Abstract
We have previously reported that the Neisseria gonorrhoeae isolates from clinical failure cases treated with cefdinir and aztreonam, β-lactams exhibited high MICs. These resistant isolates were clearly separated from the isolates exhibiting a low level of resistance to β-lactams as shown by the MIC distribution of cefozopran. Restriction fragment length polymorphism DNA typing revealed that the outbreak of cefozopran-resistant isolates in Kitakyushu, Japan, occurred as a result of clonal spread.
As a result of the absence of strains of Neisseria gonorrhoeae resistant to the expanded spectrum of cephems, the National Committee for Clinical Laboratory Standards (NCCLS) (8) has not defined the breakpoint MICs of expanded-spectrum cephems such as cefixime (CFM), cefpodoxime (CPD), cefepime (FEP), etc. A previous study reported the incidence of clinical failures in gonococcal urethritis treated with cefdinir (CDR) or aztreonam (ATM) (1). For the N. gonorrhoeae isolates from such clinical failure cases, high-level MICs of CDR, ATM, and other β-lactams were observed. In order to investigate the prevalence of these resistant isolates in Kitakyushu, Japan, we examined 54 N. gonorrhoeae isolates from different cases occurring during 1999 for susceptibility to a variety of antimicrobial agents. Forty of 54 strains were isolated from male patients with gonococcal urethritis, while the remaining isolates were from female patients with gonococcal cervicitis. Identification of N. gonorrhoeae and testing for production of β-lactamase were performed by ID-test-HN-20 Rapid (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) using colonies cultured on Thayer-Martin Agar Base, Modified (Nissui Pharmaceutical Co.). The MICs of various antimicrobials were determined by the twofold serial agar dilution method on BBL GC Agar Base (Becton Dickinson and Co., Cockeysville, Md) with 1% BBL IsoVitalX enrichment (Becton Dickinson Europe, Meylan, France) according to the guidelines of the NCCLS (8). The antimicrobial agents used in this study were purchased from or provided by the corresponding companies.
The MIC distribution of cefozopran (CZO) for N. gonorrhoeae isolates was divided into two groups. The MICs for the high-level resistance group (8 to 16 μg/ml) were more than 16 times greater than the MICs for the susceptible and low-level resistance groups (<0.5 μg/ml). The MICs of CZO were correlated with those of CDR, CPD, cefpirome (CPI), FEP, ATM, cefuroxime, cefotiam, ceftizoxime (ZOX), CFM, and cefcapene (CPN). The MICs of CZO correlated poorly with those of penicillin (PEN), cefmetazole, flomoxef, and cefodizime (CDZ) despite the fact that all 17 CZO-resistant isolates for these four agents belonged to the high-level MIC group. CFM, CDZ and ceftriaxone (CRO) exhibited lower MICs but the resistant isolates belonged to the group with reduced susceptibility to these three agents. These new resistant strains were clearly divided into two groups by the MIC distribution of CZO, with all of the CZO-resistant isolates exhibiting either resistance or a reduced susceptibility to all β-lactams tested. Clinical failures caused by these resistant isolates occurred in three patients treated with CDR or ATM (Table 1). For these 17 isolates, high-level MICs for penicillins, narrow- and expanded-spectrum cephalosporins, cephamycins, the majority of broad-spectrum oral cephalosporins, and aztreonam were observed, together with a reduced susceptibility to most broad-spectrum parenteral cephalosporins. In the measurement of MIC and the disk diffusion test, these resistant isolates can be identified easily with CZO. CZO has been rarely used in gonococcal infection, but we consider that it may well be profitable to use CZO to define this resistant organism. We therefore called these isolates CZO-resistant N. gonorrhoeae (CZRNG). These strains did not produce β-lactamase. CZRNG accounted for 32% (17/54) of all isolates tested. The MICs of CDZ, CRO, and spectinomycin (SPT) showed that the isolates were susceptible, while CFM, cefotaxime (CTX), and ZOX retained good activity. To other β-lactams, the isolates were resistant or had a reduced susceptibility. All of the 17 CZRNG isolates were resistant to ciprofloxacin (CIP), with MICs of this drug ranging between 0.125 and 64 μg/ml. Six of 17 isolates exhibited high resistance to ciprofloxacin (MICs > 2 μg/ml). All 17 isolates were resistant to tetracycline (MIC, 1 to 4 μg/ml). The MICs of minocycline (MIN) for 15 of the 17 isolates were greater than 0.5 μg/ml. The MIC of erythromycin for one isolate was 0.125 μg/ml, while MICs for the other isolates had values of 4 to 16 μg/ml. Thus, 17 CZRNG isolates were multiresistant strains.
TABLE 1.
Clinical data of 17 cefozopran-resistant N. gonorrhoeae isolatesa
| Strain no. | Hospital IDb | Age (yr) and sex of patientc | First treatment
|
Final treatment
|
||||
|---|---|---|---|---|---|---|---|---|
| Agentsd | Regimene | Clinical outcome | Agent(s) | Regimen | Clinical outcome | |||
| SNG27 | Ue | 27, M | CDR | 100 mg × 3, p.o., 3 days | Failure | CRO | 1 g i.v., single dose | Cure |
| SPX | 100 mg, 3 days | |||||||
| SNG28 | Ya | 24, M | SPT | 2 g i.m., single dose | Cure | |||
| DOX | 100 mg × 2, p.o., 7 days | |||||||
| SNG32 | Ar | 19, F | CM | Suppository, 2 times | Not tested | |||
| SNG33 | UO | 29, M | AZT | 1 g i.v., single dose | Failure | SPT | 2 g i.m., single dose | Cure |
| SNG46 | Ya | 21, M | SPT | 2 g i.m., single dose | Cure | |||
| DOX | 100 mg × 2, p.o., 7 days | |||||||
| SNG50 | Ni | 19, M | CDR | 100 mg × 3, p.o., 3 days | Failure | SPT | 2 g i.m., single dose | NF |
| SNG52 | An | 22, F | AMO | 375 mg × 4, p.o., 7 days | Failure | CDZ | 1 g i.v., single dose | Cure |
| SNG53 | Kr | 24, M | CRO | 1 g i.v., single dose | Cure | |||
| SNG54 | AY | 42, M | LVX | 100 mg × 3, p.o. | Failure | CDZ | 1 g i.v., single dose | Cure |
| SNG57 | BM | 36, M | Unknown | |||||
| SNG65 | Ni | 19, M | CDR | 100 mg × 3, 3 days | NFf | |||
| SNG70 | BM | 33, M | Unknown | |||||
| SNG74 | Ni | 30, M | SPT | 2 g i.m., single dose | Cure | |||
| MIN | 100 mg × 2, 7 days | |||||||
| SNG75 | Ni | 27, M | SPT | 2 g i.m., single dose | Cure | |||
| MIN | 100 mg × 2, p.o., 7 days | |||||||
| SNG76 | Ni | 29, M | CDZ | 1 g i.v., single dose | Cure | |||
| SNG79 | Ni | 26, M | CDZ | 1 g i.v., single dose | Cure | |||
| SNG81 | An | 19, M | CDZ | 1 g i.v., single dose | Cure | |||
Including isolates from clinical failure cases treated with CDR and AZT. Data for SNG27 and SNG33 have been previously reported (1).
Blind names of hospitals.
M, male; F, female.
CDR, cefdinir; DOX, doxycycline; MIN, minocycline; AMO, amoxicillin; SPX, sparfloxacin.
p.o., orally; i.m., intramuscularly; i.v., intravenously.
NF, not followed.
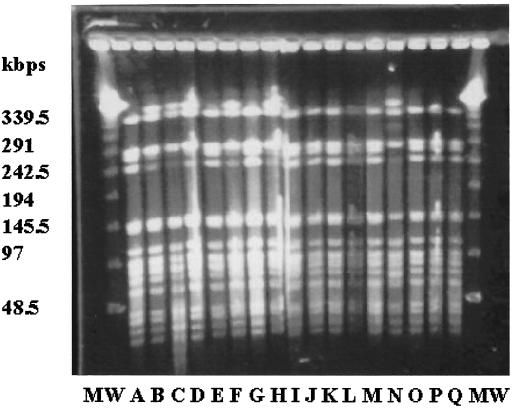
We therefore performed restriction fragment length polymorphism (RFLP) analysis as described by Struelens et al. (10) using a CHEFF Mapper pulsed-field gel electrophoresis system (Nippon Bio-Rad Laboratories, Tokyo, Japan). Figure 1 and Table 2 present the results of the macrorestriction genomic DNA analysis by SpeI carried out with 17 CZRNG strains. The 17 strains were isolated over a period of 8 months (May to December) from 17 cases at nine hospitals that were scattered within a radius of 40 km. Although the 17 CZRNG strains were isolated from multiple patients, RFLP analysis clearly divided the isolates into only two groups. The RFLP patterns by SpeI of the two groups differed by only one band. Molecular analysis software (Molecular Analyst Fingerprinting Plus; Nippon Bio-Rad Laboratories) indicated that the two groups were greater than 90% similar. The 17 CZRNG isolates underwent further RFLP typing by NheI (5), which produced identical RFLP patterns for all of the 17 CZRNG isolates. These results indicate that the outbreak of CZRNG isolates in Kitakyushu, Japan, occurred as a result of clonal spread.
FIG. 1.
The SpeI restriction patterns of chromosomal DNAs from 17 cefozopran-resistant Neisseria gonorrhoeae isolates. MW, λ DNA ladders; A, SNG27; B, SNG28; C, SNG32; D, SNG33; E, SNG46; F, SNG50; G, SNG52; H, SNG53; I, SNG54; J, SNG57; K, SNG65; L, SNG70; M, SNG74; N, SNG75; O, SNG76; P, SNG79; Q, SNG81.
TABLE 2.
DNA typing patterns and antimicrobial susceptibilities of 17 cefozopran resistant N. gonorrhoeae isolates
| Strai nno. | RFLP pattern | MICa (μg/ml)
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEN | CXM | CMZ | CTX | ZOX | CDZ | CZO | CRO | CFM | CPD | CDR | CIP | MIN | TET | SPT | ||
| SNG27b | A | 4 | 16 | 32 | 1 | 1 | 0.25 | 16 | 0.125 | 0.25 | 4 | 1 | 0.5 | 1 | 4 | 16 |
| SNG28 | B | 2 | 8 | 16 | 1 | 2 | 0.25 | 16 | 0.125 | 0.25 | 4 | 1 | 0.25 | 0.5 | 2 | 16 |
| SNG32 | B | 8 | 16 | 32 | 0.5 | 0.5 | 0.125 | 16 | 0.125 | 0.25 | 4 | 1 | 0.25 | 0.5 | 4 | 16 |
| SNG33b | B | 4 | 16 | 16 | 1 | 1 | 0.125 | 16 | 0.125 | 0.25 | 4 | 1 | 4 | 1 | 4 | 16 |
| SNG46 | A | 4 | 8 | 16 | 1 | 2 | 0.25 | 16 | 0.125 | 0.5 | 4 | 1 | 0.25 | 1 | 2 | 32 |
| SNG50 | B | 4 | 16 | 16 | 0.5 | 2 | 0.125 | 16 | 0.125 | 0.5 | 4 | 1 | 2 | 1 | 4 | 32 |
| SNG52 | A | 4 | 16 | 16 | 0.5 | 1 | 0.125 | 16 | 0.125 | 0.25 | 4 | 1 | 2 | 1 | 4 | 32 |
| SNG53 | B | 4 | 16 | 32 | 1 | 1 | 0.25 | 16 | 0.125 | 0.25 | 4 | 1 | 64 | 1 | 4 | 32 |
| SNG54 | A | 4 | 16 | 32 | 2 | 1 | 0.25 | 16 | 0.25 | 0.5 | 4 | 1 | 0.5 | 1 | 2 | 32 |
| SNG57 | A | 4 | 16 | 32 | 1 | 1 | 0.125 | 16 | 0.125 | 0.25 | 4 | 1 | 64 | 1 | 4 | 32 |
| SNG65 | A | 4 | 16 | 16 | 1 | 1 | 0.25 | 16 | 0.125 | 0.25 | 4 | 1 | 2 | 0.5 | 4 | 32 |
| SNG70 | A | 1 | 2 | 2 | 0.125 | 0.5 | 0.015 | 8 | 0.031 | 0.125 | 0.5 | 1 | 0.125 | 0.125 | 1 | 32 |
| SNG74 | A | 4 | 8 | 8 | 1 | 1 | 0.25 | 16 | 0.063 | 0.25 | 2 | 1 | 0.125 | 0.5 | 2 | 32 |
| SNG75 | B | 2 | 8 | 8 | 0.5 | 1 | 0.063 | 8 | 0.063 | 0.125 | 1 | 1 | 0.125 | 0.25 | 1 | 32 |
| SNG76 | A | 2 | 16 | 16 | 0.5 | 1 | 0.063 | 8 | 0.031 | 0.125 | 2 | 1 | 0.25 | 0.5 | 2 | 32 |
| SNG79 | A | 4 | 16 | 32 | 1 | 1 | 0.125 | 16 | 0.125 | 0.25 | 2 | 1 | 0.5 | 0.5 | 4 | 32 |
| SNG81 | A | 4 | 16 | 32 | 1 | 2 | 0.25 | 16 | 0.125 | 0.25 | 2 | 1 | 0.5 | 1 | 4 | 32 |
| ATCC49226 | 0.25 | 1 | 1 | 2 | 0.063 | 0.015 | 0.031 | 0.125 | 0.015 | 0.063 | 0.031 | 0.004 | 0.5 | 1 | 32 | |
Abbreviations: PEN, benzylpenicillin; CXM, cefuroxime; CMZ, cefmetazole; CTX, cefotaxime; ZOX, ceftizoxime; CDZ, cefodizime; CZO, cefozopran; CRO, ceftriaxone; CFM, cefixime; CPD, cefpodoxime; CIP, ciprofloxacin; TET, tetracycline; SPT, spectinomycin.
MICs and patient data for this isolate have been previously reported (1).
The CZRNG strain was first isolated at a hospital in Kitakyushu, Japan, in May, 1999. After that, the frequency of patients suffering from CZRNG rapidly increased such that the ratio of CZRNG among N. gonorrhoeae isolates reached 45.5% (5/11) during the month of December 1999. To our surprise, the genomic RFLP analysis revealed that the outbreak of CZRNG isolates occurred due to clonal spread. In Japan, most patients suffering from N. gonorrhoeae are treated with oral antimicrobials such as penicillins, broad-spectrum cephems, fluoroquinolones, and tetracyclines. Therefore, the emergence of CZRNG isolates resistant to cephems, fluoroquinolones, and tetracyclines is of serious concern. In the cases of gonococcal infection caused by CZRNG, the administration of 1 g of CRO, 1 g of CDZ, or 2 g of SPT was effective (Table 1). In Japan, CRO has not been recognized for use in gonococcal infections, and we have therefore treated patients with gonococcal infections with CDZ or SPT. Although SPT-resistant isolates were not isolated in this study, there have been reports of SPT-resistant N. gonorrhoeae isolates in other areas (6, 7). We therefore recommend that CDZ be used as the first-line treatment for gonococcal infection.
It has been reported that the mechanisms of low-level resistance to β-lactams, including cephems, involved the mutation of the porin encoded at the penB locus (4) and the decreased affinity of β-lactams to PBP-2 (2, 3, 9). Recently, we have discovered that many codons of the PBP-2 gene (penA) of the CZRNG isolates were different from those of the other susceptible isolates (data not shown). This area is under further investigation since this may be a potential resistance mechanism of CZRNG.
REFERENCES
- 1.Akasaka S, Muratani T, Yamada Y, Inatomi H, Takahashi K, Matsumoto T. Emergence of cephems and aztreonam high-resistant Neisseria gonorrhoeae that does not produce β-lactamase. J Infect Chemother. 2001;7:49–50. doi: 10.1007/s101560170034. [DOI] [PubMed] [Google Scholar]
- 2.Chalkley L J, van Vuuren S, Ballard R C, Botha P L. Characterization of penA and tetM resistance genes of Neisseria gonorrhoeae isolated in southern Africa—epidemiological monitoring and resistance development. S Afr Med J. 1995;85:775–780. [PubMed] [Google Scholar]
- 3.Dougherty T J, Koller A E, Tomasz A. Competition of β-lactam antibiotics for the penicillin-binding proteins of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1981;20:109–114. doi: 10.1128/aac.20.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gill M J, Simjee S, Al-Hattawi K, Robertson B D, Easmon C S F, Ison C A. Gonococcal resistance to β-lactams and tetracycline involves mutation in loop 3 of the porin encoded at the penB locus. Antimicrob Agents Chemother. 1998;42:2799–2803. doi: 10.1128/aac.42.11.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harnett N, Brown S, Terroa R, Krishnan C, Pauze M, Yeung K-H. High-level tetracycline-resistant Neisseria gonorrhoeae in Ontario, Canada—Investigation of a cluster of isolates, showing chromosomally mediated resistance to penicillin combined with plasmid-mediated resistance to tetracycline. J Infect Dis. 1977;176:1269–1276. doi: 10.1086/514122. [DOI] [PubMed] [Google Scholar]
- 6.Ison C A, Gendney J, Easmon C S F. Chromosomal resistance of gonococci to antibiotics. Genitourin Med. 1987;63:239–243. doi: 10.1136/sti.63.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joesoef M R, Knapp J S, Idajadi A, Linnan M, Barakbah Y, Kamboji A, O'hanley P, Moran J S. Antimicrobial susceptibilities of Neisseria gonorrhoeae strains isolated in Surabaya, Indonesia. Antimicrob Agents Chemother. 1994;38:2530–2533. doi: 10.1128/aac.38.11.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing, 9th Informational Supplement M7–A4 (M100–S9). Wayne, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 9.Spratt B G. Hybrid penicillin-binding proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature. 1988;332:173–176. doi: 10.1038/332173a0. [DOI] [PubMed] [Google Scholar]
- 10.Struelens M J, Rost F, Deplano A, Maas A, Schwam V, Serruys E, Cremer M. Pseudomonas aeruginosa and Enterobacteriaceae bacteremia after biliary endoscopy: an outbreak investigation using DNA macrorestriction analysis. Am J Med. 1993;95:489–498. doi: 10.1016/0002-9343(93)90331-i. [DOI] [PubMed] [Google Scholar]