ABSTRACT
Objective
To study the impact of the COVID‐19 outbreak and subsequent lockdown on the incidence, associated causes, and modifiable factors of stillbirth.
Methods
An analytical case‐control study was performed comparing stillbirths from March to September 2020 (cases) and March to September 2019 (controls) in a tertiary care center in India. Modifiable factors were observed as level‐I, level‐II, and level‐III delays.
Results
A significant difference in the rate of stillbirths was found among cases (37.4/1000) and controls (29.9/1000) (P = 0.045). Abruption in normotensive women was significantly higher in cases compared to controls (P = 0.03). Modifiable factors or preventable causes were noted in 76.1% of cases and 59.6% of controls; the difference was highly significant (P < 0.001, relative risk [RR] 1.8). Level‐II delays or delays in reaching the hospital for delivery due to lack of transport were observed in 12.7% of cases compared to none in controls (P < 0.006, RR 47.7). Level‐III delays or delays in providing care at the facility were observed in 31.3% of cases and 11.5% of controls (P < 0.001, RR 2.7).
Conclusion
Although there was no difference in causes of stillbirth between cases and controls, level‐II and level‐III delays were significantly impacted by the pandemic, leading to a higher rate of preventable stillbirths in pregnant women not infected with COVID‐19.
Keywords: causes of stillbirth, level of delay, lockdown, pandemic, stillbirth
Short abstract
The COVID‐19 pandemic has caused more stillbirths due to the delay in pregnant women reaching hospital and denial or suboptimal care at facilities than the infection itself.
1. INTRODUCTION
The COVID‐19 pandemic is unabated. Unlike other elective medical and surgical problems for which care can be deferred during the pandemic, pregnancies and childbirths continue.
Studies have shown that the number of intrauterine deaths and stillbirths have increased during the COVID‐19 pandemic for multiple reasons, the main one being reduced access to healthcare services. 1 , 2 The imposition of lockdown to prevent the spread of the pandemic led to the shutdown of healthcare services and a shift of focus to the prevention and treatment of COVID‐19 infections. This resulted in the disruption of routine care and monitoring of pregnant women. 2 Psychological fear because of the pandemic further prevented pregnant women from seeking care and consulting healthcare providers as well as reluctance on the part of care providers to provide care. 3 For the less fortunate living below the poverty line, access to proper nutrition and supplements during the pandemic also contributed to the problem.
The rate of perinatal mortality is a sensitive indicator of the quality of care provided to women in pregnancy, during childbirth, and to the newborns in the first week of life. 4 As part of an ongoing study funded by the WHO South‐East Asia Regional Office (SEARO; Neonatal‐Perinatal Database Network) in selected hospitals of South‐East Asian countries, the data on neonatal and perinatal outcomes are collected at the study hospital. The aim of the present study was to compare data on stillbirths occurring before the pandemic with those during the pandemic to determine the impact of the COVID‐19 outbreak and subsequent lockdown on the incidence of stillbirth, its associated causes, and modifiable factors. This was done to understand the sociodemographic mechanisms that come into play in times of a pandemic, for providing insights to the policy makers for future planning.
2. MATERIALS AND METHODS
The present study is an analytical case‐control study, performed after ethical clearance from the institute's Ethical Committee for Human Research (ECHR). All infants delivered after 20 weeks of gestation showing no signs of life after birth were considered stillborn. All stillborn infants delivered at the institute during the study period of March to September 2020 were included in the study (cases). Gestational age was calculated according to the last menstrual period or first‐trimester ultrasound if the last menstrual period was not known. The antenatal record of each case was reviewed, and all relevant clinical findings and investigations were recorded in a stillbirth proforma especially designed for this purpose. The contributory cause of death was classified under the International Classification of Diseases [ICD]‐10 PM system adopted by WHO in 2016 for use in classifying perinatal mortality. 5 The ICD PM classification system uses a layered approach to categorize perinatal mortality (including stillbirth) based on the time of death (antepartum or intrapartum), the fetal cause of death, and/or contributing maternal condition. The total number of live births were recorded each week from the existing healthcare facility registers (labor ward, admission discharge, and operation theatre registers). On a weekly basis, healthcare providers reviewed all stillbirths in the preceding week. One most relevant contributory maternal condition and one fetal cause were attributed to each stillbirth in these review meetings. Hypertensive disorder of pregnancy was taken as any rise in blood pressure of 140/90 mm Hg and above on two occasions, 4 hours apart. Pre‐eclampsia was defined according to 2013 guidelines from the American College of Obstetricians and Gynecologists. 6 Fetal growth restriction (FGR) was diagnosed when the birth weight was less than the 10th centile for the gestational age according to the Intergrowth 21 chart. 7
Apart from direct causes, modifiable causes were also determined based on antenatal history and the details of the critical events related to stillbirth. The causes were divided into levels of delay: level I if the women arrived late due to not recognizing the need for care; level II due to failure to reach the hospital for treatment due to lack of transport facilities; and level III due to inadequate care by the provider. 8 Each cause was statistically analyzed using the Fisher exact test to calculate the P value and by paired t‐test to compare the means. The relative risk (RR) of the significant variable was also calculated. Statistical analysis was performed using SPSS version 20, and P < 0.05 was considered significant.
3. RESULTS
From March to September 2019, there were 6161 deliveries and 184 stillbirths (29.9/1000) whereas between March to September 2020, there were 3610 deliveries and 134 stillbirths (37.4/1000 deliveries). There was a significant increase in the stillbirth rate during the COVID‐19 pandemic (P = 0.045) (Figure 1).
FIGURE 1.
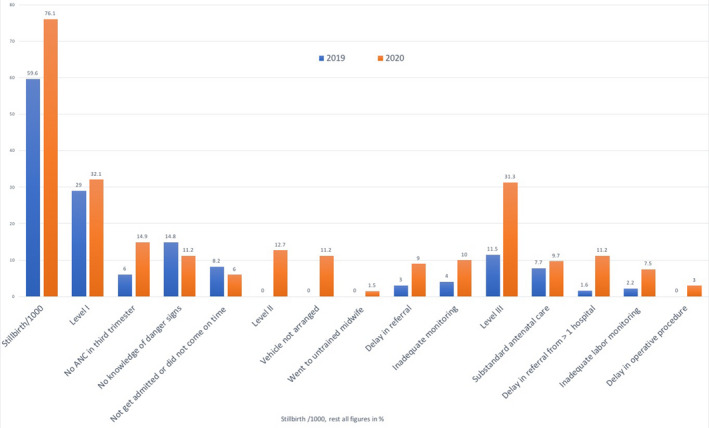
The comparative study of total stillbirth and the details of modifiable causes in controls (2019) and cases (2020)
Most of the women in both cases and controls were aged 23–27 years and were primigravidae. No significant difference in maternal age (P = 0.121), gravidity (P = 0.288), and number of abortions (P = 0.193) were observed between the cases and controls (Table 1). There were significantly fewer antenatal visits among cases compared to controls (P = 0.048). Significantly more women among the cases had a history of previous cesarean delivery (24/134, 17.9% vs 14/183, 7.7%, P = 0.014).
TABLE 1.
Epidemiological profile of cases and controls with stillbirths. a
| Variable | Cases in 2020 (n = 134) | Controls in 2019 (n = 183) | P value |
|---|---|---|---|
| Maternal age (years) | |||
| 18–22 | 36 (26.9) | 29 (15.8) | 0.121 |
| 23–27 | 42 (31.3) | 83 (45.4) | |
| 28–32 | 40 (29.9) | 52 (28.4) | |
| 33–37 | 11 (8.2) | 17 (9.3) | |
| >37 | 5 (3.7) | 2 (1.1) | |
| Gravidity | |||
| 1 | 54 (40.3) | 62 (34.4) | 0.288 |
| 2 | 43 (32.1) | 51 (28.4) | |
| 3 | 21 (15.7) | 29 (16.4) | |
| >3 | 16 (11.9) | 37 (20.8) | |
| Number of abortions | |||
| 1 | 25 (18.7) | 36 (19.7) | 0.193 |
| 2 | 2 (1.5) | 15 (8.2) | |
| ≥3 | 2 (1.5) | 1 (0.5) | |
| Number of antenatal visits | |||
| 0 | 17 (12.7) | 26 (14.2) | 0.048 |
| 1–2 | 69 (51.5) | 74 (39.9) | |
| 3–4 | 28 (20.9) | 48 (25.7) | |
| >4 | 20 (14.9) | 35 (18.6) | |
| Previous LSCS | 24 (17.9) | 14 (7.7) | 0.014 |
Abbreviation: LCSC, lower segment cesarean section.
Values are given as number (percentage).
Table 2 illustrates the details of the period of gestation, the weight of the baby, and the mode of delivery among the cases and controls. There were significantly more babies born at 30–40 weeks of gestation among the cases (103/134, 76.9%) compared to the controls (122/183, 66.7%) (P < 0.001). Similarly, significantly more babies weighed more than 3000 g among the cases than among the controls (22/134, 16.4% vs 13/183, 7.1%; P < 0.001). Lower segment cesarean section (14/134, 10.4% vs 5/183, 2.7%) and laparotomy for ruptured uterus (6/134, 4.5% vs 1/134, 0.5%) were performed in significantly more cases compared to controls (P < 0.001). There was no significant difference in the incidence of intrapartum (P = 0.105) or intramural stillbirth (P = 0.237) between the two groups.
TABLE 2.
Delivery details of cases and controls undergoing delivery of stillborns a
| Variables |
Cases Stillbirths in 2020 (n = 134) |
Controls Stillbirths in 2019 (n = 183) |
P value |
|---|---|---|---|
| Total deliveries | 3610 | 6161 | |
| Stillbirths | 134 (3.74) | 183 (2.99) | 0.045 |
| Gestational age at delivery (weeks) | |||
| 20+1–30+0 | 24 (17.9) | 58 (31.7) | <0.001 |
| 30+1–40+0 | 103 (76.9) | 122 (66.7) | |
| >40+0 | 7 (5.2) | 3 (1.6) | |
| Mode of delivery | |||
| Vaginal | 114 (85.1) | 175 (95.6) | <0.001 |
| LSCS | 14 (10.4) | 5 (2.7) | |
| Laparotomy for ruptured uterus | 6 (4.5) | 1 (0.5) | |
| Sex | |||
| Male | 66 (49.3) | 99 (54.1) | 0.426 |
| Female | 68 (50.7) | 84 (55.9) | |
| Birth weight (g) | |||
| 501–1000 | 21 (15.7) | 58 (31.7) | <0.001 |
| 1001–2000 | 47 (35.1) | 57 (31.1) | |
| 2001–3000 | 44 (32.8) | 59 (32.2) | |
| >3000 | 22 (16.4) | 13 (7.1) | |
| Type of stillbirth regarding timing | |||
| Antepartum | 88 (65.7) | 136 (74.3) | 0.105 |
| Intrapartum | 46 (34.3) | 47 (25.7) | |
| Type of stillbirth regarding place of death | |||
| Intramural | 21 (15.7) | 20 (10.9) | 0.237 |
| Extramural | 113 (84.3) | 163 (89.1) | |
Abbreviation: LCSC, lower segment cesarean section.
Values are given as number (percentage).
The causes associated with stillbirth are given in Table 3. The causes were classified according to ICD‐10 PM coding. Among the maternal conditions, the maternal medical and surgical conditions (M4) were comparable in the cases and controls (49/134, 36.2% and 68/183, 37.2%, respectively). It was observed that the complications of placenta, cord, and membranes (M1) were higher in the cases compared to the controls (22/134, 16.4% and 23/183, 12.6% respectively), but the difference was not statistically significant. Placental abruption was higher among the cases than the controls (16/134, 12% and 13/183, 7%, respectively) but the difference was not statistically significant (P = 0.169). However, the incidence of abruption in normotensive women was significantly higher in cases than in controls (P = 0.003). The relative risk of having abruption without hypertension was 3.69 among the cases.
TABLE 3.
Causes of stillbirth according to ICD‐10 PM coding observed in cases and controls
| Causes according to ICD‐10 PM coding | Cases in 2020 (n = 134) | Controls in 2019 (n = 183) | P value |
|---|---|---|---|
| Maternal conditions associated with fetal death | |||
| M1: Complications of placenta, cord & membranes | 22 (16.4) | 23 (12.6) | 0.334 |
| Abruption with hypertension | 4 (3.0) | 12 (6.6) | 0.169 |
| Abruption without hypertension | 12 (9.0) | 1 (0.50 | |
| Placental previa | 2 (1.5) | 2 (1.1) | 1.000 |
| Cord prolapse | 4 (3.0) | 4 (2.2) | 0.724 |
| Cord round the neck | 0 (0) | 4 (2.2) | 0.140 |
| M2: Maternal complications of pregnancy | 24 (17.9) | 30 (16.4) | 0.763 |
| Multiple pregnancy | 4 (3.0) | 2 (1.1) | 0.245 |
| PROM | 4 (3.0) | 8 (4.4) | 0.569 |
| Preterm labor | 5 (3.7) | 3 (1.6) | 0.289 |
| Oligohydramnios | 11 (8.2) | 14 (7.7) | 1.000 |
| Polyhydramnios | 0 (0) | 3 (1.6) | 0.265 |
| M3: Other complications of labor and delivery | |||
| Malpresentation/malposition | 2 (1.5) | 2 (1.1) | 1.000 |
| M4: Maternal medical & surgical conditions; noxious influences | 49 (36.6) | 68 (37.2) | 1.000 |
| Hypertension | 18 (13.4) | 26 (14.2) | 0.871 |
| Infection | 7 (5.2) | 10 (5.5) | 1.000 |
| Diabetes | 14 (10.4) | 19 (10.4) | 1.000 |
| IHCP | 5 (3.7) | 10 (5.5) | 0.596 |
| Maternal nutritional disorder | 5 (3.7) | 3 (1.6) | 0.289 |
| M4: No maternal high risk | 17 (12.7) | 60 (32.8) | <0.001 |
| Fetal deaths: main cause | |||
| A1/I 1: Birth defect | 17 (12.7) | 26 (10.9) | 0.742 |
| A2: Infection | 4 (3.0) | 5 (2.7) | 0.749 |
| A3: Antepartum hypoxia | 32 (23.9) | 35 (19.1) | 0.331 |
| A4/I5: Other specified disorder: hydrops | 1 (0.7) | 3 (1.6) | 0.640 |
| A5/I6: Disorder related to fetal growth | 40 (29.9) | 59 (32.2) | 0.807 |
| A6/I7: Unspecified cause of death/ other causes | 41 (30.6) | 55 (30.1) | 1.000 |
Abbreviations: ICD, International Classification of Diseases; IHCP, intrahepatic cholestasis of pregnancy; LCSC, lower segment cesarean section; PROM, premature rupture of membranes.
Values are given as number (percentage).
Regarding the fetal conditions leading to stillbirth, there was no statistical difference among the cases and controls (P > 0.05). Disorders related to fetal growth were the most common cause in both cases and controls (40/134, 29.9% and 59/183, 32.2%, respectively). All 134 mothers included in the analysis among the cases were screened for COVID‐19 infection by reverse transcription polymerase chain reaction (RT PCR) at admission: only 2/134 (1.5%) of them were positive for COVID‐19. In one case, there was severe acute respiratory distress leading to hypoxia and acidosis leading to intrauterine fetal death, and in the other, there was FGR and the mother had only a mild fever.
The modifiable factors or preventable causes were noted in 102/134 (76.1%) cases and 109/183 (59.6%) controls. The difference was highly significant (P < 0.001) (Table 4). The level‐I delay or the delay in recognizing the need for care was the most common modifiable factor in both groups (43/134, 32.1% of the cases and 53/183, 29.1% of the controls); however, no antenatal check‐up in the third trimester was observed in 29/134 (21.6%) cases compared to 11/183 (6%) controls (P < 0.001). There was no significant difference in the knowledge of danger signs between the cases and controls (P = 0.359). The delay in reaching the health facility (level‐II delay) was seen only during the period of the pandemic in 17/134 (12.7%) cases. There were 3 (2.2%) deliveries at the entrance of the hospital due to the delay in arrival at the facility with resultant birth trauma to the baby.
TABLE 4.
Details of modifiable causes and associated levels of delay in cases and controls experiencing stillbirths a
| Modifiable factors | Cases in 2020 (n = 134) | Controls in 2019 (n = 183) | P value | RR | 95% CI |
|---|---|---|---|---|---|
| Modifiable factors | 102 (76.1) | 109 (59.6) | <0.001 | 1.81 | 1.54–2.30 |
| Delay in recognizing need for care (level I) | 43 (23.1) | 53 (29.0) | 0.548 | 1.13 | 0.79–1.55 |
| No ANC in third trimester | 20 (14.9) | 11 (6.0) | <0.001 | 3.61 | 1.31–2.18 |
| No knowledge of danger signs | 15 (11.2) | 27 (14.8) | 0.359 | 0.72 | 0.42–1.37 |
| Could not get admitted or did not come on time due to family or social issues | 8 (6.0) | 15 (8.2) | 0.432 | 0.71 | 0.31–1.64 |
| Delay in reaching the hospital for delivery due to lack of conveyance (level II) | 17 (12.7) | 0 (0) | 0.006 | 47.72 | 2.89–786.35 |
| Vehicle not arranged, hence came with IUD or delivered at the hospital gate | 15 (11.2) | 0 (0) | 0.007 | 44.96 | 1.69–484.23 |
| Went to untrained care provider for delivery as there was no conveyance | 2 (1.5) | 0 (0) | 0.090 | 12.26 | 0.66–225.93 |
| Delay in receiving care (level III) | 42 (31.3) | 21 (11.5) | <0.001 | 2.70 | 1.70–4.38 |
| Substandard antenatal care | 13 (9.7) | 14 (7.7) | 0.518 | 1.30 | 0.61–2.61 |
| Delay in referral from more than one hospital | 15 (11.2) | 3 (1.6) | 0.002 | 6.81 | 2.01–23.11 |
| Inadequate monitoring in labor | 10 (7.5) | 4 (2.2) | 0.034 | 3.45 | 0.62–2.61 |
| Delay in undertaking operative procedure | 4 (3.0) | 0 (0) | 0.090 | 12.22 | 1.09–10.65 |
Abbreviations: ANC, antenatal clinic; CI, confidence interval; IUD, intrauterine death; RR, relative risk.
Values are given as number (percentage) unless otherwise specified.
The delay in providing care at the facility by the provider (level‐III delay) was observed in 42/134 (31.3%) cases, compared to 21/183 (11.5%) controls (P < 0.001). The women were referred to multiple hospitals in 15/134 (11.2%) cases compared to 3/183 (1.6%) controls (P = 0.002). Suboptimal care during labor in the initial weeks of the pandemic due to wearing of personal protective equipment (PPE), inability to listen to the fetal heart sounds while in PPE, and shortage of staff were the modifiable factors observed in significantly more cases compared to controls (P = 0.034). There was a delay in undertaking operative procedures due to the following of COVID‐19 protocols in operation theatres with consequent stillbirth in 4/134 (3%) cases.
The highest relative risk of having a stillbirth was due to delay in reaching the hospital (RR 47.70), delay in undertaking operative procedure (RR 12.20), and delay due to the patient being denied services and referred to more than one hospital before reaching our hospital (RR 6.80) (Table 4).
4. DISCUSSION
The present study highlights the impact of the COVID‐19 outbreak on the rate of stillbirths and its related reasons by comparing and analyzing the data of stillbirths from a tertiary hospital between COVID‐19 and pre‐COVID‐19 periods. The pandemic has resulted in a significantly higher incidence of stillbirths not due to COVID‐19 infection per se, but due to delays in care at all levels. The facilities were significantly impacted due to the lockdown and fear among both the pregnant women and healthcare providers during the pandemic resulting in many preventable stillbirths. The rates of stillbirth in low‐income countries are tenfold higher compared to those in high‐income countries. 9 It is important to understand how the pandemic has impacted the rates of stillbirth in high‐ and low‐income countries.
The WHO SEARO Neonatal‐Perinatal Database Network study aims to establish a framework to assess the burden of stillbirths and neonatal deaths in low‐income countries. The information generated regarding the modifiable factors contributing to stillbirths and neonatal deaths provides the decision‐makers with guidance for changes in policy. The ICD‐10 classification system was adopted for the classification of perinatal deaths (ICD‐10 PM) to facilitate more accurate and uniform reporting of causes to enable comparison within and between settings. 6 The most common maternal cause contributing to stillbirth in previous studies has been hypertension, abruption, and diabetes in pregnancy. 10 , 11 , 12 Even during the pandemic, hypertension remained the most common cause of stillbirth. Among the fetal causes, disorders related to fetal growth were the most common. The causes of stillbirth were comparable in both groups, except there were significantly more cases of abruption in normotensive women during the pandemic compared to the previous year. This could possibly be related to nutritional deficiencies due to a lack of proper nutrition and supplements during the COVID‐19 period.
Only 1.5% of women among the cases were positive for COVID‐19, implying that COVID‐19 infection did not contribute substantially to the increase in the rate of stillbirth in the study population. Fetal complications of COVID‐19 as reported in the literature include miscarriage (2%), FGR (10%), and preterm birth (39%). 13
The COVID‐19 pandemic has affected maternal and neonatal health services all over the world. Studies have identified the substantial impact of the COVID‐19 pandemic on mortality due to disruption of the healthcare delivery in obstetric patients. 2 The overall number of deliveries in our hospital from March to September decreased from 6161 to 3610 (41.4%) during the pre‐COVID‐19 and COVID‐19 periods included for analysis. Similar reductions of 33% in institutional births were reported during the Ebola virus disease outbreak in Liberia. 14
More than three‐quarters of women among the cases had a preventable stillbirth, compared to half in the control group. The modifiable factors identify missed opportunities, building momentum for the behavior change. This fact was brought forth by applying the scale suggested by Thaddeus et al. 8 for estimating preventable maternal deaths to stillbirth evaluation. The delay due to the inability to recognize the need for care and the patient not considering the need to visit a facility is a level‐I delay. The lack of knowledge of danger signs and inability to be admitted to hospital due to the social cause was not significantly different in the two groups. The knowledge of danger signs is expected to be provided during antenatal visits and is very pertinent as women may take the symptoms as pregnancy and childbirth‐related phenomena and may not seek care urgently. Hence, the precious time after the trigger event is lost. 15 , 16 In a review of factors associated with stillbirth in low‐ and middle‐income countries by Aminu et al., 17 poverty, lack of education, maternal age and lack of antenatal care were the most important modifiable factors.
It was observed that women did not attend antenatal clinics either due to fear of contracting COVID‐19 infection or due to lack of transport in significantly more cases compared to the controls (P < 0.001). The level‐II delay or delay in reaching the hospital due to lack of transport was observed only during the pandemic period, a phenomenon that was not observed in recent years in the study hospital, which is in the center of the city. Women went to local unskilled birth attendants for advice instead. Level‐III delays or provider‐related causes such as the denial of care by multiple hospitals before reaching the study hospital were observed in a significantly greater number of women. This was because some facilities were converted into exclusive COVID‐19 hospitals with no information to pregnant women booked there and a shortage of staff because healthcare providers were becoming infected themselves. Suboptimal and delayed care was observed at the facility among cases due to challenges such as: the inability to hear the fetal heartbeat by stethoscope while wearing PPE, limited numbers of CTG machines and Dopplers, delays in the decision to start cesarean delivery for fetal distress due to the extra time taken to mobilize patients from COVID‐19 suspect areas to the operation theatre and donning of PPE by healthcare providers, and the shortage of blood and blood products in blood banks due to a drastic fall in routine donations of blood.
The main limitation of the present study was its retrospective nature. The strength of the present study was the uniformity in the collection of data of all stillbirths under the WHO SEARO Neonatal‐Perinatal Database Network study and comparative study design.
5. CONCLUSION
The present study brings to light the untold story of the impact of the COVID‐19 pandemic on stillbirths due to COVID‐19‐related causes such as delays in reaching the hospital, denial of care, and suboptimal care leading to preventable stillbirths. More infants have been lost due to the lockdown than the COVID‐19 infection per se.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
MK: planning and co‐designing, carrying out the work, analysis of data and writing the manuscript; MP: concept, design, planning and co‐writing the manuscript; RY: conducting the work, revising the manuscript; RB: conducting the work, analysis of data; MS: conducting the work, revising the manuscript; VC: conducting the work, revising the manuscript; NJ: conducting the work, revising the manuscript; DM: conducting the work, analysis of data.
ACKNOWLEDGMENTS
We are grateful to WHO SEARO for funding the research and thankful to Mr Yogember Negi, the computer data operator, for helping in the collection of data.
REFERENCES
- 1. Khalil A, Kalafat E, Benlioglu C, et al. SARS‐CoV‐2 infection in pregnancy: a systematic review and meta‐analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;25: 10.1016/j.eclinm.2020.100446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ashish KC, Gurung R, Kinney MV, et al. Effect of the COVID‐19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study. Lancet. 2020;8(10):e1273–e1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O’Brien P, Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID‐19 pandemic. JAMA. 2020;324(7):705–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shrestha M, Manandhar D, Dhakal S, Nepal N. Two year audit of perinatal mortality at Kathmandu Medical College Teaching Hospital. Kathmandu Univ Med J (KUMJ). 2006;4:176–181. [PubMed] [Google Scholar]
- 5. Allanson ER, Tunçalp Ӧ, Gardosi J, et al. Giving a voice to millions: developing the WHO application of ICD‐10 to deaths during the perinatal period: ICD‐PM. BJOG‐Int J Obstet Gy. 2016;123(12):1896–1899. 10.1111/1471-0528.14243 [DOI] [PubMed] [Google Scholar]
- 6. Moussa HN, Leon MG, Marti A, et al. Pregnancy outcomes in women with preeclampsia superimposed on chronic hypertension with and without severe features. Am J Perinatol. 2017;34(4):403–408. [DOI] [PubMed] [Google Scholar]
- 7. Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross‐Sectional Study of the INTERGROWTH‐21st Project. Lancet. 2014;384:857–868. [DOI] [PubMed] [Google Scholar]
- 8. Thaddeus S, Maine D. Too far to walk: maternal mortality in context. Soc Sci Med. 1994;38(8):1091–1110. [DOI] [PubMed] [Google Scholar]
- 9. Pandey B, Hamdi I, George S, Pandey R. Perinatal mortality in Nizwa Hospital. Oman Med J. 2002;19:52–55. [Google Scholar]
- 10. Mammaro A, Carrara S, Cavaliere A, et al. Hypertensive disorders of pregnancy. J Prenatal Med. 2009;3(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- 11. McClure EM, Goldenberg RL. Stillbirth in developing countries: a review of causes, risk factors and prevention strategies. J Matern Fetal Neonatal Med. 2009;22(3):183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ngoc NTN, Merialdi M, Abdel‐Aleem H, et al. Causes of stillbirths and early neonatal deaths: data from 7993 pregnancies in six developing countries. Bull World Health Organ. 2006;84(9):699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dashraath P, Wong JLJ, Lim MXK, et al. Coronavirus disease 2019 (COVID‐19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222(6):521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iyengar P, Kerber K, Howe CJ, Dahn B. Services for mothers and newborns during the Ebola outbreak in Liberia: the need for improvement in emergencies. PLoS Curr. 2015;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khandale SN, Kedar K. Analysis of maternal mortality: a retrospective study at tertiary care centre. Int J Reprod Contracept Obstet Gynecol. 2017;6(4):1610–1613. [Google Scholar]
- 16. Paul B, Mohapatra B, Kar K. Maternal deaths in a tertiary health care centre of Odisha: an in‐depth study supplemented by verbal autopsy. Indian J Comm Med. 2011;36:213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aminu M, Unkels R, Mdegela M, Utz B, Adaji S, van den Broek N. Causes of and factors associated with stillbirth in low‐ and middle‐income countries: a systematic literature review. BJOG 2014; 121(Suppl. 4):141–153. [DOI] [PubMed] [Google Scholar]


