Abstract
Background
The coronavirus disease 2019 (COVID‐19) pandemic caused by severe acute respiratory syndrome coronavirus 2 has had dramatic effects on the pregnant population worldwide, increasing the risk of adverse perinatal outcomes.
Objective
To assess the incidence of antepartum stillbirth (aSB) during the COVID‐19 pandemic in Austria.
Methods
We collected epidemiological data from the Austrian Birth Registry and compared the rate of aSB (i.e., fetal death at or after 24+0 gestational weeks) during the pandemic period (March–December 2020) and in the respective pre‐pandemic months (2015–2019).
Results
In total, 65 660 pregnancies were included, of which 171 resulted in aSB at 33.7 ± 4.8 gestational weeks. During the pandemic, the aSB rate increased from 2.49‰ to 2.60‰ (P = 0.601), in contrast to the significant decline in preterm deliveries at or before 37 gestational weeks from 0.61‰ to 0.56‰ (relative risk [RR] 0.93; 95% confidence interval [CI] 0.91–0.96; P < 0.001). During the first lockdown, the aSB rate significantly increased from 2.38‰ to 3.52‰ (P = 0.021), yielding an adjusted odds ratio of 1.57 (95% CI 1.08–2.27; P = 0.018). The event of aSB during the COVID‐19 pandemic was strongly related with increased fetal weight and maternal obesity.
Conclusion
In Austria, there has been an overall increase in the incidence of aSB during the pandemic with a significant peak during the first lockdown.
Keywords: antepartum stillbirth, coronavirus disease 2019, epidemiology, fetal death, perinatal mortality
1. INTRODUCTION
In March 2020, the World Health Organization declared coronavirus disease 2019 (COVID‐19)—caused by the the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)—a global pandemic 1 and ever since, the disease has had profound direct and indirect effects on the pregnant population worldwide. On the one hand, the direct effects of SARS‐CoV‐2 have caused a wide spectrum of symptoms in pregnant women in the case of infection, leading to a mild to severe maternal disease with increased risk for pulmonary disease and need for intensive care. 2 , 3 On the other hand, placental infections threaten fetal well‐being, contributing to both placental dysfunction and vertical transmission to the fetus. 4
The indirect effect of the pandemic may hypothetically involve the individual's adoption of poor lifestyle habits due to social distancing, lockdowns, and redundancy, fostering unfavorable behaviors, such as smoking, high‐caloric intake, and becoming more sedentary. 5 Also health‐seeking behavior rapidly decreased, resulting in less frequent clinical check‐ups. Lastly, closures and reductions in the public healthcare system with disruptions to follow‐up visits and regular obstetric monitoring significantly impacted the management of both low‐ and high‐risk pregnancies. 6
We hypothesized that the restrictions in healthcare services and changes in prenatal care in Austria during the pandemic have negatively impacted perinatal outcomes, as reflected by the prevalence of antepartum stillbirth. We, therefore, conducted a population‐based study to assess the antepartum stillbirth rate—defined as fetal death at or after 24+0 gestational weeks, during the pandemic (March–December 2020) and in the pre‐pandemic era (matched months 2015–2019).
2. MATERIALS AND METHODS
2.1. Data collection and study design
The Austrian Birth Registry prospectively collects maternal demographic and perinatal data from the obstetrical database viewpoint (General Electric Company) of all maternity units in Austria, including registered home deliveries. For this study, we retrieved data from the Austrian Birth Registry during two distinct time periods: from March 16, 2020 to April 13, 2020 (reflecting the first lockdown phase in Austria), and from March 1, 2020 to December 31, 2020 (reflecting the pandemic era in Austria). Data were compared with the analogue periods between 2015 and 2019. We included all singleton live and stillborn deliveries at or after 24+0 gestational weeks. We excluded multiple pregnancies, intrapartum and perinatal deaths, and stillborn fetuses following late terminations of pregnancy and those with congenital anomalies (Figure 1). After a data check for integrity and consistency, all patient data were de‐identified and the database was frozen before analysis.
FIGURE 1.
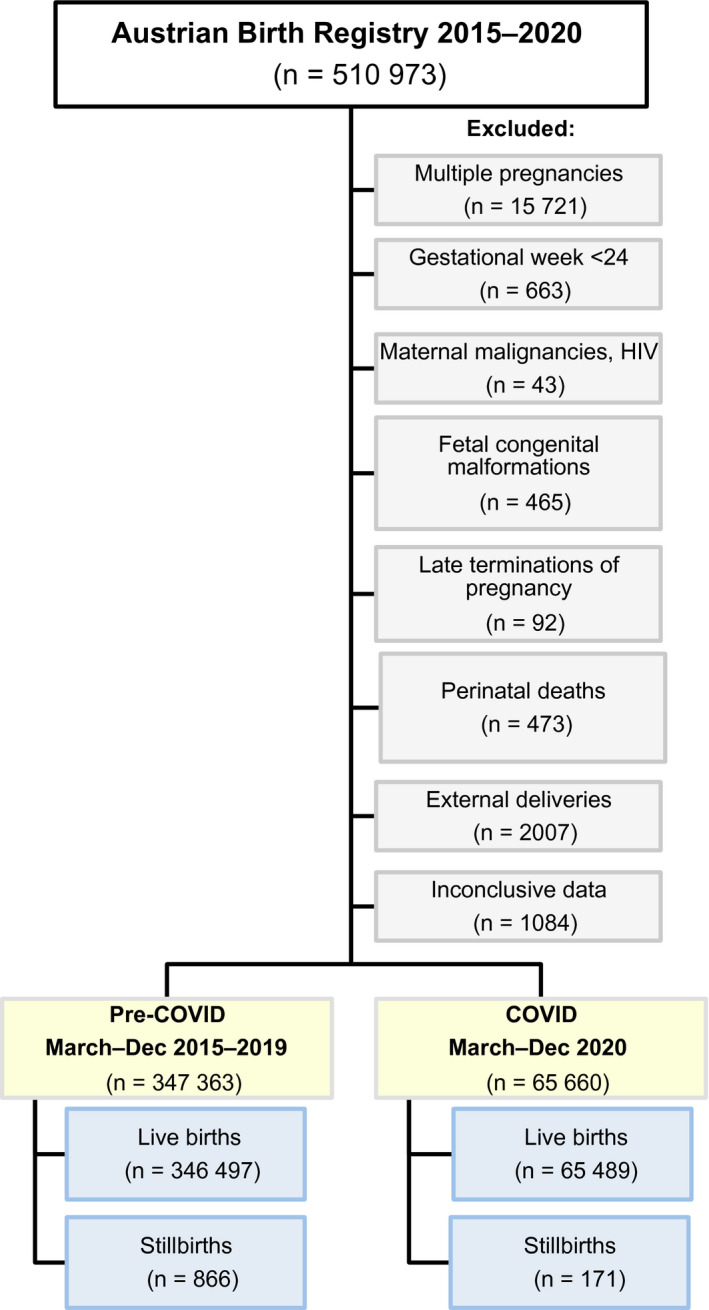
Flowchart on the selection of the study population from the Austrian Birth Registry between 2015 and 2020
2.2. Definitions
In Austria, the government issued three lockdown phases to reduce the spread of SARS‐CoV‐2. The first lockdown was undertaken from March 16 to April 13, 2020 (i.e.,, 27 days), the second lockdown was from November 17 to December 6, 2020 (i.e., 19 days), and the third lockdown was from December 26, 2020 to February 7, 2021 (i.e., 41 days). As the first lockdown was the most tight and severe phase in this country, our analyses refer to this phase only.
2.3. Statistical analysis
Categorical data are presented as absolute (n) and relative (%) frequencies. We compared categorical data with the χ2 test, and continuous data with an unpaired t test. Log‐binomial regression and logistic regression were performed to assess risk ratios (RR) and odds ratios (OR). All ratios and difference measures are accompanied by a 95% confidence interval (CI). A two‐sided P value less than 0.05 represents statistical significance. The analyses were performed using STATA 16 (StataCorp LLC, College Station, TX, USA).
2.4. Ethical permission
The study was approved by the Ethics Committee of the Medical University of Vienna (Registration number 1637/2020) and complied with the principles as outlined in the Declaration of Helsinki and Good Clinical Practice guidelines. Participants' written consent was not required as per the Austrian Federal Act (Protection of Personal Data Regulation, §46, Paragraph 1; 2000).
2.5. Dissemination to participants and related patient and public communities
Patients and the public were not involved in the design, conduct, reporting, interpretation, and dissemination of the results of this study.
3. RESULTS
3.1. Baseline population characteristics
After consideration of the inclusion and exclusion criteria (Figure 1), during the pandemic between March and December 2020, a total of 65 660 singleton deliveries were registered in Austria at a mean ± standard deviation gestational age of 39.0 ± 1.7 weeks, of which 33 831 (51.5%) newborns were male and 31 805 (48.4%) were female (0.1% unreported sex; n = 24).
In the pre‐pandemic era between March and December 2015–2019, the total study population consisted of 347 363 deliveries of singletons at a mean gestational age of 39.0 ± 1.8 weeks, of which 178 533 (51.4%) newborns were male and 168 720 (48.6%) were female (0.1% of not reported sex, n = 110). Mean maternal age was 30.5 ± 5.3 years at the time of delivery, with a mean body mass index (BMI; calculated as weight in kilograms divided by the square of height in meters) of 23.9 ± 4.8 kg/m2.
During the pandemic and in comparison with the preceding years, the total pregnant female population was older (30.7 ± 5.3 years; P < 0.001), had a higher BMI (24.3 ± 5.3; P < 0.001), and a higher parity (25.8% versus 22.8%; P < 0.001). Likewise, mean newborn weight and length were higher during the pandemic than in the previous years (3364 ± 546 g vs. 2247 ± 521 g and 50.9 ± 3.3 cm vs. 50.7 ± 2.7 cm, respectively; P < 0.001).
3.2. Antepartum stillbirth rate during the COVID‐19 pandemic in Austria
The rate of preterm delivery at or before 37 gestational weeks decreased from 6.1% to 5.6% (RR 0.93; 95% CI 0.90–0.96) during the pandemic compared with the pre‐pandemic era (P < 0.001), the stillbirth rate increased from 2.49‰ to 2.60‰ (RR 1.1; 95% CI 1.06–1.17; P = 0.601; Table 1).
TABLE 1.
Risk for stillbirth and preterm birth in Austria during the pandemic period (March to December 2020) in comparison with the same time period of the preceding years
| Events (per 1000 births) | Odds ratio (95% confidence interval) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre‐COVID‐19 | COVID‐19 | |||||||
| Births in March to December 2015–2019 | Births in March to December 2020 | Unadjusted | P value | Adjusted | P value | |||
| (n = 347 363) | (n = 65 660) | |||||||
| n | ‰ | n | ‰ | |||||
| Stillbirth | 866 | 2.49 | 171 | 2.60 | 1.04 (0.89–1.23) | 0.601 | 1.03 (0.86–1.23) a | 0.751 |
| 1.05 (0.87–1.26) b | 0.644 | |||||||
| Preterm birth (<37+0 weeks) | 21 196 | 61.02 | 3695 | 56.27 | 0.93 (0.90–0.96) | <0.001 | ||
| Extremely preterm (<28+0 weeks) | 936 | 44.16 | 173 | 46.82 | ||||
| Very preterm (28+0 to 31+6 weeks) | 1912 | 90.21 | 336 | 90.93 | ||||
| Moderately preterm (32+0 to 36+6 weeks) | 18 348 | 865.64 | 3186 | 862.25 | ||||
Adjusted for maternal age, parity, body mass index.
Adjusted for maternal age, parity, body mass index, and smoking status.
In particular, during the first lockdown phase, the risk of experiencing stillbirth increased to RR 1.48 (95% CI 1.06–1.06; P = 0.022), and to RR 1.56 (95% CI 1.08–2.27; P = 0.018) after adjustment for maternal age, BMI, and nicotine consumption (Table 2), resulting in an increased stillbirth rate from 2.38 to 3.52 per 1000 live births between March and April 2020, compared with the preceding years (P = 0.021; Figure 2).
TABLE 2.
Risk for stillbirth and preterm birth in Austria during the lockdown period (March to April 2020) in comparison to the same time period of the preceding years
| Events (per 1000 births) | Odds ratio (95% confidence interval) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre‐COVID−19 | COVID−19 | |||||||
| Births in March to April 2015–2019 | Births in March to April 2020 | Unadjusted | P value | Adjusted | P value | |||
| (n = 66 334) | (n = 12 517) | |||||||
| n | ‰ | n | ‰ | |||||
| Stillbirth | 158 | 2.38 | 44 | 3.52 | 1.48 (1.06–2.07) | 0.022 | 1.45 (1.00–2.10) a | 0.05 |
| 1.57 (1.08–2.27) b | 0.018 | |||||||
| Preterm birth (<37+0 weeks) | 3985 | 60.07 | 716 | 57.2 | 1.00 (0.88–1.14) | 0.213 | ||
| Extremely preterm (<28+0 weeks) | 213 | 53.45 | 40 | 57.2 | ||||
| Very preterm (28+0 to 31+6 weeks) | 397 | 99.62 | 68 | 94.97 | ||||
| Moderately preterm (32+0 to 36+6 weeks) | 3375 | 846.93 | 608 | 849.16 | ||||
Adjusted for maternal age, parity, body mass index.
Adjusted for maternal age, parity, body mass index, and smoking status.
FIGURE 2.
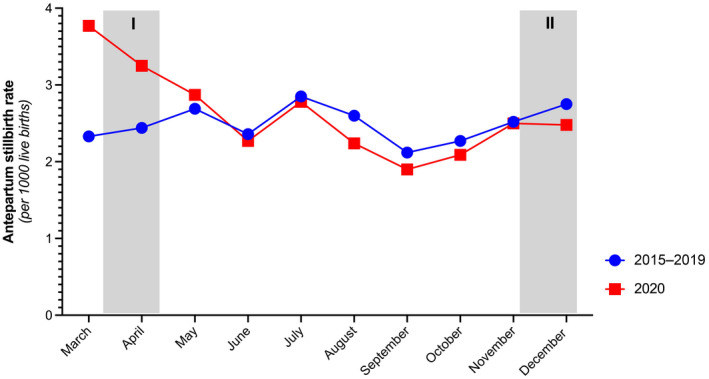
Monthly antepartum stillbirth rates per 1000 live births during the COVID‐19 pandemic (March to December 2020) and the preceding years (2015–2019) in Austria. Grey column signifies the first (I) lockdown (March 16 to April 13, 2020) and the second (II) lockdown (November 17 to December 6, 2020) in Austria
3.3. Risk factors for experiencing antepartum stillbirth in Austria
Table 3 shows the fetal and maternal characteristics in antepartum stillbirth events during the pandemic and pre‐pandemic eras.
TABLE 3.
Fetomaternal characteristics of stillbirth events in Austria from March to December, and from March to April, respectively, during the pandemic period and the preceding years a
| Pandemic months March to December | Lockdown months March to April | |||||
|---|---|---|---|---|---|---|
| Pre‐COVID−19 (2015–2019) | COVID−19 (2020) | P value | Pre‐COVID−19 (2015–2019) | COVID−19 (2020) | P value | |
| Fetal | ||||||
| Stillbirth events | 866 | 171 | 158 | 44 | ||
| Gestational week | 32.9 ± 5.0 | 33.7 ± 4.8 | 0.085 b | 32.5 ± 4.8 | 33.1 ± 4.9 | 0.426 b |
| Sex | ||||||
| Male | 436 (50.3%) | 95 (55.6%) | 0.424 c | 84 (53.2%) | 20 (45.5%) | 0.556 c |
| Female | 429 (49.5%) | 76 (44.4%) | 73 (46.2%) | 24 (54.5%) | ||
| Unknown | 1 (0.1%) | 1 (0.6%) | ||||
| Weight (g) | 1984 ± 989 | 2141 ± 1016 | 0.072 b | 1924.9 ± 1006.4 | 2026.2 ± 984.8 | 0.491 b |
| Length (cm) | 43.5 ± 7.5 | 44.7 ± 7.5 | 0.057 b | 43.0 ± 7.4 | 43.6 ± 7.5 | 0.578 b |
| Maternal | ||||||
| Age (years) | 30.6 ± 5.9 | 31.0 ± 5.5 | 0.581 b | 30.8 ± 6.0 | 30.5 ± 5.0 | 0.918 b |
| Parity | ||||||
| Nullipara | 418 (48.3%) | 90 (52.6%) | 0.219 c | 70 (44.3%) | 25 (56.8%) | 0.311 c |
| Primipara | 227 (26.2%) | 34 (19.9%) | 46 (29.1%) | 11 (25.0%) | ||
| Multipara | 221 (25.5%) | 47 (27.5%) | 42 (26.6%) | 8 (18.2%) | ||
| Body Mass Index (kg/m2) | 24.5 ± 4.7 | 25.0 ± 6.1 | 0.882 b | 24.7 ± 4.3 | 23.4 ± 5.3 | 0.036 b |
| WHO BMI Classification | ||||||
| Underweight | 36 (5.1%) | 10 (7.0%) | 0.007 c | 4 (3.1%) | 3 (8.3%) | 0.167 c |
| Normal weight | 394 (55.9%) | 83 (58.0%) | 74 (57.8%) | 26 (72.2%) | ||
| Pre‐obesity | 190 (27.0%) | 22 (15.4%) | 35 (27.3%) | 4 (11.1%) | ||
| Obesity I | 62 (8.8%) | 16 (11.2%) | 12 9.4%) | 2 (5.6%) | ||
| Obesity II | 15 (2.1%) | 7 (4.9%) | 2 (1.6%) | |||
| Obesity III | 8 (1.1%) | 5 (3.5%) | 1 (0.8%) | 1 (2.8%) | ||
| Nicotine | 88 (10.2%) | 8 (4.9%) | 0.032 c | 20 (12.7%) | 3 (6.8%) | 0.276 c |
| Previous fetal loss | 2 (0.2%) | 3 (1.8%) | 0.009 c | 1 (2.3%) | 0.057 c | |
| Gestational diabetes | 25 (2.9%) | 9 (5.3%) | 0.111 c | 2 (1.3%) | 2 (4.5%) | 0.167 c |
| Other pregnancy risks | 22 (2.5%) | 5 (33.3%) | <0.001 c | 4 (2.5%) | 15 (34.1%) | <0.001 c |
Abbreviations: BMI, body mass Index (calculated as weight in kilograms divided by the square of height in meters); COVID‐19, coronavirus disease 2019; WHO, World Health Organization.
Values are presented as number, mean ± standard deviation, or as number (percentage).
Unpaired t test with level of significance P < 0.05.
χ2 test with level of significance P < 0.05.
The event of antepartum stillbirth during the pandemic was strongly related to increased fetal weight (RR 1.00, 95% CI 1.00–1.00; P < 0.001) and obesity class III (i.e., BMI ≥ 40.0: RR 2.54, 95% CI 1.00–6.40; P = 0.049), whereas previous fetal loss (RR 2.31, 95% CI 0.94–5.69; P = 0.069), other obstetric risk factors (RR 0.72, 95% CI 0.51–1.03; P = 0.072), obesity class II (i.e., 35.0–39.0: RR 1.69, 95% CI 0.76–3.74; P = 0.200), smoking (RR 1.33, 95% CI 0.65–2.71; P = 0.432), nulliparity (OR 1.01, 95% CI 0.69–1.49; P = 0.95), and preterm deliveries (OR 0.77, 95% CI 0.47–1.26; P = 0.305) were non‐influential.
4. DISCUSSION
During the COVID‐19 pandemic in Austria, overall obstetric characteristics had become significantly unfavorable in terms of concomitant pregnancy risk factors, advanced maternal age, and both maternal and fetal weight. Throughout the pandemic starting from March 2020, the Austrian stillbirth rate increased slightly by 1.1 cases per 100 live births, yet, during the first lockdown phase from March to April 2020, the stillbirth rate had significantly increased by 11.4 cases per 100 live births, culminating in the highest rate since the implementation of the Austrian Birth Registry, surpassing the peak of 3.05‰ in the year 2011. In identifying epidemiological characteristics for stillbirths in Austria during the pandemic, the adverse perinatal outcome was strongly associated with maternal and fetal weight, yet women experiencing stillbirth were found to more frequently have other obstetric risk factors and to more frequently have had previous fetal losses.
Our findings suggest that the nationwide lockdown with rapid disruption of healthcare services had a profound effect on the general well‐being of pregnant women and the surveillance of their pregnancies. The changes in the Austrian healthcare system might have also decreased potential numbers of iatrogenic prematurity to avoid adverse perinatal outcome, especially in the case of severely restricted fetuses, but the increase in stillbirth rates seems to act as a testimony for possible preventable fetal losses, if there had been sufficient opportunities to adequately monitor and manage high‐risk pregnancies, which had been state of the art during the pre‐COVID era.
The worldwide restrictions aimed at preventing COVID‐19 transmission seem to have exacerbated antenatal health across Europe, although, to date, the data are controversial. Based on a case series from the UK Obstetric Surveillance System study noting three stillbirths in 265 women with SARS‐CoV‐2 infection, 7 the incidence of stillbirth among women affected by COVID‐19 has been extrapolated to 11.5 per 1000 total births, reflecting a three‐fold increase compared with the national rate of 4.1 per 1000 live births in the UK. 8 As a reaction to gather more robust evidence, institutional, regional and national data have been analyzed. In a large London‐based tertiary referral center, the comparison of antepartum stillbirths (above 22 weeks of gestation and excluding terminations of pregnancy) from October 2019 to January 2020 and February to June 2020 resulted in a four‐fold institutional increase in the local stillbirth rate from 1.19‰ to 6.98‰ (OR 5.79, 95% CI 1.54–10.1; P = 0.01), 9 with a constantly high incidence of 14.2‰ throughout the following months. 10 To counterbalance these local data, a further UK study assessed regional and national hospitalization data from England, including antepartum stillbirths above 24 weeks of gestation, during the pandemic from April to June 2020 and in the pre‐pandemic period between 2016 and 2019, concluding neither a regional nor a national increase in stillbirth rates in England. 11 These data support the findings of another institutional study from the UK showing no statistical difference in rates of stillbirths or decrease in preterm births. 12 Further data published on stillbirth rates in Europe continued to reflect the conflicting evidence. A regional study from Italy observed a three‐fold increase from 1.07‰ to 3.23‰ between March and May 2020 versus 2019. 13 In contrast, in Denmark, stillbirth rates declined from a pre‐pandemic rate of 3.3‰ to 2.7‰ during the lockdown from April to May 2020 (adjusted OR 0.78, 95% CI 0.57–1.06) with no compensatory increase in the rate of preterm delivery. 14 Likewise, Spanish data confirm no increase either (OR 0.9, 95% CI 0.37–2.18) 15 as do the population data from Germany, showing a pre‐pandemic stillbirth rate of 4.24‰ between January and July 2019 compared with 4.15‰ between the respective months during the COVID‐19 pandemic. 16
Recent meta‐analyses on population stillbirth rates during the pandemic and historical cohorts from the pre‐pandemic era show that, in high‐income countries, stillbirth rates, unrelated to maternal SARS‐CoV‐2 infection, remained stable, as shown in the synthesis of 21 studies from 18 countries (OR 1.08, 95% CI 0.94–1.23; P = 1.23; adjusted OR 1.06, 95% CI 0.81–1.38); in a further 14 studies from nine countries (OR 1.38, 95% CI 0.94–2.02; P = 0.099; I2 = 52%) 18 ; or another analysis including 12 studies (OR 1.113, 95% CI 0.834–1.485). 19 In accordance with our findings, an increase in birth weight was reported by six studies (mean difference 17 g, 95% CI 7–28 g) during the pandemic period. 17
However, we agree that the incidence of antepartum stillbirth is still alarmingly high, and that “there has never been a more urgent time” to conceal the direct and indirect drivers of increased stillbirth rates during the pandemic. 20
The major strength of our study is the high quality of its validated data derived from a nationwide birth registry. Our strict inclusion and exclusion criteria resulted in a homogeneous and concise cohort of antepartum fetal deaths only, excluding late terminations of pregnancy and fetal congenital malformations, which may have led to stillbirth irrespective of the pandemic. Furthermore, the present study design comparing two distinct time periods, reduced potential seasonal variances that could influence the overall stillbirth rates.
Our study is limited by its retrospective design with potentially missing data, errors in maternal data collection across study sites, and the failure to control for recall bias. Of note, we lack insight into the exact causes of fetal death in individual cases, and also, no data were available on the maternal SARS‐CoV‐2 infection status before, during, and after the event of stillbirth. However, for the latter, the low numbers of pregnant women in Austria who had COVID‐19 during the study period, and particularly during the first lockdown phase, need to be considered.
In conclusion, in Austria, there has been an overall rise in the antepartum stillbirth rate during the COVID‐19 pandemic. Particularly during the first lockdown phase, there has been a significant incidence peak, associated with maternal and fetal weight. This should be considered with regard to future perinatal management, in order to maintain high‐quality perinatal care during the ongoing COVID‐19 pandemic.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
We confirm that all authors fulfilled all conditions required for authorship, as all authors contributed to the conception, planning, and carrying out of the research. DAM and AF conceived the study. DAM wrote the first draft of this paper. DAM, KW, HK, and AF designed the study. SN, HH, and HL acquired the data. DAM, SN, and HL conducted the statistical analyses. All authors contributed to critically revising the paper for important intellectual content and approved the final version to be published. All authors accept responsibility for the article as published.
ACKNOWLEDGMENTS
DAM acknowledges the support of Prof. DDr. J.C. Huber.
Muin DA, Neururer S, Falcone V, et al. Antepartum stillbirth rates during the COVID‐19 pandemic in Austria: A population‐based study. Int J Gynecol Obstet. 2022;156:459–465. doi: 10.1002/ijgo.13989
REFERENCES
- 1. World Health Organization: WHO's COVID‐19 response [Online]. Available: http://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/interactive‐timeline.
- 2. Favre G, Pomar L, Qi X, Nielsen‐Saines K, Musso D, Baud D. Guidelines for pregnant women with suspected SARS‐CoV‐2 infection. Lancet Infect Dis. 2020;20:652‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palmrich P, Roessler B, Wisgrill L, et al. Multiprofessional perinatal care in a pregnant patient with acute respiratory distress syndrome due to COVID‐19. BMC Pregnancy Childbirth, 2021;21:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong YP, Khong TY, Tan GC. The effects of COVID‐19 on placenta and pregnancy: what do we know so far? Diagnostics. 2021;11(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hillyard M, Sinclair M, Murphy M, Casson K, Mulligan C. The impact of COVID‐19 on the physical activity and sedentary behaviour levels of pregnant women with gestational diabetes. PLoS One. 2021;16:e0254364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goyal M, Singh P, Singh K, Shekhar S, Agrawal N, Misra S. The effect of the COVID‐19 pandemic on maternal health due to delay in seeking health care: experience from a tertiary center. Int J Gynaecol Obstet. 2021;152:231‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Knight M, Bunch K, Vousden N, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS‐CoV‐2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Magee LA, Khalil A, von Dadelszen P. Covid‐19: UK Obstetric Surveillance System (UKOSS) study in context. BMJ. 2020;370:m2915. [DOI] [PubMed] [Google Scholar]
- 9. Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O'Brien P, Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID‐19 pandemic. JAMA. 2020;324:705‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khalil A, von Dadelszen P, Ugwumadu A, Draycott T, Magee LA. Effect of COVID‐19 on maternal and neonatal services. Lancet Glob Health. 2021;9:e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stowe J, Smith H, Thurland K, Ramsay ME, Andrews N, Ladhani SN. Stillbirths during the COVID‐19 pandemic in England, April‐June 2020. JAMA. 2021;325:86‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vimalesvaran S, Shetty M, Khashu M. Letter to the Editor on the original article "Lack of changes in preterm delivery and stillbirths during COVID‐19 lockdown in a European region" by Juan Arnaez. Eur J Pediatr. 2021;1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Curtis M, Villani L, Polo A. Increase of stillbirth and decrease of late preterm infants during the COVID‐19 pandemic lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106(4):456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pasternak B, Neovius M, Söderling J, et al. Preterm birth and stillbirth during the COVID‐19 pandemic in sweden: a nationwide cohort study. Ann Intern Med. 2021;174(6):873‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arnaez J, Ochoa‐Sangrador C, Caserío S, et al. Lack of changes in preterm delivery and stillbirths during COVID‐19 lockdown in a European region. Eur J Pediatr. 2021;180(6):1997‐2002. doi: 10.1007/s00431-021-03984-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kniffka MS, Nitsche N, Rau R, Kühn M. Stillbirths in Germany: on the rise, but no additional increases during the first COVID‐19 lockdown. Int J Gynaecol Obstet. 2021. doi: 10.1002/ijgo.13832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang J, D’Souza R, Kharrat A, et al. COVID‐19 pandemic and population‐level pregnancy and neonatal outcomes: a living systematic review and meta‐analysis. Acta Obstet Gynecol Scand. 2021;100(10):1756‐1770. doi: 10.1111/aogs.14206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chmielewska B, Barratt I, Townsend R, et al. Effects of the COVID‐19 pandemic on maternal and perinatal outcomes: a systematic review and meta‐analysis. Lancet Glob Health. 2021;9(6):e759‐e772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mohan M, Appiah‐Sakyi K, Oliparambil A, Pullattayil AK, Lindow SW & Konje JC. A meta‐analysis of global stillbirth rates during the COVID‐19 pandemic. Authorea June 23, 2021. doi: 10.22541/au.162441694.43272789/v1 [DOI] [PMC free article] [PubMed]
- 20. Homer CSE, Leisher SH, Aggarwal N, et al. Counting stillbirths and COVID 19‐there has never been a more urgent time. Lancet Glob Health. 2021;9:e10‐e11. [DOI] [PMC free article] [PubMed] [Google Scholar]


