Abstract
Immunotherapy combined with chemotherapy has recently changed the first-line treatment of several cancers. We performed a systematic review and meta-analysis to assess the efficacy and safety of programmed cell death 1 (PD-1) inhibitor plus chemotherapy as a first-line treatment for advanced esophageal cancer. Data were collected from eligible studies searched from PubMed, Web of Science, Cochrane Library, Embase, and meeting abstracts. The pooled hazard ratios (HRs) for overall survival (OS) and progression-free survival (PFS) and the pooled odds ratios (ORs) for objective response rate and treatment-related adverse events (TRAEs) were estimated to assess the efficacy and safety of PD-1 inhibitor plus chemotherapy versus chemotherapy. We performed several subgroup analyses to explore the variables affecting immunotherapy efficacy in esophageal cancer. The 5-point Jadad scoring system, the bias risk assessment and sensitivity analyses were used to evaluate the quality of the meta-analysis. Compared with the chemotherapy group, the OS (HR=0.70; P<0.01) and PFS (HR=0.62; P<0.01) were significantly longer and the objective response rate (OR=2.07; P<0.01) was significantly higher in the PD-1 inhibitor plus chemotherapy group. An OS benefit was observed in patients regardless of histology or programmed cell death 1 ligand 1 combined positive score. OS and PFS were generally consistent across subgroups by clinical features. In safety analyses, PD-1 inhibitor plus chemotherapy had a significantly higher incidence of TRAEs (OR=1.85; P<0.01), but there was no significant difference in grade 3 or higher TRAEs (OR=1.24; P=0.05). Compared with chemotherapy, PD-1 inhibitor plus chemotherapy improves antitumor activity and controllable adverse events in the first-line treatment of advanced esophageal cancer.
Key Words: PD-1 inhibitor, esophageal cancer, first-line treatment, chemotherapy
BACKGROUND
The incidence and mortality of esophageal cancer (EC) rank seventh and sixth among all malignant tumors, respectively, and >500,000 people die of EC every year.1 Esophageal squamous cell carcinoma (ESCC) is predominant in China, while esophageal adenocarcinoma (EAC) is common in Western countries.2–4 The diagnosis typically occurs in patients with locally advanced unresectable or metastatic disease, and systemic chemotherapy is the first choice.5 Although the application of surgery, radiotherapy, chemotherapy, and targeted therapy in the comprehensive treatment of cancer is constantly updated, the 5-year survival rate of EC is still low worldwide, at ~30%–40%.6 A large number of studies are being conducted to explore new treatment modalities to improve the survival of patients with EC.7,8
In recent years, immunotherapy has continuously made new breakthroughs in the treatment of various tumor types.9,10 Immune checkpoint inhibitors have been used in a large number of clinical studies on EC and have achieved certain results.11–13 Programmed death-1 (PD-1) is an important immunosuppressive molecule that inhibits T cell activation by binding with programmed death ligand 1 (PD-L1).14 Inhibition of the PD-1/PD-L1 pathway has shown significant survival benefits in multiple tumor therapies.15 PD-1 inhibitor, representative drugs of immunotherapy, have rapidly entered the field of EC treatment, from single-drug second-line treatment to first-line treatment with combined chemotherapy in unresectable locally advanced or metastatic EC.16 Keynote-181 demonstrated superior efficacy of the PD-1 inhibitor pembrolizumab compared with chemotherapy in the treatment of relapsed or metastatic EC.17 In the Attraction-03 trial and Escort trial, PD-1 inhibitor showed effective antitumor activity in patients with advanced ESCC.13,18 Our previous meta-analysis revealed that PD-1 inhibitor significantly prolonged overall survival (OS) compared with chemotherapy as second-line or later therapy in patients with EC.19
At the same time, immunotherapy is being explored as a first-line treatment for advanced EC. The benefit of combining PD-1 inhibitor therapy with chemotherapy has been demonstrated in several studies. Keynote 590 is the first phase 3 study to show a survival benefit from this combination in the first-line treatment of EC.20 The efficacy of immunotherapy combined with chemotherapy has also been further confirmed in Checkmate 648, and Escort-1st trials21,22 and studies such as Jupiter-06 and Orient-15 have also shown survival benefits.23,24 However, the treatment-related adverse events (TRAEs) caused by immunotherapy should not be ignored.
Currently, there are no meta-analyses exploring the safety and efficacy of PD-1 inhibitors plus chemotherapy in the first-line treatment of advanced EC. Thus, we conducted this meta-analysis, which systematically combines all prospective clinical study data to compare the efficacy and safety of PD-1 inhibitor plus chemotherapy as a first-line treatment for patients with advanced EC. We performed a comprehensive analysis of the current data published from clinical trials to inform decision making and enable the development of optimal first-line treatment strategies for those patients.
METHODS
Search Strategy
Comprehensive searches for articles published in English were carried out in PubMed, Web of Science, Cochrane Library, and Embase to collect all relevant citations. The date of the latest search was September 18, 2021. Meeting abstracts were also searched in the American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO). The following keywords were used for the search: (“immune checkpoint inhibitor” OR “ICI” OR “immunotherapy” OR “PD-1” OR “Nivolumab” OR “Pembrolizumab” OR “SHR-1210” OR “Camrelizumab” OR “Tislelizumab” OR “Toripalimab” OR “JS001” OR “Sintilimab”) AND (“esophageal” OR “esophagus” OR “oesophageal” OR “oesophagus”) AND (“cancer” OR “carcinoma” OR “tumor” OR “neoplasm”). The literature search was performed independently by 2 authors (M.X. and Yalan Y.). All searched results were evaluated according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Selection Criteria
The inclusion criteria were as follows: (1) randomized clinical trials; (2) random assignment of PD-1 inhibitor plus chemotherapy or chemotherapy; (3) previously untreated, locally advanced, unresectable or metastatic EC; and (4) studies containing one or all of the following outcomes of interest: OS, progression-free survival (PFS), objective response rate (ORR), and TRAEs.
The exclusion criteria were as follows: (1) the clinical trial was designed for the perioperative treatment or second-line or later therapy; (2) the study was an observational study, editorial, study protocol, commentary, review or case report; and (3) for duplicated or overlapping data sets, only the most recent information was included.
The primary screening was performed by reading the titles and abstracts of the studies to select relevant articles. The full texts of relevant articles were retrieved for eligibility. All the previous work was independently performed by 2 authors to select studies for inclusion in the systematic review by searching the databases (L.G. and Y.C.). Disagreements were resolved by discussion with all authors.
Data Extraction
The following study characteristics were extracted from each eligible study: authors, publication year, trial name and phase, number of patients, treatment strategy, ORR, OS, PFS, frequency of TRAEs, and some basic information, such as age, sex, region, Eastern Cooperative Oncology Group performance status (ECOG PS), histologic type, and PD-L1 status. Two authors independently extracted data with an information sheet (M.X. and Yuanyuan Y.). Discrepancies were resolved by discussion with all authors.
Statistical Analysis
The pooled hazard ratios (HRs) for OS and PFS and the pooled odds ratios (ORs) for ORR and TRAEs were estimated to assess the efficacy and safety of PD-1 inhibitor plus chemotherapy versus conventional chemotherapy. We performed several subgroup analyses to explore the variables affecting immunotherapy efficacy for EC. We used the Cochran Q test and Higgins I 2 statistic to evaluate heterogeneity. When high heterogeneity was detected (I 2 >50%), a random-effects model was adopted; otherwise, a fixed-effects model was adopted. The quality of the included trials was assessed in accordance with the 5-point Jadad scoring system.25 The risk of bias of the selected trials was evaluated by using the Cochrane Collaboration Tool.26 Sensitivity analyses were performed to evaluate the robustness of the combined outcomes. The meta-analysis was conducted according to the Cochrane handbook for systematic reviews of interventions, and forest plots were generated using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) and Stata 12.0 (Stata Corporation). All reported P-values are 2-sided, and P<0.05 was considered statistically significant. The work was done independently by 2 authors (Y.L. and F.W.). Disagreements were resolved by discussion with all authors.
RESULTS
Search Results
The literature screening process for this study is shown by a flow diagram (Fig. 1). A total of 567 records were retrieved. A total of 146 records were excluded due to duplicates, 412 records were excluded because they met the exclusion criteria, 3 studies were excluded due to a lack of comparative data, and 6 randomized clinical studies compared the efficacy and adverse events of PD-1 inhibitor plus chemotherapy with placebo plus chemotherapy as a first-line treatment for advanced or metastatic EC.20–24,27
FIGURE 1.
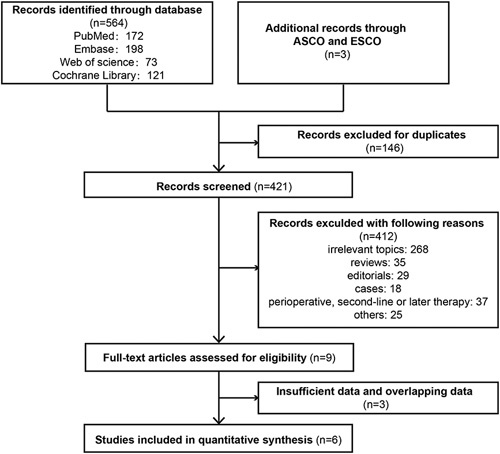
Flowchart of the study selection process for the meta-analysis. ASCO indicates American Society of Clinical Oncology; ESMO, European Society for Medical Oncology.
The main characteristics of the included trials are summarized in Table 1. All studies were randomized controlled trials (RCTs) published in 2021. The trials included a total of 3374 EC patients. Four trials enrolled patients with ESCC,21–24 and 1 trial enrolled patients with ESCC and EAC.20 Checkmate 649 reported the results for nivolumab plus chemotherapy versus chemotherapy alone in advanced or metastatic gastric, gastro-esophageal junction, or EAC, and we also extracted the patients’ data with EAC.27
TABLE 1.
Characteristics of the Studies Included in the Meta-Analysis
| Reference | Clinal Trials | Phase | Histology | Treatment Regimen | Patient Number | Median Follow-up (mo) | mOS (mo) (95% CI) | mPFS (mo) (95% CI) | ORR (%) (95% CI) | mDOR (mo) (95% CI) | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chau et al21 | Checkmate 648 | III | ESCC | Nivolumab+chemotherapy | 321 | 12.9 | 13.2 (11.1–15.7) | 5.8 (5.6–7.0) | 47.0 (42.0–53.0) | 5.7 (0.1–30.6) | 3 |
| Chemotherapy | 324 | 12.9 | 10.7 (9.4–11.9) | 5.6 (4.3–5.9) | 27.0 (22.0–32.0) | 3.4 (0.0–19.5) | |||||
| Sun et al20 | Keynote 590 | III | ESCC, EAC | Pembrolizumab+chemotherapy | 373 | 22.6 | 12.4 (10.5–14.0) | 6.3 (6.2–6.9) | 45 (39.9–50.2) | 8.3 (1.2+, 31.0+) | 5 |
| Placebo+chemotherapy | 376 | 22.6 | 9·8 (8.8–10.8) | 5.8 (5.0–6.0) | 29.3 (24.7–34.1) | 6.0 (1.5+, 25.0+) | |||||
| Luo et al22 | ESCORT-1st | III | ESCC | Camrelizumab+chemotherapy | 298 | 10.8 | 15.3 (12.8–17.3) | 6.9 (5.8–7.4) | 72.1 (66.7–77.2) | 7.0 (6.1–8.9) | 5 |
| Placebo+chemotherapy | 298 | 10.8 | 12.0 (11.0–13.3) | 5.6 (5.5–5.7) | 62.1 (56.3–67.6) | 4.6 (4.3–5.5) | |||||
| Shen et al24 | Orient-15 | III | ESCC | Sintilimab+chemotherapy | 327 | 11.4 | 16.7 (14.8–21.7) | 7.2 (7.0–9.6) | 66.1 | 9.7 (7.1–13.7) | 4 |
| Placebo+chemotherapy | 332 | 11.4 | 12.5 (11.0–14.5) | 5.7 (5.5–6.8) | 45.5 | 6.9 (5.6–7.2) | |||||
| Xu et al23 | Jupiter-06 | III | ESCC | Toripalimab+chemotherapy | 257 | 7.4 | 17.0 (14.0–NA) | 5.7 (5.6–7.0) | NA | NA | 4 |
| Placebo+chemotherapy | 257 | 7.3 | 11.0 (10.4–12.6) | 5.5 (5.2–5.6) | NA | NA | |||||
| Janjigian et al27 | Checkmate 649 | III | EAC | Nivolumab+chemotherapy | 103 | 13.1 | 12.3 | NA | NA | NA | 3 |
| Chemotherapy | 108 | 11.1 | 11.3 | NA | NA | NA |
CI indicates confidence interval; EAC, esophageal adenocarcinoma; ESCC, esophageal squamous cell carcinoma; mDOR, median duration of response; mOS, median overall survival; mPFS, median progression-free survival; NA, not available; ORR, objective response rate.
Efficacy Outcomes of PD-1 Inhibitor Plus Chemotherapy
The pooled HRs of OS and PFS and the pooled OR of ORR were used to assess the efficacy of PD-1 inhibitor plus chemotherapy in first-line EC treatment. In terms of OS benefit, PD-1 inhibitor plus chemotherapy led to a 30% reduction in the risk of death compared with chemotherapy (HR=0.70; 95% CI: 0.64–0.77, P<0.01), and there was no obvious heterogeneity (I 2=0%, P=0.571) (Fig. 2A). The pooled HR of PFS showed that PD-1 inhibitor plus chemotherapy significantly reduced the risk of disease progression compared with chemotherapy (HR=0.62; 95% CI: 0.57–0.68, P<0.01; heterogeneity: I 2=46.6%, P =0.112) (Fig. 2B). In addition, the difference in ORR benefit was significant between the PD-1 inhibitor plus chemotherapy group and the chemotherapy group (OR=2.07; 95% CI: 1.76–2.43, P<0.01; heterogeneity: I 2=24.5%, P =0.264) (Fig. 2C).
FIGURE 2.
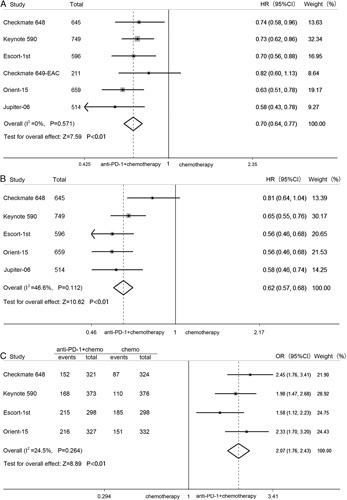
Pooled HRs for overall survival (A) and progression-free survival (B) and pooled odds ratio for objective response rate (C) in advanced esophageal cancer treated with PD-1 inhibitor plus chemotherapy versus chemotherapy. CI indicates confidence interval; HR, hazard ratio; OR, odds ratio; PD-1, programmed cell death 1.
Associations of Histology and PD-L1 Expression Status With OS
Five studies had OS results for squamous cell carcinoma, and 2 studies had OS results for adenocarcinoma. The difference in OS benefit across histology subgroups showed no significant trend (P=0.279) (Fig. 3A). Two studies assessed the PD-L1 combined positive score (CPS). In the subgroup with PD-L1 CPS <10%, the pooled HR of OS was 0.76, and the OS benefit was greater in patients with a PD-L1 CPS of at least 10% (HR=0.63). However, there was no statistically significant difference in terms of PD-L1 expression level (P=0.145) (Fig. 3B).
FIGURE 3.
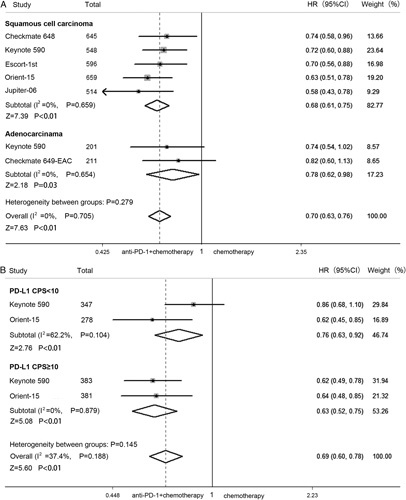
Forest plot of HRs by histologic type (A) and PD-L1 expression (B) comparing overall survival in patients who received PD-1 inhibitor plus chemotherapy versus chemotherapy. CI indicates confidence interval; CPS, combined positive score; EAC, esophageal adenocarcinoma; HR, hazard ratio; PD-L1, programmed cell death 1 ligand 1.
Subgroup Analyses by Clinical Features
We performed subgroup analyses according to some basic information, including age, sex, and ECOG PS. There was no significant interaction between the treatment effect in terms of OS and clinical features (age: P=0.236; sex: P=0.340; ECOG: P=0.593) (Fig. 4A). Similarly, the PFS benefit of PD-1 inhibitor plus chemotherapy compared with chemotherapy did not vary significantly across subgroups (age: P=0.922; sex: P=0.390; ECOG PS: P=0.319) (Fig. 4B).
FIGURE 4.
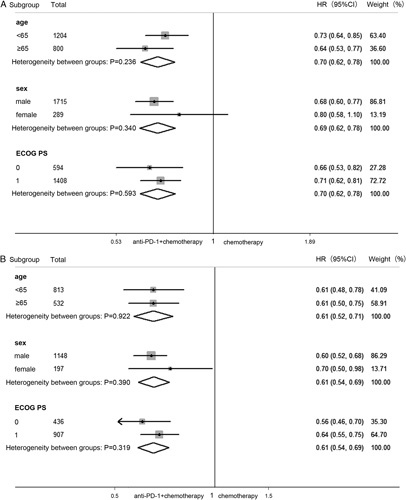
Forest plot of hazard ratio in subgroup analysis by clinical information comparing overall survival (A) and progression-free survival (B) in patients who received PD-1 inhibitor plus chemotherapy versus chemotherapy. CI indicates confidence interval; HR, hazard ratio; ECOG PS, Eastern Cooperative Oncology Group performance status; PD-1, programmed cell death 1.
Safety Evaluation of PD-1 Inhibitor Plus Chemotherapy
The pooled OR of TRAEs was 1.85 (95% CI: 1.21–2.84, P<0.01; heterogeneity: I 2=9.9%, P=0.350), which showed that PD-1 inhibitor plus chemotherapy can increase the incidence of TRAEs compared with chemotherapy (Fig. 5A). In terms of grade 3 or higher TRAEs, there was no significant difference, but there was a near-significant trend (OR=1.24; 95% CI: 1.00–1.55, P=0.05) (Fig. 5B).
FIGURE 5.
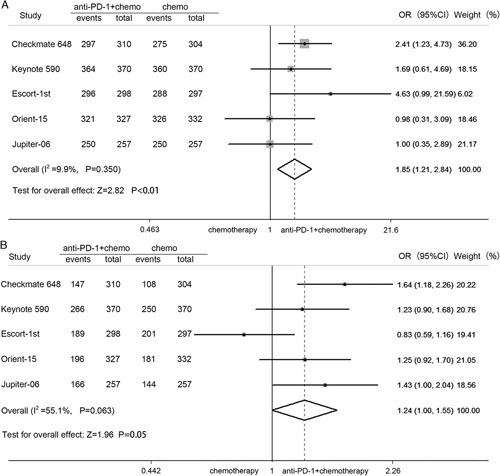
Pooled OR for the incidence of all treatment-related adverse events (A) and grade 3 or higher treatment-related adverse events (B). CI indicates confidence interval; OR, odds ratio; PD-1, programmed cell death 1.
Assessment of Study Quality and Sensitivity Analysis
All trials included in this study were multicenter, randomized clinical trials, and the Jadad score ranged from 3 to 5, indicating that the quality was high (Table 1). The bias risk of the included studies is shown in Figure 6F. All trials performed random sequence generation. Four trials were double blinded. Two trials were open label, and therefore, these studies had performance bias and detection bias. Two studies did not report all data, so they had a risk of reporting bias. Nevertheless, all studies were determined to have a low risk of attrition bias and other bias.
FIGURE 6.
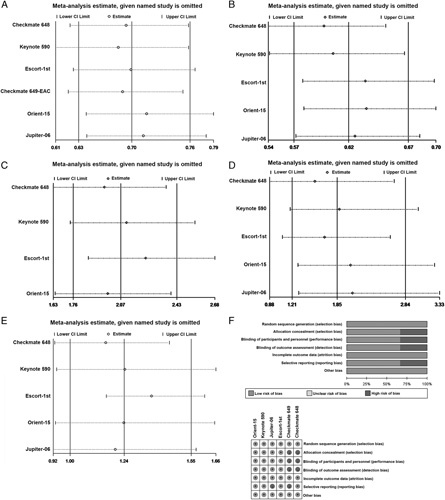
Sensitivity analysis of the hazard ratios of overall survival (A) and progression-free survival (B) and the odds ratio for objective response rate (C), treatment-related adverse events (TRAEs) (D), and grade 3 or higher TRAEs (E). The risk of bias was evaluated by using Review Manager 5.3 (F). CI indicates confidence interval.
Sensitivity analysis was performed to evaluate the stability of our meta-analysis findings. The results showed that our meta-analysis was robust in terms of the pooled HRs for OS (Fig. 6A) and PFS (Fig. 6B) and the pooled ORs for ORR (Fig. 6C), TRAEs (Fig. 6D) and grade 3 or higher TRAEs (Fig. 6E). No significant deviation from the overall results was detected.
DISCUSSION
Chemotherapy and targeted therapy did not improve the survival of patients with EC, and clinical trials of PD-1 inhibitors in the treatment of EC have been gradually carried out.28,29 In terms of the results, immunotherapy achieved a major breakthrough from the back line to the first line for patients with advanced EC.16,30 Our previous meta-analysis revealed that PD-1 inhibitors significantly prolonged OS compared with chemotherapy in previous systemic therapy patients with EC. Studies on perioperative immunotherapy for EC are also beginning to recruit patients. In 2021, there were multiple studies reporting the results of immunotherapy as first-line therapy for advanced EC. Keynote 590 announced global population data in the Lancet and was the first phase 3 study show that a survival benefit could be achieved, changing the landscape of first-line treatment for EC.20 The results from Escort-1st, published in JAMA, were a critical turning point in the treatment of ESCC.22 The results of nivolumab plus chemotherapy versus chemotherapy from Checkmate 648 were reported in 2021 ASCO meeting.21 Orient-15 and Jupiter-06 were global, randomized, double-blind studies that evaluated the efficacy and safety of PD-1 inhibitor plus chemotherapy versus chemotherapy as first-line treatment in advanced ESCC and published the main data in 2021 ESMO meeting.23,24 Survival data related to EC were extracted from Checkmate 649.27 Based on these data, a meta-analysis of prospective clinical trials was conducted. These included trials, which were all registered with ClinicalTrials.gov, were multicenter, randomized, phase 3 studies. Although some research results have yet to be published in a journal, the data presented at the ESMO or ASCO meeting met the meta-analysis criteria. To the best of our knowledge, this is the first meta-analysis that focused on investigating the survival benefits of PD-1 inhibitor plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced EC.
Recent studies have shown that PD-1 inhibitor plus chemotherapy significantly improve the survival benefit of first-line treatment for advanced non–small cell lung carcinoma.31 In our meta-analysis, we first compared the efficacy of PD-1 inhibitor plus chemotherapy versus chemotherapy as first-line therapy in advanced EC patients. OS, PFS, and ORR were selected as the primary endpoints. The results showed that PD-1 inhibitor plus chemotherapy significantly prolonged OS and PFS and improved the ORR in advanced EC. PD-1 inhibitor plus chemotherapy was associated with a 30% reduction in the risk of death, a 38% reduction in the risk of disease progression, and 2.07 times the probability of achieving an objective response compared with standard chemotherapy as first-line treatment. These results showed that immunotherapy plus chemotherapy had good clinical efficacy, so this strategy is a good choice for advanced EC.
Previous studies have shown that the expression level of PD-L1 can serve as a predictive biomarker in cancer immunotherapy.32 Therefore, we conducted a subgroup analysis to clarify the association between OS benefits and different PD-L1 expression levels. The results showed a potentially better OS benefit in patients with a baseline PD-L1 CPS of 10 or higher than in patients with a PD-L1 CPS of <10, but the test for interaction was not statistically significant. The same results were found in the Escort-1st study, which assessed PD-L1 expression with a cutoff value of 1%. Histologic types have an effect on the survival of patients with EC,33 and we also conducted a subgroup analysis to explore the OS difference between squamous cell carcinoma and adenocarcinoma. The OS benefit in patients with squamous cell carcinoma was superior to that in patients with adenocarcinoma, but there was no significant difference. These findings were consistent with data from previous studies where patients with EC, typically with squamous cell carcinoma histology, derived a greater treatment benefit from immunotherapy.17,34 The US Food and Drug Administration (FDA) approved pembrolizumab plus platinum and fluoropyrimidine-based chemotherapy for the treatment of certain patients with locally advanced or metastatic EC, regardless of PD-L1 expression.
We also performed subgroup analyses by clinical features, which may be related to the efficacy of immunotherapy.35–37 The OS and PFS benefits were similar in patients younger than 65 years and 65 years or older and patients with ECOG PS scores of 0 and 1. However, we found that greater OS and PFS benefits were achieved with PD-1 inhibitor plus chemotherapy for male patients than for female patients, but there was no significant difference. A recent meta-analysis also found that the relative benefit of immunotherapy was greater in male cancer patients than in female patients.37 This finding also raises a clinically important question of whether immunotherapy has greater efficacy in males with advanced EC than in females with advanced EC, which needs further study.
Despite the success and ongoing promise of PD-1 inhibitors in advanced cancer, TRAEs, which have emerged as frequent complications of checkpoint blockade, remain a constraint of this type of therapy.38–40 Therefore, we analyzed the incidence of total TRAEs and grade 3 or higher TRAEs. Regarding the safety profile, PD-1 inhibitor plus chemotherapy was significantly associated with an increased risk of developing TRAEs; however, no significant difference was found in the incidence of grade 3 or higher TRAEs. All of these studies showed an acceptable safety profile in patients treated with PD-1 inhibitor plus chemotherapy.
Our results demonstrated that the combination of a PD-1 inhibitor and chemotherapy can be considered a new first-line treatment in patients with advanced EC. Although some studies were searched from ASCO and ESMO meetings, the topic of this paper is still novel, and high-quality data were included in the meta-analysis, which provides a new direction for the first-line treatment of advanced EC.
CONCLUSIONS
OS, PFS, and ORR were all significantly improved in PD-1 inhibitor plus chemotherapy versus chemotherapy, with a manageable safety profile as first-line therapy in patients with advanced EC. PD-1 inhibitor plus chemotherapy should be considered for patients with unresectable, metastatic EC in the first-line setting.
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
This research was supported by the National Natural Science Funds of China (No. 81672442), Innovation Scientists and Technicians Troop Construction Projects of Henan Province the Scientific (YXKC2020017) and technological projects in Henan Province (No. SBGJ202002080).
All authors have declared that there are no financial conflicts of interest with regard to this work.
Footnotes
Y.L.: statistical analysis, writing-original draft, assessment of study quality. M.X.: literature searching, data extraction. L.G.: literature screening, assessment of study quality. Yalan Y.: literature searching. Y.C.: literature screening. Yuanyuan Y.: data extraction. F.W.: statistical analysis, writing-review and editing, funding acquisition.
Contributor Information
Yao Lu, Email: zdluyao@163.com.
Mengli Xu, Email: xumengli0912@163.com.
Lulu Guan, Email: guanll1231@163.com.
Yalan Yang, Email: yangyl15@163.com.
Yu Chen, Email: 763664331@qq.com.
Yuanyuan Yang, Email: 1344873681@qq.com.
Feng Wang, Email: zzuwangfeng@zzu.edu.cn.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;0:1–41. [DOI] [PubMed] [Google Scholar]
- 2. Short MW, Burgers KG, Fry VT. Esophageal cancer. Am Fam Physician. 2017;95:22–28. [PubMed] [Google Scholar]
- 3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. [DOI] [PubMed] [Google Scholar]
- 4. Domper Arnal MJ, Ferrandez Arenas A, Lanas Arbeloa A. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21:7933–7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:855–883. [DOI] [PubMed] [Google Scholar]
- 6. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. [DOI] [PubMed] [Google Scholar]
- 7. Zhang X, Jia J, Lu M, et al. Nimotuzumab plus paclitaxel and cisplatin as a 1(st)-line treatment for esophageal cancer: long term follow-up of a phase II study. J Cancer. 2019;10:1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Y, Wu X, Hao D, et al. Neoadjuvant nimotuzumab plus chemoradiotherapy compared to neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma. Oncotarget. 2019;10:4069–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gotwals P, Cameron S, Cipolletta D, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17:286–301. [DOI] [PubMed] [Google Scholar]
- 10. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doi T, Piha-Paul SA, Jalal SI, et al. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol. 2018;36:61–67. [DOI] [PubMed] [Google Scholar]
- 12. Huang J, Xu B, Mo H, et al. Safety, activity, and biomarkers of SHR-1210, an anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. 2018;24:1296–1304. [DOI] [PubMed] [Google Scholar]
- 13. Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506–1517. [DOI] [PubMed] [Google Scholar]
- 14. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schizas D, Charalampakis N. Immunotherapy for esophageal cancer: a 2019 update. Immunotherapy. 2020;12:203–218. [DOI] [PubMed] [Google Scholar]
- 17. Kojima T, Shah MA. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38:4138–4148. [DOI] [PubMed] [Google Scholar]
- 18. Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21:832–842. [DOI] [PubMed] [Google Scholar]
- 19. Lu Y, Guan L, Xu M, et al. The efficacy and safety of antibodies targeting PD-1 for treatment in advanced esophageal cancer: a systematic review and meta-analysis. Transl Oncol. 2021;14:101083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun J-M, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759–771. [DOI] [PubMed] [Google Scholar]
- 21. Chau I, Doki Y, Ajani JA, et al. Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): first results of the CheckMate 648 study. J Clin Oncol. 2021;39:LBA4001. [Google Scholar]
- 22. Luo H, Lu J, Bai Y, et al. Effect of camrelizumab vs. placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326:916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu RH, Wang F, Cui C, et al. 1373MO JUPITER-06: a randomized, double-blind, phase III study of toripalimab versus placebo in combination with first-line chemotherapy for treatment naive advanced or metastatic esophageal squamous cell carcinoma (ESCC). Ann Oncol. 2021;32:S1041. [Google Scholar]
- 24. Shen L, Lu ZH, Wang JY, et al. LBA52 Sintilimab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced or metastatic esophageal squamous cell cancer: first results of the phase III ORIENT-15 study. Ann Oncol. 2021;32:S1330. [Google Scholar]
- 25. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 26. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirano H, Kato K. Systemic treatment of advanced esophageal squamous cell carcinoma: chemotherapy, molecular-targeting therapy and immunotherapy. Jpn J Clin Oncol. 2019;49:412–420. [DOI] [PubMed] [Google Scholar]
- 29. Alsina M, Moehler M, Lorenzen S. Immunotherapy of esophageal cancer: current status, many trials and innovative strategies. Oncol Res Treat. 2018;41:266–271. [DOI] [PubMed] [Google Scholar]
- 30. Vivaldi C, Catanese S, Massa V, et al. Immune checkpoint inhibitors in esophageal cancers: are we finally finding the right path in the mist? Int J Mol Sci. 2020;21:1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou Y, Chen C, Zhang X, et al. Immune-checkpoint inhibitor plus chemotherapy versus conventional chemotherapy for first-line treatment in advanced non-small cell lung carcinoma: a systematic review and meta-analysis. J Immunother Cancer. 2018;6:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–856. [DOI] [PubMed] [Google Scholar]
- 33. Xi M, Yang Y, Zhang L, et al. Multi-institutional analysis of recurrence and survival after neoadjuvant chemoradiotherapy of esophageal cancer: impact of histology on recurrence patterns and outcomes. Ann Surg. 2019;269:663–670. [DOI] [PubMed] [Google Scholar]
- 34. Shah MA, Bennouna J, Shen L, et al. Pembrolizumab (MK-3475) for previously treated metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: phase II KEYNOTE-180 study. J Clin Oncol. 2016;34:TPS189. [Google Scholar]
- 35. Yang F, Wang YC, Nowakowski GS, et al. Association of sex, age and ECOG performance status with cancer immunotherapy efficacy in randomized controlled trials. J Clin Oncol. 2019;37:6952. [Google Scholar]
- 36. Jiang Y, Su Z. Cancer immunotherapy efficacy and patients’ age: a systematic review and meta-analysis. Ann Oncol. 2019;30:466P. [Google Scholar]
- 37. Conforti F, Pala L, Bagnardi V, et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol. 2018;19:737–746. [DOI] [PubMed] [Google Scholar]
- 38. Anderson R, Jing Y, Liu J, et al. Multi-omics prediction of immune-related adverse events during checkpoint immunotherapy. Support Care Cancer. 2020;11:4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abdel-Wahab N, Alshawa A, Suarez-Almazor ME. Adverse events in cancer immunotherapy. Nat Commun. 2017;995:155–174. [DOI] [PubMed] [Google Scholar]
- 40. Blidner AG, Choi J, Cooksley T, et al. Cancer immunotherapy-related adverse events: causes and challenges. Support Care Cancer. 2020;28:6111–6117. [DOI] [PubMed] [Google Scholar]


