Abstract
Background
Chemotherapy‐induced thrombocytopenia (CIT) is common during treatment with antineoplastic therapies and may adversely impact chemotherapy dose intensity. There is no approved therapy for CIT. In our recent phase II randomized study, romiplostim led to correction of platelet counts in 85% of treated patients and allowed resumption of chemotherapy, with low rates of recurrent CIT in the first two cycles or 8 weeks of chemotherapy. However, there is a lack of long‐term data on the efficacy and safety of romiplostim in CIT.
Objectives
To analyze efficacy and safety of romiplostim in the patients in the phase 2 study, who received romiplostim for ≥1 year.
Patients/Methods
Twenty‐one patients remained on romiplostim for ≥1 year. We analyzed the effect of romiplostim on platelet counts, absolute neutrophil counts, and hemoglobin, as well as impact on ongoing chemotherapy. We also tracked venous or arterial thrombotic events.
Results
During the study period, romiplostim was effective in preventing reduction of chemotherapy dose intensity due to CIT. Fourteen of the 20 (70%) analyzable patients experienced no episode of CIT, 4 subjects experienced a single chemotherapy dose delay due CIT, and 2 patients required a chemotherapy dose reduction. Platelet counts were preserved throughout the duration of the extension analysis. One patient experienced a proximal deep vein thrombosis, and one patient experienced multiple tumor‐related ischemic events.
Conclusions
Long‐term use of romiplostim for treatment of CIT was effective and safe, with no evidence of resistance or increased risk of thrombosis.
Keywords: cancer, chemotherapy, romiplostim, thrombocytopenia, thrombopoietin
Essentials.
Chemotherapy‐induced thrombocytopenia is common, with no approved treatment.
Romiplostim is effective and safe for treatment of chemotherapy‐induced thrombocytopenia.
In long‐term use (≥1 year) romiplostim remains effective, with no evidence of resistance.
Venous thromboembolism is not more frequent than anticipated.
1. INTRODUCTION
Suppression of hematopoiesis is a common adverse effect of chemotherapy and, if severe or protracted, may lead to delay or dose reduction of cancer treatment. 1 , 2 Reduced relative dose intensity of chemotherapy may adversely impact optimal management of the malignancy and may decrease progression‐free and overall survival. 1 , 3 , 4 , 5 , 6 , 7 , 8 , 9 Granulocyte colony‐stimulating factor (filgrastim and pegfilgrastim) are approved by the US Food and Drug Administration to reduce the incidence of chemotherapy‐induced neutropenia and infection. 10 However, there is no approved therapy to treat or prevent chemotherapy‐induced thrombocytopenia (CIT), an important unmet clinical need. 1 , 2
Thrombopoietin (TPO) is the primary hematopoietic growth factor regulating thrombopoiesis. 11 TPO binds to the TPO receptor (c‐mpl, CD 110), leading to activation of the Janus kinase/signal transducer and activator of transcription pathway, stimulation of megakaryocyte proliferation and differentiation, and increased platelet production. 11 Thrombopoietin receptor agonists (TPO‐RAs) (romiplostim, eltrombopag, avatrombopag and lusutrombopag) are second generation thrombopoietic agents, in that they activate the TPO receptor, but do not contain the actual structure of TPO. TPO‐RAs are approved for the management of various thrombocytopenic states, including immune thrombocytopenia, aplastic anemia, and periprocedural thrombocytopenia, particularly in patients with chronic liver disease, yet none of the TPO‐RAs are approved for treatment of CIT. 3 , 12 , 13 , 14 , 15 , 16 , 17 An increasing body of evidence indicates that romiplostim may be of benefit in the treatment of CIT. 8 , 18
Our recently published phase 2 clinical trial demonstrated that romiplostim successfully corrected CIT within 3 weeks in 85% of treated subjects. 8 A secondary end point was resumption of chemotherapy with romiplostim maintenance for at least two cycles or 8 weeks without recurrent CIT. Of the 44 subjects who resumed chemotherapy with weekly romiplostim support, just 3 patients (6.8%) experienced chemotherapy dose reduction or dose delay due to recurrent CIT within the two cycles or 8 weeks. 8
The most recent iteration of the National Comprehensive Cancer Network Guidelines for Hematopoietic Growth Factors endorses the consideration of use of romiplostim as one option for management of CIT, 19 and therefore there is a need for data on efficacy and toxicity in patients maintained on long‐term romiplostim for CIT. The phase 2 protocol specified that “patients who received romiplostim and demonstrated clinical benefit and resumed chemotherapy could continue romiplostim treatment as long as it was felt to be beneficial.” 8 In this extension analysis, we assessed the patients who corrected their platelet count within 3 weeks, resumed chemotherapy, and remained on romiplostim for 12 months or longer. We assessed the maintenance of adequate platelet counts and avoidance of episodes of CIT leading to delay or dose reduction of chemotherapy. Safety was assessed by tracking thrombosis and development of secondary hematologic malignancy.
2. METHODS
This is a long‐term efficacy and safety analysis of subjects in a previously published phase 2 randomized clinical trial (ClinicalTrials.gov identifier: NCT02052882). 8 The study was conducted at Memorial Sloan Kettering Cancer Center (MSK) and approved by the Institutional Review Board/Privacy Board. Written informed consent was obtained from all participants. The study was performed in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. The MSK Data and Safety Monitoring Committee provided oversight of the study.
Of 60 enrolled subjects in the phase 2 trial, 59 received romiplostim either as up‐front therapy or in the crossover design. Forty‐four participants (75%) achieved platelet correction within 3 weeks of initiation of romiplostim treatment, either in romiplostim up front or crossover and resumed chemotherapy with continuation of weekly romiplostim. Patients remained on romiplostim for as long as both the oncologist and the participant felt it was beneficial. This analysis is of the 21 subjects meeting eligibility who were treated for ≥1 year (between May 15, 2014, and December 22, 2020). Data were collected from 1 month before initiation of romiplostim to 1 month after the last dose of the drug.
The extracted data included complete blood count, chemotherapy doses and dates, romiplostim doses and dates, body weight, cancer diagnosis, age, sex, date of death, and thrombotic events. The mean romiplostim doses and lab values are calculated by month of study participation.
The chemotherapy participants received during the study was extracted from the electronic medical record (EMR). This includes cytotoxic chemotherapy as well as antineoplastic agents with thrombocytopenia as a known toxicity. Biological agents without thrombocytopenia as a known toxicity were excluded. Details of outpatient oral chemotherapy agents (ie, capecitabine) could not be reliably captured from the EMR and therefore were excluded from this analysis. If a dose of chemotherapy was delayed by ≥7 days or dose reduced by >20% in the presence of a platelet count <100 × 109/L at the time of scheduled chemotherapy, it was designated CIT. We did not differentiate thrombocytopenia in the presence of other cytopenia or other adverse events. If delay or dose reduction of chemotherapy occurred in the setting of platelet count ≥100 × 109/L, we did not adjudicate the reason for the delay or dose reduction of chemotherapy.
Thrombotic events that occurred during the study and up to 1 month after the last romiplostim dose were captured in the EMR, by extracting all contrast imaging studies and vascular Doppler ultrasound reports. The reports were then reviewed individually by two of the study investigators.
3. RESULTS AND DISCUSSION
Thirty‐eight patients from the phase 2 study of romiplostim corrected their platelet counts and tolerated 2 cycles or 8 weeks of chemotherapy with maintenance romiplostim. 8 Of these patients, 17 discontinued romiplostim before 12 months for the following reasons: death or transfer to hospice (n = 4), discontinuation of chemotherapy for reasons unrelated to CIT (n = 10), enrollment on a new chemotherapy clinical trial (n = 1), transfer of care to outside our institution (n = 1), and poor compliance (n = 1). None of these 17 patients discontinued romiplostim due to romiplostim resistance or side effects. One patient experienced a calf vein thrombosis but remained in the study.
This report focuses on the 21 patients on romiplostim for ≥12 months. Demographics of the 21 participants who received romiplostim for ≥12 months are listed in Table 1. The most common cancers were colorectal and breast. All 21 subjects had metastatic disease. Median age was 53 years at enrollment, with 12 men and 9 women. Chemotherapeutic agents and number of patients receiving each agent are listed in Table 2.
TABLE 1.
Characteristics of patients on romiplostim for ≥12 months
| Age, y | |
| Median | 53 |
| Range | 32‐78 |
| Sex | |
| Male | 12 |
| Female | 9 |
| Race, n | |
| White | 18 |
| Asian | 1 |
| Patient refused to answer | 1 |
| Other | 1 |
| Ethnicity, n | |
| Non‐Hispanic | 21 |
| Cancer diagnosis, n | |
| Breast | 6 |
| Colorectal | 6 |
| Ovarian | 2 |
| Pancreatic | 2 |
| Other a | 5 |
| Cancer stage, n | |
| Metastatic | 21 |
| Months in study | |
| 12‐17 | 7 |
| 18‐23 | 2 |
| 24‐29 | 7 |
| 30‐35 | 2 |
| 36‐41 | 2 |
| 42‐47 | 0 |
| 48‐53 | 1 |
Other cancer diagnoses include cholangiocarcinoma, gastroesophageal junction, liver and pancreatic, lung, mesothelioma, and sarcoma.
TABLE 2.
Chemotherapy exposure in patients on long‐term romiplostim
| Chemotherapy drug a | Number of patients |
|---|---|
| Fluorouracil | 9 |
| Irinotecan | 7 |
| Carboplatin | 6 |
| Gemcitabine | 6 |
| Oxaliplatin | 6 |
| Paclitaxel | 4 |
| Doxorubicin | 3 |
| Pemetrexed | 3 |
| Ado‐trastuzumab emtansine | 2 |
| Cisplatin | 2 |
| Floxuridine | 2 |
| Vinorelbine | 1 |
| Dacarbazine | 1 |
| Docetaxel | 1 |
| Eribulin | 1 |
| Mitomycin | 1 |
| Tipiracil‐trifluridine | 1 |
| Unknown cytotoxic b | 1 |
Excludes oral chemotherapy or investigational drugs. Also excludes biologicals without a known association with thrombocytopenia.
One patient received chemotherapy at an outside hospital, and details of chemotherapy administration was not available.
No patient discontinued romiplostim therapy due to an adverse event or due to futility. Twelve patients received romiplostim for ≥24 months, and three patients received romiplostim for >3 years. Two patients remained on romiplostim at the time of the data lock and are now on a commercial romiplostim product. At the preparation of this article, the longest exposure was 53 months.
Of the 21 participants, we have treatment records on 20. One participant, a 53‐year‐old man with metastatic pancreatic cancer, received romiplostim at our center but chemotherapy at an outside facility. We were unable to obtain details of chemotherapy treatment. However, during 29 months in the study, with weekly platelet counts and romiplostim, he never had a platelet count <100 × 109/L; therefore, we can reasonably assume he did not experience recurrent CIT. Fourteen of the 20 analyzable participants (70%) experienced no episodes of CIT leading to dose reduction or delay in chemotherapy for the duration of study participation (Table 3). Four participants (one each of breast, gastroesophageal junction, rectal, cholangiocarcinoma) experienced a single episode of CIT leading to chemotherapy dose delay, which were successfully managed with increasing the dose of romiplostim. These participants were able to maintain full‐dose chemotherapy with titrated romiplostim without further episodes of CIT. Two participants (colorectal and pancreas) had dose reduction of chemotherapy due to CIT. Chemotherapy was maintained at the reduced dose, without recurrent CIT or need for additional chemotherapy dose reduction.
TABLE 3.
Details of patients who experienced one or more episodes of reduced chemotherapy RDI in the setting of CIT
| Study number | Cancer type | Age, (Year) | Total number of doses of romiplostim | Doses of romiplostim at time of reduced RDI | Number of total chemo doses a | Number of doses impacted by CIT b | Chemotherapy associated with RDI |
|---|---|---|---|---|---|---|---|
| 2 | Breast | 47 | 200 | 37 | 22 | 1 | Gemcitabine |
| 12 | Rectal | 29 | 129 | 91 | 140 | 1 | Fluorouracil |
| 19 | Colorectal | 41 | 110 | 51 | 111 | 10 c | Fluorouracil |
| 26 | Pancreatic | 74 | 47 | 14 | 61 | 8 c |
Fluorouracil Gemcitabine |
| 50 | GE Junction | 75 | 93 | 6 | 12 | 1 | Carboplatin |
| 53 | Cholangiocarcinoma | 40 | 61 | 49 | 46 | 1 | Carboplatin |
Abbreviations: CIT, chemotherapy‐induced thrombocytopenia; RDI, relative dose intensity.
Does not include oral or investigational agents.
Includes one patient who had CIT only in the context of pancytopenia.
Subsequently remained on reduced dose.
Of the 20 patients in the extension cohort for whom chemotherapy records are complete, 12 experienced at least one change in the chemotherapy regime. The mean time from initiation of romiplostim until the first change in chemotherapy was 349 (range, 74‐583) days. None of the chemotherapy changes were due to CIT.
The mean monthly platelet counts, depicted in Figure 1, remained within the target range throughout the period of analysis. At 36 months and beyond, only three participants were receiving romiplostim, accounting for the greater variability of platelet values and mean monthly romiplostim doses. The mean romiplostim doses were in the range of 3 to 5 μg/kg through 35 months. The dose of romiplostim was titrated on the basis of the platelet count while the patients were on chemotherapy. Therefore, if platelet counts were preserved without romiplostim, the dose would gradually have been reduced to 0 μg/kg. This was not observed. There was a nonsignificant increase in mean romiplostim dose in months 24 to 35 compared with the first 24 months. There were insufficient participants at month 36 and beyond to allow for reliable interpretation of platelet counts and mean romiplostim doses.
FIGURE 1.
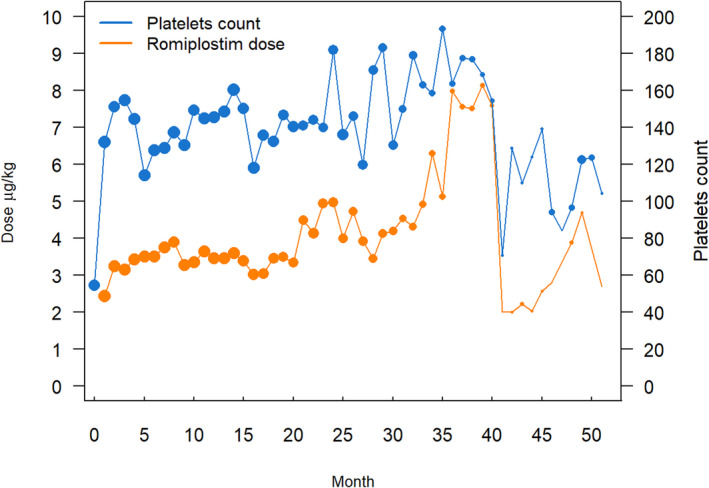
Mean monthly romiplostim dose and mean monthly platelet counts while in the study. The size of the data point on the graph reflects the number of values. Twenty‐two patients were in the study at 12 months, 12 patients at 24 months, and only 3 patients at 36 months or longer
Hemoglobin (Hgb) levels and absolute neutrophil counts (ANCs) were analyzed to assess for potential adverse impact of long‐term use of romiplostim on other hematopoietic lineages (Figure 2). Both Hgb and ANC remained stable through month 35. As with romiplostim dose and platelet counts, the variability beyond 35 months reflects the small number of participants and lab values.
FIGURE 2.
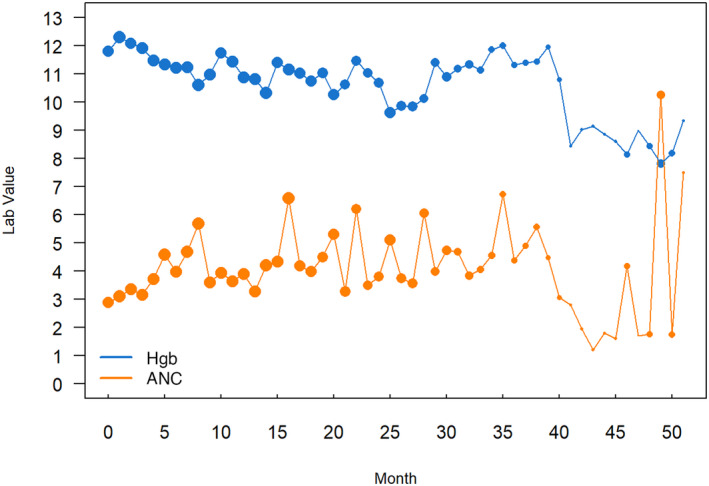
Mean monthly Hgb levels (gm/dL) and absolute neutrophil counts (×109/L) while in the study. ANC, absolute neutrophil count; Hgb, hemoglobin
There was no evidence of marrow fibrosis, based on stable platelet counts, Hgb, ANC, and absence of leukoerythroblastosis in any patient. However, surveillance bone marrow biopsies were not performed unless clinically indicated. Finally, no participant developed a secondary malignancy.
We assessed the occurrence of venous thromboembolism (VTE) as well as arterial/ischemic events. One participant developed a deep vein thrombosis (DVT)—a 57‐year‐old man with metastatic colon cancer who developed a proximal lower‐extremity DVT in the 12th month of romiplostim therapy. His platelet count was 146 × 109/L at the time. He received anticoagulation with enoxaparin and remained in the study. No patient experienced a pulmonary embolism. One participant experienced a superficial thrombus in the context of central line placement and cellulitis.
One participant of note experienced multiple ischemic thrombotic events. At the time of enrollment, she was 51 years old with widely metastatic non–small cell lung cancer. Before enrollment, she was known to be heterozygous for prothrombin G20210A with a remote history of DVT. She is a never smoker. During the study, she experienced multiple tumor‐related vascular ischemic events. At 5 months, splenic and renal infarcts of indeterminate age were incidentally noted on routine imaging. She had liver metastases at the time. She also demonstrated two separate instances of cerebral infarction in the context of brain metastases. Her tumor had wild‐type KRAS, but exhibited ALK‐EML4 fusion and MET amplification, both associated with a significant increase in VTE rates. 20 , 21 Her platelet count was 23 × 109/L at the time of the splenic and renal infarct and 210 × 109/L and 21 × 109/L at the time of the two cerebral vascular events. The subject and her oncologist decided to continue romiplostim, as her cancer was responding well to ongoing chemotherapy, and without romiplostim, thrombocytopenia precluded her from receiving optimal anticoagulation. Despite her recurrent thrombotic events, the patient is one of four participants alive, on romiplostim for 45 months, at the time of preparation of this article.
VTE 22 , 23 and arterial thrombosis 24 are well‐known complications of metastatic cancer as well as chemotherapy. Event rates are a function of cancer type, metastatic status, chemotherapy, clinical parameters within the Khorana Score, and comorbidities. 22 , 23 One subject was diagnosed with a DVT, and one subject experienced multiple arterial events, which is within expected rates of thrombosis in patients with advanced malignancy. For example, Khorana and colleagues reported that 12.6% of patients in a solid tumor cohort experienced a VTE within 12 months. 25 But we acknowledge that there is some concern that TPO‐RAs may themselves increase the risk of thrombosis. 26 None of the patients received prophylactic anticoagulation.
We are unable to assess a potential impact on overall survival or cancer progression. However, of the 44 patients in the phase 2 study who resumed chemotherapy with continuation of weekly romiplostim, 21 (48%) were alive at 12 months and 12 (27%) were alive at 24 months, which is a reassuring signal for this heavily pretreated population with metastatic cancer.
In this analysis of long‐term romiplostim use in CIT, we confirmed the drug’s long‐term efficacy and safety. We found sustained maintenance of platelet counts with a minimal burden of recurrent CIT. In addition, no participants developed resistance to romiplostim, clinical evidence of marrow fibrosis, or secondary hematologic malignancy. Finally, the rates of thrombosis were not higher than published rates in this patient population.
Our previously published study demonstrated a high rate of response to romiplostim in patients with solid tumors with CIT. In this study, our findings suggest that if patients do respond, they are likely to respond long term with little risk related to the therapy. This supportive care option may allow for continued cancer therapy in situations where CIT is otherwise limiting.
There are some limitations to our analysis. Our institution’s EMR documents all relevant details of parenteral chemotherapy administered in our infusion clinics. However, the details on oral or investigational chemotherapy could not be reliably extracted from our EMR; therefore, data collected were deemed incomplete and excluded from our analysis. This analysis also is limited to the patients who were eligible for the initial phase 2 trial and corrected their platelet count within 3 weeks. 8 Within these limitations, the extension study provides reassurance for long‐term efficacy and safety of romiplostim treatment for CIT.
RELATIONSHIP DISCLOSURE
None of the authors have any conflicts of interest to report.
AUTHOR CONTRIBUTIONS
GAS contributed the concept and oversaw all aspects of the study and manuscript preparation. SY was primarily responsible for biostatistics and data analysis. CRW, JO, and LG were primarily responsible for data extraction. CRW, JO, LG, and GAS wrote the first draft of the manuscript. All authors contributed to design, analysis, writing, and review of the final version of the manuscript.
ACKNOWLEDGMENTS
This study was supported by Amgen as an Investigator Initiated Study. Amgen had no role in the design, conduct, analysis, or reporting of the study. Support was also from the National Institutes of Health/National Cancer Institute Cancer Center Support Grant No. P30 CA008748.
Wilkins CR, Ortiz J, Gilbert LJ, et al. Romiplostim for chemotherapy‐induced thrombocytopenia: Efficacy and safety of extended use. Res Pract Thromb Haemost. 2022;6:e12701. doi: 10.1002/rth2.12701
Handling Editor: Dr Michelle Sholzberg
REFERENCES
- 1. Al‐Samkari H, Soff GA. Clinical challenges and promising therapies for chemotherapy‐induced thrombocytopenia. Expert Rev Hematol. 2021;14(5):437‐448. doi: 10.1080/17474086.2021.1924053 [DOI] [PubMed] [Google Scholar]
- 2. Leader A, Hofstetter L, Spectre G. Challenges and advances in managing thrombocytopenic cancer patients. J Clin Med. 2021;10(6):1169. doi: 10.3390/jcm10061169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al‐Samkari H, Grace RF, Kuter DJ. The role of romiplostim for pediatric patients with immune thrombocytopenia. Ther Adv Hematol. 2020;11:2040620720912992. doi: 10.1177/2040620720912992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Denduluri N, Patt DA, Wang Y, et al. Dose delays, dose reductions, and relative dose intensity in patients with cancer who received adjuvant or neoadjuvant chemotherapy in community oncology practices. J Natl Compr Canc Netw. 2015;13:1383‐1393. doi: 10.6004/jnccn.2015.0166 [DOI] [PubMed] [Google Scholar]
- 5. Elting LS, Rubenstein EB, Martin CG, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy‐induced thrombocytopenia. J Clin Oncol. 2001;19:1137‐1146. doi: 10.1200/JCO.2001.19.4.1137 [DOI] [PubMed] [Google Scholar]
- 6. Havrilesky LJ, Reiner M, Morrow PK, Watson H, Crawford J. A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit Rev Oncol Hematol. 2015;93:203‐210. doi: 10.1016/j.critrevonc.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 7. Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncology. 2015;29:282‐294. [PubMed] [Google Scholar]
- 8. Soff GA, Miao Y, Bendheim G, et al. Romiplostim treatment of chemotherapy‐induced thrombocytopenia. J Clin Oncol. 2019;37:2892‐2898. doi: 10.1200/JCO.18.01931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al‐Samkari H, Nagalla S. Efficacy and safety evaluation of avatrombopag in immune thrombocytopenia: analyses of a phase III study and long‐term extension. Platelets. 2022;33(2):257‐264. doi: 10.1080/09537104.2021.1881952 [DOI] [PubMed] [Google Scholar]
- 10. Becker PS, Griffiths EA, Alwan LM, et al. NCCN guidelines insights: hematopoietic growth factors, version 1.2020. J Natl Compr Canc Netw. 2020;18:12‐22. doi: 10.6004/jnccn.2020.0002 [DOI] [PubMed] [Google Scholar]
- 11. Kuter DJ. The biology of thrombopoietin and thrombopoietin receptor agonists. Int J Hematol. 2013;98:10‐23. doi: 10.1007/s12185-013-1382-0 [DOI] [PubMed] [Google Scholar]
- 12. Afdhal NH, Dusheiko GM, Giannini EG, et al. Eltrombopag increases platelet numbers in thrombocytopenic patients with HCV infection and cirrhosis, allowing for effective antiviral therapy. Gastroenterology. 2014;146:442‐452.e1. doi: 10.1053/j.gastro.2013.10.012 [DOI] [PubMed] [Google Scholar]
- 13. Al‐Samkari H, Marshall AL, Goodarzi K, Kuter DJ. The use of romiplostim in treating chemotherapy‐induced thrombocytopenia in patients with solid tumors. Haematologica. 2018;103:e169‐e172. doi: 10.3324/haematol.2017.180166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheloff AZ, Al‐Samkari H. Avatrombopag for the treatment of immune thrombocytopenia and thrombocytopenia of chronic liver disease. J Blood Med. 2019;10:313‐321. doi: 10.2147/JBM.S191790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagrebetsky A, Al‐Samkari H, Davis NM, Kuter DJ, Wiener‐Kronish JP. Perioperative thrombocytopenia: evidence, evaluation, and emerging therapies. Br J Anaesth. 2019;122:19‐31. doi: 10.1016/j.bja.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 16. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376:1540‐1550. doi: 10.1056/NEJMoa1613878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al‐Samkari H, Kuter DJ. Optimal use of thrombopoietin receptor agonists in immune thrombocytopenia. Ther Adv Hematol. 2019;10:2040620719841735. doi: 10.1177/2040620719841735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al‐Samkari H, Parnes AD, Goodarzi K, Weitzman JI, Connors JM, Kuter DJ. A multicenter study of romiplostim for chemotherapy‐induced thrombocytopenia in solid tumors and hematologic malignancies. Haematologica. 2021;106(4):1148‐1157. doi: 10.3324/haematol.2020.251900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Comprehensive Cancer Network . Hematopoietic Growth Factors. NCCN Guidelines Version 1.2022. 2022. https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf
- 20. Dunbar A, Bolton KL, Devlin SM, et al. Genomic profiling identifies somatic mutations predicting thromboembolic risk in patients with solid tumors. Blood. 2021;137:2103‐2113. doi: 10.1182/blood.2020007488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu VW, Zhao JJ, Gao Y, et al. Thromboembolism in ALK+ and ROS1+ NSCLC patients: a systematic review and meta‐analysis. Lung Cancer. 2021;157:147‐155. doi: 10.1016/j.lungcan.2021.05.019 [DOI] [PubMed] [Google Scholar]
- 22. Connors JM. Prophylaxis against venous thromboembolism in ambulatory patients with cancer. N Engl J Med. 2014;370:2515‐2519. doi: 10.1056/NEJMra1401468 [DOI] [PubMed] [Google Scholar]
- 23. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy‐associated thrombosis. Blood. 2008;111:4902‐4907. doi: 10.1182/blood-2007-10-116327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Navi BB, Sherman CP, Genova R, et al. Mechanisms of ischemic stroke in patients with cancer: a prospective study. Ann Neurol. 2021;90(1):159‐169. doi: 10.1002/ana.26129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high‐risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119:648‐655. doi: 10.1002/cncr.27772 [DOI] [PubMed] [Google Scholar]
- 26. Ghanima W, Cooper N, Rodeghiero F, Godeau B, Bussel JB. Thrombopoietin receptor agonists: ten years later. Haematologica. 2019;104:1112‐1123. doi: 10.3324/haematol.2018.212845 [DOI] [PMC free article] [PubMed] [Google Scholar]


