111. 10.1016/j.ajogmf.2020.100111. Accessed April 18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Juusela A, Nazir M, Gimovsky M. Two cases of COVID‐19 related cardiomyopathy in pregnancy. Am J Obstet Gynecol MFM. 2020;100113. 10.1016/j.ajogmf.2020.100113. Accessed April 18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Detailed search strategy.


